Abstract
Epidemiological and clinical studies have shown a consistent association of psoriasis with systemic metabolic disorders including an increased prevalence of diabetes, obesity and cardiovascular disease. Psoriasis is accompanied by systemic inflammation and low levels of high-density lipoprotein (HDL)-cholesterol. Recent studies provided clear evidence that psoriasis affects HDL composition and function. HDL isolated from psoriatic patients showed a significantly impaired capability to mobilize cholesterol from macrophages, a crucial step in reverse cholesterol transport and markedly lower paraoxonase activity, a protein that co-transports with HDL in serum with well-known anti-atherogenic properties. Of particular interest, successful anti-psoriatic therapy significantly improved HDL composition and function independently of serum HDL-cholesterol levels. These novel findings suggest that the conventional approaches of evaluating cardiovascular risk in psoriasis may be in need of refinement. As these data argue for a loss of beneficial activities of HDL in psoriatic patients, altered HDL functionality should be considered when evaluating the lipid status of patients.
Keywords: psoriasis, high-density lipoprotein, proteomics, inflammation, cholesterol efflux, cardiovascular disease
Introduction
Psoriasis is a widespread chronic inflammatory disease which affects about 2 – 3% of the population. Conventional cardiovascular risk factors such as obesity, hypertension and diabetes are more prevalent in psoriasis, but the association with cardiovascular events persists even after adjusting for these factors in large, population-based studies (1,2). Interestingly, the genetic control of psoriasis is relatively distinct from that of metabolic syndrome and coronary artery disease (3). A large number of studies have assessed serum lipid levels in patients with psoriasis (4-6). Increased odds of raised triglyceride levels and serum glucose were seen in individuals with psoriasis independent of the effects of obesity (7) and decreased HDL cholesterol was observed in patients with psoriasis (4-6). Lipid alterations induced by anti-psoriatic treatment resulted in contradictory results so far. A significant increase in triglyceride levels after antitumor necrosis factor-α therapy (infliximab) were reported in two studies (8,9). Decreased HDL-cholesterol concentrations in psoriatic patients during infliximab therapy were reported in one study (9), whereas others reported no change (8) or even significant increases in HDL-cholesterol levels during therapy (10).
Studies in animals have consistently provided evidence that HDL is beneficial on numerous processes involved in atherosclerosis, mainly by mediating the removal of cholesterol from lipid-laden macrophages and other cells in a process called reverse cholesterol transport. In addition, there is mounting evidence that HDL exerts additional anti-atherosclerotic effects, such as anti-oxidative activities (11). However, the causal mechanisms by which HDL impacts cardiovascular health remain complex and not fully understood. This is highlighted by the surprising observation that therapies based on increasing HDL-cholesterol in humans have been largely unsuccessful and genetic analysis failed to show a causal association between genetically raised plasma HDL-cholesterol levels and risk for myocardial infarction prompting the suggestion that the composition and function of HDL may be more important to disease outcome than the quantity of HDL itself (12,13).
Circulating HDL-cholesterol concentrations provide limited information regarding atheroprotective functionality of HDL
Given that the functional heterogeneity inherent to plasma HDL is in large part driven by its compositional diversity, these changes are not revealed by measurement of HDL-cholesterol concentrations (14-16). Circulating HDL-cholesterol concentrations provide no information regarding the anti-inflammatory, anti-oxidant, anti-thrombotic, and endothelial function promoting activities of HDL, despite increasing evidence supporting the clinical significance of its pleiotropic functions (16-19). It is becoming increasingly apparent that direct measures of HDL composition and metrics of functionality are needed. In line with that assumption is the recent observation that the inverse relationship of HDL cholesterol with cardiovascular mortality is markedly weakened in patients with coronary artery disease (20) and that HDL cholesterol concentration is not an appropriate biomarker in the secondary prevention setting. Abnormalities in lipoprotein particle size were seen in patients with psoriasis (21,22). Of particular interest, vascular inflammation was observed to be associated with decreased concentration of large HDL particles and increased concentration of small LDL and HDL particles. The association of total HDL particle concentration and small HDL particles with vascular inflammation remained robust after multivariate analysis adjusting for traditional cardiovascular risk factors, including age, gender, blood pressure, and LDL and HDL cholesterol (21). These findings suggest that HDL composition, but not HDL cholesterol, may be linked with vascular inflammation in psoriasis. Therefore, the usefulness of considering HDL-cholesterol for cardiovascular risk stratification seems limited in such patients and more informative markers for HDL are needed.
