Abstract
Introduction
Metabolic control of phenylketonuria (PKU) and compliance with the low-phenylalanine (phe) diet are frequently assessed by measuring blood phe concentrations in dried blood spots (DBS) collected by patients instead of plasma phe concentrations.
Objective
Our objective was to investigate the difference in blood phe concentrations in DBS collected by subjects and analyzed using either a validated newborn screening tandem mass spectrometry (MS/MS) protocol or ion-exchange chromatography (IEC) compared to plasma phe concentrations obtained simultaneously and analyzed using IEC.
Design
Three to four fasting blood samples were obtained from 29 subjects with PKU, ages 15–49 years. Capillary blood was spotted on filter paper by each subject and the DBS analyzed using both MS/MS and IEC. Plasma was isolated from venous blood and analyzed using IEC.
Results
Blood phe concentrations in DBS analyzed using MS/MS are 28% ± 1% (n = 110, p < 0.0001) lower than plasma phe concentrations analyzed using IEC resulting in a blood phe concentration of 514 ± 23 μmol/L and a plasma phe concentration of 731 ± 32 μmol/L (mean ± SEM). This discrepancy is larger when plasma phe is > 600 μmol/L. Due to the large variability across subjects of 13.2%, a calibration factor to adjust blood phe concentrations is not recommended. Analysis of DBS using IEC reduced the discrepancy to 15 ± 2% lower phe concentrations compared to plasma analyzed using IEC (n = 38, p = 0.0001). This suggests that a major contributor to the discrepancy in phe concentrations is the analytical method.
Conclusion
Use of DBS analyzed using MS/MS to monitor blood phe concentrations in individuals with PKU yields significantly lower phe levels compared to plasma phe levels analyzed using IEC. Optimization of current testing methodologies for measuring phe in DBS, along with patient education regarding the appropriate technique for spotting blood on filter paper is needed to improve the accuracy of using DBS to measure phe concentrations in PKU management.
Abbreviations: PKU, phenylketonuria; pah, phenylalanine hydroxylase; phe, phenylalanine; AAA, amino acid analyzer; MS/MS, tandem mass spectrometry; IEC, ion-exchange chromatography; tyr, tyrosine; DBS, dried blood spot
Keywords: Bland–Altman, Amino acid analyzer, Phenylalanine analytical methods, Newborn screening
Highlights
-
•
Phe concentration in dried blood spots is significantly lower than plasma phe.
-
•
Blood phe concentration cannot be adjusted due to large variability across subjects.
-
•
Analysis of dried blood spots using IEC instead of MS/MS improves accuracy.
-
•
Plasma phe concentration using IEC is the most accurate for metabolic monitoring in PKU.
1. Introduction
Phenylketonuria (PKU, OMIM 261600), an autosomal recessive metabolic disorder caused by loss of function mutations of the gene encoding phenylalanine hydroxylase (EC 1.14.16.1, PAH), is characterized by hyperphenylalaninemia due to an inability to convert phenylalanine (phe) to tyrosine (tyr). Untreated PKU is typically characterized by elevated blood phe concentrations and severe cognitive impairment. Introduction of a low-phe diet shortly after birth and maintained lifelong is necessary to prevent cognitive impairment, seizures, eczema, behavior abnormalities, maternal PKU syndrome and other symptoms associated with untreated PKU [1]. The low-phe diet provides the cornerstone of PKU management by reducing phe levels and its metabolites in body fluids and protecting the brain [2], [3]. Current recommended treatment for individuals with PKU of all ages in the United States [4] includes a low-phe diet with a goal of maintaining “generic” blood phe concentrations in the range of 120–360 μmol/L “specifically referencing the values obtained by classic venipuncture and amino acid analyzer (AAA) or HPLC analysis” (personal communication, Jerry Vockley). Thus, regular monitoring of blood phe levels to monitor dietary compliance and help assure good metabolic control is an essential aspect of the clinical care for PKU.
Several testing methodologies are available to measure concentrations of phe and tyr for identification, diagnosis and management of PKU. Determination of the free amino acid profile in deproteinized plasma samples using ion-exchange chromatography (IEC) with an AAA is considered the gold standard for diagnosis and management of PKU, as well as other disorders of amino acid metabolism [5]. Newborn screening, implemented in the United States in the 1960s, currently uses tandem mass spectrometry (MS/MS) to measure phe in dried blood spots (DBS) as a means of identifying patients at risk for PKU [6]. Measurement of phe concentrations in DBS with MS/MS has several advantages over plasma analysis because it is easier to obtain and transport than liquid specimens, the DBS sample preparation is minimal, phe and tyr are stable in DBS [7], and MS/MS offers a short analysis time and lower costs. Because of these advantages, many clinics are measuring phe concentrations in DBS specimens using newborn screening MS/MS protocols as a means for monitoring metabolic control in PKU patients.
