Abstract
Evidence has supported obesity as a risk factor for both benign prostate hyperplasia (BPH) and prostate cancer (PCa). Obesity causes several mechanisms including increased intra-abdominal pressure, altered endocrine status, increased sympathetic nervous activity, increased inflammation process, and oxidative stress, all of which are favorable in the development of BPH. In PCa, there are several different mechanisms, such as decreased serum testosterone, peripheral aromatization of androgens, insulin resistance, and altered adipokine secretion caused by inflammation, which may precipitate the development of and even cause high-grade PCa. The role of obesity in prostatitis still remains unclear. A greater understanding of the pathogenesis of prostate disease and adiposity could allow the development of new therapeutic markers, prognostic indicators, and drug targets. This review was made to help better understanding of the association between central obesity and prostate diseases, such as prostatitis, BPH, and PCa.
Keywords: Benign Prostate Hyperplasia, Obesity, Prostate Cancer, Prostatitis
1. Introduction
Obesity has risen alarmingly within the past few years, with an incidence of approximately one case in every three adults in the USA, compared with one in six adults 2 decades ago.1 Global prevalence of obesity in men in Europe and Asia ranges from 4% to 30%.2, 3, 4, 5, 6 According to the Riset Kesehatan Dasar7 (Indonesia) in 2007, the prevalence of obesity in populations above 15 years of age was 10.3%. This number has grown approximately 20% within less than 5 years.8 This geographic pattern can be explained, at least in part, by different socioeconomic conditions, as well as by lifestyle (sedentary lifestyle and lack of physical activity) and nutritional factors (western diet).
It is well known that obesity is associated with an increased risk of heart disease and diabetes. Most of these excess deaths were attributed to coronary artery disease, diabetes, and kidney disease. There are no clear indications that the obesity prevalence is returning back to healthier levels.9 Recent studies have also looked into its relationship with prostate disease. 10, 11, 12 The aim of this study is to evaluate the association between central obesity and prostate disease [prostatitis, benign prostate hyperplasia (BPH), and prostate cancer (PCa)].
2. Definition of obesity
The World Health Organization and The National Institutes of Health define overweight as a body mass index (BMI) of greater than 25 kg/m2, and obesity as BMI of greater than 30 kg/m2, whilst in Indonesia, according to the Riset Kesehatan Dasar7 by the Health Ministry in 2010, overweight is defined as a BMI of between 25 kg/m2 and 27 kg/m2and obesity is more than 27.1 kg/m2.8 Though easy to determine, BMI has its limitations, such as the inability to both differentiate between fat and muscle, and measure fat distribution in the body (since fat in different regions may contribute different functions), especially in elderly individuals.13, 14, 15
Central obesity is known to be more dangerous than general obesity because it is able to produce hormonal and systemic modifications leading to inflammation. Waist circumference (WC) in determining visceral adiposity has been used in various studies as an alternative measurement and definition of central obesity.16 WC can be measured either at the narrowest torso circumference or at the midpoint between the lower ribs and iliac crest.17 This relatively practical application has led to the wide use of WC in many studies as a tool to measure central obesity.18, 19, 20 The measurement of WC, which represents abdominal adipose tissue, has been reported to increase at a faster rate than BMI, which counts the body's entire weight including both adipose tissue and muscle mass. In addition, the adverse health consequences of obesity may be underestimated by trends of BMI.21, 22 Central obesity can be diagnosed if WC is larger than 90 cm in men and 80 cm in women.23
There is evidence linking the amount of adipose tissue to the level of both acute and chronic inflammation.24, 25, 26, 27, 28, 29, 30 However, there are certain types of adipose tissue, which vary from different adipose tissue location and composition, that are strongly correlated with inflammation.6, 19, 21, 22, 24, 31, 32 Adipose tissue is divided into two categories: visceral (abdominal/central) adipose tissue (VAT) and subcutaneous (peripheral) adipose tissue (SAT).25 VAT has a distinct characteristic in terms of function and inflammation compared to SAT. VAT is mainly composed of omental, mesenteric fat, and retroperitoneal fat masses by delineation along the dorsal borderline of the intestines and the ventral surface of the kidney.30 Adipocytes from VAT appear to have reduced capacity for lipogenesis and greater capacity for lipolysis than SAT cells, with VAT containing more proinflammatory and angiogenic cytokines than SAT.21 Fox et al24 demonstrated that the VAT compartment is metabolically active, secreting such vasoactive substances as inflammatory markers and adipocytokines, which may contribute to its role in the inflammation process. Alterations in plasma lipoprotein levels, particularly increased triglyceride and decreased high density lipoprotein levels, has also been associated with VAT distribution.30 Because obesity often associates with a sedentary lifestyle, and physical activity decreases the risk of prostate problems, this could suggest novel prevention and treatment strategies through weight loss and lifestyle changes.33
3. Obesity and prostatitis
Prostatitis is a common but poorly defined condition, without clear diagnostic criteria and treatment approaches.34 Age, race, and geographic region have been identified by several studies to be a significant risk factor for chronic prostatitis.35, 36, 37 Prostatitis is found in approximately 10–14% of men of all ages and racial origin and nearly 50% of men at some point in their life encounter this condition.5, 19, 37, 38, 39, 40, 41, 42
There is currently no evidence about the relationship between obesity and prostatitis. Although it still remains unclear, there are few studies reviewing this relationship between obesity and prostatitis using the BMI parameter. Wallner et al12 showed that increased BMI has a protective effect on prostatitis risk after adjustment for age.12 The odds of having a history of prostatitis were decreased by approximately 68% in obese men (BMI > 30 kg/m2) when compared with men with a BMI of 25 kg/m2. This result is likely due to the influence of physical activity on this relationship. This study revealed that the odds of developing prostatitis in men who were vigorously physically active were 67%. In the same study, a larger percentage (57.8%) of the men characterized as obese were found to engage in vigorous exercise compared with overweight (4.8%) and normal weight men (22.4%). Unfortunately, this particular study did not state the type of exercise performed by their participants. Studies regarding this subject are paramount in determining the risk of prostatitis in people with obesity. Thus many studies relating obesity and prostatitis are still needed and might be enlightening.