Proteomics technology have extended our knowledge of proteins carried by HDL
Latest progresses in proteomics technology have extended our knowledge of proteins carried by HDL (14,16,18,23). In addition to HDL-associated proteins with well-established functions in lipid transport, HDL-proteins exist with numerous other functions including serine protease inhibitors, proteins involved in the immune response, as well as numerous members of the complement system and its associated proteolysis inhibitors like apoJ and vitronectin (14). Also clearly represented are acute-phase response proteins such as SAA and LPS-binding protein. Surprisingly, HDL also carries proteins involved in heme and iron metabolism such as hemoglobin, transferrin and hemopexin as well as those with a host of additional and enigmatic functions ranging from platelet regulation to vitamin binding and transport (23-26). More recently, several research groups have started to assess whether HDL proteomic composition is altered in coronary artery disease (23,27,28) chronic kidney disease (26,29), rheumatoid arthritis (30), type 2 diabetes (31), psoriasis (25) and aging (24). All studies reported multiple alterations in the protein composition of HDL.
Psoriasis alters HDL composition
It is of particular interest to assess the effects of psoriasis on HDL composition and function, as the disease is generally characterized by typical lesions on the skin, but also affects the entire organism by maintaining a low-grade systemic inflammatory status (32).
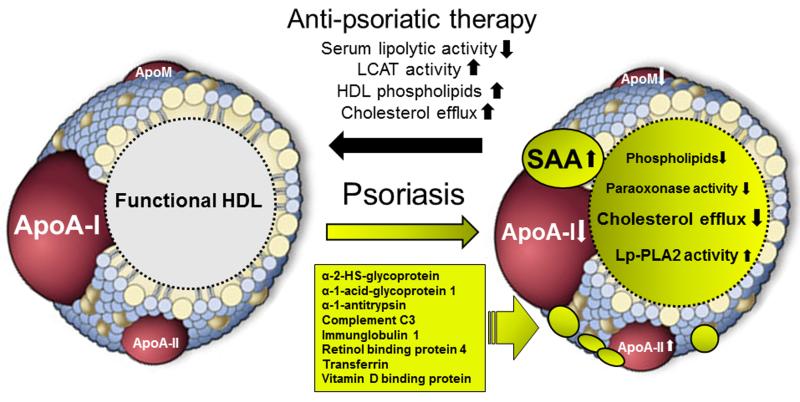
In recent studies shotgun proteomic profiling and biochemical analyses were applied to investigate psoriasis-associated alterations in the HDL proteome and lipid composition (13,25). As summarized in the table and depicted in the figure, psoriatic HDL contains markedly reduced levels of apoA-I and apoM, whereas apoA-II and several acute phase proteins like SAA, α-1-antitrypsin, prothrombin and α-1-acid-glycoprotein 1 are significantly increased. In addition, proteins involved in complement activation like complement C3 and hemoglobin and its scavenger protein haptoglobin are also enriched in psoriatic HDL. Psoriasis is not only associated with a remodelled HDL proteome, marked alterations in the phospholipid and cholesterol content of psoriatic HDL were observed.
Table. Proteomic alterations of HDL from psoriatic patients.
| Proteins | Known or suggested functions | Alteration |
|---|---|---|
| apoA-I |
|
⇩ |
| apoA-II |
|
⇧ |
| apoM |
|
⇩ |
| apoF |
|
⇩ |
| α-2-HS-glycoprotein |
|
⇧ ⇧ |
| α-1-acid-glycoprotein 1 |
|
⇧ ⇧ |
| α-1-antitrypsin |
|
⇧ ⇧ |
| Complement C3 |
|
⇧ ⇧ |
| IgG-1 |
|
⇧ ⇧ |
| Platelet basic protein |
|
⇧ |
| Prothrombin |
|
⇧ |
| Retinol BP 4 |
|
⇧ ⇧ |
| Serum amyloid A1 |
|
⇧ ⇧ |
| Serum amyloid A4 |
|
⇩ ⇩ |
| Transferrin |
|
⇧ ⇧ |
| Vitamin D BP |
|
⇧ ⇧ |
Shown are HDL-associated proteins which were significantly altered in psoriasis (25).
