Abstract
Knowledge of macrophages in steady-state and diseased tissue is rapidly expanding, propelled by improved diagnostic capacity to detect and monitor cells in their native environments. In this review, we discuss implications for ischaemic heart disease and examine innate immune cell pathways that increase systemic leucocyte supply after myocardial infarction (MI). Acute MI alters the macrophage phenotype and supply chain from tissue resident to blood monocytes sourced from haematopoietic organs. That blood leucocytosis closely associates with cardiovascular mortality provides a strong motivation to understand why and how organ ischaemia alters cellular immunity.
Keywords: Atherosclerosis, Myocardial infarction, Haematopoiesis, Monocyte, Macrophage, Bone marrow, Spleen, Proliferation
Introduction
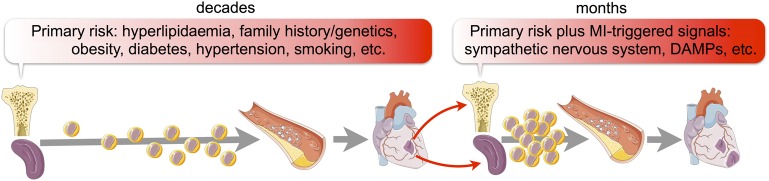
Historic and contemporary clinical data illustrate that secondary ischaemic event rates are high. Several large studies report that within the first year after an index event, the frequency of death, myocardial infarction (MI), or stroke is around 8–12% even though patients are enrolled in clinical trials in which they are well-monitored and treated according to guidelines.1–4 Softer endpoints (i.e. any ischaemia) occur even more frequently and may reach a rate of one in every two patients.5–7 These unacceptably high secondary event rates document (i) that ischaemic heart disease complications are frequent after a first MI and (ii) the need to improve secondary prevention in MI patients. Preclinical data8 suggest that acute MI may change the disease trajectory of atherosclerosis and accelerate plaque development by expanding the systemic leucocyte pool (Figure 1). In addition, clinical data suggest that the innate immune system is more active in patients with recurrent events.9 Yet inflammatory pathways are not among clinically established therapeutic targets. The growing knowledge of inflammation's role in ischaemic heart disease contrasts with the absence of drugs that specifically aim at the immune system to reduce secondary event rates, possibly due to disappointing results in earlier clinical studies that targeted inflammatory cytokines.10 We believe, however, that recent basic science progress provides new opportunities: now is not the time to be discouraged. In our review, we discuss data indicating the emerging role of—and future therapeutic opportunities associated with—myeloid cells in patients with atherosclerosis and organ ischaemia.
Figure 1.
Processes leading to secondary ischaemia. Several risk factors, especially hyperlipidaemia, increase production of leucocytes which give rise to a first ischaemic event. This ischaemic event itself accelerates haematopoiesis, for instance via increased sympathetic nervous system activity, leading to a second within a short time span after the first.
Resident immune cells in the steady-state cardiovascular system
The advent of highly sensitive assays, which rely on antibody detection of cell surface markers by flow cytometry and macrophage-specific expression of fluorescent proteins, in conjunction with three-dimensional microscopy techniques that detect delicate cellular structures, has recently demonstrated the presence of a sizable leucocyte population in the steady-state heart11–13 and arteries.14,15 Tissue-resident macrophages and dendritic cells are the most numerous leucocytes therein. The steady-state heart also contains a dense network of macrophages that nestle between myocytes and are distinct from other stromal cells such as fibroblasts.11–13 Macrophage frequency was estimated by flow cytometry to be ∼500 cells per milligram in murine myocardium, a number that fluctuates depending on the retrieval protocol. Immuno-staining clinical myocardial samples documented cardiac macrophages in humans.12 Further, CD11c fluorescent reporter systems documented the presence of myeloid cells, thought to be dendritic cells, in cardiac valves and in the murine aorta.15 Because certain tissue-resident macrophages, for instance in the lung (www.immgen.org), also express CD11c at high levels, there is debate as to whether these vascular residents are dendritic cells or macrophages. Perhaps this distinction will be informed by specific cellular tasks; however, macrophages and dendritic cells have many overlapping functions. Viewed together with the rapidly evolving knowledge on tissue-resident macrophages in other organs,16,17 the cells' abundant presence in cardiovascular organs is not surprising. Despite increased data on cellular abundance and phenotype,18,19 cardiac and vascular macrophages' precise tissue-specific functions are mostly unknown. First insights suggest that cardiac macrophages, as in other tissues, ingest dying stromal cells and pathogens.12,13 In other tissues, resident macrophages pursue highly specific tasks such as regulating temperature, modulating glucose metabolism, and forming progenitor niches,20 and macrophages that are components of cardiovascular organs likely have similarly specific functions.