4. Obesity and BPH
BPH is the most common nonmalignant medical condition of the prostate occurring in middle aged and older men.43 BPH is a histological diagnosis associated with unregulated proliferation of connective tissue, smooth muscle, and glandular epithelium within the prostatic transition zone. Cellular proliferation leads to increased prostate volume and increased stromal smooth muscle tone.44 According to a recent epidemiological study, BPH affects 70% of men aged 60–69 years old and 80% of those ≥70 years old.42
Many studies have shown that obese men have a higher risk for BPH, as shown in Table 1.19, 33, 45, 46, 47, 48, 49, 51 Even though there are many standards, according to patients' characteristics (e.g., race) in categorizing patients' BMI and WC to diagnose obesity, all studies have resulted in the same conclusion that obesity, central or general, is a risk factor for BPH and worsened urinary symptoms. Increased WC was significantly and positively associated with prostate volume, serum prostate-specific antigen, and International Prostate Symptom Score, and therefore worsened the patients lower urinary tract symptoms.19
Table 1.
The Risk of Benign Prostate Hyperplasia Relating to Obesity in Various Studies Based on Obesity Status (Body Mass Index and Waist Circumference).
| Study reference | Population studied (n) | Control (n) | Findings |
|---|---|---|---|
| Lee et al19 |
|
|
Higher WC related to worse BPH symptoms (OR = 1.68, P = 0.002) |
| Giovannucci et al45 |
|
|
Abdominal obesity associated with BPH (OR = 2.38, 95% CI 1.42–3.99 |
| Wang et al48 |
|
|
The only independent risk factor of BPH is abdominal overweight/obesity (OR 2.112, 95% CI 1.284–3.47, P = 0.003) |
| Rohrmann et al51 |
|
|
Higher WC was more likely to have LUTS (OR = 1.48, 95% CI 0.87–2.54). |
| Parsons et al33 |
|
|
Odds ratio for BPH
|
| Xie et al49 |
|
|
|
| Lee et al46 |
|
|
Higher BMI (≥ 25 kg/m2) and central obesity were at significantly increased risk of BPH (OR = 4.88, P = 0.008) |
| Penson et al47 |
|
|
Worsened BPH symptoms were significantly associated with a BMI of ≥ 35 kg/m2 (OR 1.38, 95% CI 1.17–1.63). |
BMI, body mass index; BPH, benign prostate hyperplasia; CI, confidence interval; LUTS, lower urinary tract symptoms; OR, odds ratio; WC, waist circumference.
There are many hypotheses that have been suggested for the effect of obesity on BPH. Central obesity exerts several systemic effects. Obesity will increase intra-abdominal pressure, which in turn increases bladder pressure and intravesical pressure, with the potential to exacerbate and cause worsened BPH symptoms.52 Another mechanism is altered endocrine status. Increased estrogen to androgen ratio due to the enzyme P450 aromatase expressed by fat tissue.53 Therefore, an adipose tissue mass will increase the aromatase activity and the conversion of the androgens to estrogens (testosterone to estradiol and androstenedione to estrone). Increased fat mass and aromatase activity reduces the testosterone concentrations and allows for the preferential deposition of abdominal adipose tissue/VAT as the positive caloric balance which results in a hypogonadal obesity cycle. Continued production of estradiol caused by fat mass accumulation may result in gonadotropin suppression, with a further reduction in the testosterone levels and the development of a progressive hypogonadal state thus favorable in the development of BPH.54 Increased sympathetic nervous activity in central obesity has been known to influence the development of BPH and the severity of urinary obstructive symptoms.38, 45 However, difficulties in measuring sympathetic nervous activity and heterogeneity in characterizing obesity may cause lack of a conclusive relationship between sympathetic nervous activity and obesity.45
Another proposed hypothesis is the inflammation process and oxidative stress.29, 55, 56, 57 Central obesity promotes microvascular disease and inflammation, which in turn contributes to ischemia, oxidative stress, and an intraprostatic environment favorable to BPH.52 Changes in regulation of programed cell death may lead to hyperplastic and precancerous transformation.40 In patients with central obesity, there is evidence of increased chronic inflammation cause by the secretion of inflammation cytokines by the visceral fat.50, 58 Inflammation is a very intricate phenomenon involving humoral (cytokines) and cellular (leucocyte, monocyte, and macrophages) elements.57 In normal mechanisms, there is a balance between proinflammatory (growth factor release and angiogenesis) and anti-inflammatory (decrease of those processes) processes, leading to inflammation resolution.59 However, in chronic inflammation, mainly consisting of chronically activated T cells and mononuclear phagocytes, there is persistence of proinflammatory factors causing disturbances in the inflammation process. This will further promote the inflammation process by releasing more progrowth cytokines as well as various other growth factors.59 T cells influence matrix formation and potential epithelial secretions. They then promote prostate stromal and epithelial proliferation/hyperplasia by inducing fibromuscular growth and by an autocrine or paracrine loop.40, 50 The amount of prostate enlargement corresponds to the extent and severity of the inflammation.39
There are several inflammation processes associated with BPH development in obese patients, such as toll-like receptor (TLR), cyclooxygenase-2 (COX-2), and macrophage inhibitory cytokine-1 (MIC-1). TLR is a transmembrane receptor responsible for the initiation of a range of host-defense mechanisms in response to microbial products28 and can be found in prostate tissue and acts as a proinflammatory cytokine. Poulain-Godefroy et al28 observed an increased expression of TLRs in VAT of approximately 1.35 to 1.4 fold compared with SAT. This expression in fat cells induces cytokine secretion, which triggers further inflammation.28
Chronic inflammation continuously produces COX-2, which modulates the production of angiogenic factors to induce angiogenesis, increases the carcinogenic potential of cells through the oxidation of procarcinogens to carcinogens, increases cell growth, and decreases apoptosis.40 Nitric oxide is one of the free radicals associated with prostatic inflammation. It also enhances COX activity, which has been detected in all inflammatory cells in the epithelium and interstitial spaces of human prostate tissue.56 Di Silverio et al39 and Hamid et al40 showed that, in human BPH tissue, COX-2 could produce a significant increase in prostate cell apoptotic inhibitory activity causing cell proliferation in the prostate. The relationship between COX-2 and central obesity in humans is still debatable; however, a study by Ghoshal et al27 have found that in mice, genetic deficiency of COX-2 resulted in a significant reduction in total body weight and percent body fat.
