Abstract
Secretory phospholipase A2 (sPLA2) generates bioactive lysophospholipids implicated in acute and chronic inflammation, but the pathophysiologic role of sPLA2 is poorly understood. Given that high-density lipoprotein (HDL) is the major substrate for sPLA2 in plasma, we investigated the effects of sPLA2-mediated modification of HDL (sPLA2-HDL) on neutrophil function, an essential arm of the innate immune response and atherosclerosis.
Treatment of neutrophils with sPLA2-HDL rapidly prevented agonist induced neutrophil activation, including shape change, neutrophil extracellular trap formation, CD11b activation, adhesion under flow and migration of neutrophils. The cholesterol-mobilizing activity of sPLA2-HDL was markedly increased when compared to native HDL, promoting a significant reduction of cholesterol-rich signaling microdomains integral to cellular signaling pathways. Moreover, sPLA2-HDL effectively suppressed agonist-induced rise in intracellular Ca2+ levels. Native HDL showed no significant effects and removing lysophospholipids from sPLA2-HDL abolished all anti-inflammatory activities.
Overall, our studies suggest that the increased cholesterol-mobilizing activity of sPLA2-HDL and suppression of rise in intracellular Ca2+ levels are likely mechanisms that counteract agonist induced-activation of neutrophils. These counterintuitive findings imply that neutrophil trafficking and effector responses are altered by sPLA2-HDL during inflammatory conditions.
Keywords: HDL, secretory phospholipase A2, lysophospholipid, neutrophil
1. Introduction
Concentrations of some secretory phospholipase A2 (sPLA2) subspecies can increase hundred-folds in plasma during acute inflammation [1]. More than one third of the mammalian PLA2 enzymes belong to the sPLA2 family, which consists of low molecular mass, Ca2+ dependent enzymes with a His–Asp catalytic dyad [2]. sPLA2 enzymes, including groups IIA, III, V and X, catalyze the hydrolysis of glycerophospholipids to lysophospholipids and free fatty acids at the sn-2 position. Considerable evidence implicates a potential role for groups IIA and V sPLA2 in cardiovascular disease and in patients with sepsis and septic shock, and increased plasma levels of sPLA2 have been observed in acute inflammatory conditions such as sepsis as well as in chronic inflammatory diseases [3-5].
Circulating levels of sPLA2-IIA and increased sPLA2 activities associate with cardiovascular risk in patients with sepsis and coronary artery disease [6]. Pathologic studies demonstrated the presence of sPLA2 isoforms in atherosclerotic lesions and myocardial regions that have sustained ischemic injury [7]. Unexpectedly, inhibition of sPLA2 in patients with acute coronary syndromes resulted in an excess rate of myocardial infarction and stroke [8] and showed no overall survival benefit in patients with severe sepsis [9]. These studies clearly suggest that the pathophysiologic roles of sPLA2 are poorly understood. Most sPLA2 isoforms have the unique capacity to bind and hydrolyze lipoproteins in distinct and specific manners, thereby producing various lipid mediators and modifying the lipid particles. In the acute phase of the inflammatory response sPLA2 is mostly associated with high-density lipoproteins (HDL) [10], which are the major source of phospholipids in plasma [11] and are important players in the innate and adaptive immunity and cardiovascular disease [12-14]. sPLA2 overexpression in apoA-I transgenic mice results in a dramatic shift of the HDL particle size toward smaller particles and virtually all plasma sPLA2 is found in the HDL fraction [15]. Surprisingly, direct effects of sPLA2 modified HDL on innate immune cells have not been assessed so far. Neutrophils are classically considered as short-lived phagocytes with the ability to release vast amounts of proteolytic enzymes and reactive oxygen species, both of which are important during bacterial infections. Thus, both exuberant and/or diminished responses by the innate immune system may worsen clinical outcomes in severely ill patients with acute and chronic liver diseases [16], airway diseases [17] or acute coronary syndrome [18]. Neutrophils play key roles in sepsis [19] and atherosclerosis [20] and were shown to aggravate endothelial dysfunction, to activate macrophages, promote foam cell formation and to contribute to weakening of the fibrous cap [20]. We therefore thought to assess whether sPLA2-treated HDL modulates neutrophil function, an essential arm of the innate immune response.
2. Materials and Methods
2.1. Materials
All laboratory reagents were from Sigma (Vienna, Austria), unless otherwise specified. Interleukin 8 (IL-8) and intracellular adhesion molecule-1 (ICAM-1) were purchased from Peprotech (London, UK). Free fatty acids (FFA), lysophosphatidylcholines (LPC) and lysophosphatydilserine 16:0 were from Avanti Polar Lipids (Birmingham, AL, USA). FFAs were dissolved in ethanol and LPCs in chloroform/methanol and stored at −20°C under argon atmosphere. Required amounts of LPC were dried under a stream of nitrogen and re-dissolved in PBS (pH 7.4). CellFix and FACS-Flow were from BD Bioscience (Vienna, Austria). Secretory phospholipase A2 (sPLA2) type III from bee venom and human recombinant secretory phospholipase A2 type V were purchased from Cayman Europe (Tallin, Estonia). Varespladib was purchased from Eubio (Vienna, Austria). Anti-human CD11b-FITC and CD16-PE antibodies were obtained from Biozym-Biotech (Vienna, Austria). Fluo-3-AM and SYTOX green were from Life Technologies (Vienna, Austria). Fixative solution was prepared by adding 9 ml distilled water and 30 ml FACS-Flow to 1 ml CellFix.
2.2. Isolation of HDL
HDL was isolated by density gradient ultracentrifugation as described [21-23]. Plasma density was adjusted with potassium bromide (Sigma, Vienna, Austria) to 1.24 g/ml and a two-step density gradient was generated in centrifuge tubes (16 × 76 mm, Beckman) by layering the density-adjusted plasma (1.24 g/ml) underneath a NaCl-density solution (1.006 g/ml). Tubes were sealed and centrifuged at 90.000 rpm for 4 hours in a 90Ti fixed angle rotor (Beckman Instruments, Krefeld, Germany). After centrifugation, the HDL-containing band was collected and desalted via PD10 columns (GE Healthcare, Vienna, Austria) and immediately used for experiments.
2.3. sPLA2 treatment of HDL
HDL was incubated in the presence of 200 ng/ml sPLA2 type III from bee venom or 400 ng/ml human recombinant type V sPLA2 in PBS containing Ca2+ and Mg2+, overnight at 37°C, in order to hydrolyse HDL-associated phospholipids.
2.4. Lysophosphatidylcholine (LPC), free-fatty acid (FFA) and lysophosphatidylserine enrichment/depletion of HDL
In order to generate LPC-, FFA-, or lysophosphatidylserine-enriched HDL, 1 mg/ml HDL was incubated with 0.6 mmol/L 16:0, 18:1, 18:2 or 20:4 FFA, with 0.6 mmol/L 16:0, 18:1, 18:2 or 20:4 LPC or with 0.6 mmol/L lysophosphatidylserine 16:0 for 2 hours at 37°C. Unbound LPCs and FFAs were removed by gel filtration and HDL-associated LPC and FFA contents were determined as described below. In some experiments, sPLA2-treated HDL was incubated in the presence or absence of 50 mg/ml albumin (1 h, 37°C) and sPLA2-HDL was re-isolated by density gradient ultracentrifugation in order to remove LPCs and FFAs. LPC and FFA contents were assessed as described below.
2.5. Determination of FFAs and LPCs
HDL-associated lipids were extracted according to Bligh and Dyer [24] and dried under a stream of nitrogen. Dried lipid extracts were resuspended in 200 μl CHCl3/MeOH (1:1, v/v) containing 1 pmol/μl of LPC 17:1 serving as internal standard. Chromatographic separation of lipids was performed by an Accela HPLC (Thermo Scientific) on a Thermo Hypersil GOLD C18, 100 × 1 mm, 1.9 μm column. Solvent A was a water solution of 1% ammonium acetate (v/v) and 0.1% formic acid (v/v) and solvent B was acetonitrile/2-propanol (5:2, v/v) supplemented with 1% ammonium acetate (v/v) and 0.1% formic acid (v/v), respectively. The gradient was run from 35% to 70% B for 4 min, then to 100% B in additional 16 min with subsequent hold at 100% for 10 min. The flow rate was 250 μl/min. Phospholipid species were determined by a TSQ Quantum ultra (Thermo Scientific) triple quadrupole instrument in positive ESI mode. The spray voltage was set to 4500 V and capillary voltage to 35 V. The LPC species were detected in a precursor ion scan on m/z 184 at 34 eV. Peak areas were calculated by QuanBrowser for all lipid species and the calculated peak areas for each species were expressed as a % of internal standard. Results are expressed as nmol/mg HDL protein. FFA content was assessed using a non-esterified fatty acids kit (Diasys, Holzheim, Germany). In some experiments the Azwell LPC Assay Kit (Hölzl Diagnostika) was used to assess the LPC content of sPLA2-HDL.
