Abstract
We report a patient with anti-epileptic treatment refractory neonatal seizures responsive to pyridoxine. Biochemical analysis revealed normal markers for antiquitin deficiency and also mutation analysis of the ALDH7A1 (Antiquitin) gene was negative. Mutation analysis of the PNPO gene revealed a novel, homozygous, presumed pathogenic mutation (c.481C > T; p.(Arg161Cys)). Measurements of B6 vitamers in a CSF sample after pyridoxine administration revealed elevated pyridoxamine as the only metabolic marker for PNPO deficiency. With pyridoxine monotherapy the patient is seizure free and neurodevelopmental outcome at the age of 14 months is normal.
Abbreviations: CSF, cerebrospinal fluid; PDE, (classic) pyridoxine dependent epilepsy; PLP, pyridoxal-5′-phosphate; PLP-DE, pyridoxal-phosphate dependent epilepsy; PNPO, pyridox(am)ine-5′-phosphate oxidase; α-AASA, alpha-aminoadipic semialdehyde dehydrogenase
Keywords: PNPO, Pyridoxine, Neonatal, Epilepsy, Pyridoxal-phosphate, Antiquitin
1. Introduction
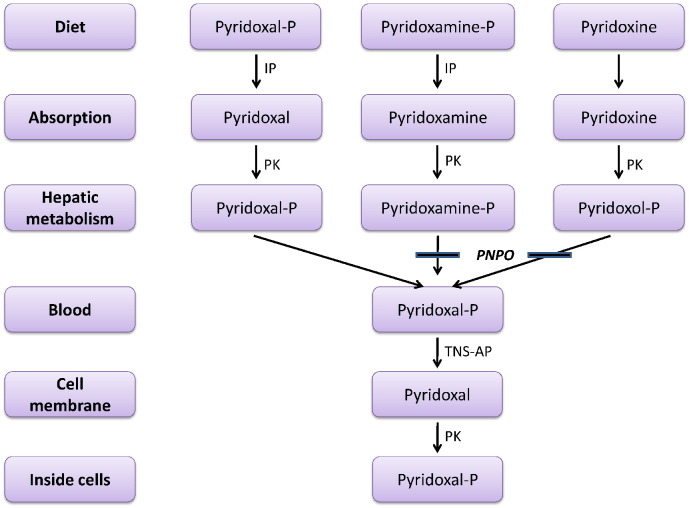
Pyridoxal-phosphate dependent epilepsy (PLP-DE) is caused by mutations in the PNPO gene leading to a deficiency of pyridox(am)ine-5′-phosphate oxidase (PNPO), which converts pyridoxine-5′-phosphate and pyridoxamine-5′-phosphate into pyridoxal-5′-phosphate (PLP), the active form of vitamin B6 (Fig. 1).
Fig. 1.
Conversion of dietary vitamin B6 to intracellular pyridoxal 5′-phosphate cofactor. IP: intestinal phosphatases; P: 5′-phosphate; PK: pyridoxal kinase; PNPO: pyridox(am)ine phosphate oxidase; TNS AP: tissue nonspecific alkaline phosphatase. A solid bar indicates an enzyme block [22].
The majority of patients with classical pyridoxine dependent epilepsy (PDE) have mutations in the ALDH7A1 (Antiquitin) gene encoding alpha-aminoadipic semialdehyde (α-AASA) dehydrogenase [1]. Deficiency of α-AASA dehydrogenase results in accelerated loss of PLP and accumulation of metabolites of the lysine degradation pathway, i.e. α-AASA, Δ1-piperideine-6-carboxylate and pipecolic acid [2].
Patients with PNPO deficiency and antiquitin deficiency present with neonatal or early-infantile onset drug-resistant seizures [3], [4], [5].
Previously it was thought that patients with PNPO deficiency respond solely to PLP and patients with antiquitin deficiency respond to both pyridoxine and PLP. However, recently patients with PNPO deficiency and pyridoxine responsiveness have been reported [6], [7], [8].
We report a neonate with a novel, homozygous and presumed pathogenic mutation in the PNPO gene with pyridoxine responsive seizures. Biomarkers for antiquitin deficiency and sequencing of the ALDH7A1 gene were normal.