For most organs, the origin of steady-state tissue-resident macrophages has been determined. Resident macrophages in the brain, lung, spleen, peritoneum, bone marrow, liver, kidney, and pancreas do not derive from circulating monocytes but rather derive from local cells that took up residence in these organs early in life. These macrophages derive from embryonic cells that originated in the yolk sac and/or foetal liver.17,21 Long-term parabiosis leads to shared circulation between two mice and thus enables us to discern monocyte- from non-monocyte-derived tissue macrophages. Parabiosis fate mapping experiments revealed that microglia, the resident macrophages in the brain, hardly rely on blood monocyte recruitment at all.22 On the opposite side of the spectrum, intestinal macrophages seem to derive mostly from blood monocytes.16 The current lack of fate mapping data for macrophages residing in the healthy arterial wall will no doubt be addressed in future studies. However, three publications do provide data on cardiac resident macrophages: two of the studies describe cardiac resident macrophages as largely independent of circulating monocytes in the steady state,12,13 while one manuscript comes to the opposite conclusion, stating that, with increasing mouse age, cardiac macrophages are replaced by monocyte-derived cells.23 Future work will address this controversy and possibly reconcile the conflicting findings.
Role of immune cells in events leading to first organ ischaemia
Macrophages residing in atherosclerotic plaque derive overwhelmingly from circulating monocytes produced in the bone marrow and the spleen,24 though local proliferation of monocyte-derived macrophages contributes dominantly to the overall number in mature murine lesions.25 Recent fate mapping data suggest that a minority of ‘macrophage-like cells’ that express certain macrophage markers may derive from smooth muscle cells. In humans, an estimated ∼18% of plaque macrophages originate from smooth muscle cells.26 The functional roles of smooth muscle cell-derived macrophages remain unresolved. Monocytes are recruited to the arterial wall via chemokine/chemokine receptor interaction (e.g. CCR2, CCR5, CX3CR1) and cellular adhesion molecules expressed by activated endothelial cells. Once in tissue, monocytes differentiate to macrophages, which then proliferate locally to give rise to more macrophages. Thus, monocyte recruitment is amplified by local macrophage proliferation. The signals that regulate macrophage proliferation in plaque are currently not well understood, but deletion of the scavenger receptor A reduced plaque macrophage proliferation.25 Monocyte and macrophage activity is often seen as detrimental to vascular health because the cells perpetuate inflammation and destabilize extracellular matrix and the endothelial layer. If vascular wall integrity is compromised, pro-thrombotic plaque components activate the clotting system through direct contact. Dying myeloid and foam cells contribute to vulnerable plaques' necrotic cores, which, together with plaque erosion, are sources of thrombotic events that lead to downstream organ ischaemia. Hyperlipidaemia, a particularly well-documented risk factor for MI, drives blood monocytosis27 and monocyte production in haematopoietic organs (other risk factors are discussed below). Compromised reverse cholesterol transport intensifies proliferation of the haematopoietic progenitor pool in the bone marrow.28 Additionally, more progenitors take up residence in the spleen and, instigated by signals such as IL-3 and GM-CSF, produce monocytes that migrate to growing atherosclerotic plaques.24
Immune cell numbers, phenotype, and function during myocardial ischaemia