BP; binding protein, apo; apoprotein ⇧ increased; ⇧⇧ highly increased; ⇩ decreased;⇩⇩ strongly decreased
Figure. Schematic illustration of HDL remodeling in psoriasis.
HDL from psoriatic patients exhibits various alterations in the proteome and lipid composition that are linked to impaired cholesterol acceptor capacity and low paraoxonase activity. Psoriatic HDL is significantly enriched in SAA1/2, apoA-II, as well as low-abundant HDL-associated proteins. These proteomic alterations were accompanied by the loss of apoA-I and apoM. In addition, psoriasis leads to profound alterations in HDL-lipid composition reflected by a decrease in HDL-phospholipids. Effective anti-psoriatic therapy increased lecithin-cholesterol acyltransferase activit (LCAT), lowers serum lipolytic activity thereby recovering HDL function.
Psoriasis affects HDL composition and function
Probably the most relevant factor regarding atheroprotection is cholesterol efflux from macrophages, although it only presents a small fraction of the overall flux through reverse cholesterol transport (33,34). The assessment of HDL-promoted cholesterol efflux has gained considerable attention in recent years, since a clinical study clearly demonstrated that cholesterol efflux capability of HDL is a better predictor of cardiovascular risk than levels of HDL-cholesterol or LDL-cholesterol (35). Isolated HDL from patients with rheumatoid arthritis or chronic kidney disease is less potent in promoting cholesterol efflux independent on serum HDL levels (29,36). A current meta-analysis revealed that mild systemic inflammation of psoriatic patients is independent of age, sex, disease severity and type (37). Interestingly, moderate psoriasis (less than 10 percent of the body affected by psoriasis) and mild systemic inflammation is sufficient to markedly affect the ability of HDL to promote cholesterol efflux from macrophages (13,22,25). Low apolipoprotein A-I, phosphatidylcholine and sphingomyelin content of psoriatic HDL were identified to be key determinants of the low cholesterol efflux capability (25). Of particular interest in this regard is the observation that HDL particle characteristics may play an important role in psoriatic vascular inflammation, as assessed by measuring macrophage activity in atherosclerotic plaques of large vessels using [18F]-fluoro-deoxyglucose-positron emission tomography (21). Concentration of small HDL particles was associated with increased overall vascular inflammation whereas total HDL particle concentration and HDL particle size were associated with decreased aortic inflammation (21).
Interestingly, significantly increased activity of lipoprotein associated phospholipase A2 (Lp-PLA2), also known as platelet-activating factor (PAF) acetyl-hydrolase, was observed in HDL form psoriasis patients (13,25). Importantly, HDL-cholesterol efflux capability negatively correlated with disease severity, whereas HDL associated Lp-PLA2 levels were positively associated (25), indicating that disease severity is linked to alterations in HDL composition and function. Previous studies have implicated that the potent phospholipid mediator PAF plays a role in the pathogenesis of psoriasis, and a significant decrease in PAF levels was observed with clinical improvement after anti-psoriatic treatment in a TGF-beta psoriatic mouse model (38) as well as human psoriasis patients (39). Therefore, increased HDL-associated Lp-PLA2 activity may inactivate PAF and contribute to clinical improvement. Intriguingly, both psoralen plus UVA (PUVA) photochemotherapy or PCA-4248, a PAF receptor antagonist, led to downregulation of PAF in the mouse model and this related to the clearance of psoriatic skin lesions. This suggests that the interruption of the known PAF autocrine loop does potentiate any anti-PAF and anti-psoriatic effect. On the other hand, data from a meta-analysis suggest that plasma levels of Lp-PLA2 are associated with an increased risk of developing cardiovascular disease (40). Therefore, the role of HDL-associated Lp-PLA2 in diseases remains unclear and needs further investigations.
Other actions of HDL, besides reverse cholesterol transport, may also play an important role in preventing atherosclerosis. Specifically, HDL-mediated anti-inflammatory activities and the ability to protect LDL from oxidation are potential important protective functions. Paraoxonase, an important HDL-associated enzyme, has been implicated in anti-oxidant and anti-inflammatory activities and is thought to hinder the development of atherosclerosis (41). Of particular interest, paraoxonase activity of HDL from subjects with moderate to severe psoriasis is decreased when compared with age and gender matched controls (13).