A limited number of studies suggest lower accuracy of phe concentrations analyzed in DBS by MS/MS compared with analysis of phe in plasma samples [8], [9], [10]. The two studies most relevant to our approach conducted analyses of venous plasma and DBS sample pairs obtained at the same time from PKU subjects where trained staff spot the filter paper cards [8], [9]. Results indicate that, when trained staff spot the filter paper cards, phe concentrations are consistently lower by 19–26% when measured in DBS and analyzed using MS/MS compared with phe concentrations in venous plasma analyzed using IEC [8], [9]. There are no reports in the literature that reflect the clinical environment where patients prick their own finger and spot the capillary blood on the filter paper. The reported discrepancies of lower blood phe levels using MS/MS compared with plasma analyzed using IEC are likely to be higher when patients, instead of trained technicians, spot the filter paper with capillary blood, consistent with evidence of home blood glucose monitoring in diabetes mellitus [11].
Our objective was to investigate the difference in blood phe concentrations in DBS collected by patients and analyzed using either a validated newborn screening MS/MS protocol or IEC compared to plasma phe concentrations obtained at the same time as DBS and analyzed using IEC. We observed that blood phe concentrations are 28% lower in DBS analyzed using MS/MS compared with plasma phe concentrations analyzed using IEC. This discrepancy was reduced to 15% lower phe concentrations compared to plasma when DBS were analyzed using IEC. Lastly, a reliable calibration factor for adjusting the blood phe levels to better reflect plasma phe concentrations cannot be determined due to the large variability across subjects of 13.2% associated with subjects spotting the filter paper cards.
2. Methods
2.1. Study participants and experimental design
The University of Wisconsin-Madison Health Sciences IRB approved the protocol as part of our clinical trial to assess the nutritional management of PKU. The trial was registered at www.clinicaltrials.gov as NCT01428258. All 29 subjects had a diagnosis of PKU that required management with low-phe medical food. The subjects included 12 males and 17 females, 27.2 ± 8.6 years of age (mean ± SD). Three to four fasting blood samples were obtained from each of the 29 subjects with PKU over a period of 10 weeks, n = 110 total sample size. Blood was collected (5 mL) into a tube with EDTA and plasma was isolated and then analyzed using IEC (plasma-IEC). Immediately after the venous puncture was performed, subjects were asked to prick their fingers and spot the capillary blood on a filter paper card for analysis of DBS using MS/MS (DBS-MS/MS). A sub-study including 16 of the 29 subjects was conducted, using the original samples, to compare phe measurement using three methods (n = 38 for each method); 1) Plasma-IEC, 2) DBS-MS/MS, and 3) blood phe extracted from DBS and analyzed using IEC (DBS-IEC). All analyses were conducted in the Wisconsin State Laboratory of Hygiene (Madison, WI) consistent with the standard of care for patients with PKU in Wisconsin. The Wisconsin State Laboratory of Hygiene is accredited by the College of American Pathologists (CAP) and participates in proficiency testing administered through CAP and the Centers for Disease Control for data quality and surveillance.
2.2. Plasma amino acid analysis using IEC
The Hitachi High-Technologies L-8900 Amino Acid Analyzer (Tokyo, Japan) and corresponding buffer components for the instrument were used for separation and quantitation of amino acids in plasma [5]. Frozen plasma samples were thawed and analyzed at the same time for each of the 3–4 samples obtained from the subjects. A 150 μL aliquot of plasma was mixed with 15 μL of a 35% sulfosalicyclic acid solution. After vortexing and centrifugation for 14,000 × g for 3 min, the supernatant was filtered using a 1 cm3 syringe with a 0.2 μm syringe filter. The eluent was mixed 1:1 with 4 nmol aminoethylcysteine internal standard and then 20 μL was injected onto the ion-exchange column. Amino acids were selectively eluted from the column by buffers of increasing pH along with a programed method of varying flow rates and temperatures. After elution, ninhydrin was mixed with the buffer–amino acid solution, heated to develop the purple color and read at 570 nm for amino acids phe and tyr. The total run time was 2.5 h per sample. The concentration of each amino acid was calculated by comparing the peak areas of the amino acid to the peak area of the internal standard, aminoethylcysteine.
2.3. Dried blood spot amino acid analysis using IEC
The Hitachi L-8900 Amino Acid Analyzer and corresponding buffer components for the instrument were used for separation and quantitation of amino acids in DBS. Four 3 mm (1/8th inch) punches (equivalent to 12.4 μL of blood) were removed from the DBS and placed into a 0.2 μm filter tube. Twenty five microliters of a 10% sulfosalicylic acid solution was added to the filter tube along with 100 μL of 4 nmol aminoethylcysteine internal standard diluted in water. The tube was vortexed for 15–20 s and then centrifuged at 14,000 × g for 3 min. to allow the eluent to flow into the collection vial. The eluent was then removed from the collection vial and placed over the filter containing the DBS. Again the tube was vortexed for 15–20 s and then centrifuged at 14,000 × g for 3 min. This step was repeated for a total of 3 times to maximize extraction of amino acids from the filter paper. Afterwards, the 20 μL solution was injected onto the ion-exchange column and amino acids were selectively eluted from the column using the same method as plasma amino acid analysis. The total run time was 2.5 h per sample. Phe and tyr were quantified by comparison of the peak areas to the internal standard, aminoethylcysteine, with adjustments made for the amount of blood contained within the DBS.
