Abstract
Background. To evaluate the long-term outcome of H. pylori eradication therapy for gastric MALT lymphoma according to the presence of H. pylori infection. Methods. We retrospectively reviewed the medical records of patients between January 2001 and June 2014. The clinicopathologic characteristics and clinical outcomes were compared between H. pylori-positive and H. pylori-negative gastric MALT lymphoma groups. Results. Fifty-four patients were enrolled: 12 H. pylori-negative and 42 H. pylori-positive patients. The tumor was located more frequently in both the proximal and distal parts of the stomach (P = 0.001), and the percentage of multiple lesions was significantly greater in the H. pylori-negative group (P = 0.046). Forty-seven patients received initial eradication therapy, and 85% (35/41) of H. pylori-positive patients and 50% (3/6) of H. pylori-negative patients achieved complete remission after eradication therapy. The presence of multiple lesions was a predictive factor for unresponsiveness to H. pylori eradication (P = 0.024). The efficacy of eradication therapy (P = 0.133), complete remission (CR) maintenance period, and relapse after eradication therapy were not significantly different between the two groups. Conclusions. H. pylori eradication therapy could be an effective first-line treatment for localized H. pylori-negative gastric MALT lymphoma, especially for single lesions.
1. Introduction
Mucosa-associated lymphoid tissue (MALT) lymphoma is a type of extranodal B-cell lymphoma; of these, gastric MALT lymphoma is the most common [1]. More than 90% of gastric MALT lymphoma cases are associated with Helicobacter pylori (H. pylori) infection, and several studies since 1993 have proven the effectiveness of eradication therapy, reporting lymphoma regression after therapy completion [2, 3]. Complete remission (CR) has been reported in approximately 80% of patients with gastric MALT lymphoma after eradication therapy, with recurrence rates of approximately 5% [4]. With such favorable clinical outcomes, eradication therapy is recommended as the first-line treatment for patients positive for H. pylori with localized gastric MALT lymphoma [5, 6]. However, little is known about the clinicopathologic characteristics and clinical course according to the H. pylori infection status in gastric MALT lymphoma. In addition, there are no established therapeutic strategies for about 5–10% of patients who have no evidence of H. pylori infection [7–9].
Therefore, the aim of the present study was to evaluate the long-term outcome of H. pylori eradication therapy for gastric MALT lymphoma according to presence of H. pylori infections and to identify predictive factors for unresponsiveness to eradication therapy.
2. Methods
2.1. Patient Population
A retrospective medical record review of patients who were histologically diagnosed with gastric MALT lymphoma at the Chungnam National University Hospital (Daejeon, Korea) between January 2001 and June 2014 was performed. The subjects were patients who had undergone consecutive follow-up for at least six months after treatment for gastric MALT lymphoma in this hospital; of the 63 enrolled patients, a total of 54 were analyzed after excluding 6 patients who were lost to follow-up and 3 who refused treatment. This study was approved by the Institutional Review Board of Chungnam National University Hospital (2015-04-003).
2.2. Diagnosis and Staging
Gastric MALT lymphoma was diagnosed according to the World Health Organization classification of lymphoid neoplasms for extranodal marginal zone B-cell lymphoma of the MALT type, and the Lugano staging system [10] was used to determine the clinical stage. Lesion shape, location, and number were analyzed based on endoscopic findings. Lesions were morphologically classified as superficial or advanced cancer-like types, and they were also classified according to their location in the stomach: proximal (cardia, fundus, upper body, and midbody) or distal (lower body, antrum, and pylorus).
2.3. Evaluation and Eradication of H. pylori Infection
H. pylori infection was confirmed with at least two of the following tests: histology, urea breath test, or rapid urease test (Hp Kit, Chongkundang Pharmaceutical, Republic of Korea). Patients with at least one positive test result were defined as H. pylori-positive, and those with all negative results were considered H. pylori-negative. For first-line eradication therapy a proton pump inhibitor- (PPI-) based triple therapy was administered for 1-2 weeks: PPI (standard dose b.i.d.), clarithromycin (0.5 g b.i.d.), and amoxicillin (1 g b.i.d.). Urea breath tests were performed in all patients at least 4–6 weeks after treatment completion to confirm H. pylori eradication. For patients with failed first-line triple therapy, a second-line eradication therapy consisting of PPI (standard dose b.i.d.), tripotassium dicitrato bismuthate (300 mg q.i.d.), metronidazole (500 mg t.i.d.), and tetracycline (500 mg q.i.d.) was administered for 1-2 weeks.