2.6. Neutrophil isolation
Human polymorphonuclear leukocytes (PMNL) were isolated as previously described [25;26], from peripheral blood of healthy volunteers according to a protocol approved by the Ethics Committee of the Medical University of Graz. Prior to blood sampling from healthy volunteers, all donors signed an informed consent form. Platelet-rich plasma was removed by centrifugation of citrated whole blood. Erythrocytes were removed by dextran sedimentation. PMNL were isolated by Histopaque gradient centrifugation. Any erythrocyte contamination of the PMNL pellet was removed by hypotonic shock lysis. The purity and viability of neutrophil preparation was greater than 95%. All functional assays of neutrophils were performed in assay buffer (PBS with Ca2+ and Mg2+, HEPES 10 mmol/L, glucose 10 mmol/L, bovine serum albumin 0.1%, pH 7.4).
2.7. Neutrophil shape change assay
Neutrophil shape change was measured as previously described [27;28]. Isolated PMNL were resuspended in assay buffer and aliquots of cells (about 3 × 105 cells per sample) were preincubated with HDL samples and then stimulated in 37°C shaking water bath with interleukin-8 (IL-8), N-formyl-methionyl-leucyl-phenylalanin (fMLP) or complement component 5a (C5a) for 4 min, with lipopolysaccharide (LPS) in the presence of 2 % serum for 90 min or with E.coli bacteria for 60 min at a final volume of 100 μl. Cells were transferred to ice and 150 μl of ice-cold fixative solution was added to terminate the reaction and maintain the change in cell shape until analysis. The samples were then analyzed on a FACScalibur flow cytometer (BD Biosciences). Eosinophils were distinguished from neutrophils according to granularity (side scatter) and by their autofluorescence in the FL-2 channel. Shape change was determined as the increase in the forward scatter property of the cell compared with vehicle stimulation.
2.8. CD11b activation
Isolated PMNL were preincubated with HDL samples and stimulated with IL-8 (3 nmol/L), fMLP (5 nmol/L) or C5a (30 nmol/L) for 4 min at 37°C in shaking water bath in the presence of FITC-conjugated Ab to the active epitope of CD11b. Cells were fixed and then analyzed by flow cytometry [29].
2.9. Neutrophil adhesion under flow conditions
Vena8 biochips (Cellix Ltd, Dublin, Ireland) were coated with 10 μg/ml intracellular adhesion molecule-1 (ICAM-1) at 4°C overnight in a humidified box. On the next day, the chips were washed twice with distilled water, blocked with 0.1 % bovine serum albumin for 30 minutes and rinsed with distilled water. Isolated PMNL were resuspended in assay buffer (containing Ca2+ and Mg2+) and treated with vehicle, HDL, or sPLA2-HDL for 15 min at 37°C. Cells (3 × 106/ml) were then perfused over the ICAM-1 coated channels at constant shear stress of 0.5 dyne cm−2 for 5 minutes using the Mirus nanopump (Cellix). Neutrophil adhesion was recorded on an Olympus IX70 fluorescence microscope and an Olympus UPIanFI-X20/0.40 lens, using a Hamamatsu ORCA-ER digital camera and the Olympus CellP software. Cell images were taken 5 minutes after the start of perfusion and adherent neutrophils were analyzed using ImageJ software (National Institutes of Health) as described before [30].
2.10. Migration assay
Migration of freshly isolated human neutrophils (1.5 × 105 cells per well) was assessed using 96-well transwell plates with a pore size of 8 μm (Corning) as described [31]. Neutrophils were preincubated with vehicle, sPLA2-HDL, HDL or PLA2 for 15 minutes at 37°C and seeded into the upper wells. Cells were allowed to migrate towards IL-8 (10 nmol/L) for 1 hour at 37°C. Cells that had migrated to the lower compartment were enumerated by flow cytometric counting for 30 s. Spontaneous migration was determined in wells containing only assay buffer. To calculate the chemotactic index, the number of cells migrated in response to IL-8 was divided by the number of spontaneously migrated cells.
2.11. Ca2+ Flux
Changes in intracellular Ca2+ levels in neutrophils were analyzed by flow cytometry as described previously [32]. PMNL were loaded with the cell membrane permeable Ca2+-sensitive dye Fluo-3-AM (2 μmol/L) in the presence of 0.02 % F-127 pluronic acid for 60 min at room temperature. Cells were then washed, stained with anti-CD16 (PE) and resuspended in assay buffer. Neutrophils were identified as CD16-positive cells.
2.12. Neutrophil extracellular traps (NET)
The kinetics of NET formation was measured as previously described [33]. Isolated human neutrophils (5 × 104 cells per well) were treated in 96-well black plates in a final volume of 200 μl in the presence of SYTOX green (5 μmol/L), a cell-impermeable nucleic acid dye. 10 nmol/L PMA was used to induce NET formation. NET formation was observed by measuring mean fluorescence (Ex 488 nm, Em 523 nm) every 10 min for 5 hours at 37°C (FlexStation II; Molecular Devices).
2.13. Cholesterol-rich microdomain (lipid raft) assessment
Isolated human neutrophils were incubated with different HDL preparations for 5 to 120 min at 37°C; subsequently cells were washed with PBS and incubated with 1 μg/ml FITC-cholera toxin B for 1 hour at room temperature. Neutrophils were fixed and lipid raft abundance was measured by flow cytometry. For fluorescence microscopy, cells were spun on glass coverslips using a Cytospin 3 centrifuge (Shandon). The cells were mounted with Vectashield mounting medium including DAPI (Szabo Scandic, Vienna, Austria) and analyzed using OLYMPUS fluorescence microscope equipped with a Hamamatsu ORCA CCD camera.
2.14. Cholesterol efflux measurement
Isolated neutrophils were labeled for 3 hours with [3H]-cholesterol (1 μCi/ml) at 37°C in the presence of 2 μg/ml Acyl-CoA:cholesterol acyltransferase (ACAT) inhibitor Sandoz 58-035. The cells were then washed three times with PBS containing 1 mg/ml bovine serum albumin by centrifugation. Efflux to the respective cholesterol acceptors was allowed to occur for 1 hour at 37°C in a total volume of 400 μl. After the efflux period, cells were pelleted and the radioactivity in cells and supernatant was measured.
2.15. Filipin staining of unesterified cholesterol
Neutrophils were incubated with HDL samples, cells were then washed with ice cold PBS, fixed on ice for 20 min, washed with PBS and stained for 1 h with filipin (100 μg/ml). Cells were washed before quantification by flow cytometry (LSR II, BD Biosciences).
2.16. Statistical analysis
Data are shown as mean ± SD for n observations. Experiments were repeated three to six times with neutrophils from different donors. Statistical analyses were performed using GraphPad Prism Version 4.03. Student’s t-test was used for experiments comparing two groups and one-way ANOVA with Tukey multiple-comparison post hoc test was used for experiments comparing three or more groups. Significance was accepted at p<0.05.