Induction of anti-inflammatory factors such as MIC-1 is an early response due to inflammation in the prostate.60 MIC-1 was down regulated in BPH tissues compared with normal prostate tissue.40 This may be caused by gland destruction by inflammatory infiltrates, followed by replacement of the stromal component in symptomatic BPH.41 However, Dostalova et al61 revealed that central obesity is associated with significantly higher serum MIC-1 levels, although the exact mechanism is still poorly understood. It is possible that expression of circulating MICs might be in a different concentration than prostatic MICs, especially in the transition zone (site of BPH).40
5. Obesity and PCa
PCa is the second most commonly diagnosed cancer and the sixth most common cause of cancer-related mortality among men worldwide.62 According to GLOBOCAN 2012,63 it was shown that PCa is the third most common cancer in men (after lung and colorectal cancer) and has around a 9.8% incidence and 8.9% mortality amongst all cancers in Indonesia.
Studies have reported the association between obesity and many cancers including PCa.64, 65, 66, 67 A detailed explanation from many studies is shown in Table 2.22, 68, 69, 70, 71, 72 Throughout many studies it is shown that obesity is a risk factor for the development of prostate cancer (odds ratio 1.097–2.47) and even the progression towards high grade disease (odds ratio 1.49–2.56).
Table 2.
The Risk of Prostate Cancer Relating to Obesity in Various Studies Based on Obesity Status (Body Mass Index and Waist Circumference).
| Study reference | Population studied (n) | Control (n) | Findings |
|---|---|---|---|
| De Nunzio et al22 |
|
|
Central adiposity was significantly associated with PCa (OR 1.66, CI 95% 1.05–2.63, P = 0.03) and high-grade disease (OR 2.56, CI 95% 1.38–4.76, P = 0.003) |
| Nemesure et al70 |
|
|
WC of ≥ 102 cm had an OR of 1.84 (95% CI 1.19–2.85) compared with those with WC of < 90 cm |
| Irani et al69 |
|
|
Obesity was significantly associated with PCa (OR 2.47, 95% CI 1.41–4.34) |
| De Nunzio et al68 |
|
|
Obesity was significantly associated with PCa (OR 1.097, 95% CI 1.029–1.171) |
| Rundle et al72 |
|
|
Obesity at the time of biopsy was associated with PCa incidence during follow-up (OR 1.57; 95% CI 1.07–2.30) |
| Park et al71 |
|
|
Obesity was significantly associated with a higher risk of detection on PCa in biopsy patients (OR = 1.446, P = 0.024) Obesity was significantly associated with a higher rate of high-grade PCa detected from the biopsy (OR = 1.498, P = 0.039) |
BMI, body mass index; CI, confidence interval; OR, odds ratio; PCa, prostate cancer; WC, waist circumference.