2.4. Response Assessment
Posttreatment response was classified into four groups based on the grading system from the European Gastro-Intestinal Lymphoma Study (EGILS) [6]. Complete remission (CR) was defined as no macroscopic findings of lymphoma and negative histologic findings in at least two subsequent follow-up investigations. Partial remission (PR) was defined as reduction of macroscopic and histologic findings. Stable disease (SD) was characterized as unmodified macroscopic and/or histologic findings. Progressive disease (PD) was defined as the worsening of macroscopic or histologic findings. Patients other than those who met the CR criteria at the follow-up of at least 6 months (H. pylori-negative patients) or 12 months (H. pylori-positive patients) after H. pylori eradication were defined nonresponders. In all patients, follow-up endoscopy with multiple biopsies was performed every 3–6 months after therapy completion until CR was achieved. In the case of CR, regular endoscopic surveillance was performed every 6–12 months.
2.5. Statistical Analysis
Each categorical variable was analyzed using chi-square or Fisher's exact tests in order to compare the baseline characteristics of H. pylori-positive and H. pylori-negative patients with gastric MALT lymphoma. The odds ratios of predictive factors of resistance to eradication therapy were analyzed with a logistic regression model. For multivariate analysis, some variables that were considered clinically significant were adjusted. All analyses were conducted using SPSS version 19.0 software (SPSS Inc., Chicago, IL, USA); P values were two-sided, and P values less than 0.05 were considered significant.
3. Results
3.1. Clinical Characteristics of H. pylori-Positive and H. pylori-Negative Groups
A total of 54 patients with gastric MALT lymphoma were enrolled in the study; of these, 42 (78%) were H. pylori-positive. The mean age (standard deviation, SD) was 58.8 (9.8) years (range: 38–82 years). The baseline characteristics according to H. pylori infection status are shown in Table 1. The lesions were located more frequently in both the proximal and distal parts of the stomach (P = 0.001), and the percentage of multiple lesions was significantly greater in the H. pylori-negative group than in the H. pylori-positive group (P = 0.046). However, other characteristics, including age, sex, endoscopic findings, and clinical stage, were not significantly different between the two groups.
Table 1.
Baseline characteristics of H. pylori-positive and H. pylori-negative gastric mucosa-associated lymphoid tissue lymphoma patients.
| Variables | H. pylori+ (n = 42) | H. pylori− (n = 12) | P value |
|---|---|---|---|
| Mean age (SD) | 58.9 (11.5) | 58.5 (6.7) | 0.651 |
| Gender (%) | 0.051 | ||
| Male | 14 (33) | 8 (67) | |
| Female | 28 (67) | 4 (33) | |
| Site of lesion (%) | 0.001 | ||
| Proximal∗ | 23 (55) | 4 (33) | |
| Distal† | 15 (36) | 2 (17) | |
| Both | 4 (9) | 6 (50) | |
| Number of lesions (%) | 0.046 | ||
| Single | 22 (52) | 2 (17) | |
| Multiple | 20 (48) | 10 (83) | |
| Endoscopic finding (%) | 0.356 | ||
| Superficial type | 37 (88) | 9 (75) | |
| Advanced cancer-like type | 5 (12) | 3 (25) | |
| Clinical stage (%) | 0.067 | ||
| I | 40 (95) | 9 (75) | |
| II or more | 2 (5) | 3 (25) |
∗Cardia, fundus, high body, or midbody. †Low body, angle, or antrum.
SD: standard deviation; H. pylori: Helicobacter pylori.