3. Results
3.1. sPLA2 modification of HDL generates particles with potent shape change inhibitory activity
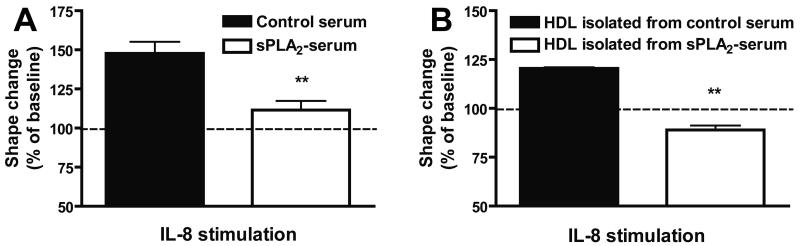
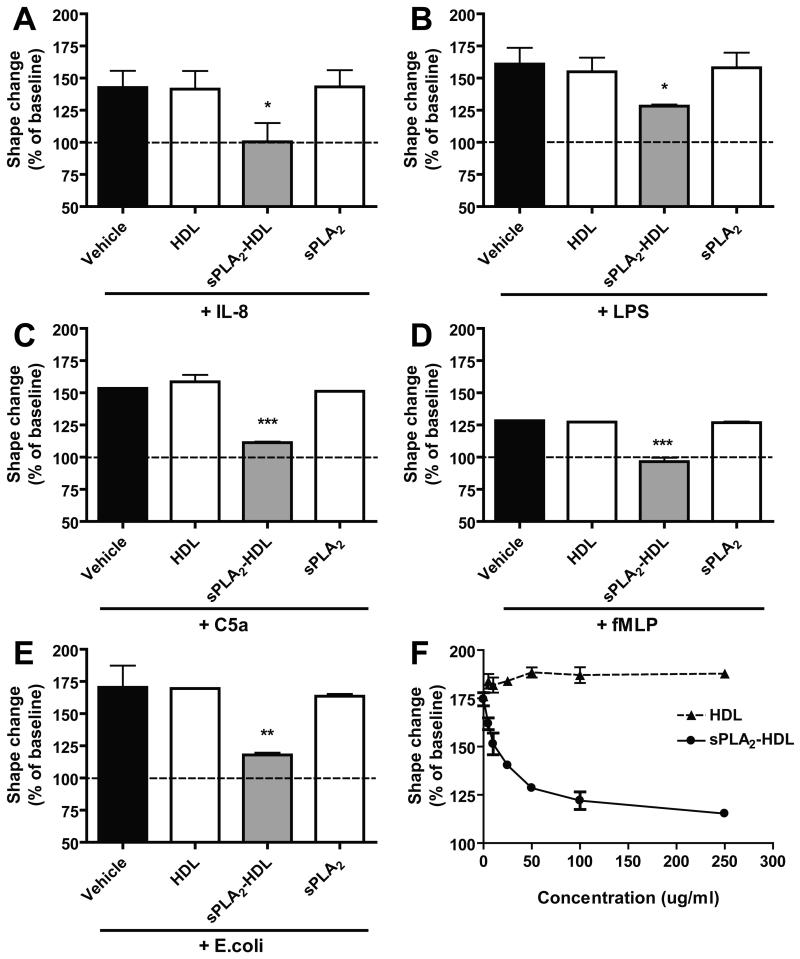
Stimulation of PMNL with chemoattractants results in rapid reorganization of the cytoskeleton and changes of their shape, which can be assessed by flow cytometry (Supplementary Figure IA to ID) [34]. These responses are important prerequisites for granulocyte adhesion, polarization, locomotion, and degranulation. To elucidate the role of sPLA2 in chemoattractant-induced shape change responses, neutrophils were added to 20 % serum pretreated for 20 hours in the absence or presence of sPLA2 type III. Cells were subsequently stimulated with interleukin 8 (IL-8) and increase in the forward scatter (shape change response) was assessed by means of flow cytometry. Surprisingly, sPLA2-treated serum almost completely reversed IL-8 induced shape change (Figure 1A). Given that HDL is the major carrier of phospholipids in plasma and the major substrate for sPLA2 [10;15], we isolated HDL from sPLA2-treated plasma by ultracentrifugation and assessed whether the HDL containing fraction shows similar effects. Indeed, HDL isolated from sPLA2 treated plasma effectively suppressed neutrophil shape change, whereas HDL isolated from control plasma showed no effect (Figure 1B). We next assessed whether direct modification of HDL with sPLA2 (sPLA2-HDL) generates particles with shape change inhibitory activity. The lysophosphatidylcholine (LPC) content of sPLA2-HDL and control HDL is shown in Supplementary Table I. We observed that sPLA2-HDL effectively inhibited the activation of neutrophils induced by various agonists (IL-8, formy-methionyl-leucyl-phenylanaline (fMLP), lipopolysaccharide (LPS), complement 5a (C5a) and E. coli bacteria) (Figure 2A to 2E and Supplementary Figure ID), whereas native HDL or addition of sPLA2 alone showed no effects. Importantly, sPLA2-HDL-induced inhibition of shape change was seen in neutrophils from all donors. Several HDL preparations from different donors showed comparable inhibitory effects after sPLA2 treatment. Notably, also low-density lipoprotein treated with sPLA2 suppressed agonist induced shape change (Supplementary Figure II). sPLA2-HDL did not impact neutrophil viability (Supplementary Figure III). These results suggest that sPLA2 induced hydrolysis of HDL-phospholipids is required for the observed effects. The inhibitory activity of sPLA2-HDL on neutrophils was dose dependent (Figure 2F).
Figure 1. sPLA2-treated serum inhibits neutrophil activation.
Freshly isolated human serum was incubated in the absence or presence of sPLA2 type III for 24 hours at 37°C. (A) Treated and untreated serum (20 % v/v) was subsequently added followed by stimulation with IL-8 (3 nmol/L) for 4 min. (B) HDL was isolated from sPLA2 treated serum and control serum and subsequently added to human neutrophils (125 μg HDL-protein/ml, 15 min at 37°C) followed by stimulation with IL-8 (3 nmol/L) for 4 min. Neutrophil shape change was assessed by flow cytometry. Values are expressed as % of baseline. The results are shown as mean ± SD of three separate experiments using PMNL from different healthy donors. Statistical analysis was performed using Student’s t-test. *p < 0.05, **p < 0.01 versus control.
Figure 2. sPLA2-treated HDL inhibits IL-8, LPS, C5a, fMLP and E. coli-induced neutrophil activation.
Isolated neutrophils were incubated with vehicle, native HDL (100 μg protein/ml), sPLA2-treated HDL (100 μg protein/ml) or sPLA2 alone for 15 min at 37°C, and stimulated with (A) IL-8 (3 nmol/L, 4 min, 37°C) (B) 1 ng/ml LPS in the presence of 2 % serum (90 min, 37°C) (C) C5a (30 nmol/L, 4 min, 37°C) (D) fMLP (5 nmol/L, 4 min, 37°C) or (E) E. coli bacteria (1 h, 37°C, the ratio of neutrophils and bacteria was 1:5). Neutrophil shape change was measured by flow cytometry. Values are expressed as % of baseline. Results shown are mean ± SD. The data represent the results from three to five experiments done using PMNL from different healthy donors. Statistical significance was assessed by one-way ANOVA with Tukey multiple-comparison post hoc test. *p < 0.05, **p < 0.01, ***p < 0.001 versus vehicle. (F) Concentration-response curve with sPLA2-treated HDL. Neutrophils were incubated with increasing concentrations of native HDL or sPLA2-treated HDL (0 up to 250 μg/ml) for 15 min at 37°C and stimulated with 1 ng/ml LPS (90 min, 37°C). Neutrophil shape change was measured by flow cytometry and results are shown as mean ± SD (n=3).
Notably, addition of sPLA2-HDL after stimulation of neutrophils (with LPS or IL-8) rapidly reversed shape change (Supplementary Figure IV).
3.2. sPLA2-generated lysophosphatidylcholines are the active moiety in HDL
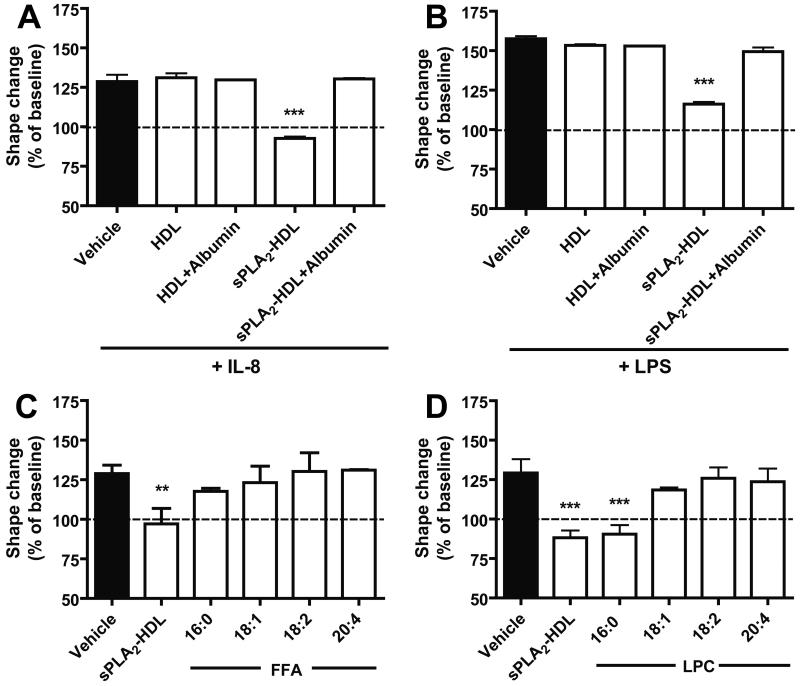
Next, we assessed whether the potent effects of sPLA2-HDL on neutrophils result from phospholipid hydrolysis products that are formed and enriched in sPLA2-HDL. Therefore, we added an excess of fatty-acid free albumin to sPLA2-HDL to remove lysophospholipids and free fatty acids and re-isolated HDL using density gradient ultracentrifugation. The lysophospholipid and FFA content following albumin incubation was effectively reduced as shown in Supplementary Figure V. We observed that the activity of sPLA2-HDL was not due to reduced particle size or other structural alterations, but rather depended on the presence of lysophospholipids and/or free fatty acids in HDL (Figure 3A and 3B). In order to investigate whether specific free fatty acids and/or lysophospholipids mediate the effects on neutrophils, we enriched HDL with different free fatty acids and lysophospholipids. We observed that the major sPLA2-HDL associated lysophospholipid LPC 16:0 was the most effective, whereas other LPCs and free fatty acids showed weak or no inhibitory effects (Figure 3C and 3D). Interestingly, besides LPC 16:0 also lysophosphatidylserine 16:0 showed potent inhibitory activity (Supplementary Figure VI).
Figure 3. Presence of LPC on sPLA2-treated HDL is necessary for the inhibition of neutrophil shape change.
