Abstract
Drusinae (Trichoptera, Limnephilidae) are highland caddisflies inhabiting high-gradient, turbulent running water and spring habitats. They are disjunctly distributed over the Eurasian mountain ranges, and the majority of species is endemic to particular mountain areas. The most diverse of three main groups of the Drusinae, the grazer clade, consists of species in which larvae feed on epiltihic biofilm and algae. In this paper we describe three previously unknown grazer-clade Drusinae larvae: Drusus krusniki Malicky 1981 (endemic to the Dinaric western Balkans), D. vernonensis Malicky 1989 (endemic to the Hellenic western Balkans), and D. vespertinus Marinković 1976 (endemic to the Dinaric western Balkans). The larvae of these species have toothless mandibles typical of the Drusinae grazer clade. Larvae and adults were unambiguously associated using molecular genetic data, i.e., the mitochondrial cytochrome oxidase I gene fragment (mtCOI3-P). Morphological characteristics of the larvae are described and the diagnostic features enabling species-level identification are illustrated. We further discuss the ecology and distribution of three Western Balkan endemic species.
Keywords: 5th instar larva, description, identification, distribution, endemism
Introduction
Drusinae are restricted to mountain springs and high-gradient, turbulent running waters in hard-substrate channels covering the Eurasian mountain ranges from the Iberian Peninsula to the Iranian Highlands. Three quarters of the known species are endemic to a single or very few mountain ranges, making the group an ideal model for studying evolutionary processes like speciation, diversification and cryptic species evolution (Schmid 1956; Kumanski 1973; Marinković-Gospodnetić 1971a, b, 1976; Sipahiler 2002; Malicky 2005a; Pauls et al. 2006; Previšić et al. 2014a). Mountain areas of the Western Balkans harbor particularly high numbers of Drusinae endemics (Vitecek et al. 2015a). The fragmented montane sky-island populations of the Drusinae are also very sensitive to global change and their species are among the most threatened by climate warming (Previšić et al. 2009, Balint et al. 2011). A molecular phylogeny of the subfamily yielded three well-supported clades that reflect the morphology and feeding ecology of larvae (Pauls et al. 2008, Graf et al. 2009, Vitecek et al. 2015b, Waringer et al. 2015): omnivorous shredders, epilithic grazers, and carnivorous filter feeders. Drusinae comprises eight genera, with the genus Drusus Stephens containing the greatest number of species (92; e.g., Malicky 2004, 2005a; Oláh et al. 2015; Vitecek et al. 2015a).
The genus Drusus was further divided into particular species groups of presumably closely related species (Schmid 1956). The Drusus bosnicus Group was first defined by Schmid (1956) based on the morphology of genitalia, comprising species from the Balkans and Central Europe. Many new species were described from the Western Balkans in the meantime, most of them small-scale endemics with highly similar genital morphology to the Drusus bosnicus Group (e.g., Marinković-Gospodnetić 1971a, b, 1976; Malicky 1989; Oláh 2010, 2011; Vitecek et al. 2015a).
Drusinae larvae exhibit high niche specificity, mostly prefer xenosaprobic to oligosaprobic headwater sections of streams or springs (Graf et al. 2008), and are comparatively easily identified. The group is therefore well-suited for water quality assessment (Barbour et al. 1999, Barbour & Yoder 2000, AQEM Consortium 2002). However, larval stages of many Drusinae species including some of the Drusus bosnicus Group are still unknown, and larvae of seven species only have been described previously [D. bosnicus Klapálek 1899, D. crenophylax Graf & Vitecek 2015a, D. klapaleki Marinković-Gospodnetić 1971b, D. medianus Marinković-Gospodnetić 1976, D. radovanovici Marinković-Gospodnetić 1971b, D. ramae Marinković-Gospodnetić 1971b and D. septentrionis (Marinković-Gospodnetić 1976); Kučinić et al. 2008, 2010, 2011a, 2011b, 2015; Vitecek et al. 2015a].
In this contribution we extend the knowledge of larval taxonomy of the Drusinae by presenting descriptions of the hitherto unknown larvae of Drusus krusniki Malicky 1981, D. vernonensis Malicky 1989, and D. vespertinus Marinković 1976. The putative larvae of these three species were associated with co-occurring adults using molecular genetic sequence data [mitochondrial cytochrome oxidase c subunit I (mtCOI3-P)]. We also highlight the most important diagnostic features enabling separation of these larvae from those of other European Drusinae species. Additionally, we discuss the ecology and distribution of the three Western Balkan endemic species and the Drusus bosnicus Group in general.
Material and methods
Sampling, collected material and taxonomy
Adults and larvae were collected by A.P., W.G. and M.K. in Bosnia and Herzegovina, Macedonia and Montenegro using a hand net and kick sampling at the following locations: Drusus krusniki: Montenegro: Alipaša’s spring, 42°33’N, 19°49’E, 942 m, 31 May 2009, sixteen 5th instar larvae; Ibra spring, 42°48′N, 20°05′E, 1271 m, 31 May 2009, nine 5th instar larvae. D. vernonensis: Macedonia: Pelister Mt. Tributary of Caparska reka, 40°58′N, 21°12′E, 2323 m, 8 July 2010, two 5th instar larvae. D. vespertinus: Bosnia and Herzegovina: Gornji Ribnik, 44°25′N, 16°49′E, 316 m, 3 June 2008, eight 5th instar larvae. Additionally, temperature loggers (Hobo Onset) logging ambient water temperature at 0200 h and 1400 h were exposed at the same sites and recovered the following year. Material intended for sequencing was stored in 100 % ethanol, the material for morphological analyses in 70% ethanol in order to keep the specimens more flexible. Information on examined voucher specimens and other Drusus species larvae used as comparative material is given in Table 1 (all in collection of the first author). The larvae were studied and photographed using a Nikon SMZ 1500 binocular microscope with DS-Fi1 camera and NIS-elements D 3.1 image stacking software for combining 8 to 42 frames in one focused image. Larval morphological nomenclature follows Wiggins (1998).
Table 1.
Information on larval Drusus specimens (fifth instar larvae) from the Western Balkans used in this paper.
| Species | Locality (country, name) | Latitude (°) | Longitude (°) | No of larvae examined | Collector |
|---|---|---|---|---|---|
| Drusus bosnicus | (BIH) Paljanska Miljacka spring | N 43.9234 | E 18.5973 | 1 | Kučinić & Previšić |
| Drusus crenophylax | (BIH) Cvrcka river | N 44.5489 | E 17.3927 | 2 | Dmitrović & Šukalo |
| Drusus klapaleki | (BIH) Toplica spring | N 43.5943 | E 18.4949 | 2 | Graf & Previšić |
| Drusus krusniki | (MNE) Alipaša’s springs, Gusinje | N 42.5501 | E 19.8259 | 16 | Previšić |
| Drusus krusniki | (MNE) Ibar spring | N 42.7975 | E 20.0904 | 9 | Previšić |
| Drusus medianus | (BIH) Plava voda spring | N 44.2303 | E 17.6717 | 1 | Kučinić & Previšić |
| Drusus radovanovici | (BIH) Sutjeska NP, stream close to Čemerno | N 43.2650 | E 18.5928 | 1 | Graf & Previšić |
| Drusus septentrionis | (BIH) Bistrica spring, Livno | N 43.8325 | E 17.0084 | 2 | Kučinić |
| Drusus serbicus | (SRB) Golija Mt, spring Ilinac | N 43.3333 | E 20.2819 | 3 | Bjelanović, Kučinić & Živić |
| Drusus vernonensis | (MKD) Pelister, tributary of Caparska reka | N 41.0148 | E 21.1741 | 2 | Previšić |
| Drusus vespertinus | (BIH) Ribnik, spring reach | N 44.4655 | E 16.8380 | 8 | Kučinić & Previšić |
Species affiliation was based on two criteria:
-
1)
Larval and adult specimens were collected at the same sites, close to the type localities of the respective species where other Drusinae species are absent or already known due to existing descriptions.
-
2)
Molecular sequence data from the mitochondrial cytochrome oxidase c subunit I (mtCOI3-P) supports conspecificity of the larvae and adults.
DNA extraction and PCR amplification
For the association of larvae and adults both published and new sequences of a 541-bp-long fragment of the mitochondrial cytochrome oxidase c subunit I (mtCOI3-P) were used. Whole genomic DNA was extracted from part of the thorax or abdomen of adult or larval specimens, respectively, using the DNEasy Blood and Tissue Kit or QIAamp DNA Micro Kit (Qiagen) according to the manufacturer’s protocol. PCR amplifications were carried out using primers S20 and Jerry (Simon et al. 1994; Pauls et al. 2006). DNA extraction and amplification were performed as outlined by Pauls et al. (2008) and Previšić et al. (2009).