There are several mechanisms most commonly proposed to explain the association between obesity and PCa, which are the insulin/insulin-like growth factor (IGF)-1 axis, altered sex hormones (decreased serum testosterone and peripheral aromatization of androgen), and adipokine signaling caused by inflammation.62, 64 Insulin alone does not induce somatic cell mutations; however, it has anabolic, antiapoptotic, and, in supraphysiological concentrations, mitotic effects. Activation of insulin on the intracellular transduction pathway may affect the growth of cancer cells, especially when high concentrations of insulin are present, as occurs in obesity.64 Central obesity and hyperinsulinemia are associated with increased circulating amounts of bioactive IGF-1, a growth factor with a recognized pathogenic role in many cancers, which in turn elevate circulating growth factors. Gallagher and LeRoith73 confirmed that growth hormone receptor is upregulated by an increase of insulin concentration in portal circulation thus increasing growth hormone receptor signaling and hepatic IGF-1 synthesis, in hyperinsulinemia state, which induces neovascularization and prevents apoptosis.64
The androgens testosterone and dihydrotestosterone have been known to have an important role in the development of the prostate gland, PCa progression, and involvement in earlier stages of PCa development. Increased serum insulin levels inhibit hepatic sex hormone-binding globulin (SHBG) synthesis. SHBG, a carrier protein that specifically binds circulating testosterone and dihydrotestosterone and reduces their availability to tissues, correlates inversely to BMI. Total testosterone bioavailability, plus chemically bound/unavailable testosterone, also correlates inversely with BMI and insulin. This relationship can be explained by long-loop feedback inhibition of pituitary luteinizing hormone secretion by bioavailable (non-SHBG bound) testosterone, which leads to reduced testicular androgen synthesis. In obese men, total testosterone, SHBG, luteinizing hormone pulse amplitude, diurnal luteinizing hormone, and bioavailable testosterone are all reduced.74 Men with PCa who have low testosterone tend towards a more aggressive phenotype. However, the low T environment in obese men may be one plausible link between obesity and aggressive PCa, although the exact mechanisms remain unknown.62
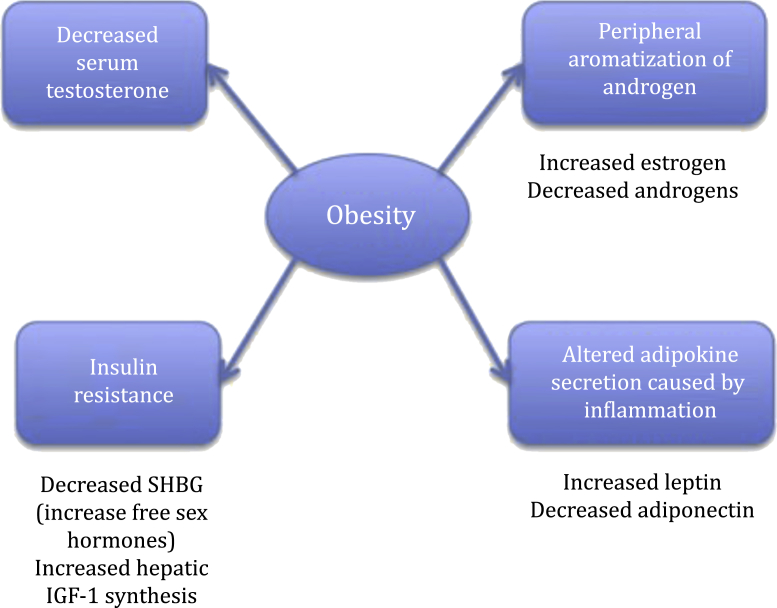
Prabhat et al31 concluded that central obesity had a higher correlation with hormonal and metabolic alterations compared with BMI, which may contribute to various mechanisms, such as chronic inflammation.31 Central adiposity, which was evaluated in European patients at risk of PCa, is a state of chronic subclinical inflammation associated with adipokine signaling such as higher levels of leptin, lower levels of adiponectin, which have, though controversially, been associated with PCa.22 Leptin, the adipocyte-derived hormone, is elevated in central obesity and exerts a predominantly protumor effect in human PCa cell lines, by promoting angiogenesis and increased sympathetic nervous system activity.75 In contrast to leptin, adiponectin has largely antitumor effects by inhibiting cancer cell growth, metastasis, inhibiting dehydrotestosterone, and it inhibits inflammation by inhibiting the activity of mature phagocytic macrophages; however, serum levels of adiponectin are reduced in central obesity.76 All of these mechanisms result in increased proliferation, decreased apoptosis, and transition from androgen dependence to androgen independence. The mechanism of how obesity might affect PCa is shown in Fig. 1.
Fig. 1.
Possible mechanisms for obesity-related prostate cancer progression. IGF-1, insulin-like growth factor-1, SHBG, sex hormone-binding globulin. Note. From “Obesity and prostate cancer: a role for adipokines” by T. Mistry, J.E. Digby, K.M. Desai, H.S. Randeva, 2007, European Urology, 52, p. 46–53. Copyright 2007, Elsevier B.V. European Association of Urology. Adapted with permission.
It is thought that androgens might promote the initiation and progression of well-differentiated PCa, while protecting against poorly differentiated cancers.74 However, an increased risk for poorly differentiated PCa has been associated with hyperinsulinemia and leptin.22, 31, 58, 73, 75, 76 A recent study indicates that obese people have low testosterone levels, raised insulin and leptin, characteristics of adiposity, which increases the risk for poorly differentiated, androgen dependent PCa.77
6. Conclusion
Central obesity is one of the modifiable risk factors in relation to prostate diseases. Adipose tissue, favoring VAT, is an important risk factor for the development of BPH and PCa. Many mechanisms have been hypothesized to explain the correlation between obesity and risk of BPH and PCa, such as increased estrogen-to-androgen ratio and increased sympathetic nervous activity, promotion of inflammation process, which in turn contributes to ischemia, oxidative stress, and an intraprostatic environment favorable to BPH and PCa. A greater understanding of the pathogenesis of prostate disease and adiposity could allow the development of new therapeutic markers, prognostic indicators, and drug targets. It may also provide scientific evidence to promote weight loss and other lifestyle modifications as beneficial adjuvant therapies for prostate diseases.
Conflicts of interest
The authors declare that there is no conflict of interests regarding the publication of this paper.