Native or sPLA2-treated HDL were incubated in the presence or absence of 50 mg/ml albumin (1 h, 37°C) and HDL was re-isolated by density gradient ultracentrifugation to remove FFA and lysophospholipids as described in Materials and Methods. 100 μg/ml HDL protein was used in the shape change assay with (A) IL-8 (3 nmol/L, 4 min) or (B) 1 ng/ml LPS (90 min, 37°C). Enrichment of HDL with FFA and LPC. Neutrophils were preincubated (15 min, 37°C) with sPLA2-HDL (100 μg/ml protein) and (C) HDL enriched with FFA (16:0, 18:1, 18:2, 20:4) or (D) HDL enriched with LPC (16:0, 18:1, 18:2, 20:4). Neutrophils were then stimulated with IL-8 (3 nmol/L, 4 min, 37°C). Neutrophil shape change was measured by flow cytometry. Values are expressed as % of baseline. Results shown are mean ± SD of three separate experiments using cells from different healthy donors. Statistical significance was assessed by one-way ANOVA with Tukey multiple-comparison post hoc test. *p < 0.05, **p < 0.01, ***p < 0.001 versus vehicle.
3.3. sPLA2-HDL suppresses CD11b activation, adhesion, migration and extracellular trap (NET) formation of neutrophils
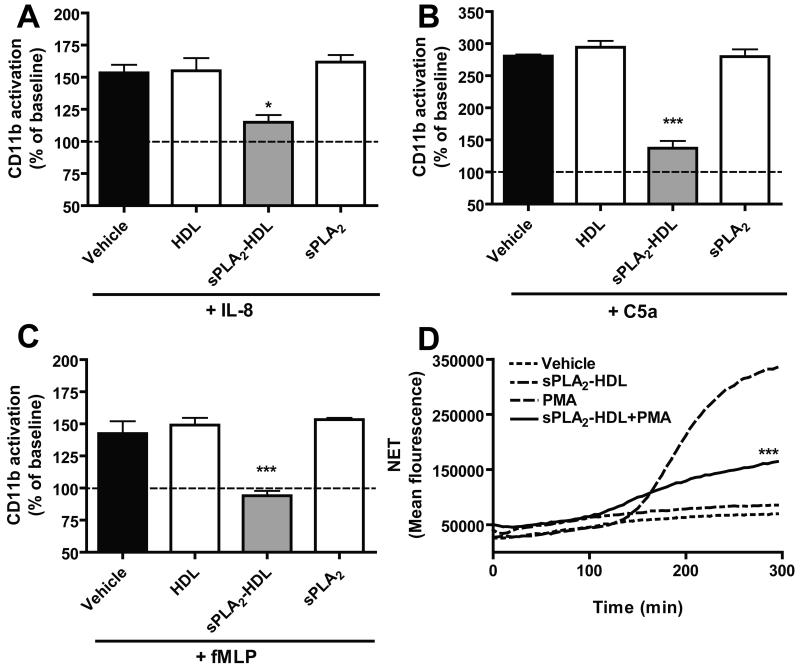
When stimulated, neutrophils display an increased activation of CD11b, which is an important integrin for leukocyte adhesion and migration. In order to further investigate the effects of sPLA2-HDL on neutrophil function, we measured CD11b activation. sPLA2-HDL potently inhibited CD11b activation induced by IL-8, fMLP and C5a (Figure 4A to 4C).
Figure 4. sPLA2-treated HDL inhibits CD11b activation in neutrophils.
Isolated neutrophils were preincubated with vehicle, HDL (100 μg protein/ml), sPLA2-HDL (100 μg protein/ml) or sPLA2 alone for 15 min at 37°C in the presence of antibody to the active epitope of CD11b and stimulated with (A) IL-8 (3 nmol/L), (B) fMLP (10 nmol/L) or (C) C5a (30 nmol/L) for 4 min at 37°C. CD11b activation was measured by flow cytometry. Vehicle treated (unstimulated) control was set as baseline and values are expressed as % over baseline. The results are shown as mean ± SD of 4 separate experiments using cells from different healthy donors. Statistical significance was assessed by one-way ANOVA with Tukey multiple-comparison post hoc test. *p < 0.05, **p < 0.01, ***p < 0.001 versus vehicle. (D) Isolated neutrophils were treated with vehicle or sPLA2-HDL (100 μg protein/ml) in the presence or absence of 10 nmol/L PMA and NET formation was measured as an increase in fluorescence of the SYTOX Green dye every 10 min for 5 hours at 37°C. Results show a representative experiment of three independent experiments. Values are expressed as mean fluorescence. *p < 0.05, **p < 0.01, ***p < 0.001 versus PMA at 5 hours time point.
Interestingly, LPS induced IL-8 production of neutrophils was not inhibited by sPLA2-HDL (Supplementary Figure VII). Neutrophils, along with other inflammatory cells, infiltrate atherosclerotic plaques. An intriguing role has recently been suggested for neutrophil extracellular trap (NET) formation in this process [35;36]. NETs are pro-inflammatory, antimicrobial structures consisting of extracellular chromatin decorated with granular and cytoplasmic proteins, such as myeloperoxidase and neutrophil elastase. Of particular interest, stimulation of NET formation with the protein kinase C activator phorbol 12-myristate 13-acetate was effectively suppressed by sPLA2-HDL (Figure 4D).
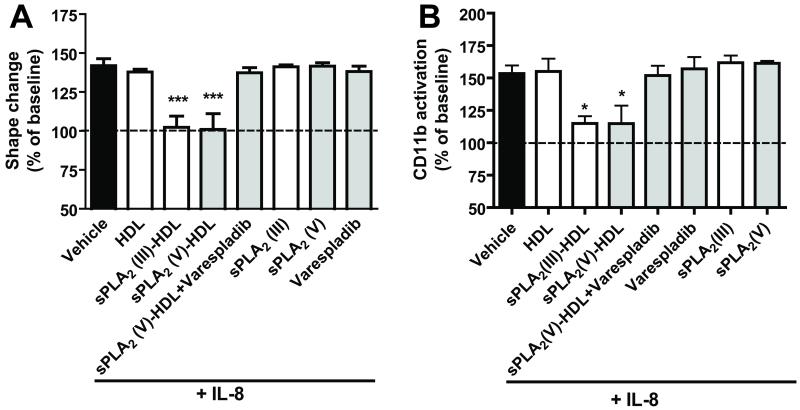
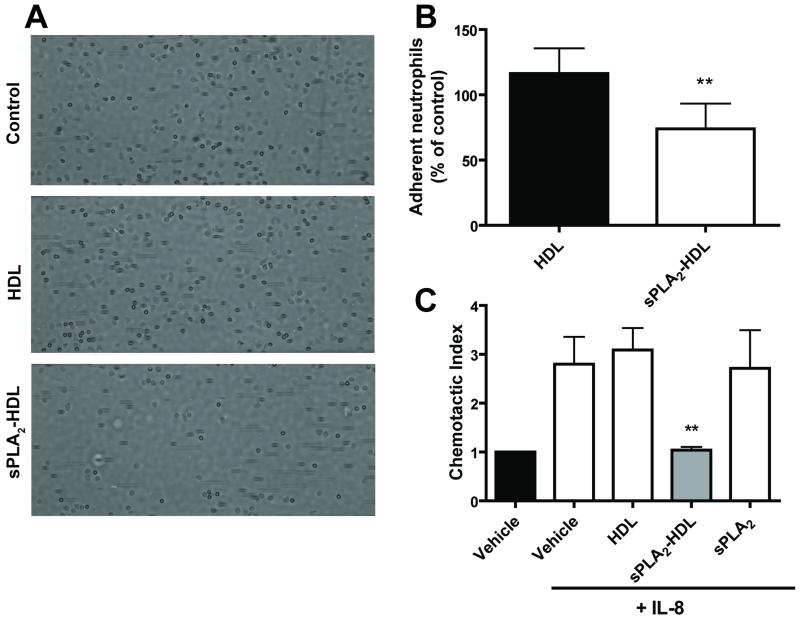
To show that the inhibition of neutrophil activation was dependent on the enzymatic activity of sPLA2, varespladib (an inhibitor of sPLA2 types IIA, V and X but not sPLA2 type III) was added during incubation of sPLA2 with HDL. In agreement with results above, varespladib prevented the effects of sPLA2 type V-treated HDL on neutrophil shape change and CD11b activation induced with IL-8 (Figure 5) and other agonists (Supplementary Figure VIII). To assess whether the sPLA2-HDL-induced inhibition of CD11b activation is associated with functional alterations, we examined neutrophil adhesion to intercellular adhesion molecule 1 coated surfaces under shear stress. sPLA2-HDL significantly reduced neutrophil adhesion under flow conditions (Figure 6A and 6B). Moreover, in accordance with these data, incubation of neutrophils with sPLA2-HDL potently inhibited neutrophil migratory response towards IL-8 (Figure 6C).
Figure 5. The sPLA2 inhibitor varespladib inhibits the effects of sPLA2-treated HDL on neutrophils.