For D. krusniki 71 published mtCOI3-P sequences from five populations (Previšić et al. 2014b; Table 2) were aligned. For D. vernonensis mtCOI3-P sequences of 3 adults (Previšić et al. 2014b; Vitecek et al. 2015a; GenBank accession nos KC881524, KP793086 and KP793087) and 3 larvae (GenBank accession nos KT598011-KT598013) from one population were aligned. For D. vespertinus mtCOI3-P sequences of 5 adults and 3 larvae from two populations were aligned (Previšić et al. 2009 and new sequences, Table 4). New sequences were edited manually using Geneious R7 (Kearse et al. 2012) and all were aligned using Muscle algorithm in Mega 6.0.1 (Tamura et al. 2013). Intraspecific uncorrected p-distances were calculated in Mega 6.0.1 for each species.
Table 2.
Minimal and maximal values of nucleotide differences and uncorrected p-distance of the mtCOI3-P gene segment within populations of Drusus krusniki (data from Previšić et al. 2014b).
| Drusus krusniki populations | Number of adult males | Number of larvae | Number of nucleotide difference (min-max) | Uncorrected p-distance (min-max) | GenBank accession nos |
|---|---|---|---|---|---|
| Alipaša springs | 11 | 2 | 0-1 | 0-0.002 | KC881401 - KC881413 |
| Biogradska rijeka | 8 | 5 | 0-6 | 0-0.011 | KC881414 - KC881426 |
| Bukovica spring | 2 | 14 | 0-2 | 0-0.004 | KC881427 - KC881442 |
| Ibar spring | 1 | 14 | 0-4 | 0-0.004 | KC881443 - KC881457 |
| Murinska rijeka | 6 | 8 | 0 | 0 | KC881458 - KC881471 |
Table 4.
Number of nucleotide differences (below diagonal) and uncorrected p-distance (above diagonal) of the mtCOI3-P gene segment within and between populations of Drusus vespertinus. For details on specimen sampling sites see Table 1.
| Population | DvRIM1 | DvRL1 | fDvs0103L | fDvs0104L | fDvs0202M | fDvs0203F | fDvs0205F | fDvs0206F | GenBank access. nos | |
|---|---|---|---|---|---|---|---|---|---|---|
| Ribnik spring, BIH | DvRIM1 | 0.002 | 0.004 | 0.004 | 0.013 | 0.013 | 0.013 | 0.013 | KT598004 | |
| Ribnik spring, BIH | DvRL1 | 1 | 0.002 | 0.002 | 0.011 | 0.011 | 0.011 | 0.011 | FJ002685 | |
| Ribnik spring, BIH | fDvs0103L | 2 | 1 | 0.004 | 0.013 | 0.013 | 0.013 | 0.013 | KT598005 | |
| Ribnik spring, BIH | fDvs0104L | 2 | 1 | 2 | 0.013 | 0.013 | 0.013 | 0.009 | KT598006 | |
| Una spring, HRV | fDvs0202M | 7 | 6 | 7 | 7 | 0 | 0.004 | 0.007 | KT598007 | |
| Una spring, HRV | fDvs0203F | 7 | 6 | 7 | 7 | 0 | 0.004 | 0.007 | KT598008 | |
| Una spring, HRV | fDvs0205F | 7 | 6 | 7 | 7 | 2 | 2 | 0.007 | KT598009 | |
| Una spring, HRV | fDvs0206F | 7 | 6 | 7 | 5 | 4 | 4 | 4 | KT598010 |
Results
Association of adults and larvae of Drusus krusniki
Comparison of haplotypes of the mtCOI3-P segment between a total of 28 adult males and 44 larvae belonging to 5 different populations of Drusus krusniki supported the association of adults and larvae. Within each particular population haplotypes differed by maximally 6 nucleotides between adults and larvae (Table 2; data from Previšić et al. 2014b).
Description of the fifth instar larva of Drusus krusniki
Biometry
Body length of final instar larva 7.1–12.3 mm, head width 1.60–1.83 mm (n = 25).
Head
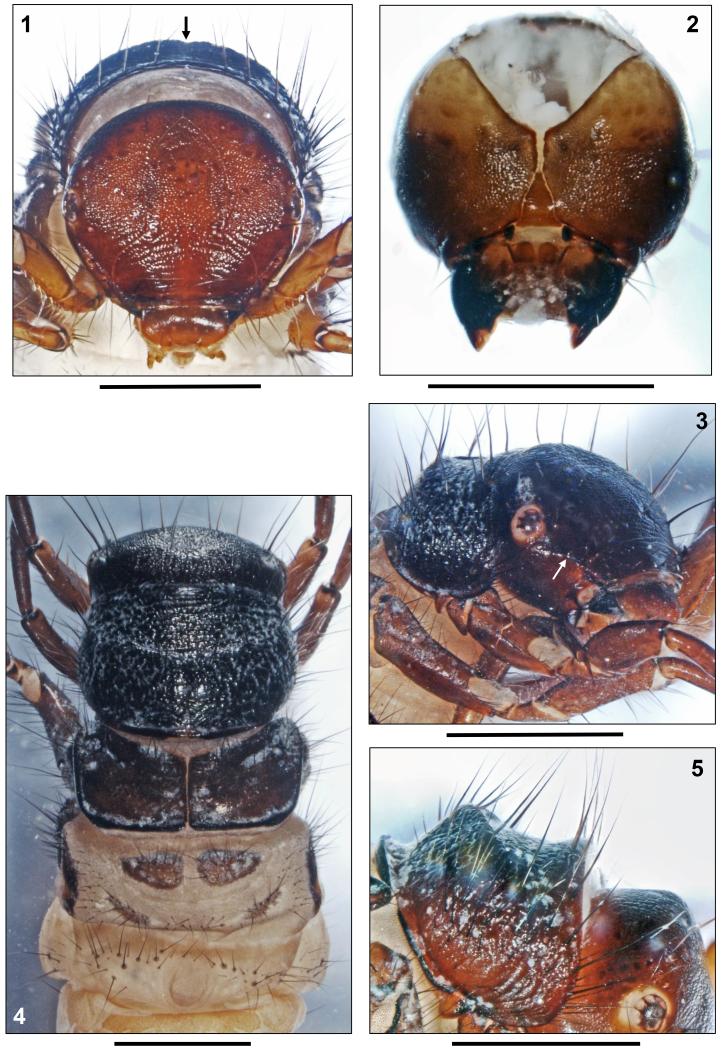
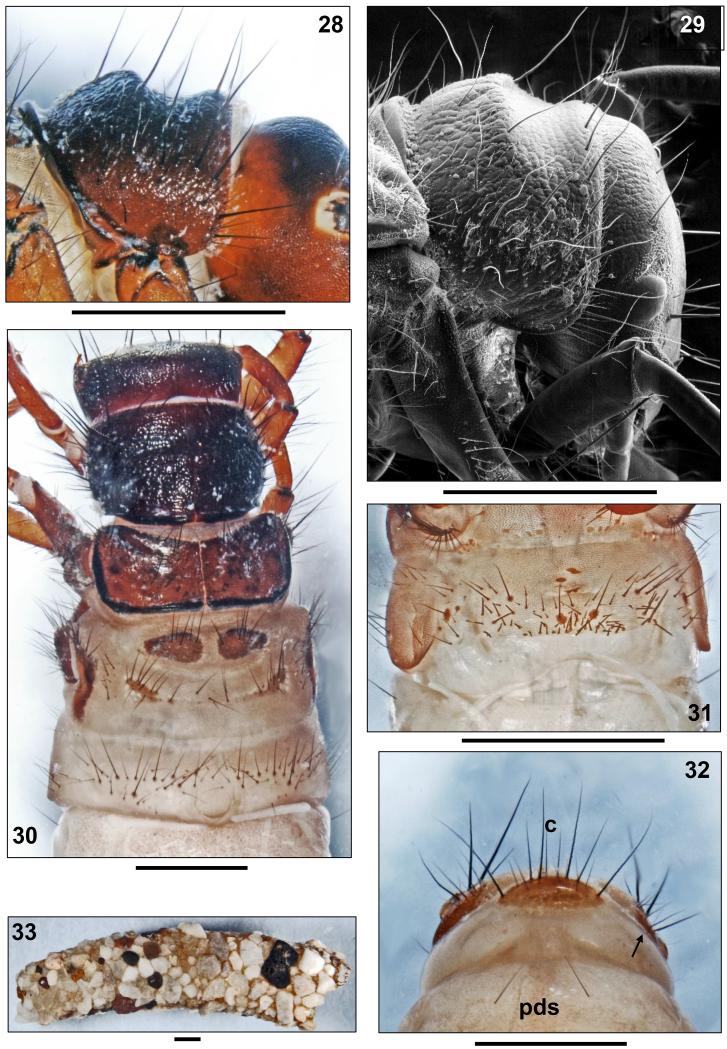
Head capsule coarsely granulated, almost circular in shape and hypognathous (Figs. 1, 3), dorsally with dark brown to blackish brown coloration and blackish muscle attachment spots. Vertex rounded (Fig. 6).Ventral parietalia sections, submentum, maxillolabial sclerites and premandibular areas yellowish brown (Figs. 2, 3). White ring around each eye (Fig. 3). In lateral view, head capsule with straight carina (approximately 0.09 mm wide) extending from anterior eye margin to frontomedian corner of parietale (Fig. 3, arrow). Head capsule with complete set of 18 pairs of primary setae (nomenclature by Wiggins 1998) and lacking any additional spines or bristles known to occur in other Drusinae