2.4. Dried blood spot amino acid analysis using MS/MS
The routine, newborn screening, non-derivatized flow-injection analysis—tandem mass spectrometry (FIA-MS/MS) method was used for quantification of phe and tyr in DBS [12]. Amino acids were extracted from a 3 mm (1/8th inch) punch of the DBS (equivalent to 3.1 μL of blood) after the addition of 100 μL of methanol containing internal standards for phe (13C6-Phenylalanine) and tyr (13C6-Tyrosine) followed by shaking at room temperature for 10 min. The AB Sciex API 4000 tandem mass spectrometer with a TurboV electrospray ionization source was operated in positive-ionization mode (Framingham, MA). The mobile phase of 80:20 acetonitrile/water containing 0.05% formic acid flowed at a rate of 0.08 mL/min for the entire specimen run time of 1.5 min. Data acquisition was performed using Analyst 1.5.2 software (AB Sciex) using a neutral loss of 46 to identify phe and tyr. The concentrations of phe and tyr were quantified using ChemoView 2.0.2 software (AB Sciex), which incorporated an adjustment for the amount of blood contained within the DBS.
2.5. Analytical performance: recovery and precision of the methods for analysis of phe in dried blood spots and plasma
2.5.1. Quality control materials
Dried blood spot quality control materials used to establish recovery and precision for assays were prepared by enriching whole blood from presumptively normal adult donors with 1500 μM, 500 μM, or 100 μM of phe and tyr. The enriched and un-enriched whole blood pools were then dispensed onto filter paper, dried overnight under ambient conditions, and stored at − 20 °C in zip-closure plastic bags containing desiccant packets. The assays to measure phe and tyr concentrations in DBS controls were performed as described above. Plasma controls containing normal (un-enriched) and high (enriched) concentrations of phe and tyr (Clinchek®-lyophilized plasma controls) were purchased from IRIS Technologies International (Olathe, Kansas). These plasma controls were analyzed using IEC per the method described above and recovery was measured at 105 ± 0.5% in both the normal and high controls.
2.5.2. Determination of recovery and precision
Recovery of phe and tyr from DBS using either method (DBS-IEC and DBS-MS/MS) was determined by comparison of the observed concentration to the enriched concentration at three levels of enrichment (1500 μM, 500 μM, or 100 μM) (Table 1). Inter-assay precision for the two DBS methods (DBS-IEC and DBS-MS/MS) and the plasma-IEC method was evaluated by analysis of quality control materials (un-enriched and high enrichment) on twenty different days (with n = 1 for each day).
Table 1.
Extraction recovery of phe and tyr from dried blood spots (DBS) using tandem mass spectrometry (MS/MS) and ion-exchange chromatography (IEC).
| Percent extraction recovery (mean ± SD) |
||||
|---|---|---|---|---|
| Phe |
Tyr |
|||
| DBS-MS/MS | DBS-IEC | DBS-MS/MS | DBS-IEC | |
| 1500 μM spiked (n = 3) | 78 ± 3% | 104 ± 2% | 76 ± 4% | 108 ± 2% |
| 500 μM spiked (n = 3) | 62 ± 3% | 90 ± 1% | 60 ± 3% | 88 ± 1% |
| 100 μM spiked (n = 3) | 66 ± 3% | 86 ± 7% | 70 ± 2% | 93 ± 1% |
2.6. Statistical analysis
The Mixed procedure of SAS 9.4 TS Level 1M0 for Windows was used for all statistical analyses (SAS Institute, 2012, Cary, NC). Discrepancies were estimated using a Bland–Altman analysis, which involved fitting a regression model of the percent differences between 2 phe concentrations using 2 methods ((A − B) / A) against the mean ((A + B) / 2), where A represents the more reliable method in the A & B comparison, i.e. A = plasma-IEC or DBS-IEC; B = DBS-MS/MS [13]. A preliminary statistical analysis to estimate discrepancies indicated that the slopes were close to and not significantly different from zero. Thus, the regression model used for the final Bland–Altman analyses was an intercepts-only model (slope equal to zero). Included in the model were random effects, subject ID and subject ID by diet interaction. Differences in dietary treatment did not have a significant effect on the method discrepancy estimates. Discrepancy estimate were expressed as the intercept ± SE. p-values < 0.05 are considered significant.