HDL was incubated with 200 ng/ml sPLA2 type III or 400 ng/ml sPLA2 type V in the presence or absence of varespladib (1 μmol/L), overnight, at 37°C. These samples were used for neutrophil treatment. Isolated neutrophils were preincubated (15 min at 37°C) with vehicle (assay buffer), HDL (100 μg protein/ml), sPLA2 (III)-HDL (100 μg protein/ml), sPLA2 (V)-HDL, sPLA2 (V)-HDL + varespladib, varespadib, sPLA2 (III) or sPLA2 (V) alone. Neutrophils were then stimulated with IL-8 (3 nmol/L, 4 min, 37°C) to assess shape change (A) or CD11b activation (B) by flow cytometry. Values are expressed as % of baseline. Results represent means ± SD (n=3). Statistical significance was assessed by one-way ANOVA with Tukey multiple-comparison post hoc test. *p < 0.05, **p < 0.01, ***p < 0.001 versus vehicle.
Figure 6. sPLA2-HDL inhibits neutrophil adhesion under flow conditions and neutrophil migration.
Isolated human neutrophils were treated with vehicle (assay buffer), HDL (100 μg protein/ml) or sPLA2-HDL (100 μg protein/ml) for 15 minutes at 37°C before perfusion over ICAM-1 coated microchannels. (A) Representative images were taken 5 minutes after the start of the perfusion with neutrophils. (B) Images were quantified by computerized image analysis. Values are expressed as % of vehicle treated control. Data are shown as mean ± SD of 6 experiments with neutrophils from different donors. Statistical analysis was performed using Student’s t-test. *p < 0.05, **p < 0.01 versus HDL. (C) Neutrophils were preincubated with vehicle, HDL (100 μg protein/ml), sPLA2-HDL (100 μg protein/ml) or sPLA2 for 15 minutes at 37°C and seeded into the upper wells. Cells were allowed to migrate towards IL-8 (10 nmol/L) for 1 hour at 37°C. Cells that had migrated to the lower chamber were enumerated by flow cytometry. The results are shown as mean ± SD of 3 separate experiments using PMNL from different healthy donors. Statistical significance was assessed by one-way ANOVA with Tukey multiple-comparison post hoc test. *p < 0.05, **p < 0.01 versus vehicle.
3.4. sPLA2-HDL suppresses agonist induced Ca2+ flux and reduces lipid raft assembly
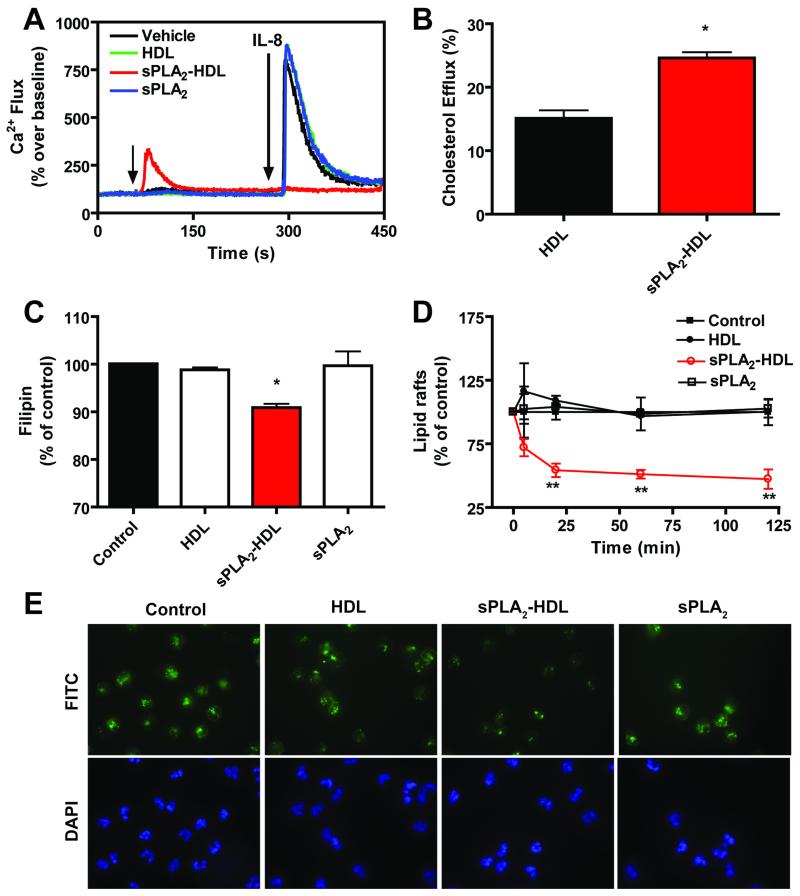
Cytoplasmic Ca2+ changes in neutrophils play a key role in a series of pathways leading to activation. Stimulation with IL-8 resulted in a marked rise in the intracellular Ca2+, which was effectively suppressed by sPLA2-HDL, whereas control HDL showed no effect (Figure 7A). Interestingly, we observed that sPLA2-HDL on its own moderately induced Ca2+ flux in unstimulated (resting) cells. To gain insights into the potential mechanism(s) involved in sPLA2-HDL effects on neutrophils, we next examined the involvement of cAMP, as previous investigations showed that lysophospholipids increase intracellular cAMP in neutrophils. However, we did not observe altered cAMP levels in neutrophils treated with sPLA2-HDL (Supplementary Figure IX). Native HDL differs from sPLA2-HDL in its lysophospholipid content, raising the possibility that the variant effects of these particles on neutrophil function may be related to their cholesterol efflux activity. Interestingly, cholesterol efflux capability of sPLA2-HDL was increased when compared to control HDL (Figure 7B), reflected by a significantly decreased membrane free cholesterol content in neutrophils as shown by filipin fluorescence staining (Figure 7C).
Figure 7. sPLA2-HDL abolishes agonist-induced Ca2+ flux and efficiently removes unesterified cholesterol from neutrophils.
(A) Baseline Ca2+ levels were measured for 1 min and then neutrophils were treated with vehicle, HDL (100 μg protein/ml), sPLA2-HDL (100 μg protein/ml) or sPLA2 for 4 min and Ca2+ flux was induced with IL-8 (10 nmol/L). Ca2+ flux was detected at room temperature by flow-cytometry as an increase in fluorescence intensity of the Ca2+-sensitive dye Fluo-3-AM in the FL-1 channel. Results show a representative of 4 independent experiments done with cells from different donors. Values are expressed as % over baseline. (B) Isolated neutrophils were preloaded with [3H]-cholesterol for 3 hours and incubated with HDL (100 μg protein/ml) or sPLA2-HDL (100 μg protein/ml protein) for 60 minutes, and percentage of cholesterol efflux was assessed. Cholesterol efflux is expressed as the radioactivity in the medium relative to total radioactivity in medium and cells. The results are shown as mean ± SD of three separate experiments using PMNL from different healthy donors. Statistical analysis was performed using Student’s t-test. *p<0.05, **p<0.01, ***p <0.001 versus HDL. (C) Neutrophils were incubated with vehicle, HDL (100 μg protein/ml), sPLA2-HDL (100 μg protein/ml), sPLA2 alone for 30 min at 37°C and stained with Filipin. The amount of unesterified cholesterol was assessed by flow-cytometry. Vehicle treated control was set at 100 % and values are expressed as % of control. Results are shown as mean ± SD (n=3). Statistical significance was assessed by one-way ANOVA with Tukey multiple-comparison post hoc test. *p < 0.05, **p < 0.01, ***p < 0.001 versus control. (D) Neutrophils were incubated with vehicle, HDL (100 μg protein/ml), sPLA2-HDL (100 μg protein/ml) or sPLA2 alone for 5 up to 120 min at 37°C. Lipid raft abundance was determined by assessing FITC-cholera toxin B stained cholesterol-containing microdomains by flow cytometry. Control was set at 100 % and values are expressed as % of vehicle-treated control. Results are shown as mean ± SD (n=3). Statistical significance was assessed by one-way ANOVA with Tukey multiple-comparison post hoc test. *p < 0.05, **p < 0.01 versus control. (E) Fluorescence microscopy analysis of lipid rafts (green staining). DAPI (blue staining) was used to visualize the nuclei. Original magnification X60 was used. Representative images from 3 independent experiments with neutrophils from different donors are shown.
Given that cholesterol-rich lipid rafts are known to play an important role in leukocyte activation, we investigated whether increased cholesterol efflux capability of sPLA2-HDL alters lipid raft abundance in neutrophils. For that purpose, FITC-labeled cholera toxin B, which binds to the raft constituent ganglioside GM1, was used to visualize lipid rafts. Indeed, we observed that sPLA2-HDL caused rapid depletion of cholera-toxin positive lipid rafts in neutrophils (Figure 7D, 7E). ABCA1 and SR-BI have been reported to localize selectively in lipid rafts or caveolae [37;38] and to be involved in cholesterol efflux. However, neither the ABCA1-inhibitor probucol [39] nor the SR-BI inhibitor BLT-1 [40] diminished sPLA2-HDL and native HDL mediated efflux from neutrophils, suggesting that the increased cholesterol efflux capability of sPLA2-HDL is independent of these receptors (Supplementary Figure X). Moreover, when cholesterol efflux experiments were performed with J774 macrophages, cholesterol efflux capability of sPLA2-HDL was not different compared with native HDL, suggesting a cell type specific effect (Supplementary Figure XI).