larvae (e.g., some Ecclisopteryx spp.). However, posterior to each eye, with oval areas (diameter approximately 0.05 × 0.09 mm) of spinules (= small spines approximately 0.03 mm long) surrounding bases of setae #15 and 16 (Figs. 6, 7; white ovals). Such spinule areas occur in most members of the Drusus bosnicus Group sensu Marinković-Gospodnetić (1971a). Frontoclypeus bell-shaped, with narrow central constriction (Fig. 1). Antennae located halfway between eye and anterior head margin at small conical dorsal outcrops of lateral carina (Figs. 1, 3,19b), each consisting of 1 short cylindrical base and 1 short flagellum. At each parietal, 10 dorsal and 2 ventral primary setae present, with primary setae # 2, 3, 7, 9, 15, and 16 long and conspicuous (Figs. 1, 3). Each side of frontoclypeus with 6 primary setae, 3 of them along anterior border. Labrum brown, with setal brush and primary setae # 1–3 at anterolateral margins; on dorsal area, setation consisting of primary setae # 4–6 (Fig. 1). Ventral apotome elongated triangular, with almost parallel sides, bell-shaped, brown, postgenal suture approximately 45% of apotome length (Fig. 2). Mandibles black with dark brown tips, lacking terminal teeth along edges as well as lacking ridges in central concavity (Figs. 2, 19b).
FIGURES 1–5.
Drusus krusniki Malicky 1981, 5th instar larva. 1, Head, dorsal (arrow: pronotal notch). 2, Head, ventral. 3, Head and prothorax, right anterolateral (arrow: antenna on lateral carina). 4, Head, thorax and first abdominal segment, dorsal. 5, Pronotum, right lateral. Scale bars: 1 mm.
FIGURES 6–14.
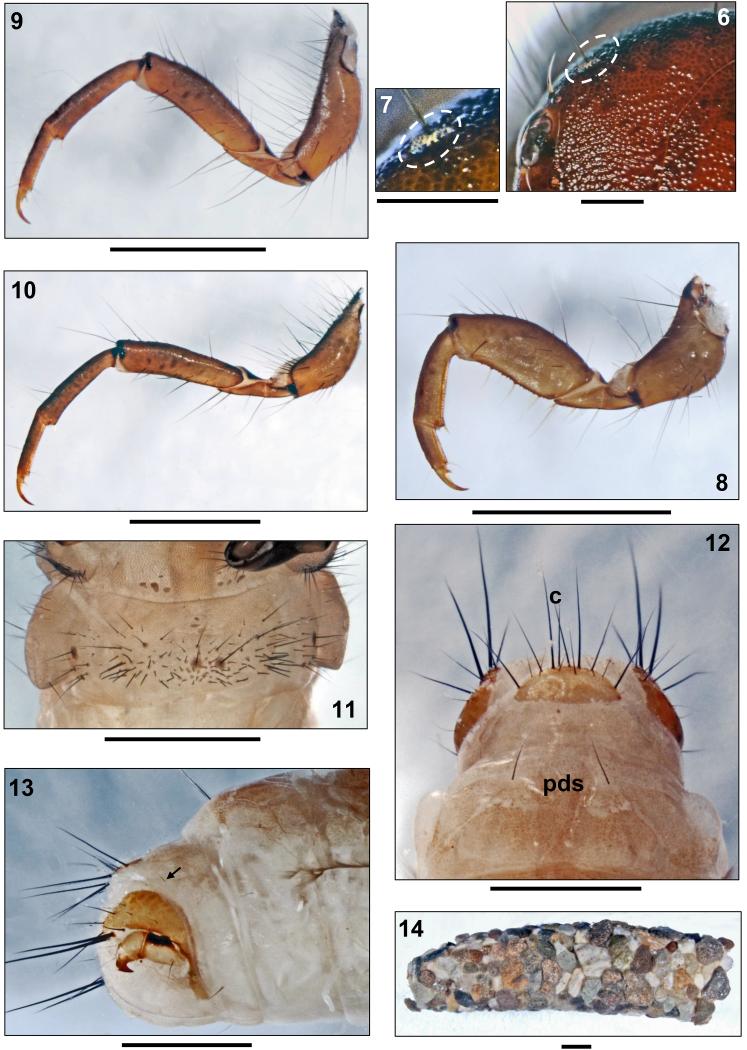
Drusus krusniki Malicky 1981, 5th instar larva. 6, Head, frontal (dotted oval: spinule field). 7, Head, detail of spinule field (dotted oval). 8, Right foreleg, anterior. 9, Right mid leg, anterior. 10, Right hind leg, anterior. 11, First abdominal sternum, ventral.12, 8th and 9th abdominal terga, dorsal (pds: posterodorsal setae; c: position of c setae). 13, Tip of abdomen, right lateral view (arrow: posterolateral seta). 14, Larval case, right lateral. Scale bars: 1 mm (except Figs. 6, 7: 0.25mm).
FIGURES 15–21.
Drusus vernonensis Malicky 1989, 5th instar larva. 15, Head, dorsal (arrows: left carina; a: antenna). 16, Head, detail of spinule field (dotted oval). 17, Head, ventral, submentum. 18, Head, pro- and mesothorax, right anterolateral (arrows: thin, long, yellowish setae). 19a, Head, lateral, carina with antenna. 19b, Drusus krusniki Malicky 1981, 5th instar larva. Head, lateral, carina with antenna. 20, Drusus vernonensis Malicky 1989, 5th instar larva. Head and pronotum, right lateral. 21, First abdominal sternum, ventral. Scale bars: 1 mm (except Figs. 16, 17, 19: 0.25mm).
Thorax
Pronotum blackish brown, very coarsely granulated (Figs. 3–5); at lateral and posterior pronotal surface, adjacent series of granuli creating ribbed structures (Fig. 5). Posterior margin thickened and darkly striped (Fig. 5). In frontal view, small dorsocentral pronotal notch present (Fig. 1, arrow). Pronotal transverse groove at end of anterior 3rd lacking. Pronotum type D (Table 3) in lateral view: median hump prominent, high, with anterior-looking crest gradually fading laterally; posterior slope straight, anterior slope concave (Fig. 5). Two setal rows along anterior border of pronotum: (1) Dense fringe of short, curved, fine, yellow setae; (2) widely-spaced, continuous row of long, straight, dark setae meeting at anterior pronotal midline (Figs. 1, 3, 5); in total, 50–60 dark setae of varying lengths distributed over each pronotal half. In addition, pronotal surface covered by high number of tiny, pale, recumbent setae (Figs. 3, 4); spines present in other Drusinae (e.g., D. trifidus) lacking. Prosternite pentangular, pale yellow, darker (light brown) along posterior border; prosternal horn present.
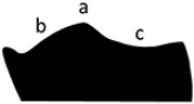
Table 3.