2. Case report
A boy was born after an uneventful pregnancy and delivery at 37 4/7 weeks' gestation to consanguineous parents of Pakistani ethnicity. Pregnancy occurred after previous miscarriages. There was no history of excessive intrauterine movements. Family history was negative for epilepsy.
One day after birth he developed focal seizures responsive to phenobarbital (20 mg/kg). On day 3 he developed generalized tonic–clonic seizures with apnea. EEG revealed a burst suppression pattern and centrally located rhythmic sharp and slow waves, consistent with status epilepticus. Initiation of bursts corresponded with an apneic event.
Therapeutic doses of phenobarbital, midazolam and lidocaine did not resolve seizure activity. Full septic workup was negative, cranial MRI was normal.
A pyridoxine trial (100 mg/iv, in one dose) on day 4 did not lead to immediate improvement of the EEG. Pending the results of biochemical analysis, pyridoxine was continued in a single dose of 15 mg/kg/day. Other anticonvulsants were tapered off. EEG a day after pyridoxine challenge showed a continuous background activity. Within days his clinical condition improved and seizures disappeared.
Biochemical testing had to be performed after pyridoxine administration because no samples were taken before treatment. Levels of pipecolic acid in cerebrospinal fluid (CSF) and plasma, as well as α-AASA in urine were normal (Table 1).
Table 1.
Biochemical profile of the patient and of two patients with antiquitin deficiency during pyridoxine treatment for comparison. α-AASA: alpha-aminoadipic semialdehyde dehydrogenase; 5-HIAA: 5-hydroxyindole-3-acetic acid; HVA: homovanillic acid; MHPG: 3-methoxy-4-hydroxyphenylglycol.
| Patient | Antiquitin patient 1 | Antiquitin patient 2 | Reference value | |
|---|---|---|---|---|
| Urine (mmol/mol kreat) | ||||
| AASA | 0.5 | 0.0–2.0 | ||
| CSF (nmol/l) | ||||
| Pipecolic acid | 0.07 | 0.00–0.10 | ||
| Pyridoxal 5′-phosphate | 21 | 178 | 44 | 11–46 |
| Pyridoxal | 297 | 11,107 | 3667 | 5–106 |
| Pyridoxine | 13,000 | 1324 | 14,101 | 5–11 |
| Pyridoxamine | 5100 | 64 | 13 | n.d. |
| Amino acids (μmol/l) | ||||
| Glycine | 8.2 | 3.0–8.3 | ||
| Threonine | 52.6 | 15.0–130.0 | ||
| Homocarnosine | 4.3 | 5.5–12.0 | ||
| Neurotransmitters (nmol/l) | ||||
| HVA | 1091 | 445–2228 | ||
| 5-HIAA | 729 | 593–1653 | ||
| MHPG | 104 | 85–306 | ||
| 3-O-methylDOPA | 299 | 108–506 | ||
| Plasma | ||||
| Pipecolic acid, μmol/l | 1.0 | 0.1–7.0 | ||
| Lactate, mmol/l | 0.9 | 0.0–2.3 | ||
| Pyruvate, mmol/l | 0.06 | 0.00–0.13 | ||
As biomarkers for antiquitin deficiency were found normal, pyridoxine was withdrawn. At this point the patient was in good clinical condition. Forty-eight hours after withdrawal he developed status epilepticus, refractory to a combination of anti-epileptic drugs but resolving after reinitiating pyridoxine.
Meanwhile, sequencing of the ALDH7A1 gene was performed, which did not identify any mutations.
Subsequently, DNA sequence analysis of the PNPO gene was carried out and revealed a novel, homozygous missense mutation (c.481C > T; p.(Arg161Cys)). Both parents were found to be carriers of the mutation, confirming homozygosity in the patient. The mutation was predicted to be pathogenic, based on the conservation of the amino acid and the in silico analysis.
In order to look for functional confirmation of PNPO deficiency, CSF and plasma amino acid profiles were analyzed, showing no significant abnormalities, apart from a slightly decreased homocarnosine in CSF. Also neurotransmitter analysis revealed a normal pattern of the biogenic amine metabolites.
However, measurement of B6 vitamers in the CSF sample revealed a strongly increased concentration of pyridoxamine. Pyridoxamine was also measured in CSF samples of two patients with antiquitin deficiency during pyridoxine treatment for comparison. In these samples only modest elevations were found (Table 1).