References
- 1.Freedland S.J., Aronson W.J., Kane C.J., Presti J.C., Jr., Amling C.L., Elashoff D. Impact of obesity on biochemical control after radical prostatectomy for clinically localized prostate cancer: a report by the Shared Equal Access Regional Cancer Hospital database study group. J Clin Oncol. 2004;22:446–453. doi: 10.1200/JCO.2004.04.181. [DOI] [PubMed] [Google Scholar]
- 2.Mohamud W.N., Musa K.I., Khir A.S., Ismail A.A., Ismail I.S., Kadir K.A. Prevalence of overweight and obesity among adult Malaysians: an update. Asia Pac J Clin Nutr. 2011;20:35–41. [PubMed] [Google Scholar]
- 3.Obesity: preventing and managing the global epidemic. Report of a World Health Organization (WHO) consultation. WHO technical report series. 2000;894:i–xii. 1–253. [PubMed] [Google Scholar]
- 4.Jitnarin N., Kosulwat V., Rojroongwasinkul N., Boonpraderm A., Haddock C.K., Poston W.S. Prevalence of overweight and obesity in Thai population: results of the National Thai Food Consumption Survey. Eat Weight Disord. 2011;16:e242–e249. doi: 10.1007/BF03327467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Florentino R.F. The burden of obesity in Asia: Challenges in assessment, prevention and management. Asia Pac J Clin Nutr. 2002;11(suppl 8):S676–S680. [Google Scholar]
- 6.Deepa M., Farooq S., Deepa R., Manjula D., Mohan V. Prevalence and significance of generalized and central body obesity in an urban Asian Indian population in Chennai, India (CURES: 47) Eur J Clin Nutr. 2009;63:259–267. doi: 10.1038/sj.ejcn.1602920. [DOI] [PubMed] [Google Scholar]
- 7.Department of Health R.I. Badan Penelitian dan Pengembangan Kesehatan . Riset Kesehatan Dasar (RISKESDAS) Department of Health R.I.; Indonesia: 2007. [Google Scholar]
- 8.Department of Health R.I. Badan Penelitian dan Pengembangan Kesehatan . Riset Kesehatan Dasar (RISKESDAS) Department of Health R.I.; Indonesia: 2010. [Google Scholar]
- 9.Chamie K., Oberfoell S., Kwan L., Labo J., Wei J.T., Litwin M.S. Body mass index and prostate cancer severity: do obese men harbor more aggressive disease on prostate biopsy? Urology. 2013;81:949–955. doi: 10.1016/j.urology.2013.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hsing A.W., Sakoda L.C., Chua S., Jr. Obesity, metabolic syndrome, and prostate cancer. Am J Clin Nutr. 2007;86:s843–s857. doi: 10.1093/ajcn/86.3.843S. [DOI] [PubMed] [Google Scholar]
- 11.Kristal A.R., Arnold K.B., Schenk J.M., Neuhouser M.L., Weiss N., Goodman P. Race/ethnicity, obesity, health related behaviors and the risk of symptomatic benign prostatic hyperplasia: results from the prostate cancer prevention trial. J Urol. 2007;177:1395–1400. doi: 10.1016/j.juro.2006.11.065. [DOI] [PubMed] [Google Scholar]
- 12.Wallner L.P., Clemens J.Q., Sarma A.V. Prevalence of and risk factors for prostatitis in African American men: the Flint Men's Health Study. Prostate. 2009;69:24–32. doi: 10.1002/pros.20846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garn S.M., Leonard W.R., Hawthorne V.M. Three limitations of the body mass index. Am J Clin Nutr. 1986;44:996–997. doi: 10.1093/ajcn/44.6.996. [DOI] [PubMed] [Google Scholar]
- 14.McLaren D.S. Three limitations of the body mass index. Am J Clin Nutr. 1987;46:121. doi: 10.1093/ajcn/46.1.121. [DOI] [PubMed] [Google Scholar]
- 15.Micozzi M.S., Albanes D. Three limitations of the body mass index. Am J Clin Nutr. 1987;46:376–377. doi: 10.1093/ajcn/46.2.376. [DOI] [PubMed] [Google Scholar]
- 16.Pongchaiyakul C., Kosulwat V., Rojroongwasinkul N., Charoenkiatkul S., Thepsuthammarat K., Laopaiboon M. Prediction of percentage body fat in rural Thai population using simple anthropometric measurements. Obes Res. 2005;13:729–738. doi: 10.1038/oby.2005.82. [DOI] [PubMed] [Google Scholar]
- 17.Pischon T., Boeing H., Weikert S., Allen N., Key T., Johnsen N.F. Body size and risk of prostate cancer in the European prospective investigation into cancer and nutrition. Cancer Epidemiol Biomarkers Prev. 2008;17:3252–3261. doi: 10.1158/1055-9965.EPI-08-0609. [DOI] [PubMed] [Google Scholar]
- 18.Park J.H., Cho B.L., Kwon H.T., Lee C.M., Han H.J. Effect of body mass index and waist circumference on prostate specific antigen and prostate volume in a generally healthy Korean population. J Urol. 2009;182:106–110. doi: 10.1016/j.juro.2009.02.130. [DOI] [PubMed] [Google Scholar]
- 19.Lee R.K., Chung D., Chughtai B., Te A.E., Kaplan S.A. Central obesity as measured by waist circumference is predictive of severity of lower urinary tract symptoms. BJU Int. 2012;110:540–545. doi: 10.1111/j.1464-410X.2011.10819.x. [DOI] [PubMed] [Google Scholar]
- 20.Janssen I., Katzmarzyk P.T., Ross R. Waist circumference and not body mass index explains obesity-related health risk. Am J Clin Nutr. 2004;79:379–384. doi: 10.1093/ajcn/79.3.379. [DOI] [PubMed] [Google Scholar]