Ischaemia profoundly changes the immune cell landscape in the heart. Within hours after onset, ischaemia causes rapid death of tissue-resident macrophages.12 At the same time, several hundred thousand myeloid cells are recruited from the blood pool each day.29 Recruitment of neutrophils and inflammatory monocytes dominates the early phase after MI. As inflammation begins to resolve on Day 4, the infarct macrophage phenotype changes to support healing. Non-inflammatory monocytes are also recruited, albeit at lower numbers.30,31 Why resident cardiac macrophages die only to be replaced by monocyte-derived macrophages recruited from blood is currently unclear, but this switch in macrophage sources occurs in concert with a profound transition in phenotype and a robust increase in macrophage numbers. The newly recruited cells have wound healing functions, including proteolysis and phagocytosis during Days 1–3 and angiogenesis and fibrosis thereafter, that resemble injury response programmes in other tissues.31 For in-depth information on the role of macrophages and other immune cells during infarct healing, we refer the interested reader to recent reviews.20,32,33
The bone marrow as a source of cells
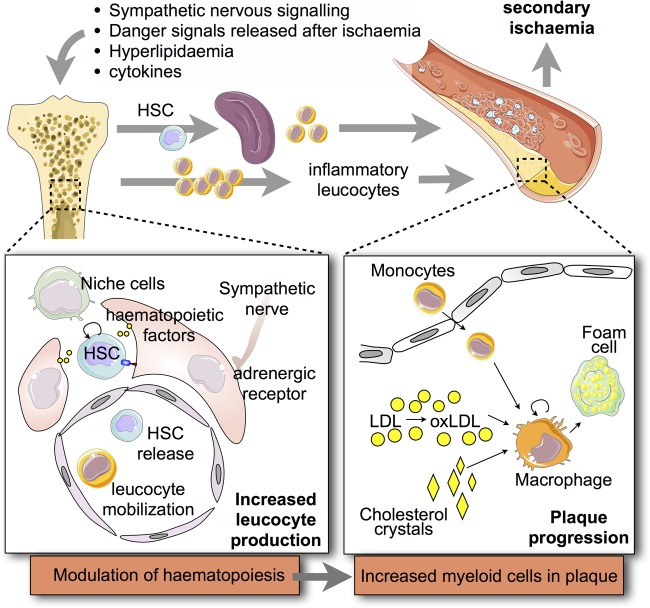
Traditionally, the bone marrow has not received attention from cardiologists and cardiovascular scientists. First interesting clinical post-MI observations include, for instance, haematopoietic progenitor cell release into the blood34 and increased bone marrow glucose uptake using 18F-FDG PET imaging signal as a surrogate.35 More recently, the haematopoietic organs have received attention as sources of leucocytes that fuel inflammation in arterial plaques and ischaemic myocardium.36 This interest is based on: (i) leucocytes' short lifespans, (ii) myeloid cells' relatively fast turnover in inflammatory foci, and (iii) the close and robust clinical association of blood myeloid numbers with cardiovascular events, post-MI heart failure, and death.37–40 Conceptually, haematopoietic tissues can be divided into (i) haematopoietic stem and progenitor cells (HSPCs) that give rise to blood leucocytes and (ii) a supporting cast of non-haematopoietic niche cells that protect HSPC and regulate their activity, especially progenitor cell proliferation, differentiation, and migration. Haematopoietic stem and progenitor cells and niche cells have been scrutinized in mice and patients with haematological disorders and infection (see relevant reviews, refs 41–43), but their role in ischaemic heart disease has only recently began to emerge. Cells that influence HSPC, with secreted factors or information transfer via ligands expressed on the cell surface, include endothelial cells, mesenchymal cells, osteoblasts, macrophages, and lymphocytes (Figure 2). Adhesion molecules such as selectins and VCAM-1 retain VLA-4 expressing HSPC in specific environments, and secreted factors such as M-CSF, GM-CSF, SCF, SDF-1, notch ligands, and angiopoietin regulate proliferation and differentiation into different blood cell lineages.41–43 Soluble factors that travel with blood, including pathogen- and damage-associated molecular patterns (PAMPs and DAMPs) that can act as ligands on toll-like receptors expressed by HSPC, likewise contribute to the acceleration of haematopoiesis in response to external stimuli such as infection. For instance, lipopolysaccharide, a component of the bacterial cell wall considered as a PAMP, binds to toll-like receptor-4 and increases HSPC proliferation.44 It is conceivable that DAMPs derived from injured cardiovascular organs likewise activate innate immune cell production.
Figure 2.
Innate immune pathways leading to increased myeloid cell production and acceleration of atherosclerosis. A number of pathways and signals increase their input on haematopoietic tissues after a first organ ischaemia. The signals can either be sensed by haematopoietic stem cells or by niche cells which instruct HSC behaviour. Altered niche haematopoietic signals may increase haematopoietic stem cell proliferation (i.e. leucocyte production) and increase their migration out of the bone marrow. As a consequence, splenic leucocyte production increases as well. Altogether, the systemic pool of innate immune cells, including plaque macrophages, expands.