Effective anti-psoriatic therapy improves HDL composition and function independently of HDL cholesterol levels
Data on whether treatment of psoriasis will improve outcomes for cardiovascular disease are still limited. However, recent data from more than 8845 patients enrolled the Kaiser Permanente Southern California health plan suggested that treatment of psoriasis with tumor necrosis factor (TNF) inhibitors was associated with a significant reduction in myocardial infarction risk and incidence rate compared to the treatment with topical agents (42). Moreover, systemic treatment with methotrexate (43) or photo(chemo)therapy, including broad-band UV-B, narrow-band UV-B or PUVA) (42) was also associated with a significant reduction of cardiovascular risk. Interestingly, a small study demonstrated recently that treatment of psoriasis utilizing anti-TNF therapy led to improvement in carotid intimal-medial thickness, an established surrogate marker of cardiovascular disease (44). In contrast, data on inhibition of the p40 subunit of IL-12/13 by biological treatment with the monoclonal antibody ustekinumab or briakinumab revealed variable results concerning cardiovascular outcome (45,46). Indeed, early reports on major adverse cardiovascular events (MACEs) suggested an increased risk for this anti-IL-12/13 treatment in psoriasis patients (45). Moreover, those IL-12/13 antibodies may not only differ in their short-term anti-psoriatic effect on the skin (47) but also in their effect on long-term vascular outcome (45,46).
A recent study open-label study of psoriasis treatment to understand the effect on HDL characteristics provided first evidence that effective anti-psoriatic therapy was associated with improvement in HDL composition and function (Figure) (13). The anti-psoriatic treatment spectrum administered to the patients of the study was broad and included adalimumab, etanercept, ustekinumab, methotrexate, cyclosporin, and fumaric acid esters alone or in combination with topical agents as well as UV-B narrowband or PUVA therapy. Intriguingly, the reduction in Psoriasis Area and Severity Index (PASI) scores after an average treatment of about one year, correlated with significantly improved cholesterol efflux capability of psoriatic HDL. Furthermore, Lp-PLA2 and paraoxonase enzyme activities were modulated in a favourable direction, supporting the notion of improvement in HDL characteristics. Anti-psoriatic therapy did not alter blood lipid levels and HDL-cholesterol levels remained significant lower than compared to the control group. Circulating C-reactive protein levels in the treatment group tended to decrease, but this did not reach statistical significance. In the bloodstream, HDL lipid composition is remodelled by several serum enzymes. Of particular interest, anti-psoriatic therapy related to (i) reduced serum lipolytic activity and (ii) significantly improved lecithin-cholesterol acyltransferase activity; whereas cholesterolester and phospholipid transfer protein activities were not altered. Anti-psoriatic therapy-induced alterations in the activity of serum enzymes resulted in increased phospholipid content and size of HDL, key determinants of the cholesterol efflux capability of HDL (48,49).
Conclusion and outlook
In summary, recent studies clearly show that the HDL proteome can change in psoriasis and these changes are related to metrics of HDL functionality. Importantly, all improvements in HDL function and composition occurred, without changing blood lipids including HDL, suggesting that conventional approaches of evaluating cardiovascular risk in psoriasis may be in need of modification. It remains to be seen whether the changes in HDL composition contribute to the disease etiology or if these changes are secondary to other processes occurring during disease progression. Given that the recent studies included only a small number of subjects, the efficiency of individual treatments on HDL composition and function cannot be given and need further investigation in a larger cohort. Therefore, the improvement of psoriatic skin lesions itself (resulting in a reduced overall inflammatory state with normalization of skin-derived systemic cytokine and chemokine levels), rather than the systemic anti-psoriatic treatment, may be responsible for the recovery of HDL composition and function. This also proposes that investigating the influence of a specific anti-psoriatic agent on HDL functionality may help identifying treatment strategies with favourable effects on long-term cardiovascular outcome.
Acknowledgments
Sources of Funding
This work was supported by the Austrian Science Fund FWF (Grant P22976-B18, W1241) and the Jubiläumsfonds of the Austrian National Bank (Grant 14853)
Footnotes
Conflict of interest
None declared.