- 21.Wang H., Wang J., Liu M.M., Wang D., Liu Y.Q., Zhao Y. Epidemiology of general obesity, abdominal obesity and related risk factors in urban adults from 33 communities of Northeast China: the CHPSNE study. BMC Public Health. 2012;12:967. doi: 10.1186/1471-2458-12-967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.De Nunzio C., Albisinni S., Freedland S.J., Miano L., Cindolo L., Finazzi Agro E. Abdominal obesity as risk factor for prostate cancer diagnosis and high grade disease: a prospective multicenter Italian cohort study. Urol Oncol. 2013;31:997–1002. doi: 10.1016/j.urolonc.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 23.WHO/SEARO . World Health Organization; Asia: 2005. Surveillance of major noncommunicable diseases in South–East Asia Region, Report of an Inter-country Consultation. [Google Scholar]
- 24.Fox C.S., Massaro J.M., Hoffmann U., Pou K.M., Maurovich-Horvat P., Liu C.Y. Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham Heart Study. Circulation. 2007;116:39–48. doi: 10.1161/CIRCULATIONAHA.106.675355. [DOI] [PubMed] [Google Scholar]
- 25.Villaret A., Galitzky J., Decaunes P., Esteve D., Marques M.A., Sengenes C. Adipose tissue endothelial cells from obese human subjects: differences among depots in angiogenic, metabolic, and inflammatory gene expression and cellular senescence. Diabetes. 2010;59:2755–2763. doi: 10.2337/db10-0398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fantuzzi G. Adipose tissue, adipokines, and inflammation. J Allergy Clin Immunol. 2005;115:911–919. doi: 10.1016/j.jaci.2005.02.023. [DOI] [PubMed] [Google Scholar]
- 27.Ghoshal S., Trivedi D.B., Graf G.A., Loftin C.D. Cyclooxygenase-2 deficiency attenuates adipose tissue differentiation and inflammation in mice. J Biol Chem. 2011;286:889–898. doi: 10.1074/jbc.M110.139139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Poulain-Godefroy O., Le Bacquer O., Plancq P., Lecœur C., Pattou F., Frühbeck G. Inflammatory role of Toll-like receptors in human and murine adipose tissue. Mediators Inflamm. 2010;2010:823486. doi: 10.1155/2010/823486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mraz M., Haluzik M. The role of adipose tissue immune cells in obesity and low-grade inflammation. J Endocrinol. 2014;222:R113–R127. doi: 10.1530/JOE-14-0283. [DOI] [PubMed] [Google Scholar]
- 30.Wajchenberg B.L. Subcutaneous and visceral adipose tissue: their relation to the metabolic syndrome. Endocr Rev. 2000;21:697–738. doi: 10.1210/edrv.21.6.0415. [DOI] [PubMed] [Google Scholar]
- 31.Prabhat P., Tewari R., Natu S.M., Dalela D., Goel A., Tandon P. Is central obesity, hyperinsulinemia and dyslipidemia associated with high-grade prostate cancer? A descriptive cross-sectional study. Indian J Urol. 2010;26:502–506. doi: 10.4103/0970-1591.74440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xi B., Liang Y., He T., Reilly K.H., Hu Y., Wang Q. Secular trends in the prevalence of general and abdominal obesity among Chinese adults, 1993–2009. Obes Rev. 2012;13:287–296. doi: 10.1111/j.1467-789X.2011.00944.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Parsons J.K., Carter H.B., Partin A.W., Windham B.G., Metter E.J., Ferrucci L. Metabolic factors associated with benign prostatic hyperplasia. J Clin Endocrinol Metab. 2006;91:2562–2568. doi: 10.1210/jc.2005-2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pontari M.A., Joyce G.F., Wise M., McNaughton-Collins M. Urologic Diseases in America Project. Prostatitis. J Urol. 2007;177:2050–2057. doi: 10.1016/j.juro.2007.01.128. [DOI] [PubMed] [Google Scholar]
- 35.Collins M.M., Stafford R.S., O'Leary M.P., Barry M.J. Distinguishing chronic prostatitis and benign prostatic hyperplasia symptoms: results of a national survey of physician visits. Urology. 1999;53:921–925. doi: 10.1016/s0090-4295(98)00636-0. [DOI] [PubMed] [Google Scholar]
- 36.Collins M.M., Meigs J.B., Barry M.J., Walker Corkery E., Giovannucci E., Kawachi I. Prevalence and correlates of prostatitis in the health professionals follow-up study cohort. J Urol. 2002;167:1363–1366. [PubMed] [Google Scholar]
- 37.Cheah P.Y., Liong M.L., Yuen K.H., Teh C.L., Khor T., Yang J.R. Chronic prostatitis: symptom survey with follow-up clinical evaluation. Urology. 2003;61:60–64. doi: 10.1016/s0090-4295(02)02081-2. [DOI] [PubMed] [Google Scholar]
- 38.Caine M., Raz S., Zeigler M. Adrenergic and cholinergic receptors in the human prostate, prostatic capsule and bladder neck. Br J Urol. 1975;47:193–202. doi: 10.1111/j.1464-410x.1975.tb03947.x. [DOI] [PubMed] [Google Scholar]