Pronotum types observed in the Drusus bosnicus group.
| Pronotum type | Lateral right profile of pronotum | Description |
|---|---|---|
| Type A |

|
Low median hump, evenly rounded (a); posterior (b) and anterior slope (c) almost straight. |
| Type B |
|
Median hump prominent, high, smoothly rounded (a); posterior slope convex (b); anterior slope concave (c). |
| Type C |
|
Median hump prominent, high, with crest gradually fading laterally (a); posterior slope convex (b); anterior slope concave (c). |
| Type D |

|
Median hump prominent, high, with crest gradually fading laterally (a); posterior slope straight (b); anterior slope concave (c). |
| Type E |
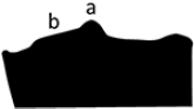
|
Median hump annular, crest-like, highest at dorsal center, gradually fading laterally (a); with semicircular step (b) directly posterior of crest center and in front of posterior pronotal rim. |
Mesonotum completely covered by 2 dark brown sclerites with black brown muscle attachment spots and slightly paler posterior and lateral sections; their anterolateral corners, lateral and posterior margins darkly sclerotized (Fig. 4). Counts for mesonotal setae are as follows: anterior setal group sa1: 12–15, posterior group sa2: 20–25, lateral group sa3: 25–30.
Metanotum partially covered by 3 pairs of yellowish brown sclerites. Anterior metanotal sclerites (sa1) very large, broadly triangular, their intermediate separation distinctly smaller than the length of each of them; each with black anterior margin; approximately 15 setae per sclerite (Fig. 4). Row of setae present between small posteromedian sclerites (sa2); 15–20 setae per sclerite. Small setal group present between each lateral (sa3) and posteromedian sclerite (sa2); sa3 scleritescrescent-shaped, dark brown at center and along dorsal border, with approximately 25–30 setae per sclerite, concentrated anteriorly (Fig. 4).
Legs yellowish brown with numerous setae on coxae, trochanters and femora; tibiae and tarsi with only small number of setae; on all femora several proximodorsal setae present (Figs. 8–10). Coxa, femur and tibia of each foreleg wider than those of mid- and hind legs. Setae present only at distal sections of trochanters. Additional setae present at both anterior and posterior faces of all femora; ventral trochanteral brush present at distal sections of fore- and midtrochanters. Forefemora each with 4 yellow ventral-edge setae, mid- and hind femora each with 3–4dark ventral edge setae. Dorsal setae only at distal third of mid- and hind tibiae (Figs. 7–9).
Abdomen
First abdominal segment with 1 dorsal and 2 lateral fleshy protuberances (Fig. 4). Dorsal setal areas sa1, sa2 and sa3 fused, thereby creating continuous transverse row of setae anterior to dorsal protuberance until dorsal section of each lateral protuberance, sharply delimited basal sclerites present for about 50% of these setae. Without setal group posterior to dorsal protuberance (Fig. 4). Posterior sclerites at lateral protuberances absent. In front of each lateral protuberance, continuous band of anterolateral setae (as Fig. 22a) linking with each dorsal and ventral sa3 setal group (Figs. 4, 11). On 1st abdominal sternum, ventral setal areas sa1, sa2 and sa3 fused, creating continuous field of setae;setal bases at central section of 1st abdominal sternum mostly small and inconspicuous except two larger bases near midline which occasionally fuse with 1–2 neighbouring smaller setal bases (Fig. 11). On 8th abdominal dorsum, two long and two very tiny posterodorsal setae (pds) present (Fig. 12, pds). Only 1 posterolateral seta on each half of 9th abdominal dorsum (Fig. 13, arrow).
FIGURES 22–27.
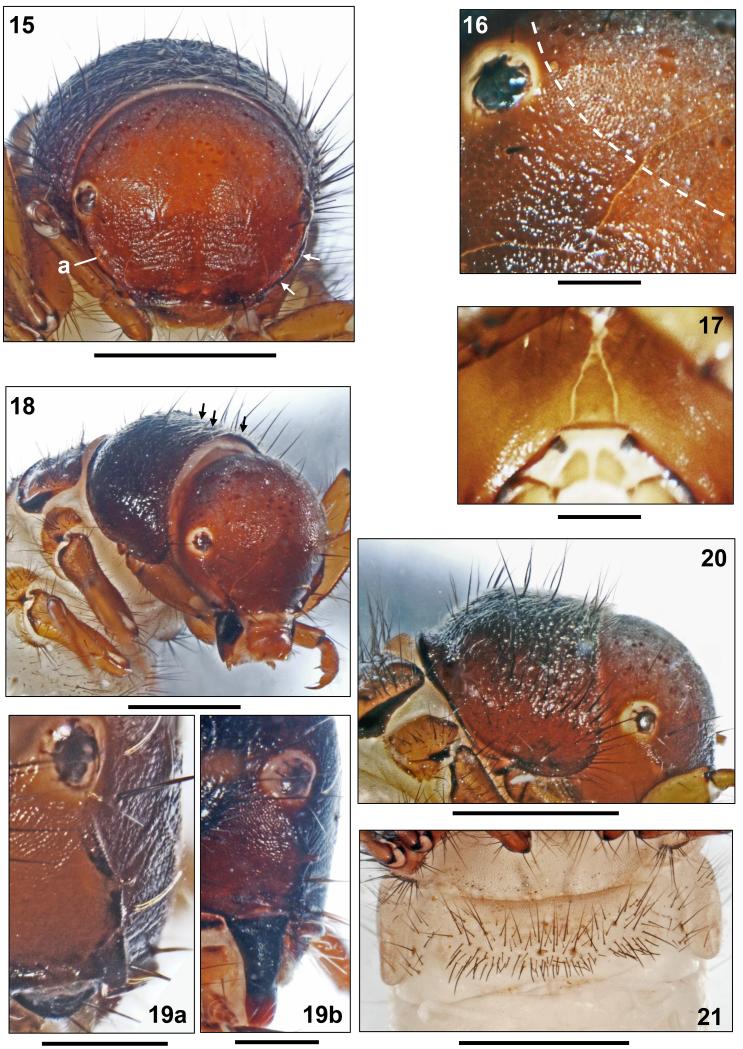
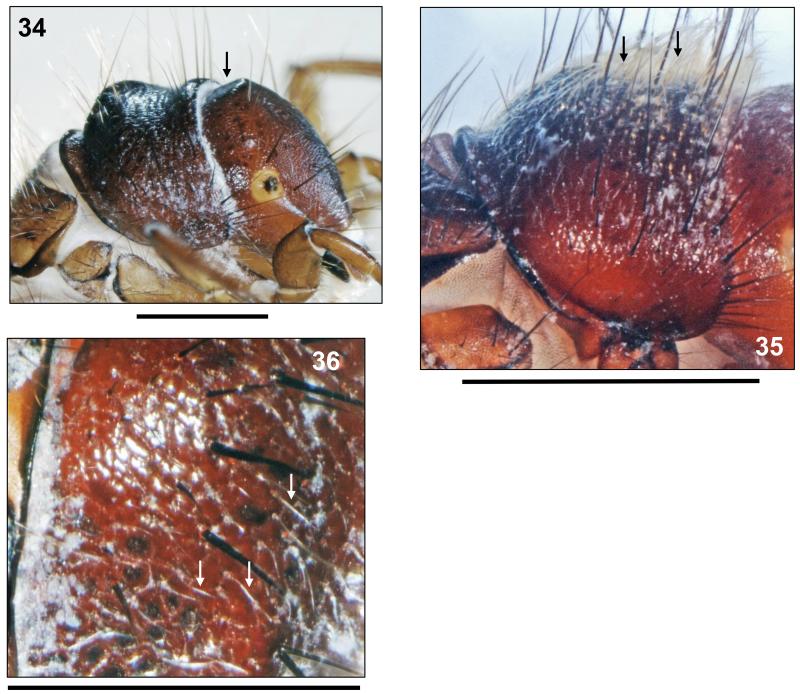
FIGURES 22–24. Drusus vernonensis Malicky 1989, 5th instar larva. 22, Metathorax and anterior half of abdomen, right lateral (a: row of setae anterior of lateral protuberance; b: small portion of lateral fringe on 2nd segment, c: start of lateral fringe on 3rd segment). 23, 8th and 9th abdominal terga, dorsal (pds: posterodorsal setae; c: position of c setae; arrow: posterolateral seta). 24, Larval case, right lateral. FIGURES 25–27. Drusus vespertinus Marinković 1976, 5th instar larva. 25, Head, dorsal (arrow: pronotal notch). 26, Head, lateral, carina with antenna. 27, Head, detail of spinule field (dotted oval). Scale bars: 1 mm (except Figs. 26, 27: 0.25 mm).