3.2. H. pylori Eradication and Response to H. pylori Eradication
The treatment response of each group of patients is shown in Table 2. The median follow-up period was 51 months (range: 7–156 months), and all patients except two had localized gastric MALT lymphoma (stage I). Of the 42 H. pylori-positive patients, 41 received eradication therapy. Of them, 35 patients achieved successful eradication of H. pylori (85%): 33 patients who received first-line triple therapy and 2 of 8 patients who received second-line quadruple eradication therapy. As a result of the eradication therapy, all of these 35 patients (100%) achieved CR. Six of 12 H. pylori-negative patients (50%) underwent eradication therapy. Three of these six patients (50%) showed CR. The median time to reach CR after the completion of eradication therapy tended to be longer in H. pylori-negative patients (6.1 months) than in H. pylori-positive patients (4.8 months). However, it was not significantly different between the two groups.
Table 2.
Clinical response to eradication therapy according to H. pylori infection status.
| Variables | H. pylori+ (n = 42) | H. pylori− (n = 12) | P value |
|---|---|---|---|
| Initial H. pylori eradication (%) | 41 (100)∗ | 6 (50)† | |
| Successful eradication (%) | 35 (85) | ||
| Response to H. pylori eradication (%) | |||
| Complete remission | 35 (85) | 3 (50) | 0.133 |
| Partial remission | 2 (5) | 0 (0) | |
| Stable disease | 4 (10) | 2 (33) | |
| Progressive disease | 0 (0) | 1 (17) | |
| Median time to achieve CR (month, SD) | 4.8 (1.1) | 6.1 (1.5) | 0.108 |
| CR maintenance period (month, SD) | 28.0 (19.1) | 32.0 (18.0) | 0.442 |
| Relapse rate after CR | 3 (9) | 1 (33) | 0.718 |
∗One patient received chemotherapy initially due to advanced stage. †Three patients underwent chemotherapy and other three patients were treated with radiotherapy initially.
SD: standard deviation; H. pylori: Helicobacter pylori; CR: complete remission.
3.3. Characteristics of Treatment Responders and Nonresponders
Among 47 patients who chose eradication therapy as first-line treatment, 38 (80.9%) showed a response, while 9 did not; the clinical characteristics of the groups are shown in Table 3. The proportion of patients with multiple lesions was significantly greater in the nonresponder group (8 patients, 89%) than in the responder group (16 patients, 42%) (P = 0.023). However, other characteristics, including age, sex, lesion site, endoscopic findings, and clinical stage, did not differ significantly between the two groups. Univariate and multivariate logistic regression analysis identified that the presence of multiple lesions was the only independent predictor of resistance to H. pylori eradication (P = 0.024) (Table 4).
Table 3.
Clinical characteristics of responder and nonresponder group after eradication therapy.
| Variables | Responder (n = 38) | Nonresponder (n = 9) | P value |
|---|---|---|---|
| Mean age (SD) | 58.7 (10.8) | 59.2 (11.4) | 0.259 |
| Gender (%) | 0.252 | ||
| Male | 12 (32) | 5 (56) | |
| Female | 26 (68) | 4 (44) | |
| H. pylori status (%) | 0.075 | ||
| Positive | 35 (92) | 6 (67) | |
| Negative | 3 (8) | 3 (33) | |
| Site of lesion (%) | 0.270 | ||
| Proximal∗ | 23 (61) | 3 (33) | |
| Distal† | 11 (29) | 5 (56) | |
| Both | 4 (10) | 1 (11) | |
| Number of lesions (%) | 0.023 | ||
| Single | 22 (58) | 1 (11) | |
| Multiple | 16 (42) | 8 (89) | |
| Endoscopic finding (%) | 0.302 | ||
| Superficial type | 32 (84) | 8 (89) | |
| Advanced cancer-like type | 6 (16) | 1 (11) | |
| Clinical stage (%) | 0.650 | ||
| I | 36 (95) | 9 (100) | |
| II or more | 2 (5) | 0 (0) |
∗Cardia, fundus, high body, or midbody. †Low body, angle, or antrum.
SD: standard deviation; H. pylori: Helicobacter pylori.
Table 4.