For a given observed discrepancy in phe concentration, the total variability is a combination of the variabilities for the subject ID, subject ID by diet interaction and residual. Therefore, the total variability across subjects was estimated by taking the square root of the sum of variance parameter estimates for subject ID, subject ID by diet interaction and residual, to calculate the standard deviation, which was converted to a percentage.
Bland–Altman analyses for tyr and the phe/tyr ratio showed that the discrepancy was dependent on the tyr value. Specifically, unlike phe concentrations, which were all lower in DBS compared to plasma, tyr concentrations measured both higher and lower in DBS compared to plasma. Thus, no reliable conclusions regarding tyr and the phe/tyr ration discrepancy could be made because the relationship between the tyr concentrations measured in DBS compared to plasma is dependent on whether the concentration of tyr was high or low, respectively. For this reason, and because of the low tyrosine concentration range (plasma tyr: 17–57 μmol/L) observed in our subjects, these analyses are not shown here.
3. Results
3.1. Extraction recovery of phe and tyr from DBS
Recovery of phe and tyr from DBS was higher with IEC compared with MS/MS at all three concentrations tested (1500 μM, 500 μM, 100 μM) (Table 1). Extraction recovery of phe ranged from 62 to 78% using DBS-MS/MS and ranged from 86 to 104% using DBS-IEC. Extraction recovery of tyr ranged from 60 to 76% using DBS-MS/MS and ranged from 88 to 108% using DBS-IEC. Recovery of phe, but not tyr, was improved with higher spiked concentrations of phe.
3.2. Precision of phe and tyr measurement
Comparison of the precision values for plasma-IEC, DBS-IEC and DBS-MS/MS for phe and tyr are presented in Table 2. The lowest inter-assay coefficient of variation (CV) resulted from plasma-IEC using high and low phe controls (1.6%). The CV for phe concentrations using DBS-IEC (3.5–5.9%) were greater than plasma-IEC, but were lower than DBS-MS/MS (11.2–11.7%). For tyr concentrations, DBS-MS/MS gave the highest inter-assay variation (10.3–12.1%). Low tyr controls resulted in CVs of 4.9% and 9.2%, respectively, for plasma-IEC and DBS-IEC, while high tyr controls resulted in CVs of 5.9% and 5.1% for plasma-IEC and DBS-IEC.
Table 2.
Precision or coefficient of variation (CV) reflecting phe and tyr measurement with three different methods.
| Phe |
Tyr |
||||||
|---|---|---|---|---|---|---|---|
|
Control level |
n |
Concentration (μM) mean ± SD |
CV (%) |
n |
Concentration (μM) mean ± SD |
CV (%) |
|
| Plasma-IEC | Low | 20 | 79 ± 1 | 1.6% | 20 | 61 ± 3 | 4.9% |
| High | 20 | 403 ± 7 | 1.6% | 20 | 223 ± 13 | 5.9% | |
| DBS-IEC | Low | 20 | 32 ± 2 | 5.9% | 20 | 38 ± 4 | 9.2% |
| High | 20 | 482 ± 17 | 3.5% | 20 | 462 ± 23 | 5.1% | |
| DBS-MS/MS | Low | 20 | 33 ± 4 | 11.2% | 20 | 33 ± 3 | 10.3% |
| High | 20 | 371 ± 43 | 11.7% | 20 | 648 ± 79 | 12.1% | |
3.3. Lower phe levels measured in DBS using MS/MS compared with plasma using IEC
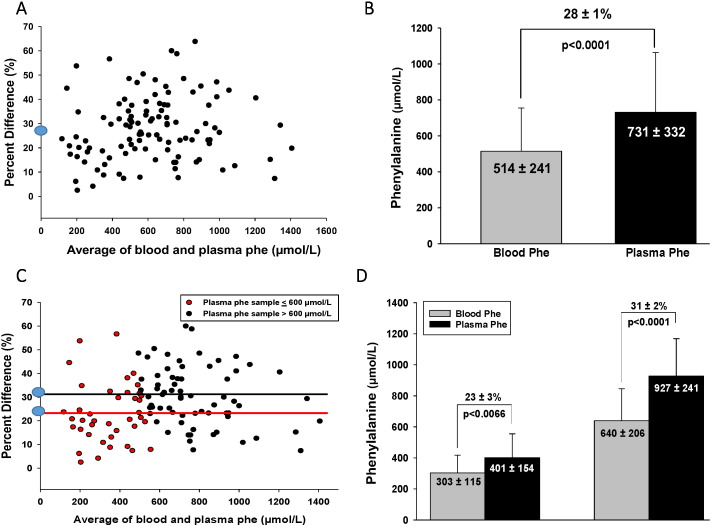