All gills single filaments. Dorsal gills present at most from 2nd segment (presegmental position) to 6th segment (postsegmental position). Ventral gills ranging from 2nd (presegmental) to 7th segment (postsegmental). Dorsolateral gills ranging from 2nd (presegmental) to 4th segment (presegmental), and ventrolateralgills ranging from 2nd (postsegmental) to 4th segment (postsegmental). Lateral fringe extending from last quarter of 2nd to middle of 8th abdominal segments (Fig. 13). Yellowish brown sclerite on 9th abdominal tergum semicircular (Fig. 12); along its posterior border, 8 long and several shorter setae present, 2 of these long setae having position of central intermediate c setae (Fig. 12c). Anal prolegs of limnephilid type, yellowish brown, with light brown muscle attachment spots. Ventral sole plate with black dorsal rim (Fig. 13).Tips of anal claws dark brown, each with 1 small accessory hook (Fig. 13).
Case
Larval case 10.3–13.3 mm long (n= 25), slightly curved, slightly conical (width at anterior opening 2.4–3.5 mm and at posterior opening 1.6–2.5 mm), consisting of mineral particles (sand grains of mixed size; Fig. 14).
Association of adults and larvae of Drusus vernonensis
Haplotypes of the mtCOI3-P segment of 3 adult males and 3 larvae of D. vernonensis collected at the same site were completely identical. Moreover, the two different stages were sampled in different years (i.e. larvae in 2009 and adults in 2010). This confirms the conspecificity of the larvae and adults of D. vernonensis collected at the Pelister Mt. in Macedonia.
Description of the fifth instar larva of Drusus vernonensis
Biometry
Body length of final instar larva 10.1–10.3 mm, head width 1.53–1.60 mm (n= 2). All morphological characters identical to those of D. krusniki except as noted below.
Head
Head capsule granulated, reddish to medium brown (Figs. 15, 18, 20), with dark brown muscle attachment spots. Large spinule field covering the dorsal sections of parietalia and frontoclypeus dorsal the eyes (Fig. 16). Antennae originating from lateral carinae (Fig. 19a). Labrum yellowish brown (Fig. 15). Ventral apotome elongated triangular, postgenal suture approximately 25–30% of apotome length (Fig. 17).
Thorax
Pronotum dark brown, without pronotal notch (Figs. 15, 18). Pronotum type A (Table 3) in lateral view: low median hump, evenly rounded; steep posterior and flat anterior slope almost straight (Fig. 20). In addition to 50–60 dark setae of varying lengths distributed over each pronotal half, pronotal surface covered by (1) tiny, pale, recumbent setae (Fig. 20), and (2) thin, long, yellowish setae concentrated at anterior center of pronotum (Fig. 18, arrows). Prosternite very weakly sclerotized, pentangular, darkest along posterior border; prosternal horn present.
Mesonotum completely covered by 2 yellowish brown sclerites. Counts for mesonotal setae are as follows: anterior setal group sa1: 16–20, posterior group sa2: 22–26, lateral group sa3: 35–40.
Anterior metanotal sclerites large, broadly triangular, each with brown anterior margin; approximately 20 setae per sclerite. Setae between posteromedian sclerites scarce; 12–15 steae per posteromedian sclerite; sa3 sclerites with approximately 25–30 setae per sclerite, concentrated anteriorly (Fig. 22). Legs yellowish brown; setation as in D. krusniki.
Abdomen
At 1st abdominal segment a small setal group may be present posterior to dorsal protuberance. Central section of 1st abdominal sternum with two larger bases near midline which occasionally fuse with 1–2 neighbouring smaller setal bases (Fig. 21). On 8th abdominal dorsum, two long, two intermediateand up to four minute posterodorsal setae (pds) present (Fig. 23, pds). Only 1 posterolateral seta on each half of 9th abdominal dorsum (Fig. 23, arrow).
All gills single filaments. Dorsal gills present at most from 2nd segment (presegmental position) to 6th segment (presegmental position). Ventral gills ranging from 2nd (presegmental) to 7th segment (presegmental). Dorsolateral gills present at 3rd segment only (presegmental), and ventrolateral gills ranging from 2nd (postsegmental) to 3rd segment (postsegmental). Lateral fringe starting at last quarter of 2nd segment (Fig. 22b), followed by a gap until mid-3rd segment (Fig. 22c); from this point extending to middle of 8th abdominal segment. Pale yellowish brown sclerite on 9th abdominal tergum semicircular (Fig. 23).
Case
Larval case 10.0–10.1 mm long (n = 2), slightly curved, slightly conical (width at anterior opening 2.7–2.9 mm and at posterior opening 1.9–2.0 mm), consisting of a mix of mineral particles (Fig. 24).
Association of adults and larvae of Drusus vespertinus
Comparison of the mtCOI sequences from one adult male D. vespertinus and three larvae collected at the Ribnik spring reach supports the association of larvae to this species as they differed by maximally 2 nucleotide positions (Table 4). Additionally, uncorrected p-distances between haplotypes from two known populations of D. vespertinus (0.9-1.3%; Table 4) agree with variability recorded in other Drusus endemics in the Western Balkans (e.g Previšić et al. 2009, 2014b).
Description of the fifth instar larva of Drusus vespertinus
Biometry
Body length of final instar larva 10.9–12.1 mm, head width 1.40–1.54 mm (n = 8). All morphological characters identical to those of D. krusniki except as noted below.
Head
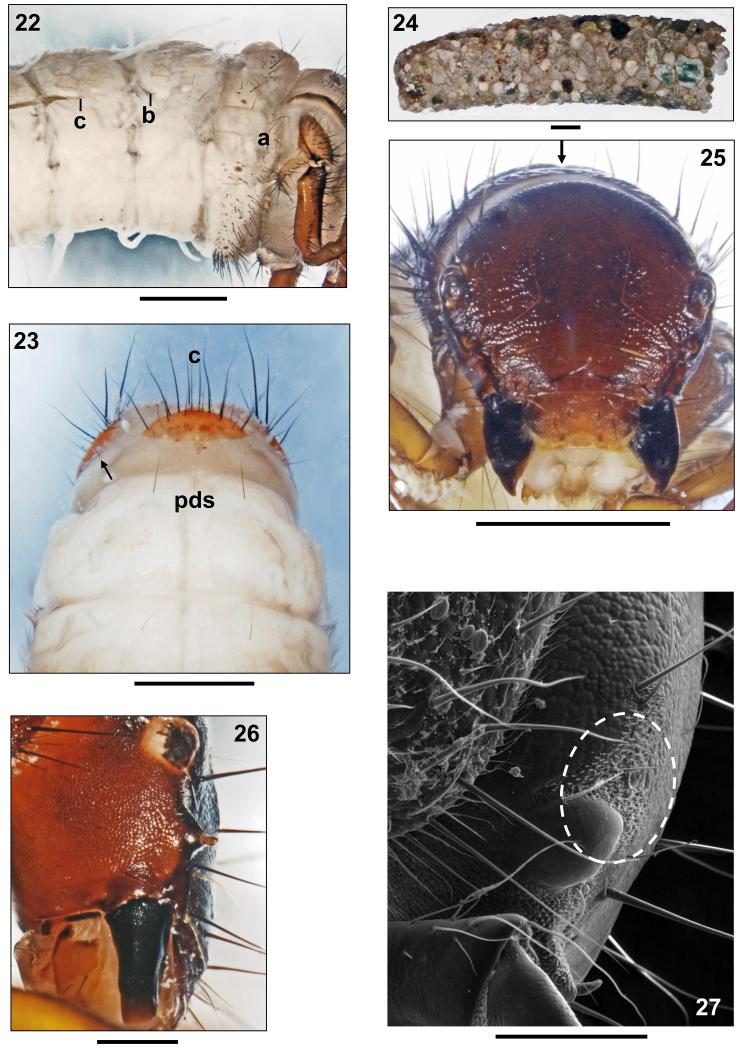
Head capsule coarsely granulated, broadly oval and hypognathous (Fig. 25), dorsally with dark brown to blackish brown coloration and blackish muscle attachment spots. Vertex rounded (Figs. 25, 28). In lateral view, head capsule with straight carina starting with a conical, slightly inward-curved antennal base approximately 0.09 mm high; anterior of the antenna the carina decreases in height to 0.02 mm (Figs. 25–27). Posterior to each eye, with oval areas of spinules (= small spines approximately 0.03 mm long) (Fig. 27; white oval). Ventral apotome scutiform, anterior half parallel-sided, posterior half triangular; postgenal suture approximately 33% of apotome length.
FIGURES 28–33.