Haematopoietic stem cells (HSC) are rare (∼1 in 10 000 bone marrow cells); mostly quiescent (<5% of these HSC cycle in the steady state); multipotent (they give rise to all blood cells), and long-lived (murine HSC can survive their hosts if transplanted into recipient mice). The most specific assays to identify and enumerate these cells are functional and rely on HSC transplantation. One successfully transplanted HSC can rescue a lethally irradiated mouse that would otherwise die of bleeding, anaemia, and leucopenia. To put it differently, if this single transferred cell is not an HSC, the mouse will not survive. Hence, either survival or the presence of blood cells in the recipient mouse determines the properties of the transferred cell. With less precision but more practicality, HSC can also be identified by their cell surface markers. Flow cytometry identifies HSC with up to 50% specificity as lineage− c-kit+ sca-1+ CD150+ CD48−. These so-called SLAM HSC45 respond to coronary ligation with increased proliferative activity in 48 h.46 Within the SLAM HSC population, most increased proliferation is observed in cells that express the chemokine receptor CCR2.46 Transcriptome analyses revealed that CCR2+ HSPC's emergence depends on the co-transcription factor Mtg16.46 CCR2+ HSC are hierarchically below long-term CCR2− HSC, they are myeloid biased and represent the migratory population that can be found outside the marrow.46 These cells differentiate to an expanding pool of more immediate monocyte precursors (MDP, CMoP) and finally differentiate into monocytes47 that rely on chemokine signals such as CCL248 and CCL749 for release into blood. The precise signalling that leads to expanded myelopoiesis after MI and, perhaps more importantly, the signals that taper the proliferative bone marrow response during resolution of infarct inflammation are incompletely understood. It is clear, however, that cell intrinsic factors, including transcription factors such as Mtg16 and PU.1, play a dominant role in activating HSC proliferation and steering production towards the myeloid cell lineage.46 Future research will reveal if MI also changes the epigenetic properties of HSPC for longer periods and whether this change increases inflammatory leucocyte supply and accelerates ischaemic heart disease.
A second major contribution to post-MI changes in bone marrow cell production stems from haematopoietic niche factors and supporting niche cells (Figure 2). We found that the bone marrow microenvironment changes substantially after MI. In general terms, factors that keep HSC quiescent in the bone marrow niche are down-regulated after ischaemic organ injury. One prominent retention factor, CXCL12 (also known as SDF-1), which is provided by mesenchymal cells, decreases significantly,8 leading to higher proliferation and decreased bone marrow retention of HSPC. This CXCL12 reduction is triggered by the post-MI increase in sympathetic nervous signalling in the bone marrow via the β3 adrenergic receptor expressed by niche cells.8 Increased noradrenaline levels may act directly on HSPC, which express β2 adrenergic receptors, but the contribution of β2 adrenergic receptor signalling remains to be tested in the setting of MI.
Extramedullary sites as sources of myeloid cells
In addition to the bone marrow and the marginal cell pool, the spleen is a source for inflammatory leucocytes. Within the first day after coronary ligation in mice and rats, the spleen releases several hundred thousand monocytes from its subcapsular red pulp into circulation.50 We determined that the spleen contributes about half of the myeloid cells that migrate to ischaemic myocardium within the first day. Splenic monocyte release is triggered by angiotensin-2 signalling through receptors expressed by splenic monocytes.50,51 The cell release is so massive that, in mice, spleen size and weight are considerably reduced shortly after coronary ligation. Whether a post-MI decrease in spleen size occurs in human patients is currently not known.
Several days after myocardial ischaemia, the spleen becomes a site of extramedullary haematopoiesis in mice.29 Triggered by sympathetic nervous system activity, the bone marrow releases increased numbers of HSPC, and this phenomenon has been observed in mice and humans after MI.8,34 This HSPC migration leads to a wave of haematopoietic progenitor seeding in the spleen (Figure 2). For instance, the number of splenic MDPs increases 24-fold in mice after MI.29 Likely, the increased HSPC supply collaborates with a more receptive splenic niche environment. Splenic CD169+ macrophages retain HSPC via VCAM-1/VLA-4 interaction.52 In addition, soluble factors such as IL-1β contribute to increased monocyte production after MI.29 As a consequence, bone marrow and splenic leucocyte production lead to monocytosis, and even though millions of these cells are recruited to the ischaemic heart, in ApoE-/- mice the newly-produced myeloid cells are recruited to atherosclerotic lesions, where they contribute to plaque growth and inflammation.8
Co-morbidities and lifestyle kindle inflammation
We have already discussed pathways involved in chronic atherosclerosis acceleration after organ ischaemia, yet similar interactions between atherosclerosis progression and other comorbidities also contribute to activation of innate immunity. Psychosocial stress, obesity, and diabetes increase the odds ratio for MI53 and have a strong inflammatory component even though pro-inflammatory effects may not be their most dominant feature. Psychosocial stress leads to increased production, release, and activation of inflammatory leucocytes (reviewed in ref. 54). In particular, stress-related sympathetic signalling increases HSPC proliferation in murine bone marrow via pathways similar to those observed after MI.55 Obviously MI is a highly stressful event. In humans, stress increases leucocyte levels and renders their phenotypes more inflammatory.56 Adipose tissue macrophages influence haematopoiesis via IL-1β,57 and hyperglycaemia in mice with diabetes can accelerate the myelopoietic output via interaction of neutrophil-derived S100A8/9 with RAGE on HSPC.58 Thus, acute MI and other comorbidities or lifestyle factors converge on a pathway of increased myelopoiesis that elevates production of neutrophils and monocytes, which then migrate to atherosclerotic lesions to pursue plaque-destabilizing roles.