Based on the Bland–Altman analysis, phe concentrations extracted from DBS measured by MS/MS were 28 ± 1% (mean ± SE, p < 0.0001) lower compared to plasma phe concentrations obtained by venipuncture and analyzed using IEC for 110 sample pairs from 29 subjects (Fig. 1A). This discrepancy resulted in a blood phe concentration of 514 ± 241 μmol/L (mean ± SD) and a plasma phe concentration of 731 ± 332 μmol/L (mean ± SD) (Fig. 1B). In order to determine if the 28% discrepancy was different when comparing high (> 600 μmol/L) and low (≤ 600 μmol/L) plasma phe concentrations, additional Bland–Altman analyses were completed. When plasma phe concentrations were ≤ 600 μmol/L (n = 41), the discrepancy between blood and plasma phe sample pairs was 23 ± 3% (mean ± SE, p < 0.0066), compared to a 31 ± 2% (mean ± SE, p < 0.0001) discrepancy when plasma phe concentrations were > 600 μmol/L (n = 69) (Fig. 1C). The 23 ± 3% discrepancy resulted in a blood phe concentration of 303 ± 115 μmol/L (mean ± SD) and a plasma phe concentration of 401 ± 154 μmol/L (mean ± SD) when plasma phe concentrations were ≤ 600 μmol/L. The 31 ± 2% discrepancy resulted in a blood phe concentration of 640 ± 206 μmol/L (mean ± SD) and a plasma phe concentration of 927 ± 241 μmol/L (mean ± SD), when plasma phe concentrations were > 600 μmol/L (Fig. 1D).
Fig. 1.
The discrepancy between blood phe concentrations extracted from DBS and analyzed using MS/MS and plasma phe concentrations obtained by venipuncture and analyzed using IEC. Percent difference in Figures A & C is defined as ((plasma phe-blood) / plasma phe) x 100. (A) Bland–Altman analysis shows a 28 ± 1% (mean ± SE, p < 0.0001) discrepancy, indicating that phe concentrations extracted from DBS measured by MS/MS are 28 ± 1% lower compared to plasma phe concentrations obtained by venipuncture and analyzed using IEC for 110 sample pairs from 29 subjects. (B) Blood phe concentration is 514 ± 241 μmol/L (mean ± SD) and plasma phe concentration is 731 ± 332 μmol/L (mean ± SD) for 110 sample pairs from 29 subjects. (C) Bland–Altman analysis shows a 23 ± 3% (mean ± SE, p < 0.0066) discrepancy between blood and plasma phe sample pairs when plasma phe ≤ 600 μmol/L (n = 41), compared to a 31 ± 2% (mean ± SE, p < 0.0001) discrepancy within blood and plasma phe sample pairs when plasma phe concentrations > 600 μmol/L (n = 69). (D) When plasma phe concentrations ≤ 600 μmol/L (n = 41), blood phe concentration is 303 ± 115 μmol/L (mean ± SD) and plasma phe concentration is 401 ± 154 μmol/L (mean ± SD) for 41 sample pairs. When plasma phe concentrations > 600 μmol/L, blood phe concentration is 640 ± 206 μmol/L (mean ± SD) and plasma phe concentration is 927 ± 241 μmol/L (mean ± SD) for 41 sample pairs.
3.4. Variability across subjects for blood and plasma phe concentration sample pairs
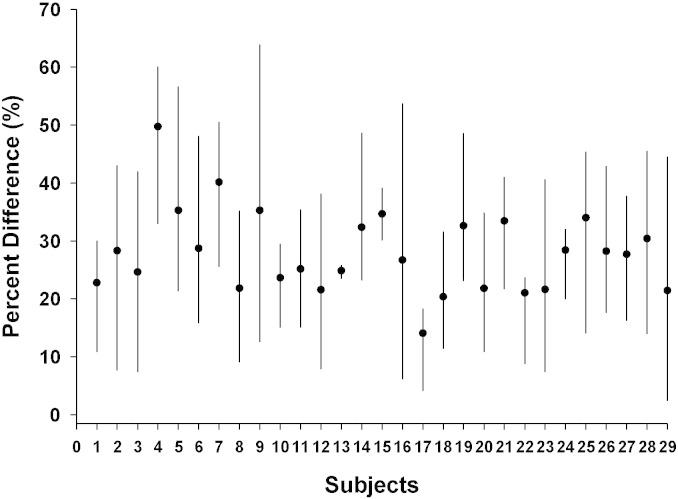
To address the use of a calibration factor based on the discrepancy in phe concentrations, total variability across subjects was calculated. Total variability was high (SD = ± 13.2%) for 110 sample pairs from 29 subjects (Fig. 2).
Fig. 2.
Heterogeneity of variability across subjects for phe measurement. Each line represents the range of percent differences from maximum to minimum within 3–4 sample pairs for each subject. Percent difference is defined as (plasma phe-blood phe)/plasma phe). x 100 The circle in the middle of each line represents the mean percent difference by subject. Total variability is high (SD = 13.2%) for 110 samples pairs from 29 subjects.