Drusus vespertinus Marinković1976, 5th instar larva. 28, 29, Pronotum, right lateral. 30, Head, thorax and first abdominal segment, dorsal. 31, First abdominal sternum, ventral. 32, 8th and 9th abdominal terga, dorsal (pds: posterodorsal setae; c: position of c setae; arrow: posterolateral seta). 33, Larval case, right lateral.Scale bars: 1 mm.
Thorax
Blackish brown pronotal surface very coarsely granulated, covered by white recumbent setae (Figs. 28–30). In frontal view, small dorsocentral pronotal notch present (Fig. 25, arrow). Pronotum type C (Table 3) in lateral view: median hump prominent, high, with edged crest gradually fading laterally; posterior slope convex, anterior slope concave (Figs. 28, 29). In total, 45–50 dark setae of varying lengths distributed over each pronotal half.
Counts for mesonotal setae are as follows: anterior setal group sa1: 15–20, posterior group sa2: 20–25, lateral group sa3: 20–25 (Fig. 30).
Anterior metanotal sclerites with 15–20 setae per sclerite (Fig. 30), posteromedian sclerites with 15–18 setae and posteromedian sclerites with approximately 20–25 setae per sclerite, concentrated anteriorly.
Fore femora each with 5–6 yellow ventral-edge setae, mid- and hind femora each with 5–6 ventral edge setae. Dorsal setae only at distal third of mid- and hind tibiae.
Abdomen
At first abdominal segment a small setal group may be present posterior to dorsal protuberance. Setal bases at central section of 1st abdominal sternum mostly small and inconspicuous except two larger bases near midline which occasionally fuse with 1–2 neighbouring smaller setal bases (Fig. 31). On 8th abdominal dorsum, two long posterodorsal setae (pds) present (Fig. 32, pds). Only 1 posterolateral seta on each half of 9th abdominal dorsum (Fig. 32, arrow).
All gills single filaments. Dorsal gills present at most from 2nd segment (presegmental position) to 7th segment (presegmental position). Ventral gills ranging from 2nd (praesegmental) to 7th segment (postsegmental). Dorsolateral gills ranging from 2nd (presegmental) to 4th segment (presegmental), and ventrolateral gills ranging from 2nd (postsegmental) to 4th segment (postsegmental). Lateral fringe extending from last quarter of 2nd to first quarter of 8th abdominal segments. Medium brown sclerite on 9th abdominal tergum semicircular and medium brown (Fig. 32); setation as in D. krusniki.
Case
Larval case 9.9–10.8 mm long (n = 8), distinctly curved, slightly conical (width at anterior opening 2.5–3.9 mm and at posterior opening 1.7–2.3 mm), consisting of mineral particles (sand grains of mixed size; Fig. 33).
Morphological separation of fifth instar larvae of Drusus krusniki, D. vernonensis and D. vespertinus from other European Trichoptera
Within the framework of the limnephilid key by Waringer & Graf (2011) and Waringer et al. (2010), Drusus krusniki, D. vernonensis and D. vespertinus are separable from other species by the following features:
-
-
gills consisting of single filaments only; dorsal gills present (Fig. 22);
-
-
metanotum covered by three pairs of small sclerites (Figs. 4, 30);
-
-
mandibles spoon-shaped (terminal teeth and central cavity ridges lacking; Figs. 19b, 26);
-
-
head capsule with fields of spinules (= small spines approximately 0.03 mm long; Figs. 6, 7, 16, 27);
-
-
anterior-row setae present near dorsal midline of pronotum (Figs. 1, 15, 25);
-
-
dorsal-edge setae restricted to distal 3rd of mid- and hind tibiae (Figs. 9, 10);
-
-
center of first abdominal sternum without large sclerotized patches or concentrations of fused basal sclerites of setae (Figs. 11, 21, 31).
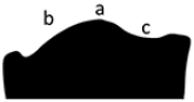
Based on this character list, Drusus krusniki, D. vernonensis and D. vespertinus key together with the other members of the Drusus bosnicus Group which have spinule fields on the head (so far, only Drusus ramae Marinković-Gospodnetić 1971b, also a member of the Drusus bosnicus Group, was found to lack such spinule fields). These species are easily separated by differences in vertex structure of the head (Figs. 28, 34), dorsal profile (Table 3; Figs. 5, 20, 28, 29), sculpturing (Fig. 5) and setation of the pronotum (Figs. 20, 35, 36) and presence/absence of a dorsocentral pronotal notch (Figs. 1, 25). A synopsis of differentiation characters in the Drusus bosnicus Group with spinule fields on the head is given in Table 5.
FIGURE 34–36.
FIGURE 34. Drusus bosnicus Klapálek 1899, 5th instar larva. Head and pronotum, right lateral (arrow: vertex flattened). FIGURE 35. Drusus radovanovici Marinković 1970, 5th instar larva. Pronotum, right lateral (arrows: thin long yellowish setae). FIGURE 36. Drusus medianus Marinković 1976, 5th instar larva. Detail of pronotum, right lateral (arrows: white recumbent setae). Scale bars: 1 mm (except Fig. 36: 0.5 mm).
Table 5.
Synopsis of characters separating the currently known Drusinae larvae (5th instars) which share the following group morphomatrix: spoon-shaped mandibles; with spinule areas at the head capsule; anterior-row setae present near dorsal pronotal midline; dorsal gills present; dorsal edge setae restricted to distal third of mid- and hind tibiae; basal sclerites of setae at first abdominal sternum not fusing into sclerotized plates or multilobed patterns. Pronotum types are defined in Table 3. Distribution in ecoregions according to Illies 1978; ER5 – Dinaric Western Balkans, ER6 – Hellenic Western Balkans.
| Species/character | Head with flat vertex (Fig. 34)? | Pronotum with thin, long, yellowish setae (Figs. 20, 35)? | Pronotum with numerous white, recumbent setae (Fig. 36)? | Pronotum type (Table 3) | Dorsocentral pronotal notch present (Figs. 1, 25)? | Distribution (ecoregions sensu Illies 1978) | References |
|---|---|---|---|---|---|---|---|
| Drusus bosnicus | yes | no | yes | C | yes | ER5 | Kučinić et al. 2015 |
| Drusus crenophylax 1 | no | no | yes | B | yes | ER5 | Vitecek et al. 2015a |
| Drusus klapaleki 1 | no | no | yes | B | yes | ER5 | Kučinić et al. 2011b |
| Drusus krusniki | no | no | yes | D | yes | ER5, ER6 | this paper |
| Drusus medianus | no | no | yes | B | no | ER5 | Kučinić et al. 2010 |
| Drusus radovanovici | no | yes | no | B | no | ER5 | Kučinić et al. 2011a |
| Drusus septentrionis | no | no | no | C | yes | ER5 | Kučinić et al. 2008 |
| Drusus serbicus | no | no | yes | E | yes | ER5 | Waringer et al. 2015 |
| Drusus vernonensis | no | yes | yes | A | no | ER6 | this paper |
| Drusus vespertinus | no | no | yes | C | yes | ER5 | this paper |
In Drusus klapaleki, white recumbent setae are distributed over the whole pronotal surface (Kučinić et al. 2011b) whereas in D. crenophylax those setae are lacking in a semicircular area anterior of the pronotal dorsal hump (Vitecek et al. 2015a).
Discussion
Larval morphology of the Western Balkans Drusus endemics
The three larvae described in the present paper bear morphological characters common to members of the Drusus bosnicus Group, such as the fields of spinules of various sizes posterior to each eye. Marinković-Gospodnetić (1971a) assigned D. bosnicus, D. klapaleki, D. plicatus Radovanović 1942, D. radovanovici and D. ramae to this group of species, based on similarities of main structures of the male genitalia. Later, D. krusniki, D. medianus, D. septentrionis and D. vespertinus were added (see discussion in Kučinić et al. 2011a). Moreover, the morphology of some Drusus species described in the recent years in Albania indicates close relatedness with the Drusus bosnicus Group (Oláh 2010, 2011) in adult morphology; larvae of these species are still unknown. Furthermore, the newly described endemic D. crenophylax and also D. serbicus Marinković-Gospodnetić 1971a share some of the common larval morphological features of this group (Vitecek et al. 2015a, Waringer et al. 2015). In all these species, including the three described herein, the spinule fields on the larval head are only absent in D. ramae, but are present in D. serbicus (Waringer et al. 2015) and the hitherto unknown larvae of Drusus krusniki, D. vernonensis and D. vespertinus. Thus, the spinules most probably represent a synapomorphic character in this Group, and may also be present in the unknown larvae of other species.