Apart from a breakthrough seizure during a viral infection with fever the patient is seizure-free with pyridoxine monotherapy (15 mg/kg/day; once daily). Neurodevelopmental outcome at the age of 14 months is normal.
3. Discussion
We describe a neonate with anti-epileptic drug treatment refractory seizures responsive to pyridoxine. Antiquitin biomarkers and ALDH7A1 sequencing were normal. The homozygous mutation found in the PNPO gene, has to our knowledge not been reported in other patients with neonatal-onset PDE. However, other pathogenic missense mutations, often involving an arginine, have been reported in PNPO deficiency [7].
Mutations in the PNPO gene lead to absent or reduced PNPO activity resulting in low levels of PLP. Administration of PLP controls seizures in the majority of patients [9].
Patients with (partial) PNPO deficiency and a transient or complete response to pyridoxine instead of PLP have been described before [7], [10], [11]. Remarkably some of these patients developed even worsening of seizure control when given PLP [6]. The majority of these pyridoxine responsive PNPO deficient patients have early onset therapy resistant seizures. Additional reported features include prematurity, low Apgar scores, irritability, respiratory distress and a parental history of infertility [7].
Plecko et al. described 11 patients with homozygous or compound heterozygous, novel PNPO mutations in a group of 31 patients with pyridoxine-responsive seizures. These patients had normal biomarkers for antiquitin deficiency and normal sequencing of the ALDH7A1 gene.
Patients with antiquitin deficiency often show an immediate response to pyridoxine administration and frequently need anti-epileptic co-medication to control seizures [5]. Our patient had a delayed pyridoxine response and is seizure free on pyridoxine monotherapy. Forty-four percent of the patients described by Plecko et al. also showed an immediate pyridoxine response and 66% of patients were seizure free with pyridoxine monotherapy [6]. The rapid recurrence of seizures after withdrawal of pyridoxine described here is highly unusual in antiquitin deficiency and was also observed in one of the patients with PNPO deficiency responsive to pyridoxine described by Plecko [5], [12].
Biochemical analysis in PNPO deficiency may reveal a reduction of PLP and pyridoxal [9], [13]. Most likely due to earlier administration of pyridoxine, we found normal values in CSF. Aromatic amino acid decarboxylase, threonine dehydratase and glycine cleavage system enzymes are PLP-dependent enzymes. Therefore secondary amino acid deficiencies in CSF like elevated glycine and threonine can be expected, but were absent in our patient. Only homocarnosine was slightly decreased, which might be a (weak) reflection of reduced activity of glutamate decarboxylase.
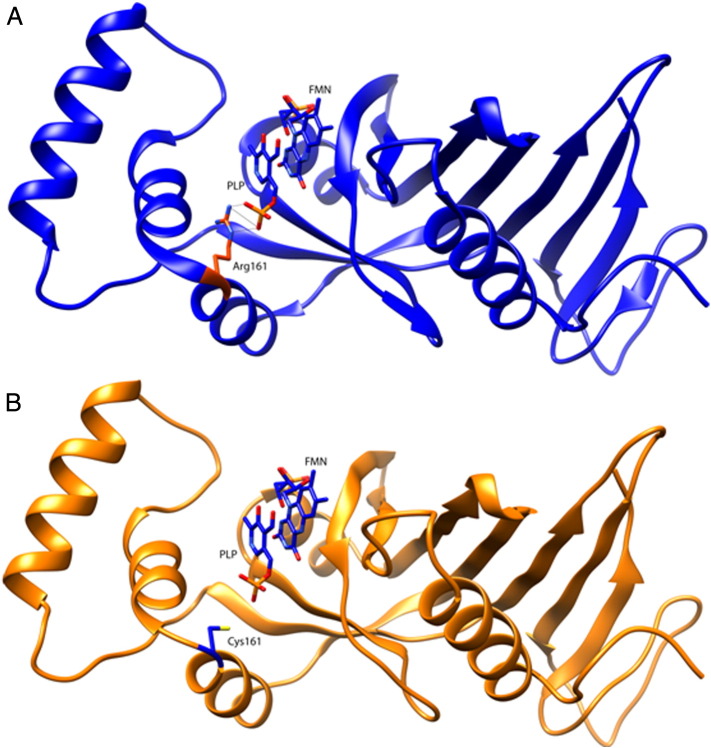
However, the elevation of pyridoxamine seems to be a convincing intrinsic biomarker for PNPO deficiency, being the direct substrate for PNPO. This shows that analyzing a CSF sample on pyridoxine treatment may even have an advantage over a pretreatment sample, because it can be considered as a pyridoxine loading test to stress the affected metabolic pathway. The marker is not fully specific, because elevations of pyridoxamine can also be seen in antiquitin deficiency, but as the latter was excluded, this disadvantage was overcome [14]. This finding therefore provided a strong argument for functional PNPO deficiency and pathogenicity of the mutation. The mutation (c.481C > T; p.(Arg161Cys)) found in the PNPO gene (17q21.32) has not been reported thus far. Arginine 161 is highly conserved in evolution and is predicted to be pathogenic by SIFT, MutationTaster and PolyPhen, which analyze the impact of sequence variations on protein function. The c.481C > T mutation has been detected once in 13,000 control alleles (Exome Variant Server, NHLBI GO Exome Sequencing Project (ESP), Seattle, WA (URL: http://evs.gs.washington.edu/EVS/) and three times in 121,300 control alleles (Exome Aggregation Consortium (ExAC) database) which suggests a pathogenic mutation. Arginine 161 is involved in the binding of PLP (Fig. 2) [15]. Another common pathogenic missense mutation i.e. Arg225His (also detected 7 times in 130,000 control alleles of the ExAc database) has been reported that is also involved in binding of PLP and is also responsive to pyridoxine supplementation [6], [9], [16].