- 39.Di Silverio F., Gentile V., De Matteis A., Mariotti G., Giuseppe V., Luigi P.A. Distribution of inflammation, pre-malignant lesions, incidental carcinoma in histologically confirmed benign prostatic hyperplasia: a retrospective analysis. Eur Urol. 2003;43:164–175. doi: 10.1016/s0302-2838(02)00548-1. [DOI] [PubMed] [Google Scholar]
- 40.Hamid A.R., Umbas R., Mochtar C.A. Recent role of inflammation in prostate diseases: chemoprevention development opportunity. Acta Med Indones. 2011;43:59–65. [PubMed] [Google Scholar]
- 41.Shigemura K., Arakawa S., Yamanaka K., Kataoka N., Yuien K., Fujisawa M. Significance of lateral biopsy specimens during transrectal ultrasound-guided prostate biopsies in Japanese men. Int J Urol. 2007;14:935–938. doi: 10.1111/j.1442-2042.2007.01865.x. [DOI] [PubMed] [Google Scholar]
- 42.Wei J.T., Calhoun E., Jacobsen S.J. Urologic diseases in America project: benign prostatic hyperplasia. J Urol. 2005;173:1256–1261. doi: 10.1097/01.ju.0000155709.37840.fe. [DOI] [PubMed] [Google Scholar]
- 43.Berry S.J., Coffey D.S., Walsh P.C., Ewing L.L. The development of human benign prostatic hyperplasia with age. J Urol. 1984;132:474–479. doi: 10.1016/s0022-5347(17)49698-4. [DOI] [PubMed] [Google Scholar]
- 44.Patel N.D., Parsons J.K. Epidemiology and etiology of benign prostatic hyperplasia and bladder outlet obstruction. Indian J Urol. 2014;30:170–176. doi: 10.4103/0970-1591.126900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Giovannucci E., Rimm E.B., Chute C.G., Kawachi I., Colditz G.A., Stampfer M.J. Obesity and benign prostatic hyperplasia. Am J Epidemiol. 1994;140:989–1002. doi: 10.1093/oxfordjournals.aje.a117206. [DOI] [PubMed] [Google Scholar]
- 46.Lee S., Min H.G., Choi S.H., Kim Y.J., Oh S.W., Kim Y.J. Central obesity as a risk factor for prostatic hyperplasia. Obesity. 2006;14:172–179. doi: 10.1038/oby.2006.21. [DOI] [PubMed] [Google Scholar]
- 47.Penson D.F., Munro H.M., Signorello L.B., Blot W.J., Fowke J.H., Urologic Diseases in America Project Obesity, physical activity and lower urinary tract symptoms: results from the Southern Community Cohort Study. J Urol. 2011;186:2316–2322. doi: 10.1016/j.juro.2011.07.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang H.H., Hsieh C.J., Lin K.J., Chu S.H., Chuang C.K., Chen H.W. Waist circumference is an independent risk factor for prostatic hyperplasia in Taiwanese males. Asian J Surg. 2011;34:163–167. doi: 10.1016/j.asjsur.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 49.Xie L.P., Bai Y., Zhang X.Z., Zheng X.Y., Yao K.S., Xu L. Obesity and benign prostatic enlargement: a large observational study in China. Urology. 2007;69:680–684. doi: 10.1016/j.urology.2006.12.030. [DOI] [PubMed] [Google Scholar]
- 50.Penna G., Fibbi B., Amuchastegui S., Cossetti C., Aquilano F., Laverny G. Human benign prostatic hyperplasia stromal cells as inducers and targets of chronic immuno-mediated inflammation. J Immunol. 2009;182:4056–4064. doi: 10.4049/jimmunol.0801875. [DOI] [PubMed] [Google Scholar]
- 51.Rohrmann S., Smit E., Giovannucci E., Platz E.A. Associations of obesity with lower urinary tract symptoms and noncancer prostate surgery in the Third National Health and Nutrition Examination Survey. Am J Epidemiol. 2004;159:390–397. doi: 10.1093/aje/kwh060. [DOI] [PubMed] [Google Scholar]
- 52.Wang S., Mao Q., Lin Y., Wu J., Wang X., Zheng X. Body mass index and risk of BPH: a meta-analysis. Prostate Cancer Prostatic Dis. 2012;15:265–272. doi: 10.1038/pcan.2011.65. [DOI] [PubMed] [Google Scholar]
- 53.Corona G., Vignozzi L., Rastrelli G., Lotti F., Cipriani S., Maggi M. Benign prostatic hyperplasia: a new metabolic disease of the aging male and its correlation with sexual dysfunction. J Endocrinol Invest. 2014;2014:329456. doi: 10.1155/2014/329456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Williams G. Aromatase up-regulation, insulin and raised intracellular oestrogens in men, induce adiposity, metabolic syndrome and prostate disease, via aberrant ER-alpha and GPER signalling. Mol Cell Endocrinol. 2012;351:269–278. doi: 10.1016/j.mce.2011.12.017. [DOI] [PubMed] [Google Scholar]
- 55.Furukawa S., Fujita T., Shimabukuro M., Iwaki M., Yamada Y., Nakajima Y. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest. 2004;114:1752–1761. doi: 10.1172/JCI21625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chughtai B., Lee R., Te A., Kaplan S. Role of inflammation in benign prostatic hyperplasia. Rev Urol. 2011;13:147–150. [PMC free article] [PubMed] [Google Scholar]