D. vernonensis is unique in that the spinule field covers large areas of the parietalia and the frontoclypeus dorsally of eye level; in all other known larvae, the spinule fields are small oval areas posterior of the eyes (Kučinić et al. 2008, 2010, 2011a, 2011b, 2015, Vitecek et al. 2015a). Additionally, larval D. vernonensis have other unique characters (Table 3) that are inconsistent with characters usually observed in Drusus bosnicus Group taxa. Interestingly, these differences are consistent with differences in male adult genitalia morphology which suggest a close relationship of D. vernonensis with species of the Drusus discophorus Group (Malicky 1989). However, the larval stages of the majority of species of the latter group are still unknown. Thus, a more comprehensive discussion on the significance of the presented larval morphological characters in “fine scale” Drusinae systematics is not possible at this point. Nevertheless, our findings are consistent with previous studies implying a close link of larval morphology, feeding ecology and phylogenetic relationships within Drusinae in general (Pauls et al. 2008, Graf et al. 2009), and more specifically within the carnivorous clade (Vitecek et al. 2015b). Hence, the improvement of the knowledge of the larval taxonomy presented in the current paper adds valuable information to an overall puzzle within the largest clade of Drusinae species, the epilithic grazers.
Distribution and ecology of Drusus krusniki, D. vernonensis and D. vespertinus
Species included in to the Drusus bosnicus Group are all endemic to the Dinaric Balkans, i.e. to the ecoregions ER5 (Dinaric Western Balkan) and ER6 (Hellenic Western Balkan) (sensu Illies 1978; Table 5). Drusus krusniki is endemic to the Western Balkans, inhabiting the southeastern parts of the Dinaric Alps in Montenegro and Kosovo (Graf et al. 2008, Previšić et al. 2014b, Gashi et al. 2015) and the Prokletje Mts. in Albania (Oláh 2010). D. vespertinus is a (micro-) endemic to the Dinaric Alps, (ER 5), known from the western part of Bosnia and Herzegovina (Marinković-Gospodnetić 1976) and Una River spring in Croatia (Kučinić et al. 2014). D. vernonensis was considered endemic to the Vernon Mountains in Greece (Malicky 2005b), but the current data show that its distribution extends northwards to the Pelister Mt. in Macedonia. However, it is an endemic species of the ecoregion 6 (Graf et al. 2008).
All these species prefer spring areas and the headwaters of cold, oxygen-rich streams with high to moderate currents at high altitudes in the mountains. D. vespertinus occurs in large karstic springs, like the spring of the river Ribnik, a tributary of the river Sana (Marinković-Gospodnetić, 1976), as well as the Una River spring (Kučinić et al. 2014). Mean annual water temperatures obtained by permanently exposed data loggers for sites inhabited by D. vernonensis (e.g., Pelister, 1805 m a.s.l, Macedonia) were 3.78°C (annual range 2.07–6.84°C) and for D. krusniki (e.g., Montenegro: Ibar spring, 1256 m a.s.l.; Alipaša’s springs, 932 m a.s.l.) 6.22°C (annual range 5.88–8.20°C) and 6.13°C (annual range 3.60–14.35°C), respectively.
Mandible morphology of Drusus krusniki, D. vernonensis and D. vespertinus larvae suggests a grazing lifestyle, with larvae feeding on biofilms and epilithic algae. The adults and larvae of Drusus krusniki, D. vernonensis and D. vespertinus were collected in late May in 2009, June and in early July. The latter species may be on the wing as early as late March since the holotype, paratypes and allotypes were collected on 26 March 1968 (Marinković-Gospodnetić, 1976). The holotype of D. krusniki was collected at the end of May (Malicky 1981) whereas only few samples of D. vernonensis exist which were obtained in June and July (Malicky 1989, 2005b). This is in accordance with the reported short summer flight period of D. vernonensis by Graf et al. (2008).
Acknowledgements
This contribution includes some of the results of the project “The Drusinae (Insecta: Trichoptera) in a world of global change” (project number P23687-B17, PI: J. Waringer) funded by the Austrian Science Fund (FWF). It was also funded by the Croatian Ministry of Science, Education and Sports (Project Nos. 119–1193080–1206 and 119–1193080–3076) and the University in Zagreb.
References
- AQEM Consortium . Manual for the Application of the AQEM System. A Comprehensive Method to Assess European Streams Using Benthic Macroinvertebrates, Developed for the Purpose of the Water Framework Directive. 2002. Version 1_0. [Google Scholar]
- Bálint M, Domisch S, Engelhardt CHM, Haase P, Lehrian S, Sauer J, Theissinger K, Pauls SU, Nowak C. Cryptic biodiversity loss linked to global climate change. Nature Climate Change. 2011;1:313–318. [Google Scholar]
- Barbour MT, Gerritsen J, Snyder BD, Stribling JB. Rapid bioassessment protocols for use in wadeable streams and rivers: Periphyton, benthic macroinvertebrates and fish. 2nd Ed. EPA 841-B-99-002. U.S. Environmental Protection Agency; Washington, D.C.: 1999. xii + 326 pp. [Google Scholar]
- Barbour MT, Yoder CO. The multimetric approach to bioassessment, as used in the United States. In: Wright JF, Sutcliffe DW, Furse MT, editors. Assessing the Biological Quality of Fresh Waters: RIVPACS and Other Techniques. Freshwater Biological Association; Ambleside, UK: 2000. pp. 281–292. [Google Scholar]
- Gashi A, Ibrahimi H, Grapci-Kotori L, Sejdiu N, Bislimi K. New Records of Drusus siveci Malicky, 1981 (Trichoptera, Limnephilidae, Drusinae) from the Balkan Peninsula, with Ecological Notes. Acta Zoologica Bulgarica. 2015;67:259–264. [Google Scholar]
- Graf W, Murphy J, Dahl J, Zamora-Muñoz C, López-Rodríguez MJ. Trichoptera. In: Schmidt-Kloiber A, Hering D, editors. Distribution and Ecological Preferences of European Freshwater Organisms. Vol. 1. Pensoft Publishers; Sofia, Moscow: 2008. p. 388. [Google Scholar]
- Graf W, Waringer J, Pauls SU. A new feeding group within larval Drusinae (Trichoptera: Limnephilidae): The Drusus alpinus Group sensu Schmid, 1956, including larval descriptions of Drusus franzi Schmid, 1956, and Drusus alpinus (Meyer-Dür, 1875) Zootaxa. 2009;2031:53–62. [PMC free article] [PubMed] [Google Scholar]
- Illies J. Limnofauna Europaea. A Checklist of the Animals Inhabiting European Inland Waters, with an Account of their Distribution and Ecology. 2nd Edition Gustav Fischer Verlag; Stuttgart: 1978. p. 552. [Google Scholar]
- Kearse M, Moir R, Wilson A, Stones-Havas S, Cheung M, Sturrock S, Buxton S, Cooper A, Markowitz S, Duran C, Thierer T, Ashton B, Mentjies P, Drummond A. Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics. 2012;28:1647–1649. doi: 10.1093/bioinformatics/bts199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klapálek F. Prilozi k poznavanju fauneTrichoptera i Neuroptera Bosne i Hercegovine. Glasnik zemaljskog muzeja u Sarajevu. 1899;11:323–337. [Google Scholar]
- Kučinić M, Previšić A, Gottstein-Matočec S, Hrašovec B, Stanić-Koštroman S, Pernek M, Delić A. Description of the larvae of Drusus radovanovici septentrionis Marinković-Gospodnetić, 1976 and Drusus croaticus Marinković-Gospodnetić, 1971 (Trichoptera: Limnephilidae) from Bosnia and Herzegovina, and Croatia. Zootaxa. 2008;1783:1–17. [Google Scholar]
- Kučinić M, Previšić A, Stanić-Koštroman S, Franjević M, Šerić Jelaska L, Delić A, Posilović H. Description of the larvae of Drusus ramae Marinković-Gospodnetić, 1971 and Drusus medianus Marinković-Gospodnetić, 1976 (Trichoptera: Limnephilidae) with some genetic data, distribution, ecological, faunal and conservation notes. Zootaxa. 2010;2484:1–24. [Google Scholar]