3.5. Attenuation of phe discrepancy with analysis of DBS using IEC
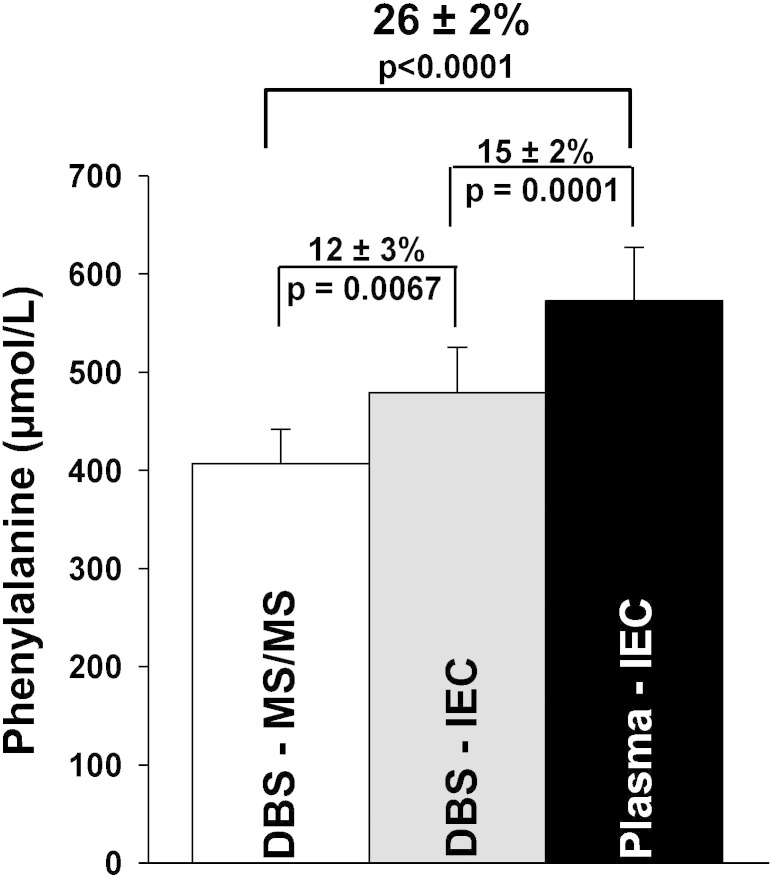
To determine whether the discrepancy between blood phe extracted from DBS and analyzed using MS/MS (DBS-MS/MS) and plasma phe obtained by venipuncture and analyzed using IEC (plasma-IEC) could be reduced, we compared phe measurement discrepancy among 3 methods (Fig. 3). Each method included 38 samples from 16 subjects. DBS-MS/MS is 26 ± 2% (mean ± SE) lower compared to plasma-IEC for these 16 subjects (p < 0.0001). Use of IEC to measure blood phe extracted from DBS reduced the discrepancy from 26 ± 2% (mean ± SE) to 15 ± 2% (mean ± SE, p = 0.0001). For DBS, MS/MS analysis yields a phe concentration that is 12 ± 3% (mean ± SE p = 0.0067) lower compared to IEC.
Fig. 3.
Estimated phe concentration measurements discrepancies using 3 different methodologies. The three methods include blood phe concentrations extracted from DBS and analyzed using MS/MS (DBS-MS/MS), blood phe concentrations extracted from DBS and analyzed using IEC (DBS-IEC), and plasma phe concentrations obtained by venipuncture and analyzed using IEC (plasma-IEC). Each method included 38 samples from 16 subjects. Blood phe concentration, extracted from DBS and analyzed using MS/MS, is 407 ± 216 μmol/L (mean ± SD). Blood phe concentration, extracted from DBS and analyzed using IEC, is 479 ± 283 μmol/L (mean ± SD). Plasma phe concentration, obtained by venipuncture and analyzed using IEC, is 573 ± 334 μmol/L (mean ± SD). DBS-MS/MS is 26 ± 2% (mean ± SE) lower compared to plasma-IEC for these 16 subjects (p < 0.0001). Use of the same analytical method, IEC, on DBS reduced the discrepancy from 26 ± 2% (mean ± SE) to 15 ± 2% (mean ± SE, p = 0.0001). For DBS, MS/MS analysis yields a phe concentration that is 12 ± 3% (mean ± SE p = 0.0067) lower compared to IEC.