4. Discussion
The studies presented here demonstrate that sPLA2 modification of HDL generates an anti-inflammatory particle that has the ability to effectively alter neutrophil function. Of particular interest is the observation that sPLA2-HDL, but not control HDL, effectively depleted the free cholesterol content of neutrophils and suppressed agonist-induced rise in intracellular Ca2+ levels. It is now well established that alterations in membrane cholesterol content cause disruption of cholesterol-rich signaling microdomains integral to cellular signaling pathways known as lipid rafts [41]. Several lines of evidence suggest that HDL regulates the plasma membrane cholesterol content through the ability to remove cholesterol and other lipid species from cells, thereby dampening plasma membrane receptor signaling [42]. Our studies suggest that the increased cholesterol-mobilizing activity of sPLA2-HDL as well as the suppression of agonist-induced rise in intracellular Ca2+ levels are likely mechanism that counteracts activation of neutrophils, resulting in an effective inhibition of CD11b activation and NET formation. CD11b mediates inflammation by regulating leukocyte adhesion and migration and has been implicated in several immune processes such as phagocytosis, cell-mediated cytotoxicity, chemotaxis and cellular activation [43]. Importantly, sPLA2-HDL efficiently inhibited neutrophil adhesion to ICAM-1 coated surfaces under shear stress and potently inhibited neutrophil migratory response towards IL-8. Although NETs are able to trap and kill bacteria and their formation is an important first-line defense against various pathogens, excessive NET production may strongly promote inflammation, leading to endothelial damage, infiltration of further neutrophils into atheromatous lesions, thrombus formation and autoimmunity [35;36].
Of particular interest, we observed that the major sPLA2-HDL associated lysophospholipid, LPC 16:0, was the most effective, whereas other LPCs and free fatty acids showed weak or no inhibitory effects. Given that groups IIA, V and X show a differential hydrolysis of molecular species of phosphatidylcholine [44], sPLA2 modified HDLs with various activities are expected to be formed under inflammatory conditions.
Notably, treatment with native HDL showed only minimal effects on neutrophil function despite its ability to efflux cholesterol. Our results suggest that bidirectional lipid flux (efflux of cholesterol is accompanied by influx of HDL-cholesterylester) mediated by control HDL results in minimal net change in the cholesterol content of the neutrophil membrane as observed by filipin staining. We assume that lysophospholipid-enrichment of HDL generates a particle that more efficiently depletes neutrophil cholesterol. In line with our finding is the observation that that lysophospholipids rapidly enter lipid rafts in the cell membrane promoting raft fission [45] and promote net cholesterol efflux [46], which might explain the increase in cholesterol efflux properties of sPLA2-HDL. In line with that notion, sPLA2-HDL preparations, but not control HDL, markedly disrupted neutrophil lipid rafts. Interestingly, the increased ability of sPLA2-HDL to mobilize cell cholesterol seems to be independent of ABCA1 and SR-BI, proteins known to mediate cholesterol efflux (Supplementary figure X). Moreover, the improved cholesterol efflux ability of sPLA2-HDL strikingly differs between cell types, given that sPLA2-HDL mediated cholesterol efflux of from J774 macrophages was not different from native HDL (Supplementary figure XI). The reason for that surprising observation is not clear, but different lipid raft content and/or lipid raft composition of freshly isolated neutrophils compared with a macrophage cell line might be an explanation.
Of particular interest is the recent observation that a large fraction of phosphatidylcholine moiety of administered reconstituted HDL is rapidly hydrolyzed by sPLA2 isoforms in blood and causes a remarkable increase of HDL-associated lysophospholipid content reaching levels of 300 μmol/L 4 hours post administration [47;48], which raises the possibility that formed lysophospholipids contributed to the potent anti-inflammatory activity of administered reconstituted HDL.
Previous studies supported an important role for a subset of sPLA2 isoforms (sPLA2-IIA, -III, -V, and -X) from initiation and progression to cardiovascular complications in the pathophysiology of atherosclerosis [49]. Hydrolysis of phosphatidylcholine in low-density lipoprotein by sPLA2 isoforms has been implicated in the promotion of atherosclerosis [50;51]. These observations have stimulated interest in the potential value of sPLA2 inhibition as a cardioprotective strategy. Initial studies demonstrated that varespladib, a nonspecific pan-sPLA2 inhibitor, reduced activity of sPLA2-IIA by more than 90%, in addition to lowering low-density-lipoprotein cholesterol and C-reactive protein in patients with stable coronary disease and acute coronary syndrome. Surprisingly, varespladib-induced sPLA2 inhibition did not reduce cardiovascular ischemic complications and resulted in an excess rate of myocardial infarction and stroke in patients with acute coronary syndromes [8]. Moreover, inhibition of sPLA2 showed no overall survival benefit in septic patients [9]. Interestingly, also a large phase III study testing an inhibitor of lipoprotein-associated phospholipase A2 has failed to lower the risk of cardiovascular events in coronary heart disease patients [52], providing further evidence that the physiologic and pathophysiologic roles of phospholipases are not well understood.
A potential limitation of the present study is that we only assessed the impact of sPLA2 on HDL functionality, while in vivo HDL particles are subjected to an array of compositional changes and factors acting in concert. Hydrolysis of HDL phospholipids by sPLA2 alters the structure and composition of HDL particles with the metabolic consequences of increased catabolism. Experimental studies have shown that the addition of LPS to plasma resulted in a marked decrease in cholesterol ester transfer protein activity, leading to changes in HDL levels considered as an adaptive response to preserve or increase HDL [53]. But also other factors alter HDL composition and function under inflammatory conditions. The concentrations of the acute phase protein SAA markedly increase during the acute-phase reaction. SAA associates with HDL, altering structure and function of HDL [54]. Interestingly, overexpression of SAA in the absence of generalized inflammation does not result in changes of HDL cholesterol or apoA-I levels in vivo [55] whereas overexpression of sPLA2 results in alterations of HDL metabolism even in the absence of generalized inflammation [56]. Although increased sPLA2 and decreased CETP activities might be beneficial for host defense in the setting of acute infection, replacement of HDL-apolipoproteins by SAA might contribute to increased incidence of cardiovascular disease [54].
In conclusion, our data provide novel evidence that the increased cholesterolmobilizing activity and suppression of rise in intracellular Ca2+ levels of sPLA2-HDL might effectively counteract agonist-induced activation of neutrophils. Our results raise the possibility that sPLA2-induced modification of HDL composition and function modulates neutrophil trafficking and effector responses during inflammation.
Supplementary Material
Acknowledgements
This work was supported by the Austrian Science Fund FWF (Grant P22976-B18 to G.M., P22521-B18 to A.H., P25633 to R.S.) and the Jubiläumsfonds of the Austrian National Bank (14263 to A.H. and 14853 to G.M.). S. Curcic was supported by the PhD-Program MolMed of the Medical University Graz. R. Frei and L. Pasterk were funded by the PhD Program DK-MOLIN (FWF - W1241).
Abbreviations
- C5a
complement component 5a
- FFA
free fatty acid
- fMLP
N-formyl-lmethionyl-l-leucyl-l-phenylalanine
- HDL
high-density lipoprotein
- ICAM-1
intracellular adhesion molecule-1
- IL-8
interleukin 8
- NET
neutrophil extracellular traps
- LPC
lysophosphatidylcholine
- PC
phosphatidylcholine
- PMNL
polymorphonuclear leukocytes
- sPLA2
secretory phospholipase A2
- sPLA2-HDL
secretory phospholipase A2 treated HDL
Footnotes
Conflict of Interest
None declared.