Fig. 2.
Structural representation of the PNPO monomer (PDB ID 1NRG). Comparative analysis of the wild-type (A) and the p.Arg161Cys containing PNPO monomer (B). Pyridoxal 5′-phosphate (PLP) using flavin mononucleotide (FMN) and Arg161 (A) or Cys161 (B) are represented in stick model. Potential hydrogen bonds are shown. This image was obtained using the UCSF Chimera package. Chimera is developed by the Resource for Biocomputing, Visualization, and Informatics at the University of California, San Francisco (supported by NIGMS P41-GM103300).
This mutation possibly allows residual PNPO activity. Increased synthesis of PLP might be achieved with addition of more pyridoxine as substrate. Considering that patients with identical mutations but a different response to pyridoxine have been reported, other factors must also contribute to pyridoxine responsiveness [7]. An alternative explanation suggests pyridoxine to have a chaperone function that prevents the mutant PNPO protein from premature decay [6], [10].
Neurodevelopmental outcome in patients with antiquitin deficiency is impaired in the majority of cases [3], [5], [17], [18]. A review of all reported patients with PNPO deficiency reported 62% of patients to have developmental disabilities ranging from severe global delay to autism spectrum disorder [4].
As only a small number of patients with PNPO deficiency and pyridoxine responsiveness have been reported thus far it is difficult to predict neurodevelopmental outcome. Outcome in reported patients, varied from severe global developmental delay to mild learning disabilities to normal development. Some patients have recurrent (febrile) seizures. Existing data suggest that prompt treatment is associated with better outcome [6], [7].
Early recognition of pyridoxine responsive PNPO deficient patients is highly important to prevent possible morbidity and even mortality. However making a diagnosis has proven to be difficult. Pyridoxine-responsive PNPO deficient patients cannot be differentiated on clinical grounds from patients with PNPO deficiency that are PLP responsive or patients with classic PDE. Seizure semiology in these defects is similar and includes all types of seizures [19]. To make matters even more complex, biochemical abnormalities in classic PDE may mimic those of PLP dependent epilepsy and up to now no pathognomonic EEG or MRI features have been identified in PNPO or antiquitin deficiency.
The paradox between diagnostic difficulty on the one hand and the need for an early diagnosis on the other hand poses implications for clinical practice.
We subscribe to the practice-recommendations formulated by Plecko et al. and suggest a standardized trial of first pyridoxine, in case of an absent response followed by an empirical trial of PLP, in all neonates and infants with anti-epileptic treatment refractory epilepsy, without awaiting results of metabolic analysis. As neonatal asphyxia has been reported in a number of patients with PNPO and antiquitin deficiency, trials should be undertaken irrespective of birth history. CSF analysis remains important and antiquitin as well as PNPO biomarkers should be measured with priority, preferably before as well as on treatment. Low CSF PLP and pyridoxal, elevated pyridoxamine, and secondary amino acid deficiencies should raise suspicion of PNPO deficiency [9]. In case of a pyridoxine response, normal antiquitin biomarkers and normal ALDH7A1 sequencing, molecular analysis of the PNPO gene should be undertaken. Pyridoxine or PLP should preferably be continued until genetic tests are completed. One should take into account that the response to pyridoxine might be delayed in PNPO deficiency.