- 57.Sciarra A., Mariotti G., Salciccia S., Autran Gomez A., Monti S., Toscano V. Prostate growth and inflammation. J Steroid Biochem Mol Biol. 2008;108:254–260. doi: 10.1016/j.jsbmb.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 58.Hsieh C.J., Wang P.W., Chen T.Y. The relationship between regional abdominal fat distribution and both insulin resistance and subclinical chronic inflammation in non-diabetic adults. Diabetol Metab Syndr. 2014;6:49. doi: 10.1186/1758-5996-6-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sandhu J.S. Prostate cancer and chronic prostatitis. Curr Urol Rep. 2008;9:328–332. doi: 10.1007/s11934-008-0056-6. [DOI] [PubMed] [Google Scholar]
- 60.Karan D., Holzbeierlein J., Thrasher J.B. Macrophage inhibitory cytokine-1: possible bridge molecule of inflammation and prostate cancer. Cancer Res. 2009;69:2–5. doi: 10.1158/0008-5472.CAN-08-1230. [DOI] [PubMed] [Google Scholar]
- 61.Dostalova I., Roubicek T., Bartlova M., Mraz M., Lacinova Z., Haluzikova D. Increased serum concentrations of macrophage inhibitory cytokine-1 in patients with obesity and type 2 diabetes mellitus: the influence of very low calorie diet. Eur J Endocrinol. 2009;161:397–404. doi: 10.1530/EJE-09-0417. [DOI] [PubMed] [Google Scholar]
- 62.Allott E.H., Masko E.M., Freedland S.J. Obesity and prostate cancer: weighing the evidence. Eur Urol. 2013;63:800–809. doi: 10.1016/j.eururo.2012.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ferlay J., Soerjomataram I., Dikshit R., Eser S., Mathers C., Rebelo M. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;36:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 64.De Pergola G., Silvestris F. Obesity as a major risk factor for cancer. J Obes. 2013;2013:291546. doi: 10.1155/2013/291546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Song X., Pukkala E., Dyba T., Tuomilehto J., Moltchanov V., Mannisto S. Body mass index and cancer incidence: the FINRISK study. Eur J Epidemiol. 2014;29:477–487. doi: 10.1007/s10654-014-9934-z. [DOI] [PubMed] [Google Scholar]
- 66.Vucenik I., Stains J.P. Obesity and cancer risk: evidence, mechanisms, and recommendations. Ann N Y Acad Sc. 2012;1271:37–43. doi: 10.1111/j.1749-6632.2012.06750.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tewari R., Rajender S., Natu S.M., Dalela D., Goel A., Goel M.M. Diet, obesity, and prostate health: are we missing the link? J Androl. 2012;33:763–776. doi: 10.2164/jandrol.111.015578. [DOI] [PubMed] [Google Scholar]
- 68.De Nunzio C., Freedland S.J., Miano L., Finazzi Agro E., Banez L., Tubaro A. The uncertain relationship between obesity and prostate cancer: an Italian biopsy cohort analysis. Eur J Surg Oncol. 2011;37:1025–1029. doi: 10.1016/j.ejso.2011.09.036. [DOI] [PubMed] [Google Scholar]
- 69.Irani J., Lefebvre O., Murat F., Dahmani L., Dore B. Obesity in relation to prostate cancer risk: comparison with a population having benign prostatic hyperplasia. BJU Int. 2003;91:482–484. doi: 10.1046/j.1464-410x.2003.04133.x. [DOI] [PubMed] [Google Scholar]
- 70.Nemesure B., Wu S.Y., Hennis A., Leske M.C. Prostate Cancer in a Black Population Study Group. Central adiposity and prostate cancer in a black population. Cancer Epidemiol Biomarkers Prev. 2012;21:851–858. doi: 10.1158/1055-9965.EPI-12-0071. [DOI] [PubMed] [Google Scholar]
- 71.Park J., Cho S.Y., Lee S.B., Son H., Jeong H. Obesity is associated with higher risk of prostate cancer detection in a Korean biopsy population. BJU Int. 2014;114:891–895. doi: 10.1111/bju.12600. [DOI] [PubMed] [Google Scholar]
- 72.Rundle A., Jankowski M., Kryvenko O.N., Tang D., Rybicki B.A. Obesity and future prostate cancer risk among men after an initial benign biopsy of the prostate. Cancer Epidemiol Biomarkers Prev. 2013;22:898–904. doi: 10.1158/1055-9965.EPI-12-0965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gallagher E.J., LeRoith D. The proliferating role of insulin and insulin-like growth factors in cancer. Trends Endocrinol Metab. 2010;21:610–618. doi: 10.1016/j.tem.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kaaks R., Stattin P. Obesity, endogenous hormone metabolism, and prostate cancer risk: a conundrum of “highs” and “lows”. Cancer Prev Res. 2010;3:259–262. doi: 10.1158/1940-6207.CAPR-10-0014. [DOI] [PubMed] [Google Scholar]
- 75.Stattin P., Soderberg S., Hallmans G., Bylund A., Kaaks R., Stenman U.H. Leptin is associated with increased prostate cancer risk: a nested case-referent study. J Clin Endocrinol Metab. 2001;86:1341–1345. doi: 10.1210/jcem.86.3.7328. [DOI] [PubMed] [Google Scholar]
- 76.Izadi V., Farabad E., Azadbakht L. Serum adiponectin level and different kinds of cancer: a review of recent evidence. ISRN Oncol. 2012;2012:982769. doi: 10.5402/2012/982769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lu K., Song X.L., Han S.L., Wang C.H., Zhong N., Qi L.F. Potential study perspectives on mechanisms and correlations between adiposity and malignancy. Asian Pac J Cancer Prev. 2014;15:1057–1060. doi: 10.7314/apjcp.2014.15.2.1057. [DOI] [PubMed] [Google Scholar]