References
- [1].Vadas P, Pruzanski W. Induction of group II phospholipase A2 expression and pathogenesis of the sepsis syndrome. Circ.Shock. 1993;39:160–167. [PubMed] [Google Scholar]
- [2].Murakami M, Lambeau G. Emerging roles of secreted phospholipase A(2) enzymes: an update. Biochimie. 2013;95:43–50. doi: 10.1016/j.biochi.2012.09.007. [DOI] [PubMed] [Google Scholar]
- [3].Nevalainen TJ. Serum phospholipases A2 in inflammatory diseases. Clin.Chem. 1993;39:2453–2459. [PubMed] [Google Scholar]
- [4].Lin MK, Farewell V, Vadas P, Bookman AA, Keystone EC, Pruzanski W. Secretory phospholipase A2 as an index of disease activity in rheumatoid arthritis. Prospective double blind study of 212 patients. J.Rheumatol. 1996;23:1162–1166. [PubMed] [Google Scholar]
- [5].Pruzanski W, Vadas P. Secretory synovial fluid phospholipase A2 and its role in the pathogenesis of inflammation in arthritis. J.Rheumatol. 1988;15:1601–1603. [PubMed] [Google Scholar]
- [6].Koenig W, Vossen CY, Mallat Z, Brenner H, Benessiano J, Rothenbacher D. Association between type II secretory phospholipase A2 plasma concentrations and activity and cardiovascular events in patients with coronary heart disease. Eur.Heart J. 2009;30:2742–2748. doi: 10.1093/eurheartj/ehp302. [DOI] [PubMed] [Google Scholar]
- [7].Rosenson RS, Hurt-Camejo E. Phospholipase A2 enzymes and the risk of atherosclerosis. Eur.Heart J. 2012;33:2899–2909. doi: 10.1093/eurheartj/ehs148. [DOI] [PubMed] [Google Scholar]
- [8].Nicholls SJ, Kastelein JJ, Schwartz GG, Bash D, Rosenson RS, Cavender MA, Brennan DM, Koenig W, Jukema JW, Nambi V, Wright RS, Menon V, Lincoff AM, Nissen SE. VISTA-16 Investigators, Varespladib and cardiovascular events in patients with an acute coronary syndrome: the VISTA-16 randomized clinical trial. JAMA. 2014;311:252–262. doi: 10.1001/jama.2013.282836. [DOI] [PubMed] [Google Scholar]
- [9].Abraham E, Naum C, Bandi V, Gervich D, Lowry SF, Wunderink R, Schein RM, Macias W, Skerjanec S, Dmitrienko A, Farid N, Forgue ST, Jiang F. Efficacy and safety of LY315920Na/S-5920, a selective inhibitor of 14-kDa group IIA secretory phospholipase A2, in patients with suspected sepsis and organ failure. Crit.Care Med. 2003;31:718–728. doi: 10.1097/01.CCM.0000053648.42884.89. [DOI] [PubMed] [Google Scholar]
- [10].Gijon MA, Perez C, Mendez E, Crespo M. Sanchez. Phospholipase A2 from plasma of patients with septic shock is associated with high-density lipoproteins and C3 anaphylatoxin: some implications for its functional role. Biochem.J. 1995;306(Pt 1):167–175. doi: 10.1042/bj3060167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Dashti M, Kulik W, Hoek F, Veerman EC, Peppelenbosch MP, Rezaee F. A phospholipidomic analysis of all defined human plasma lipoproteins. Sci.Rep. 2011;1:139. doi: 10.1038/srep00139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Catapano AL, Pirillo A, Bonacina F, Norata GD. HDL in innate and adaptive immunity. Cardiovasc.Res. 2014;103:372–383. doi: 10.1093/cvr/cvu150. [DOI] [PubMed] [Google Scholar]
- [13].Marsche G, Saemann MD, Heinemann A, Holzer M. Inflammation alters HDL composition and function: implications for HDL-raising therapies. Pharmacol.Ther. 2013;137:341–351. doi: 10.1016/j.pharmthera.2012.12.001. [DOI] [PubMed] [Google Scholar]
- [14].Birner-Gruenberger R, Schittmayer M, Holzer M, Marsche G. Understanding high-density lipoprotein function in disease: Recent advances in proteomics unravel the complexity of its composition and biology. Prog.Lipid Res. 2014;56C:36–46. doi: 10.1016/j.plipres.2014.07.003. [DOI] [PubMed] [Google Scholar]
- [15].Tietge UJ, Maugeais C, Lund-Katz S, Grass D, deBeer FC, Rader DJ. Human secretory phospholipase A2 mediates decreased plasma levels of HDL cholesterol and apoA-I in response to inflammation in human apoA-I transgenic mice. Arterioscler.Thromb.Vasc.Biol. 2002;22:1213–1218. doi: 10.1161/01.atv.0000023228.90866.29. [DOI] [PubMed] [Google Scholar]
- [16].Leber B, Spindelboeck W, Stadlbauer V. Infectious complications of acute and chronic liver disease. Semin.Respir.Crit.Care.Med. 2012;33:80–95. doi: 10.1055/s-0032-1301737. [DOI] [PubMed] [Google Scholar]
- [17].Donnelly LE, Barnes PJ. Defective phagocytosis in airways disease. Chest. 2012;141:1055–1062. doi: 10.1378/chest.11-2348. [DOI] [PubMed] [Google Scholar]
- [18].Guasti L, Dentali F, Castiglioni L, Maroni L, Marino F, Squizzato A, Ageno W, Gianni M, Gaudio G, Grandi AM, Cosentino M, Venco A. Neutrophils and clinical outcomes in patients with acute coronary syndromes and/or cardiac revascularisation. A systematic review on more than 34,000 subjects. Thromb.Haemost. 2011;106:591–599. doi: 10.1160/TH11-02-0096. [DOI] [PubMed] [Google Scholar]
- [19].Medzhitov R, Janeway C., Jr Innate immunity. N.Engl.J.Med. 2000;343:338–344. doi: 10.1056/NEJM200008033430506. [DOI] [PubMed] [Google Scholar]
- [20].Soehnlein O. Multiple roles for neutrophils in atherosclerosis. Circ.Res. 2012;110:875–888. doi: 10.1161/CIRCRESAHA.111.257535. [DOI] [PubMed] [Google Scholar]
- [21].Holzer M, Trieb M, Konya V, Wadsack C, Heinemann A, Marsche G. Aging affects high-density lipoprotein composition and function. Biochim.Biophys.Acta. 2013;1831:1442–1448. doi: 10.1016/j.bbalip.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Holzer M, Zangger K, El-Gamal D, Binder V, Curcic S, Konya V, Schuligoi R, Heinemann A, Marsche G. Myeloperoxidase-derived chlorinating species induce protein carbamylation through decomposition of thiocyanate and urea: novel pathways generating dysfunctional high-density lipoprotein. Antioxid.Redox Signal. 2012;17:1043–1052. doi: 10.1089/ars.2011.4403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Holzer M, Wolf P, Inzinger M, Trieb M, Curcic S, Pasterk L, Weger W, Heinemann A, Marsche G. Anti-psoriatic therapy recovers high-density lipoprotein composition and function. J.Invest.Dermatol. 2014;134:635–642. doi: 10.1038/jid.2013.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].BLIGH EG, DYER WJ. A rapid method of total lipid extraction and purification. Can.J.Biochem.Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- [25].Schratl P, Royer JF, Kostenis E, Ulven T, Sturm EM, Waldhoer M, Hoefler G, Schuligoi R, Lippe IT, Peskar BA, Heinemann A. The role of the prostaglandin D2 receptor, DP, in eosinophil trafficking. J.Immunol. 2007;179:4792–4799. doi: 10.4049/jimmunol.179.7.4792. [DOI] [PubMed] [Google Scholar]
- [26].Balenga NA, Aflaki E, Kargl J, Platzer W, Schroder R, Blattermann S, Kostenis E, Brown AJ, Heinemann A, Waldhoer M. GPR55 regulates cannabinoid 2 receptor-mediated responses in human neutrophils. Cell Res. 2011;21:1452–1469. doi: 10.1038/cr.2011.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Luschnig-Schratl P, Sturm EM, Konya V, Philipose S, Marsche G, Frohlich E, Samberger C, Lang-Loidolt D, Gattenlohner S, Lippe IT, Peskar BA, Schuligoi R, Heinemann A. EP4 receptor stimulation down-regulates human eosinophil function. Cell Mol.Life Sci. 2011;68:3573–3587. doi: 10.1007/s00018-011-0642-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Sabroe I, Hartnell A, Jopling LA, Bel S, Ponath PD, Pease JE, Collins PD, Williams TJ. Differential regulation of eosinophil chemokine signaling via CCR3 and non-CCR3 pathways. J.Immunol. 1999;162:2946–2955. [PubMed] [Google Scholar]
- [29].Konya V, Blattermann S, Jandl K, Platzer W, Ottersbach PA, Marsche G, Gutschow M, Kostenis E, Heinemann A. A Biased Non-Galphai OXE-R Antagonist Demonstrates That Galphai Protein Subunit Is Not Directly Involved in Neutrophil, Eosinophil, and Monocyte Activation by 5-Oxo-ETE. J.Immunol. 2014;192:4774–4782. doi: 10.4049/jimmunol.1302013. [DOI] [PubMed] [Google Scholar]
- [30].Konya V, Philipose S, Balint Z, Olschewski A, Marsche G, Sturm EM, Schicho R, Peskar BA, Schuligoi R, Heinemann A. Interaction of eosinophils with endothelial cells is modulated by prostaglandin EP4 receptors. Eur.J.Immunol. 2011;41:2379–2389. doi: 10.1002/eji.201141460. [DOI] [PubMed] [Google Scholar]