Translational observations
Clinical evidence for translating the above experimental findings is not easy to obtain because human haematopoietic organs are difficult to sample in vivo. Assessing blood and using imaging may help bridge the preclinical and clinical spaces. Clinical studies34,35 showed that acute MI leads to an increased number of HSPC circulating in blood, and these results parallel the description of post-MI HSPC release due to reduced retention factors on Day 4 after coronary ligation in mice.8 Several clinical imaging studies reported post-MI activation of haematopoietic tissues and increased 18F-FDG-uptake in non-culprit lesions.59–61 In one retrospective analysis, 18F-FDG uptake in the spleen and bone marrow correlated positively with the cardiovascular event rate following imaging.60 Further, accelerated coronary plaque growth after first MI was documented with coronary imaging in humans.62 These studies were mostly small, and the surrogate imaging parameters were not specific to the immune pathways elaborated in mice. Thus, more specific, larger, and prospective studies should interrogate the importance and translatability of these preclinical pathways in humans with ischaemic heart disease.
Opportunities and hurdles for drug development
In this review, we summarize emerging data on innate immune pathways that accelerate ischaemic heart disease after a first ischaemic event. The clinical data supporting post-MI leucocytosis have been reproduced many times over, and the leucocytosis' association with cardiovascular mortality is strong. Despite mounting preclinical evidence on the pathways that alert haematopoiesis after MI, many still await translational verification in human patients. An additional challenge for developing therapeutics lies in the nature of the innate immune response, which is not specific to ischaemic injury and protects patients against infections and, to some degree, is likely necessary to enabling cardiac repair and avoiding post-MI heart failure. Data on monocyte or macrophage depletion show that post-MI recovery is worse if inflammatory myeloid cells are either over- or undersupplied, making a measured approach necessary. Perhaps we can identify pathways that lead to overproduction of inflammatory monocytes and target those exuberant responses without sacrificing steady-state haematopoiesis. For example, inhibiting post-MI traffic of HSPC from the bone marrow to the spleen8 would reduce splenic but not bone marrow leucocyte production. This could be achieved by neutralizing HSPC's capacity to be released from the marrow, migrate in blood, seed, and/or proliferate in the spleen. Suitable neutralization avenues include antibodies for adhesion molecules and chemokines, small molecule inhibitors for chemokine receptors and RNA interference to silencing targets inside myeloid cells and their progenitors, including chemokine receptors and transcription factors.
Authors' contributions
M.N./F.S. drafted the manuscript, and made critical revision of the manuscript for key intellectual content.
Funding
This work was supported by grants from the US National Institutes of Health R01HL117829, R01NS084863, the MGH Research Scholar Award (to M.N.), and R01HL095612, R56AI104695, and the Howard M. Goodman Fellowship (to F.K.S.).
Conflict of interest: none declared.
Acknowledgements
We thank Kaley Joyes, PhD, for editing the manuscript.