Predictive factor for resistance to H. pylori eradication by logistic regression analysis.
| Variables | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | |
| Age (elderly) | 1.12 (0.81–1.45) | 0.289 | ||
| Gender (male) | 1.26 (0.69–1.83) | 0.325 | ||
| H. pylori status (negative) | 5.83 (0.95–35.10) | 0.057 | 4.71 (0.89–31.58) | 0.073 |
| Number of lesions (multiple) | 8.22 (1.21–51.48) | 0.019 | 7.83 (1.16–49.81) | 0.024 |
| Location of lesion (proximal) | 0.29 (0.06–1.42) | 0.127 | ||
H. pylori: Helicobacter pylori; OR: odds ratio; CI: confidence interval.
3.4. Treatments and Outcomes in H. pylori-Positive and H. pylori-Negative Patients
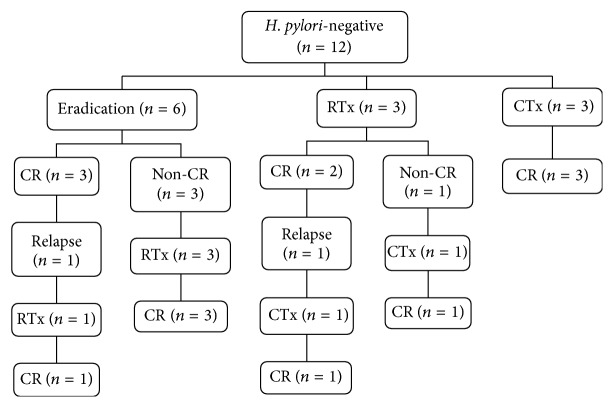
Posttreatment clinical outcomes in H. pylori-positive and H. pylori-negative patients with gastric MALT lymphoma are shown in Figures 1 and 2. Of 42 H. pylori-positive patients, one patient received chemotherapy due to advanced-stage disease. Six H. pylori-positive patients received radiotherapy, those who did not respond to eradication therapy. Three H. pylori-negative patients received chemotherapy, and the remaining three patients received radiotherapy. All H. pylori-positive and H. pylori-negative patients who received chemotherapy achieved CR. All H. pylori-positive patients who received radiotherapy achieved CR. However, one H. pylori-negative patient who had no response to radiotherapy received additional chemotherapy and achieved CR. Comparison of the overall CR rate of each treatment modality was as follows: eradication therapy resulted in a CR rate of 81% (38/47), radiotherapy resulted in 93% (14/15), and chemotherapy resulted in 100% (6/6).
Figure 1.
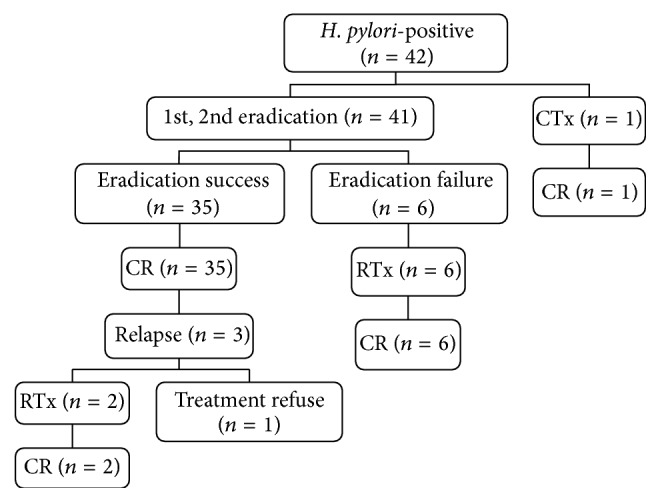
Clinical outcome of Helicobacter pylori-positive gastric mucosa-associated lymphoid tissue (MALT) lymphoma. CR: complete remission; RTx: radiotherapy; CTx: chemotherapy.
Figure 2.
Clinical outcome of Helicobacter pylori-negative gastric mucosa-associated lymphoid tissue (MALT) lymphoma. CR: complete remission; RTx: radiotherapy; CTx: chemotherapy.