- [31].Sturm GJ, Schuligoi R, Sturm EM, Royer JF, Lang-Loidolt D, Stammberger H, Amann R, Peskar BA, Heinemann A. 5-Oxo-6,8,11,14-eicosatetraenoic acid is a potent chemoattractant for human basophils. J.Allergy Clin.Immunol. 2005;116:1014–1019. doi: 10.1016/j.jaci.2005.08.001. [DOI] [PubMed] [Google Scholar]
- [32].Heinemann A, Ofner M, Amann R, Peskar BA. A novel assay to measure the calcium flux in human basophils: effects of chemokines and nerve growth factor. Pharmacology. 2003;67:49–54. doi: 10.1159/000066786. [DOI] [PubMed] [Google Scholar]
- [33].Kirchner T, Moller S, Klinger M, Solbach W, Laskay T, Behnen M. The impact of various reactive oxygen species on the formation of neutrophil extracellular traps. Mediators Inflamm. 2012;2012:849136. doi: 10.1155/2012/849136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Schratl P, Heinemann A. Differential involvement of Ca2+ and actin filament in leukocyte shape change. Pharmacology. 2009;83:131–140. doi: 10.1159/000186498. [DOI] [PubMed] [Google Scholar]
- [35].Doring Y, Drechsler M, Wantha S, Kemmerich K, Lievens D, Vijayan S, Gallo RL, Weber C, Soehnlein O. Lack of neutrophil-derived CRAMP reduces atherosclerosis in mice. Circ.Res. 2012;110:1052–1056. doi: 10.1161/CIRCRESAHA.112.265868. [DOI] [PubMed] [Google Scholar]
- [36].Doring Y, Manthey HD, Drechsler M, Lievens D, Megens RT, Soehnlein O, Busch M, Manca M, Koenen RR, Pelisek J, Daemen MJ, Lutgens E, Zenke M, Binder CJ, Weber C, Zernecke A. Auto-antigenic protein-DNA complexes stimulate plasmacytoid dendritic cells to promote atherosclerosis. Circulation. 2012;125:1673–1683. doi: 10.1161/CIRCULATIONAHA.111.046755. [DOI] [PubMed] [Google Scholar]
- [37].Peng Y, Akmentin W, Connelly MA, Lund-Katz S, Phillips MC, Williams DL. Scavenger receptor BI (SR-BI) clustered on microvillar extensions suggests that this plasma membrane domain is a way station for cholesterol trafficking between cells and high-density lipoprotein. Mol.Biol.Cell. 2004;15:384–396. doi: 10.1091/mbc.E03-06-0445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Drobnik W, Borsukova H, Bottcher A, Pfeiffer A, Liebisch G, Schutz GJ, Schindler H, Schmitz G. Apo AI/ABCA1-dependent and HDL3-mediated lipid efflux from compositionally distinct cholesterol-based microdomains. Traffic. 2002;3:268–278. doi: 10.1034/j.1600-0854.2002.030404.x. [DOI] [PubMed] [Google Scholar]
- [39].Favari E, Zanotti I, Zimetti F, Ronda N, Bernini F, Rothblat GH. Probucol inhibits ABCA1-mediated cellular lipid efflux. Arterioscler.Thromb.Vasc.Biol. 2004;24:2345–2350. doi: 10.1161/01.ATV.0000148706.15947.8a. [DOI] [PubMed] [Google Scholar]
- [40].Nieland TJ, Penman M, Dori L, Krieger M, Kirchhausen T. Discovery of chemical inhibitors of the selective transfer of lipids mediated by the HDL receptor SR-BI. Proc.Natl.Acad.Sci.U.S.A. 2002;99:15422–15427. doi: 10.1073/pnas.222421399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Simons K, Ikonen E. Functional rafts in cell membranes. Nature. 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- [42].Feig JE, Shamir R, Fisher EA. Atheroprotective effects of HDL: beyond reverse cholesterol transport. Curr.Drug Targets. 2008;9:196–203. doi: 10.2174/138945008783755557. [DOI] [PubMed] [Google Scholar]
- [43].Solovjov DA, Pluskota E, Plow EF. Distinct roles for the alpha and beta subunits in the functions of integrin alphaMbeta2. J.Biol.Chem. 2005;280:1336–1345. doi: 10.1074/jbc.M406968200. [DOI] [PubMed] [Google Scholar]
- [44].Pruzanski W, Lambeau L, Lazdunsky M, Cho W, Kopilov J, Kuksis A. Differential hydrolysis of molecular species of lipoprotein phosphatidylcholine by groups IIA, V and X secretory phospholipases A2. Biochim.Biophys.Acta. 2005;1736:38–50. doi: 10.1016/j.bbalip.2005.07.005. [DOI] [PubMed] [Google Scholar]
- [45].Tanaka T, Sano R, Yamashita Y, Yamazaki M. Shape changes and vesicle fission of giant unilamellar vesicles of liquid-ordered phase membrane induced by lysophosphatidylcholine. Langmuir. 2004;20:9526–9534. doi: 10.1021/la049481g. [DOI] [PubMed] [Google Scholar]
- [46].Hara S, Shike T, Takasu N, Mizui T. Lysophosphatidylcholine promotes cholesterol efflux from mouse macrophage foam cells. Arterioscler.Thromb.Vasc.Biol. 1997;17:1258–1266. doi: 10.1161/01.atv.17.7.1258. [DOI] [PubMed] [Google Scholar]
- [47].Levels JH, Pajkrt D, Schultz M, Hoek FJ, van Tol A, Meijers JC, van Deventer SJ. Alterations in lipoprotein homeostasis during human experimental endotoxemia and clinical sepsis. Biochim.Biophys.Acta. 2007;1771:1429–1438. doi: 10.1016/j.bbalip.2007.10.001. [DOI] [PubMed] [Google Scholar]
- [48].Drew BG, Carey AL, Natoli AK, Formosa MF, Vizi D, Reddy-Luthmoodoo M, Weir JM, Barlow CK, van Hall G, Meikle PJ, Duffy SJ, Kingwell BA. Reconstituted high-density lipoprotein infusion modulates fatty acid metabolism in patients with type 2 diabetes mellitus. J.Lipid Res. 2011;52:572–581. doi: 10.1194/jlr.P012518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Mallat Z, Lambeau G, Tedgui A. Lipoprotein-associated and secreted phospholipases A(2) in cardiovascular disease: roles as biological effectors and biomarkers. Circulation. 2010;122:2183–2200. doi: 10.1161/CIRCULATIONAHA.110.936393. [DOI] [PubMed] [Google Scholar]
- [50].Sato H, Kato R, Isogai Y, Saka G, Ohtsuki M, Taketomi Y, Yamamoto K, Tsutsumi K, Yamada J, Masuda S, Ishikawa Y, Ishii T, Kobayashi T, Ikeda K, Taguchi R, Hatakeyama S, Hara S, Kudo I, Itabe H, Murakami M. Analyses of group III secreted phospholipase A2 transgenic mice reveal potential participation of this enzyme in plasma lipoprotein modification, macrophage foam cell formation, and atherosclerosis. J.Biol.Chem. 2008;283:33483–33497. doi: 10.1074/jbc.M804628200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Boyanovsky BB, van der Westhuyzen DR, Webb NR. Group V secretory phospholipase A2-modified low density lipoprotein promotes foam cell formation by a SR-A- and CD36-independent process that involves cellular proteoglycans. J.Biol.Chem. 2005;280:32746–32752. doi: 10.1074/jbc.M502067200. [DOI] [PubMed] [Google Scholar]
- [52].O'Donoghue ML, Braunwald E, White HD, Steen DP, Lukas MA, Tarka E, Steg PG, Hochman JS, Bode C, Maggioni AP, Im K, Shannon JB, Davies RY, Murphy SA, Crugnale SE, Wiviott SD, Bonaca MP, Watson DF, Weaver WD, Serruys PW, Cannon CP, SOLID-TIMI 52 Investigators Effect of darapladib on major coronary events after an acute coronary syndrome: the SOLID-TIMI 52 randomized clinical trial. JAMA. 2014;312:1006–1015. doi: 10.1001/jama.2014.11061. [DOI] [PubMed] [Google Scholar]
- [53].Masucci-Magoulas L, Moulin P, Jiang XC, Richardson H, Walsh A, Breslow JL, Tall A. Decreased cholesteryl ester transfer protein (CETP) mRNA and protein and increased high density lipoprotein following lipopolysaccharide administration in human CETP transgenic mice. J.Clin.Invest. 1995;95:1587–1594. doi: 10.1172/JCI117832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Artl A, Marsche G, Lestavel S, Sattler W, Malle E. Role of serum amyloid A during metabolism of acute-phase HDL by macrophages. Arterioscler.Thromb.Vasc.Biol. 2000;20:763–772. doi: 10.1161/01.atv.20.3.763. [DOI] [PubMed] [Google Scholar]
- [55].Hosoai H, Webb NR, Glick JM, Tietge UJ, Purdom MS, de Beer FC, Rader DJ. Expression of serum amyloid A protein in the absence of the acute phase response does not reduce HDL cholesterol or apoA-I levels in human apoA-I transgenic mice. J.Lipid Res. 1999;40:648–653. [PubMed] [Google Scholar]
- [56].Tietge UJ, Maugeais C, Cain W, Grass D, Glick JM, de Beer FC, Rader DJ. Overexpression of secretory phospholipase A(2) causes rapid catabolism and altered tissue uptake of high density lipoprotein cholesteryl ester and apolipoprotein A-I. J.Biol.Chem. 2000;275:10077–10084. doi: 10.1074/jbc.275.14.10077. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.