4. Discussion
Ongoing monitoring of blood phe concentrations to assess dietary compliance and metabolic control is an essential component of the treatment of PKU. Measurement of phe concentrations in DBS by MS/MS using newborn screening protocols is a common practice because of the convenience and lower cost as compared with measuring phe concentrations in plasma using IEC. Previous reports where trained staff spot venous or capillary blood on filter paper indicate that phe concentrations are significantly lower by 19–26% compared with plasma phe concentrations determined using IEC [8], [9]. Herein, we report for the first time the discrepancy in phe concentrations when subjects spot their own blood on filter paper, as occurs in the clinical setting, using a well-controlled sampling procedure with determination of variability across subjects and valid Bland–Altman statistical analyses [13]. We note that blood phe concentrations in DBS eluates analyzed with MS/MS are 28% lower compared to plasma phe concentrations obtained simultaneously and analyzed with IEC, p < 0.0001. Thus, use of DBS analyzed using newborn screening protocols to monitor phe concentrations may obscure evaluation of metabolic control in patients with PKU.
Our results suggest multiple factors contribute to the consistently lower phe concentrations obtained using DBS compared to plasma. For example, we observed a reduction in the discrepancy to 15% when the DBS-IEC protocol was used instead of the DBS-MS/MS protocol. The discrepancy may be due to differences in instrumentation demonstrated by the lower precision of MS/MS compared to IEC shown in this paper and by Allard et al. [14], or extraction procedures. At this time, we chose to maintain the current newborn screening protocol for extraction and analysis of phe from DBS in order to align with existing clinical practices. Other factors that may influence the discrepancy include the milieu of the sample (venous blood, capillary blood or plasma), heterogeneity of the collected sample, and sample volume. Similar to diabetes monitoring, where blood glucose concentrations are noted to be 11% lower compared to plasma [15], lower phe concentrations in blood reflect composition differences, such as the presence of clotting factors like fibrinogen in blood and the possible inclusion of lymph when the blood sample is obtained with a lancet. When the capillary blood is obtained and spotted on filter paper, heterogeneity and volume of the collected sample varies due to the location of the punch taken from the DBS (center vs. periphery) and filter paper spotting technique (one large central spot vs. multiple small spots). Variation in sample volume is also influenced by hematocrit, hemoglobin concentration, and hydration. Related to sample quality and patient technique, Skeie et al. demonstrated better precision of blood glucose measurements using home glucose meters performed by laboratory technicians compared to patients, as determined by CVs of 2.5–5.9% for technicians compared to 7–20% for patients [11].
Given the 2014 PKU Management Guidelines for the narrow phe reference range, 120–360 μmol/L [4], there are several clinical considerations in using phe concentrations obtained from DBS to monitor metabolic control in PKU. First, due to the large variability of 13.2% across subjects that we observed, use of a calibration factor based on the discrepancies is not recommended. Thus, predictions of plasma phe concentrations based on adjusting the blood phe concentrations obtained from DBS with the discrepancy values would not likely be accurate. Second, awareness and effective communication to clinicians as to how the samples are obtained and analyzed for phe are essential for temporal comparison of phe concentrations. Third, repeated patient education on proper techniques for finger prick and filter paper spotting when obtaining DBS at home is essential to minimize analytical error. Fourth, it may be prudent to obtain a plasma phe concentration using IEC when DBS phe levels approach the upper limit of the current recommended treatment goals for PKU of 360 μmol phe/L.
This paper suggests that the use of IEC, rather than MS/MS, to measure blood phe concentrations obtained from DBS reduced the discrepancy by approximately one-half to 15 ± 2% compared to plasma-IEC. Considering the convenient, less invasive sample acquisition approach of DBS compared to venipuncture, analysis of DBS using IEC may be a practical solution [14]. An exploration of modifications to the extraction protocol for DBS is needed to maximize efficiency. Additionally, adjustments to the IEC method by modifying flow rates or buffer compositions, should be considered to allow for reduction in sample analysis times.
In conclusion, we observed that blood phe concentrations were 28% lower in DBS analyzed using a validated newborn screening MS/MS protocol compared with the gold standard of venous plasma phe concentrations analyzed using IEC. Use of a calibration factor is not recommended due to the large variability across subjects. These findings highlight the need for structured patient education on proper finger prick and filter paper spotting techniques when obtaining DBS in order to minimize analytical error in the measurement of blood phe concentrations. Lastly, further research is needed to identify and evaluate how to effectively implement practical solutions to improve metabolic monitoring of PKU utilizing the convenience of DBS and with future consideration of home phe monitoring for PKU [16].
Funding
This work was supported by Department of Health and Human Services grants R01 FD003711 from the FDA Office of Orphan Products Development to Ney, P30-HD-03352, and by the Clinical and Translational Science Award (CTSA) program, through the NIH National Center for Advancing Translational Sciences (NCATS), grant UL1TR000427. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or FDA.
Acknowledgments
We thank all of our PKU subjects for their participation in this study. We thank Dr. Sandra van Calcar for input during the initial stages of this study and Dr. Harvey Levy and Fran Rohr for assistance with sample collection from subjects at Boston Children's Hospital.