3.5. Recurrence of MALT Lymphoma
During the follow-up period, three patients in the H. pylori-positive group relapsed at a median of 13 months (range: 6–27 months) after remission without evidence of reinfection. Of them, one patient refused alternative treatment owing to old age, and the other two patients received radiotherapy and achieved CR. One H. pylori-negative patient who had undergone eradication therapy relapsed at 17 months after CR; this patient achieved CR after radiotherapy and is currently undergoing follow-up. One patient who had shown CR after radiotherapy relapsed at 13 months but achieved CR after chemotherapy. In summary, the relapse rate was 11% (4/38) after eradication therapy and 7% (1/15) after radiotherapy and there was no relapse (0/6) after chemotherapy. There was no statistical difference in the CR maintenance period or the relapse rate after CR between the H. pylori-positive and negative groups (Table 2).
4. Discussion
In the current study, 85% (35/41) of H. pylori-positive patients and 50% (3/6) of H. pylori-negative patients achieved CR after eradication. It has been demonstrated that eradication therapy has a high efficacy (approximately 80%) in H. pylori-positive gastric MALT lymphoma [5]. The high response rate observed in our study can be explained in part by the fact that the majority of enrolled patients (78%) were H. pylori-positive and that a high proportion (95%) of the patients had early-staged lesions. Our present data reconfirm the fact that H. pylori eradication should be the first-line treatment for localized H. pylori-positive gastric MALT lymphoma.
On the other hand, the efficacy of eradication therapy in H. pylori-negative gastric MALT lymphoma has not yet been fully determined [11]. A multicenter cohort study in Japan reported a 13.6% (6/44) response rate after eradication [12]. In addition, a systematic review with pooled analysis including 11 studies with 110 patients with early-stage H. pylori-negative MALT lymphoma revealed a 15.5% (17/110; 95% confidence interval, 8.7–22.2) CR rate [11]. However, many studies included in this pooled analysis enrolled a limited number of patients, and there were a wide variety of CR rates between the studies. Therefore, the effectiveness of H. pylori eradication therapy for H. pylori-negative gastric MALT lymphoma remains to be elucidated. The 2010 EGILS Group recommendations for the treatment of H. pylori-negative gastric MALT lymphoma state that patients “can also undergo anti-H. pylori treatment” [6]. The 2013 European Society of Medical Oncology guidelines recommend eradication in H. pylori-negative MALT lymphoma cases, albeit consideration of oncological treatments is needed [13]. Although the number of H. pylori-negative patients that underwent eradication therapy was small (n = 6) in our study, the 50% CR rate observed after eradication therapy in H. pylori-negative patients supports its use in this patient group. Furthermore, the present study demonstrated comparable results in terms of the median time to achieve CR after the completion of eradication therapy, the CR maintenance period, and the relapse rate between the H. pylori-positive and H. pylori-negative gastric MALT lymphoma groups. Hence, it would seem reasonable to perform eradication therapy before oncologic therapies in localized H. pylori-negative gastric MALT patients. Eradication therapy is expected to produce promising results not only in initial treatment responses, but also in long-term follow-up for H. pylori-negative MALT lymphoma patients. Further studies are necessary to confirm these results.
Research on the clinicopathologic characteristics of H. pylori-negative MALT lymphoma is limited and has shown inconsistent results. In the present study, no significant differences in age, sex, endoscopic findings, or clinical stage that were found in previous studies [14, 15] were observed between the two groups. Interestingly, our study showed that H. pylori-negative gastric MALT lymphomas more frequently involve both proximal and distal parts of the stomach (P = 0.001) and have a higher frequency of multiple lesions compared to the H. pylori-positive group (P = 0.046). Some studies have indicated that although gastric MALT lymphomas can occur in any region of the stomach, they are frequently located in the distal portion of the stomach, suggesting that they are associated with the localization of the highest concentration of colonized H. pylori organisms and acquired lymphoid tissue [7, 16, 17]. Further studies with a larger number of patients are needed to verify this difference.
Some predictive factors for resistance to H. pylori eradiation in gastric MALT lymphoma have been demonstrated, such as the absence of H. pylori infection [8, 14, 15, 18], advanced stage [8, 14], a diffuse large B-cell lymphoma component [9, 19–21], proximal location [16, 22], endoscopic nonsuperficial type [18, 19], deep invasion of lymphoma in the gastric wall [20, 23], and t(11;18)/API2-MALT1 translocation [14, 22]. Our results are in line with the earlier studies, which reported that multiple locations are associated with nonresponse to therapy [11]. Though a study with a larger sample size is needed, the presence of multiple lesions should be taken into consideration before eradication therapy in patients with gastric MALT lymphoma.