As a paradoxical reaction has been observed in confirmed PNPO deficiency when pyridoxine was replaced by PLP and because treatment with PLP can cause liver toxicity, continuing pyridoxine in a clear pyridoxine response is preferable [6], [7], [20], [21].
Study sponsorship or funding
None.
Acknowledgments
The authors would like to thank the Exome Aggregation Consortium and the groups that provided exome variant data for comparison. A full list of contributing groups can be found at http://exac.broadinstitute.org/about.
Finally, we thank the parents for allowing us to present their child's results.
Footnotes
B. Jaeger, G.S. Salomons, E.A. Struys, N.G. Abeling, M. Simas-Mendes, V.G. Geukers and B.T. Poll-The report no disclosures.
Contributor Information
B. Jaeger, Email: b.jaeger@amc.uva.nl.
N.G. Abeling, Email: n.g.abeling@amc.uva.nl.
G.S. Salomons, Email: g.salomons@vumc.nl.
E.A. Struys, Email: e.struys@vumc.nl.
M. Simas-Mendes, Email: m.mendes@vumc.nl.
V.G. Geukers, Email: v.g.geukers@amc.uva.nl.
B.T. Poll-The, Email: b.t.pollthe@amc.uva.nl.
References
- 1.Mills P.·.B., Struys E., Jakobs C., Plecko B., Baxter P., Baumgartner M. Mutations in antiquitin in individuals with pyridoxine-dependent seizures. Nat. Med. 2006;12:307–309. doi: 10.1038/nm1366. [DOI] [PubMed] [Google Scholar]
- 2.Stockler S., Plecko B., Gospe S., Coulter-Mackie M., Connolly M., van Karnebeek C. Pyridoxine dependent epilepsy and antiquitin deficiency: clinical and molecular characteristics and recommendations for diagnosis, treatment and follow-up. Mol. Genet. Metab. 2011;104:48–60. doi: 10.1016/j.ymgme.2011.05.014. [DOI] [PubMed] [Google Scholar]
- 3.Bok L., Halbertsma F., Houterman S., Wevers R., Vreeswijk C., Jakobs C. Long-term outcome in pyridoxine-dependent epilepsy. Dev. Med. Child Neurol. 2012;54:849–854. doi: 10.1111/j.1469-8749.2012.04347.x. [DOI] [PubMed] [Google Scholar]
- 4.Guerin A., Aziz A.S., Mutch C., Lewis J., Go C.·.Y., Mercimek-Mahmutoglu S. Pyridox(am)ine-5-Phosphate oxidase deficiency treatable cause of neonatal epileptic encephalopathy with burst suppression: case report and review of the literature. J. Child Neurol. 2015;30:1218–1225. doi: 10.1177/0883073814550829. [DOI] [PubMed] [Google Scholar]
- 5.Mills P.·.B., Footitt E.J., Mills K.A., Tuschl K., Aylett S., Varadkar S. Genotypic and phenotypic spectrum of pyridoxine-dependent epilepsy (ALDH7A1 deficiency) Brain. 2010;133:2148–2159. doi: 10.1093/brain/awq143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Plecko Paul, Mills Clayton, Paschke Maier. Pyridoxine responsiveness in novel mutations of the PNPO gene. Neurology. 2014;82:1425–1433. doi: 10.1212/WNL.0000000000000344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mills P.·.B., Camuzeaux S.·.S., Footitt E.J., Mills K.A., Gissen P., Fisher L. Epilepsy due to PNPO mutations: genotype, environment and treatment affect presentation and outcome. Brain. 2014;137:1350–1360. doi: 10.1093/brain/awu051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ware T., Pitt J., Freeman J. Pyridoxine responsiveness in novel mutations of the PNPO gene. Neurology. 2015;84:329. doi: 10.1212/WNL.0000000000001147. [DOI] [PubMed] [Google Scholar]