References
- 1.Yeung H, Takeshita J, Mehta NN, et al. Psoriasis severity and the prevalence of major medical comorbidity: a population-based study. JAMA Dermatol. 2013;149:1173–1179. doi: 10.1001/jamadermatol.2013.5015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gerdes S, Osadtschy S, Buhles N, Baurecht H, Mrowietz U. Cardiovascular biomarkers in patients with psoriasis. Exp Dermatol. 2014;23:322–325. doi: 10.1111/exd.12381. [DOI] [PubMed] [Google Scholar]
- 3.Gupta Y, Moller S, Zillikens D, Boehncke WH, Ibrahim SM, Ludwig RJ. Genetic control of psoriasis is relatively distinct from that of metabolic syndrome and coronary artery disease. Exp Dermatol. 2013;22:552–553. doi: 10.1111/exd.12192. [DOI] [PubMed] [Google Scholar]
- 4.Tobin AM, Veale DJ, Fitzgerald O, et al. Cardiovascular disease and risk factors in patients with psoriasis and psoriatic arthritis. J Rheumatol. 2010;37:1386–1394. doi: 10.3899/jrheum.090822. [DOI] [PubMed] [Google Scholar]
- 5.Gottlieb AB, Dann F. Comorbidities in patients with psoriasis. Am J Med. 2009;122:1150.e1–1150.e9. doi: 10.1016/j.amjmed.2009.06.021. [DOI] [PubMed] [Google Scholar]
- 6.Friedewald VE, Cather JC, Gelfand JM, et al. AJC editor's consensus: psoriasis and coronary artery disease. Am J Cardiol. 2008;102:1631–1643. doi: 10.1016/j.amjcard.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 7.Langan SM, Seminara NM, Shin DB, et al. Prevalence of metabolic syndrome in patients with psoriasis: a population-based study in the United Kingdom. J Invest Dermatol. 2012;132:556–562. doi: 10.1038/jid.2011.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Castro KR, Aikawa NE, Saad CG, et al. Infliximab induces increase in triglyceride levels in psoriatic arthritis patients. Clin Dev Immunol. 2011;2011:352686. doi: 10.1155/2011/352686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Antoniou C, Dessinioti C, Katsambas A, Stratigos AJ. Elevated triglyceride and cholesterol levels after intravenous antitumour necrosis factor-alpha therapy in a patient with psoriatic arthritis and psoriasis vulgaris. Br J Dermatol. 2007;156:1090–1091. doi: 10.1111/j.1365-2133.2007.07835.x. [DOI] [PubMed] [Google Scholar]
- 10.Spanakis E, Sidiropoulos P, Papadakis J, et al. Modest but sustained increase of serum high density lipoprotein cholesterol levels in patients with inflammatory arthritides treated with infliximab. J Rheumatol. 2006;33:2440–2446. [PubMed] [Google Scholar]
- 11.STARK GR. On the Reversible Reaction of Cyanate with Sulfhydryl Groups and the Determination of Nh2-Terminal Cysteine and Cystine in Proteins. J Biol Chem. 1964;239:1411–1414. [PubMed] [Google Scholar]
- 12.Voight BF, Peloso GM, Orho-Melander M, et al. Plasma HDL cholesterol and risk of myocardial infarction: a mendelian randomisation study. Lancet. 2012;380:572–580. doi: 10.1016/S0140-6736(12)60312-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holzer M, Wolf P, Inzinger M, et al. Anti-psoriatic therapy recovers high-density lipoprotein composition and function. J Invest Dermatol. 2014;134:635–642. doi: 10.1038/jid.2013.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shah AS, Tan L, Long JL, Davidson WS. Proteomic diversity of high density lipoproteins: our emerging understanding of its importance in lipid transport and beyond. J Lipid Res. 2013;54:2575–2585. doi: 10.1194/jlr.R035725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kontush A, Lhomme M, Chapman MJ. Unraveling the complexities of the HDL lipidome. J Lipid Res. 2013;54:2950–2963. doi: 10.1194/jlr.R036095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marsche G, Saemann MD, Heinemann A, Holzer M. Inflammation alters HDL composition and function: Implications for HDL-raising therapies. Pharmacol Ther. 2013;137:341–351. doi: 10.1016/j.pharmthera.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 17.deGoma EM, deGoma RL, Rader DJ. Beyond high-density lipoprotein cholesterol levels evaluating high-density lipoprotein function as influenced by novel therapeutic approaches. J Am Coll Cardiol. 2008;51:2199–2211. doi: 10.1016/j.jacc.2008.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Annema W, von Eckardstein A. High-density lipoproteins. Multifunctional but vulnerable protections from atherosclerosis. Circ J. 2013;77:2432–2448. doi: 10.1253/circj.cj-13-1025. [DOI] [PubMed] [Google Scholar]