A variety of relapse rates after CR in patients who have undergone H. pylori eradication have been reported. In a prospective, multicenter trial from Europe comprising 120 patients, 3% (3/96) of patients relapsed within 4 months, 5 months, and 24 months, respectively, after a median follow-up of 75 months [24]. In another retrospective, multicenter study enrolling 60 patients, 30.9% (13/42) recurred within a median time of 19 months (range, 3–41 months) [25]. In our study, relapse occurred in 10.5% (4/38) of patients with a median follow-up period of 51 months. Of note, lymphoma relapse occurred within a median of 17 months, with the last relapse occurring at 27 months. According to a previous study, gastric MALT lymphoma recurrence could be observed up to 131 months after CR [11]. In this regard, long-term, careful follow-up is mandatory for all patients.
Gastric MALT lymphoma progresses slowly, and its prognosis tends to be favorable [26]. Thus, eradication therapy is often administered as an initial treatment before oncologic therapy in H. pylori-negative patients. The slow disease progression and indolence of H. pylori mean that treatment delay due to a lack of response to initial eradication therapy does not greatly affect patient prognosis. In fact, one study reported that all five H. pylori-negative patients who had no response to eradication therapy reached regression after radiotherapy [15], a finding similar to the current study, in which all three patients who had no response to eradication therapy achieved CR without recurrence after radiotherapy. Considerations of stomach preservation and morbidity for the treatment of localized gastric MALT lymphoma have recently diminished the role of surgery in favor of radiotherapy or chemotherapy, with satisfactory results [27, 28]. Likewise, the treatment success rate in the present study was 93% for radiotherapy and 100% for chemotherapy in all patients, regardless of their H. pylori infection status, and recurrence was low (radiotherapy: 7%, chemotherapy: 0%). Therefore, in patients with gastric MALT lymphoma, radiotherapy or chemotherapy should be the first choice for those who have experiences with therapy failure or in those who are predicted to have resistance to eradication therapy.
This study had several limitations. First, selection bias was possible owing to the small sample size of H. pylori-negative gastric MALT lymphoma patients. It was difficult to enroll many patients because of the low incidence of gastric MALT lymphoma. Second, we were unable to study the status of the t(11;18)/API2-MALT1 translocation, which is known to be an important factor for predicting eradication therapy response in patients with gastric MALT lymphoma. Third, there was a possibility that some treatment responders were categorized as nonresponders; in some patients, the evaluation of response to therapy occurred too soon after eradication therapy, and it was difficult to apply the “watch and wait” strategy to all patients.
In conclusion, H. pylori eradication therapy could be an effective first-line treatment option in localized H. pylori-negative gastric MALT lymphoma, especially for patients with single lesions. Initial treatment responses as well as long-term outcomes in H. pylori-negative gastric MALT lymphoma are comparable to those in H. pylori-negative lymphoma.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publishing of this paper.