- Kučinić M, Previšić A, Graf W, Šerić Jelaska L, Stanić-Koštroman S, Waringer J. Larval description, genetic and ecological features of Drusus radovanovici radovanovici Marinković-Gospodnetić, 1971 (Trichoptera: Limnephilidae) with some phylogenetic and taxonomic data on the bosnicus group in the Balkan Peninsula. Deutsche Entomologische Zeitschrift. 2011a;58:136–153. [Google Scholar]
- Kučinić M, Previšić A, Stanić-Koštroman S, Graf W, Franjević M, Posilović H, Waringer J. Morphological and ecological features of Drusus larvae from the bosnicus group on the Balkan Peninsula with description of the larva of Drusus klapaleki Marinković-Gospodnetić, 1976. Zoosymposia. 2011b;5:244–254. [Google Scholar]
- Kučinić M, Delić A, Ćuk R, Previšić A, Mihoci I, Žganec K, Cerjanec D, Vučković I. The first finding of Drusus bosnicus Group (Insecta, Trichoptera, Limnephilidae) in Croatia with some notes on diversity, distribution and ecology of genus Drusus in Croatia and in Dinaric karst of the Balkan Peninsula. Natura Croatia. 2014;23:265–377. [Google Scholar]
- Kučinić M, Previšić A, Graf W, Mihoci I, Šoufek M, Stanić-Koštroman S, Suvad L, Vitecek S, Waringer J. Larval description of Drusus bosnicus Klapálek 1899 (Trichoptera: Limnephilidae), with distributional, molecular and ecological features. Zootaxa. 2015;3957:85–97. doi: 10.11646/zootaxa.3957.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumanski K. Die Unterfamilie Drusinae (Trichoptera) in Bulgarien. Tijdschrift voor Entomologie. 1973;116:107–121. [Google Scholar]
- Malicky H. Weiteres Neues über Köcherfliegen aus dem Mediterrangebiet (Trichoptera) Entomofauna. 1981;2:335–355. [Google Scholar]
- Malicky H. Ein neuer Drusus aus Nord-Griechenland (Trichoptera, Limnephilidae) Entomologische Zeitung. 1989;99:303–304. [Google Scholar]
- Malicky H. Atlas of European Trichoptera. 2nd Edition Springer; 2004. p. 359. [Google Scholar]
- Malicky H. Ein kommentiertes Verzeichnis der Köcherfliegen (Trichoptera) Europas und des Mediterrangebietes. Linzer biologische Beiträge. 2005a;37:533–596. [Google Scholar]
- Malicky H. Die Köcherfliegen Griechenlands. Denisia. 2005b;17:1–240. [Google Scholar]
- Marinković-Gospodnetić M. The species of the genus Drusus in Yugoslavia. Godišnjak biološkog Instituta Univerziteta u Sarajevu. 1971a;24:105–109. [Google Scholar]
- Marinković-Gospodnetić M. New species of Trichoptera from a Bosnia and Herzegovina. Bulletin Scientifique (Sectiona A) 1971b;15(5–6):144–145. [Google Scholar]
- Marinković-Gospodnetić M. In: Malicky H, editor. The differentiation of Drusus species of the group bosnicus; Proceedings of the First International Symposium on Trichoptera; 1976; The Hague: Dr. W. Junk; pp. 77–85. [Google Scholar]
- Oláh J. New species and new records of Palaearctic Trichoptera in the material of the Hungarian Natural History Museum. Annales historico-naturales Musei nationalis hungarici. 2010;102:65–117. [Google Scholar]
- Oláh J. New species and records of Balkan Trichoptera. Folia historico-naturalia Musei Matraensis. 2011;35:111–121. [Google Scholar]
- Oláh J, Chvojka P, Coppa G, Godunko JR, Lodovici O, Majecka K, Majecki J, Szczęsny B, Urbanič G, Valle M. Limnephilid taxa revised by speciation traits: Rhadicoleptus, Isogamus, Melampophylax genera, Chaetopteryx rugulosa, Psilopteryx psorosa species groups, Drusus bolivari, Annitella kosciuszkii species complexes (Trichoptera: Limnephilidae) Opuscula Zoologica Budapest. 2015;46:3–117. [Google Scholar]
- Pauls SU, Lumbsch HT, Haase P. Phylogeography of the montane caddisfly Drusus discolor: Evidence for multiple refugia and periglacial survival. Molecular Ecology. 2006;15:2153–2169. doi: 10.1111/j.1365-294X.2006.02916.x. [DOI] [PubMed] [Google Scholar]
- Pauls SU, Graf W, Haase P, Lumbsch HT, Waringer J. Grazers, shredders and filtering carnivores – The evolution of feeding ecology in Drusinae (Trichoptera: Limnephilidae): Insights from a molecular phylogeny. Molecular Phylogenetics and Evolution. 2008;46:776–791. doi: 10.1016/j.ympev.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Previšić A, Walton C, Kučinić M, Mitrikeski PT, Kerovec M. Pleistocene divergence of Dinaric Drusus endemics (Trichoptera, Limnephilidae) in multiple microrefugia within the Balkan Peninsula. Molecular Ecology. 2009;18:634–647. doi: 10.1111/j.1365-294X.2008.04046.x. [DOI] [PubMed] [Google Scholar]
- Previšić A, Graf W, Vitecek S, Kučinić M, Bálint M, Keresztes L, Pauls SU, Waringer J. Cryptic diversity of caddisflies in the Balkans: the curious case of Ecclisopteryx species (Trichoptera: Limnephilidae) Arthropod Systematics and Phylogeny. 2014a;72:309–329. [PMC free article] [PubMed] [Google Scholar]
- Previšić A, Schnitzler J, Kučinić M, Graf W, Ibrahimi H, Kerovec M, Pauls SU. Microscale vicariance and diversification of western Balkan caddisflies linked to karstification. Freshwater Science. 2014b;33:250–262. doi: 10.1086/674430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radovanović M. Über zwei neue Trichopteren-Arten aus Mazedonien. Zoologischer Anzeiger. 1942;140:183–190. [Google Scholar]
- Schmid F. La sous-famille des Drusinae (Trichoptera, Limnophilidae) Memoires de l’Institut Royal des Sciences Naturelles de Belgique, 2. Serie. 1956;55:1–92. [Google Scholar]
- Simon C, Frati F, Beckenbach A. Evolution, weighting and phylogenetic utility of mitochondrial gene sequences and a compilation of conserved polymerase chain reaction primers. Annals of the Entomological Society of America. 1994;87:651–701. [Google Scholar]
- Sipahiler F. Hadimina torosensis, new genus and new species of Drusinae from southern Turkey (Trichoptera: Limnephilidae). (Proceedings of the 10th International Symposium on Trichoptera); 2002; Nova Supplementa Entomologica; pp. 239–248. [Google Scholar]
- Stephens JF. Illustrations of British entomology; or, a synopsis of indigenous insects: containing their generic and specific distinctions; with an account of their metamorphoses, times of appearance, localities, food, and economy, as far as practicable. Vol. VI. Mandibulata. Baldwin &Cradock; London: 1836-1837. p. 240. [Google Scholar]
- Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Molecular Biology and Evolution. 2013;30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitecek S, Previšić A, Kučinić M, Bálint M, Keresztes L, Waringer J, Pauls SU, Malicky H, Graf W. Description of a unique Wormaldia from Sardinia and a new Drusus from the Western Balkans (Trichoptera: Philopotamidae; Limnephilidae) ZooKeys. 2015a;496:85–103. doi: 10.3897/zookeys.496.9169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitecek S, Graf W, Previšić A, Kučinić M, Oláh J, Bálint M, Keresztes L, Pauls SU, Waringer J. A hairy case: The evolution of filtering carnivorous Drusinae (Limnephilidae, Trichoptera) Molecular Phylogenetics and Evolution. 2015b;93:249–260. doi: 10.1016/j.ympev.2015.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waringer J, Graf W, Pauls SU, Previšić A, Kučinić M. A larval key to the Drusinae species of Austria, Germany, Switzerland and the dinaric western Balkan. Denisia. 2010;29:383–406. [PMC free article] [PubMed] [Google Scholar]
- Waringer J, Graf W. Atlas of Central European Trichoptera Larvae. Erik Mauch Verlag; Dinkelscherben: 2011. p. 468. [Google Scholar]
- Waringer J, Graf W, Balint M, Kučinić M, Pauls SU, Previšić A, Keresztes L, Ibrahimi H, Živić I, Bjelanović K, Krpač V, Vitecek S. Larval morphology and phylogenetic position of Drusus balcanicus, D. botosaneanui, D. serbicus and D. tenellus (Trichoptera: Limnephilidae: Drusinae) European Journal of Entomology. 2015;112:344–361. doi: 10.14411/eje.2015.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiggins GB. Larvae of the North American Caddisfly Genera (Trichoptera) 2nd Edition University of Toronto Press; Toronto: 1998. p. 457. [Google Scholar]