References
- 1.NIH Phenylketonuria (PKU): screening and management. NIH Consens. Statement. 2000;17(3):1–33. [PubMed] [Google Scholar]
- 2.MacLeod E.L., Ney D.M. Nutritional management of phenylketonuria. Ann. Nestle Eng. 2010;68:58–69. doi: 10.1159/000312813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Singh R.H., Rohr F., Frazier D., Cunningham A., Mofidi S., Ogata B. Recommendations for the nutrition management of phenylalanine hydroxylase deficiency. Genet. Med. 2014;16(2):121–131. doi: 10.1038/gim.2013.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vockley J., Andersson H.C., Antshel K.M., Braverman N.E., Burton B.K., Frazier D.M. Phenylalanine hydroxylase deficiency: diagnosis and management guideline. Genet Med. 2014;16(2):188–200. doi: 10.1038/gim.2013.157. [DOI] [PubMed] [Google Scholar]
- 5.Le Boucher J., Charret C., Coudray-Lucas C., Giboudeau J., Cynober L. Amino acid determination in biological fluids by automated ion-exchange chromatography: performance of Hitachi L-8500A. Clin. Chem. 1997;43(8 Pt 1):1421–1428. [PubMed] [Google Scholar]
- 6.Ceglarek U., Muller P., Stach B., Buhrdel P., Thiery J., Kiess W. Validation of the phenylalanine/tyrosine ratio determined by tandem mass spectrometry: sensitive newborn screening for phenylketonuria. Clin. Chem. Lab. Med. 2002;40(7):693–697. doi: 10.1515/CCLM.2002.119. [DOI] [PubMed] [Google Scholar]
- 7.Strnadova K.A., Holub M., Muhl A., Heinze G., Ratschmann R., Mascher H. Long-term stability of amino acids and acylcarnitines in dried blood spots. Clin. Chem. 2007;53(4):717–722. doi: 10.1373/clinchem.2006.076679. [DOI] [PubMed] [Google Scholar]
- 8.Gregory C.O., Yu C., Singh R.H. Blood phenylalanine monitoring for dietary compliance among patients with phenylketonuria: comparison of methods. Genet Med. 2007;9(11):761–765. doi: 10.1097/GIM.0b013e318159a355. [DOI] [PubMed] [Google Scholar]
- 9.Groselj U., Murko S., Zerjav Tansek M., Kovac J., Trampus Bakija A., Repic Lampret B. Comparison of tandem mass spectrometry and amino acid analyzer for phenylalanine and tyrosine monitoring—implications for clinical management of patients with hyperphenylalaninemia. Clin. Biochem. 2015;48(1–2):14–18. doi: 10.1016/j.clinbiochem.2014.09.014. [DOI] [PubMed] [Google Scholar]
- 10.Grunert S.C., Brichta C.M., Krebs A., Clement H.W., Rauh R., Fleischhaker C. Diurnal variation of phenylalanine and tyrosine concentrations in adult patients with phenylketonuria: subcutaneous microdialysis is no adequate tool for the determination of amino acid concentrations. Nutr. J. 2013;12:60. doi: 10.1186/1475-2891-12-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Skeie S., Thue G., Nerhus K., Sandberg S. Instruments for self-monitoring of blood glucose: comparisons of testing quality achieved by patients and a technician. Clin. Chem. 2002;48(7):994–1003. [PubMed] [Google Scholar]
- 12.Chace D.H., Kalas T.A., Naylor E.W. Use of tandem mass spectrometry for multianalyte screening of dried blood specimens from newborns. Clin. Chem. 2003;49(11):1797–1817. doi: 10.1373/clinchem.2003.022178. [DOI] [PubMed] [Google Scholar]
- 13.Altman D.G., Bland J.M. Measurement in medicine; the analysis of method comparison studies. Statistician. 1983;32:307–317. [Google Scholar]
- 14.Allard P., Cowell L.D., Zytkovicz T.H., Korson M.S., Ampola M.G. Determination of phenylalanine and tyrosine in dried blood specimens by ion-exchange chromatography using the Hitachi L-8800 analyzer. Clin. Biochem. 2004;37(10):857–862. doi: 10.1016/j.clinbiochem.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 15.Sacks D.B., Arnold M., Bakris G.L., Bruns D.E., Horvath A.R., Kirkman M.S. Guidelines and recommendations for laboratory analysis in the diagnosis and management of diabetes mellitus. Diabetes Care. 2011;34(6):e61–e99. doi: 10.2337/dc11-9998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Silva V., Oldham C.D., May S.W. l-Phenylalanine concentration in blood of phenylketonuria patients: a modified enzyme colorimetric assay compared with amino acid analysis, tandem mass spectrometry, and HPLC methods. Clin. Chem. Lab. Med. 2010;48(9):1271–1279. doi: 10.1515/CCLM.2010.271. [DOI] [PubMed] [Google Scholar]