References
- 1.Nakamura S., Matsumoto T., Iida M., Yao T., Tsuneyoshi M. Primary gastrointestinal lymphoma in Japan: a clinicopathologic analysis of 455 patients with special reference to its time trends. Cancer. 2003;97(10):2462–2473. doi: 10.1002/cncr.11415. [DOI] [PubMed] [Google Scholar]
- 2.Weber D. M., Dimopoulos M. A., Anandu D. P., Pugh W. C., Steinbach G. Regression of gastric lymphoma of mucosa-associated lymphoid tissue with antibiotic therapy for Helicobacter pylori . Gastroenterology. 1994;107(6):1835–1838. doi: 10.1016/0016-5085(94)90828-1. [DOI] [PubMed] [Google Scholar]
- 3.Siavoshi F., Malekzadeh R., Daneshmand M., Ashktorab H. Helicobacter pylori endemic and gastric disease. Digestive Diseases and Sciences. 2005;50(11):2075–2080. doi: 10.1007/s10620-005-3010-1. [DOI] [PubMed] [Google Scholar]
- 4.Stolte M., Bayerdörffer E., Morgner A., et al. Helicobacter and gastric MALT lymphoma. Gut. 2002;50(3):19–24. doi: 10.1136/gut.50.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dreyling M., Thieblemont C., Gallamini A., et al. Esmo consensus conferences: guidelines on malignant lymphoma. Part 2: marginal zone lymphoma, mantle cell lymphoma, peripheral T-cell lymphoma. Annals of Oncology. 2013;24(4):857–877. doi: 10.1093/annonc/mds643. [DOI] [PubMed] [Google Scholar]
- 6.Ruskoné-Fourmestraux A., Fischbach W., Aleman B. M. P., et al. EGILS consensus report. Gastric extranodal marginal zone B-cell lymphoma of MALT. Gut. 2011;60(6):747–758. doi: 10.1136/gut.2010.224949. [DOI] [PubMed] [Google Scholar]
- 7.Steinbach G., Ford R., Glober G., et al. Antibiotic treatment of gastric lymphoma of mucosa-associated lymphoid tissue. An uncontrolled trial. Annals of Internal Medicine. 1999;131(2):88–95. doi: 10.7326/0003-4819-131-2-199907200-00003. [DOI] [PubMed] [Google Scholar]
- 8.Ruskoné-Fourmestraux A., Lavergne A., Aegerter P. H., et al. Predictive factors for regression of gastric MALT lymphoma after anti-Helicobacter pylori treatment. Gut. 2001;48(3):297–303. doi: 10.1136/gut.48.3.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nakamura S., Matsumoto T., Suekane H., et al. Predictive value of endoscopic ultrasonography for regression of gastric low grade and high grade MALT lymphomas after eradication of Helicobacter pylori . Gut. 2001;48(4):454–460. doi: 10.1136/gut.48.4.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rohatiner A., d'Amore F., Coiffier B., et al. Report on a workshop convened to discuss the pathological and staging classifications of gastrointestinal tract lymphoma. Annals of Oncology. 1994;5(5):397–400. doi: 10.1093/oxfordjournals.annonc.a058869. [DOI] [PubMed] [Google Scholar]
- 11.Zullo A., Hassan C., Ridola L., et al. Eradication therapy in Helicobacter pylori-negative, gastric low-grade mucosa-associated lymphoid tissue lymphoma patients: a systematic review. Journal of Clinical Gastroenterology. 2013;47(10):824–827. doi: 10.1097/mcg.0b013e318286ff72. [DOI] [PubMed] [Google Scholar]
- 12.Nakamura S., Sugiyama T., Matsumoto T., et al. Long-term clinical outcome of gastric MALT lymphoma after eradication of Helicobacter pylori: a multicentre cohort follow-up study of 420 patients in Japan. Gut. 2012;61(4):507–513. doi: 10.1136/gutjnl-2011-300495. [DOI] [PubMed] [Google Scholar]
- 13.Zucca E., Copie-Bergman C., Ricardi U., Thieblemont C., Raderer M., Ladetto M. Gastric marginal zone lymphoma of MALT type: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Annals of Oncology. 2013;24(6):144–148. doi: 10.1093/annonc/mdt343.mdt343 [DOI] [PubMed] [Google Scholar]
- 14.Sumida T., Kitadai Y., Hiyama T., et al. Antibodies to Helicobacter pylori and CagA protein are associated with the response to antibacterial therapy in patients with H. pylori-positive API2-MALT1-negative gastric MALT lymphoma. Cancer Science. 2009;100(6):1075–1081. doi: 10.1111/j.1349-7006.2009.01139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Akamatsu T., Mochizuki T., Okiyama Y., Matsumoto A., Miyabayashi H., Ota H. Comparison of localized gastric mucosa-associated lymphoid tissue (MALT) lymphoma with and without Helicobacter pylori infection. Helicobacter. 2006;11(2):86–95. doi: 10.1111/j.1523-5378.2006.00382.x. [DOI] [PubMed] [Google Scholar]