- 19.Triolo M, Annema W, Dullaart RP, Tietge UJ. Assessing the functional properties of high-density lipoproteins: an emerging concept in cardiovascular research. Biomark Med. 2013;7:457–472. doi: 10.2217/bmm.13.35. [DOI] [PubMed] [Google Scholar]
- 20.Silbernagel G, Schottker B, Appelbaum S, et al. High-density lipoprotein cholesterol, coronary artery disease, and cardiovascular mortality. Eur Heart J. 2013;34:3563–3571. doi: 10.1093/eurheartj/eht343. [DOI] [PubMed] [Google Scholar]
- 21.Yu Y, Sheth N, Krishnamoorthy P, et al. Aortic vascular inflammation in psoriasis is associated with HDL particle size and concentration: a pilot study. Am J Cardiovasc Dis. 2012;2:285–292. [PMC free article] [PubMed] [Google Scholar]
- 22.Mehta NN, Li R, Krishnamoorthy P, et al. Abnormal lipoprotein particles and cholesterol efflux capacity in patients with psoriasis. Atherosclerosis. 2012;224:218–221. doi: 10.1016/j.atherosclerosis.2012.06.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vaisar T, Pennathur S, Green PS, et al. Shotgun proteomics implicates protease inhibition and complement activation in the antiinflammatory properties of HDL. J Clin Invest. 2007;117:746–756. doi: 10.1172/JCI26206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Holzer M, Trieb M, Konya V, Wadsack C, Heinemann A, Marsche G. Aging affects high-density lipoprotein composition and function. Biochim Biophys Acta. 2013;1831:1442–1448. doi: 10.1016/j.bbalip.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Holzer M, Wolf P, Curcic S, et al. Psoriasis alters HDL composition and cholesterol efflux capacity. J Lipid Res. 2012;53:1618–1624. doi: 10.1194/jlr.M027367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weichhart T, Kopecky C, Kubicek M, et al. Serum Amyloid A in Uremic HDL Promotes Inflammation. J Am Soc Nephrol. 2012;23:934–947. doi: 10.1681/ASN.2011070668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alwaili K, Bailey D, Awan Z, et al. The HDL proteome in acute coronary syndromes shifts to an inflammatory profile. Biochim Biophys Acta. 2011;1821:405–415. doi: 10.1016/j.bbalip.2011.07.013. [DOI] [PubMed] [Google Scholar]
- 28.Riwanto M, Rohrer L, Roschitzki B, et al. Altered activation of endothelial anti- and proapoptotic pathways by high-density lipoprotein from patients with coronary artery disease: role of high-density lipoprotein-proteome remodeling. Circulation. 2013;127:891–904. doi: 10.1161/CIRCULATIONAHA.112.108753. [DOI] [PubMed] [Google Scholar]
- 29.Holzer M, Birner-Gruenberger R, Stojakovic T, et al. Uremia Alters HDL Composition and Function. J Am Soc Nephrol. 2011;22:1331–1341. doi: 10.1681/ASN.2010111144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Watanabe J, Charles-Schoeman C, Miao Y, et al. Proteomic profiling following immunoaffinity capture of HDL: Association of acute phase proteins and complement factors with pro-inflammatory HDL in rheumatoid arthritis. Arthritis Rheum. 2012;64:1828–1837. doi: 10.1002/art.34363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gordon SM, Davidson WS, Urbina EM, et al. The effects of type 2 diabetes on lipoprotein composition and arterial stiffness in male youth. Diabetes. 2013;62:2958–2967. doi: 10.2337/db12-1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Feingold KR, Grunfeld C. Psoriasis: It's More Than Just The Skin. J Lipid Res. 2012;53:1427–1429. doi: 10.1194/jlr.E029330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rader DJ, Alexander ET, Weibel GL, Billheimer J, Rothblat GH. The role of reverse cholesterol transport in animals and humans and relationship to atherosclerosis. J Lipid Res. 2009;50(Suppl):S189–S194. doi: 10.1194/jlr.R800088-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Toth PP, Barter PJ, Rosenson RS, et al. High-density lipoproteins: a consensus statement from the National Lipid Association. J Clin Lipidol. 