- 9.Levtova A., Camuzeaux S., Laberge A.-M.M., Allard P., Brunel-Guitton C., Diadori P. Normal cerebrospinal fluid pyridoxal 5′-phosphate level in a PNPO-deficient patient with neonatal-onset epileptic encephalopathy. JIMD Rep. 2015;22:67–75. doi: 10.1007/8904_2015_413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pearl P., Gospe S. Pyridoxine or pyridoxal-5′-phosphate for neonatal epilepsy: the distinction just got murkier. Neurology. 2014;82:1392–1394. doi: 10.1212/WNL.0000000000000351. [DOI] [PubMed] [Google Scholar]
- 11.Pearl P., Hyland K., Chiles, McGavin C., Yu Y., Taylor D. Partial pyridoxine responsiveness in PNPO deficiency. JIMD Rep. 2013;9:139–142. doi: 10.1007/8904_2012_194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schmitt B., Baumgartner M., Mills P., Clayton P., Jakobs C., Keller E. Seizures and paroxysmal events: symptoms pointing to the diagnosis of pyridoxine-dependent epilepsy and pyridoxine phosphate oxidase deficiency. Dev. Med. Child Neurol. 2010;52:e133–e142. doi: 10.1111/j.1469-8749.2010.03660.x. [DOI] [PubMed] [Google Scholar]
- 13.Ormazabal A., Oppenheim M., Serrano M., García-Cazorla A., Campistol J., Ribes A. Pyridoxal 5′-phosphate values in cerebrospinal fluid: reference values and diagnosis of PNPO deficiency in paediatric patients. Mol. Genet. Metab. 2008;94:173–177. doi: 10.1016/j.ymgme.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 14.Footitt E.J., Heales S.J., Mills P.·.B., Allen G.F., Oppenheim M., Clayton P.T. Pyridoxal 5′-phosphate in cerebrospinal fluid; factors affecting concentration. J. Inherit. Metab. Dis. 2011;34:529–538. doi: 10.1007/s10545-011-9279-7. [DOI] [PubMed] [Google Scholar]
- 15.Lek M., Karczewski K., Minikel E., Samocha K., Banks E., Fennell T. Analysis of protein-coding genetic variation in 60,706 humans. bioRxiv. 2015:030338. doi: 10.1038/nature19057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Musayev F.·.N., Di Salvo M.L., Ko T.-P.·.P., Schirch V., Safo M.K. Structure and properties of recombinant human pyridoxine 5′-phosphate oxidase. Protein Sci. 2003;12:1455–1463. doi: 10.1110/ps.0356203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Basura G.J., Hagland S.·.P., Wiltse A.M., Gospe S.M. Clinical features and the management of pyridoxine-dependent and pyridoxine-responsive seizures: review of 63 North American cases submitted to a patient registry. Eur. J. Pediatr. 2009;168:697–704. doi: 10.1007/s00431-008-0823-x. [DOI] [PubMed] [Google Scholar]
- 18.Baxter P. Pyridoxine-dependent and pyridoxine-responsive seizures. Dev. Med. Child Neurol. 2001;43:416–420. doi: 10.1017/s0012162201000779. [DOI] [PubMed] [Google Scholar]
- 19.Veerapandiyan A., Winchester S., Gallentine W., Smith E., Kansagra S., Hyland K. Electroencephalographic and seizure manifestations of pyridoxal 5′-phosphate-dependent epilepsy. Epilepsy Behav. 2011;20:494–501. doi: 10.1016/j.yebeh.2010.12.046. [DOI] [PubMed] [Google Scholar]
- 20.Sudarsanam A., Singh H., Wilcken B., Stormon M., Arbuckle S., Schmitt B. Cirrhosis associated with pyridoxal 5′-phosphate treatment of pyridoxamine 5′-phosphate oxidase deficiency. JIMD Rep. 2014;17:67–70. doi: 10.1007/8904_2014_338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Coman D., Lewindon P., Clayton P., Riney K. PNPO deficiency and cirrhosis: expanding the clinical phenotype? JIMD Rep. 2015 doi: 10.1007/8904_2015_456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saudubray J.-M., Berghe G., Walter J.H. Inborn Metabolic Diseases. fifth ed. Springer-Verlag; Berlin Heidelberg: 2012. Disorders of neurotransmission; p. 417. [Google Scholar]