- 16.Kim J. S., Chung S. J., Choi Y. S., et al. Helicobacter pylori eradication for low-grade gastric mucosa-associated lymphoid tissue lymphoma is more successful in inducing remission in distal compared to proximal disease. British Journal of Cancer. 2007;96(9):1324–1328. doi: 10.1038/sj.bjc.6603708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wotherspoon A. C. Gastric lymphoma of mucosa-associated lymphoid tissue and Helicobacter pylori . Annual Review of Medicine. 1998;49:289–299. doi: 10.1146/annurev.med.49.1.289. [DOI] [PubMed] [Google Scholar]
- 18.Takenaka R., Yokota K., Mizuno M., et al. Serum antibodies to Helicobacter pylori and its heat-shock protein 60 correlate with the response of gastric mucosa-associated lymphoid tissue lymphoma to eradication of H. pylori . Helicobacter. 2004;9(3):194–200. doi: 10.1111/j.1083-4389.2004.00225.x. [DOI] [PubMed] [Google Scholar]
- 19.Nakamura S., Matsumoto T., Suekane H., et al. Long-term clinical outcome of Helicobacter pylori eradication for gastric mucosa-associated lymphoid tissue lymphoma with a reference to second-line treatment. Cancer. 2005;104(3):532–540. doi: 10.1002/cncr.21152. [DOI] [PubMed] [Google Scholar]
- 20.Chen L.-T., Lin J.-T., Tai J. J., et al. Long-term results of anti-Helicobacter pylori therapy in early-stage gastric high-grade transformed MALT lymphoma. Journal of the National Cancer Institute. 2005;97(18):1345–1353. doi: 10.1093/jnci/dji277. [DOI] [PubMed] [Google Scholar]
- 21.Nakamura T., Seto M., Tajika M., et al. Clinical features and prognosis of gastric MALT lymphoma with special reference to responsiveness to H. pylori eradication and API2-MALT1 status. The American Journal of Gastroenterology. 2008;103(1):62–70. doi: 10.1111/j.1572-0241.2007.01521.x. [DOI] [PubMed] [Google Scholar]
- 22.Zullo A., Hassan C., Cristofari F., et al. Effects of Helicobacter pylori eradication on early stage gastric mucosa-associated lymphoid tissue lymphoma. Clinical Gastroenterology and Hepatology. 2010;8(2):105–110. doi: 10.1016/j.cgh.2009.07.017. [DOI] [PubMed] [Google Scholar]
- 23.Sackmann M., Morgner A., Rudolph B., et al. Regression of gastric MALT lymphoma after eradication of Helicobacter pylori is predicted by endosonographic staging. Gastroenterology. 1997;113(4):1087–1090. doi: 10.1053/gast.1997.v113.pm9322502. [DOI] [PubMed] [Google Scholar]
- 24.Wündisch T., Thiede C., Morgner A., et al. Long-term follow-up of gastric MALT lymphoma after Helicobacter pylori eradication. Journal of Clinical Oncology. 2005;23(31):8018–8024. doi: 10.1200/jco.2005.02.3903. [DOI] [PubMed] [Google Scholar]
- 25.Andriani A., Miedico A., Tedeschi L., et al. Management and long-term follow-up of early stage H. pylori-associated gastric MALT-lymphoma in clinical practice: an Italian, multicentre study. Digestive and Liver Disease. 2009;41(7):467–473. doi: 10.1016/j.dld.2008.09.009. [DOI] [PubMed] [Google Scholar]
- 26.Psyrri A., Papageorgiou S., Economopoulos T. Primary extranodal lymphomas of stomach: clinical presentation, diagnostic pitfalls and management. Annals of Oncology. 2008;19(12):1992–1999. doi: 10.1093/annonc/mdn525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gobbi P. G., Corbella F., Valentino F., et al. Complete long-term response to radiotherapy of gastric early-stage marginal zone lymphoma resistant to both anti-Helicobacter pylori antibiotics and chemotherapy. Annals of Oncology. 2009;20(3):465–468. doi: 10.1093/annonc/mdn668. [DOI] [PubMed] [Google Scholar]
- 28.Raderer M., Chott A., Drach J., et al. Chemotherapy for management of localisd high-grade gastric B-cell lymphoma: how much is necessary? Annals of Oncology. 2002;13(7):1094–1098. doi: 10.1093/annonc/mdf178. [DOI] [PubMed] [Google Scholar]