2013;7:484–525. doi: 10.1016/j.jacl.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 35.Khera AV, Cuchel M, de la Llera-Moya M, et al. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N Engl J Med. 2011;364:127–135. doi: 10.1056/NEJMoa1001689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Charles-Schoeman C, Lee YY, Grijalva V, et al. Cholesterol efflux by high density lipoproteins is impaired in patients with active rheumatoid arthritis. Ann Rheum Dis. 2012;71:1157–1162. doi: 10.1136/annrheumdis-2011-200493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dowlatshahi EA, van der Voort EA, Arends LR, Nijsten T. Markers of systemic inflammation in psoriasis: a systematic review and meta-analysis. Br J Dermatol. 2013;169:266–282. doi: 10.1111/bjd.12355. [DOI] [PubMed] [Google Scholar]
- 38.Singh TP, Huettner B, Koefeler H, et al. Platelet-activating factor blockade inhibits the T-helper type 17 cell pathway and suppresses psoriasis-like skin disease in K5.hTGF-beta1 transgenic mice. Am J Pathol. 2011;178:699–708. doi: 10.1016/j.ajpath.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Izaki S, Yamamoto T, Goto Y, et al. Platelet-activating factor and arachidonic acid metabolites in psoriatic inflammation. Br J Dermatol. 1996;134:1060–1064. [PubMed] [Google Scholar]
- 40.Rosenson RS, Hurt-Camejo E. Phospholipase A2 enzymes and the risk of atherosclerosis. Eur Heart J. 2012;33:2899–2909. doi: 10.1093/eurheartj/ehs148. [DOI] [PubMed] [Google Scholar]
- 41.Bhattacharyya T, Nicholls SJ, Topol EJ, et al. Relationship of paraoxonase 1 (PON1) gene polymorphisms and functional activity with systemic oxidative stress and cardiovascular risk. JAMA. 2008;299:1265–1276. doi: 10.1001/jama.299.11.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu JJ, Poon KY, Channual JC, Shen AY. Association between tumor necrosis factor inhibitor therapy and myocardial infarction risk in patients with psoriasis. Arch Dermatol. 2012;148:1244–1250. doi: 10.1001/archdermatol.2012.2502. [DOI] [PubMed] [Google Scholar]
- 43.Churton S, Brown L, Shin TM, Korman NJ. Does treatment of psoriasis reduce the risk of cardiovascular disease? Drugs. 2014;74:169–182. doi: 10.1007/s40265-013-0173-5. [DOI] [PubMed] [Google Scholar]
- 44.Jokai H, Szakonyi J, Kontar O, et al. Impact of effective tumor necrosis factor-alfa inhibitor treatment on arterial intima-media thickness in psoriasis: results of a pilot study. J Am Acad Dermatol. 2013;69:523–529. doi: 10.1016/j.jaad.2013.06.019. [DOI] [PubMed] [Google Scholar]
- 45.Ryan C, Leonardi CL, Krueger JG, et al. Association between biologic therapies for chronic plaque psoriasis and cardiovascular events: a meta-analysis of randomized controlled trials. JAMA. 2011;306:864–871. doi: 10.1001/jama.2011.1211. [DOI] [PubMed] [Google Scholar]
- 46.Tzellos T, Kyrgidis A, Zouboulis CC. Re-evaluation of the risk for major adverse cardiovascular events in patients treated with anti-IL-12/23 biological agents for chronic plaque psoriasis: a meta-analysis of randomized controlled trials. J Eur Acad Dermatol Venereol. 2013;27:622–627. doi: 10.1111/j.1468-3083.2012.04500.x. [DOI] [PubMed] [Google Scholar]
- 47.Inzinger M, Weger W, Salmhofer W, Wolf P. Differential response of chronic plaque psoriasis to briakinumab vs. ustekinumab. Acta Derm Venereol. 2012;92:357–358. doi: 10.2340/00015555-1243. [DOI] [PubMed] [Google Scholar]
- 48.Davidson WS, Rodrigueza WV, Lund-Katz S, Johnson WJ, Rothblat GH, Phillips MC. Effects of acceptor particle size on the efflux of cellular free cholesterol. J Biol Chem. 1995;270:17106–17113. doi: 10.1074/jbc.270.29.17106. [DOI] [PubMed] [Google Scholar]
- 49.Davidson WS, Gillotte KL, Lund-Katz S, Johnson WJ, Rothblat GH, Phillips MC. The effect of high density lipoprotein phospholipid acyl chain composition on the efflux of cellular free cholesterol. J Biol Chem. 1995;270:5882–5890. doi: 10.1074/jbc.270.11.5882. [DOI] [PubMed] [Google Scholar]