References
- 1. Roe MT, Ohman EM. A new era in secondary prevention after acute coronary syndrome. N Engl J Med 2012;366:85–87. [DOI] [PubMed] [Google Scholar]
- 2. Alexander JH, Lopes RD, James S, Kilaru R, He Y, Mohan P, Bhatt DL, Goodman S, Verheugt FW, Flather M, Huber K, Liaw D, Husted SE, Lopez-Sendon J, De Caterina R, Jansky P, Darius H, Vinereanu D, Cornel JH, Cools F, Atar D, Leiva-Pons JL, Keltai M, Ogawa H, Pais P, Parkhomenko A, Ruzyllo W, Diaz R, White H, Ruda M, Geraldes M, Lawrence J, Harrington RA, Wallentin L. Apixaban with antiplatelet therapy after acute coronary syndrome. N Engl J Med 2011;365:699–708. [DOI] [PubMed] [Google Scholar]
- 3. Mega JL, Braunwald E, Wiviott SD, Bassand JP, Bhatt DL, Bode C, Burton P, Cohen M, Cook-Bruns N, Fox KA, Goto S, Murphy SA, Plotnikov AN, Schneider D, Sun X, Verheugt FW, Gibson CM. Rivaroxaban in patients with a recent acute coronary syndrome. N Engl J Med 2012;366:9–19. [DOI] [PubMed] [Google Scholar]
- 4. Wallentin L, Becker RC, Budaj A, Cannon CP, Emanuelsson H, Held C, Horrow J, Husted S, James S, Katus H, Mahaffey KW, Scirica BM, Skene A, Steg PG, Storey RF, Harrington RA, Freij A, Thorsen M. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2009;361:1045–1057. [DOI] [PubMed] [Google Scholar]
- 5. Goldstein JA, Demetriou D, Grines CL, Pica M, Shoukfeh M, O'Neill WW. Multiple complex coronary plaques in patients with acute myocardial infarction. N Engl J Med 2000;343:915–922. [DOI] [PubMed] [Google Scholar]
- 6. Jernberg T, Hasvold P, Henriksson M, Hjelm H, Thuresson M, Janzon M. Cardiovascular risk in post-myocardial infarction patients: nationwide real world data demonstrate the importance of a long-term perspective. Eur Heart J 2015;36:1163–1170. [DOI] [PubMed] [Google Scholar]
- 7. Fox KA, Carruthers KF, Dunbar DR, Graham C, Manning JR, De Raedt H, Buysschaert I, Lambrechts D, Van de Werf F. Underestimated and under-recognized: the late consequences of acute coronary syndrome (GRACE UK-Belgian Study). Eur Heart J 2010;31:2755–2764. [DOI] [PubMed] [Google Scholar]
- 8. Dutta P, Courties G, Wei Y, Leuschner F, Gorbatov R, Robbins CS, Iwamoto Y, Thompson B, Carlson AL, Heidt T, Majmudar MD, Lasitschka F, Etzrodt M, Waterman P, Waring MT, Chicoine AT, van der Laan AM, Niessen HW, Piek JJ, Rubin BB, Butany J, Stone JR, Katus HA, Murphy SA, Morrow DA, Sabatine MS, Vinegoni C, Moskowitz MA, Pittet MJ, Libby P, Lin CP, Swirski FK, Weissleder R, Nahrendorf M. Myocardial infarction accelerates atherosclerosis. Nature 2012;487:325–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Biasucci LM, Liuzzo G, Grillo RL, Caligiuri G, Rebuzzi AG, Buffon A, Summaria F, Ginnetti F, Fadda G, Maseri A. Elevated levels of C-reactive protein at discharge in patients with unstable angina predict recurrent instability. Circulation 1999;99:855–860. [DOI] [PubMed] [Google Scholar]
- 10. Mann DL, McMurray JJ, Packer M, Swedberg K, Borer JS, Colucci WS, Djian J, Drexler H, Feldman A, Kober L, Krum H, Liu P, Nieminen M, Tavazzi L, van Veldhuisen DJ, Waldenstrom A, Warren M, Westheim A, Zannad F, Fleming T. Targeted anticytokine therapy in patients with chronic heart failure: results of the Randomized Etanercept Worldwide Evaluation (RENEWAL). Circulation 2004;109:1594–1602. [DOI] [PubMed] [Google Scholar]
- 11. Pinto AR, Paolicelli R, Salimova E, Gospocic J, Slonimsky E, Bilbao-Cortes D, Godwin JW, Rosenthal NA. An abundant tissue macrophage population in the adult murine heart with a distinct alternatively-activated macrophage profile. PLoS One 2012;7:e36814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Heidt T, Courties G, Dutta P, Sager HB, Sebas M, Iwamoto Y, Sun Y, Da Silva N, Panizzi P, van der Laan AM, Swirski FK, Weissleder R, Nahrendorf M. Differential contribution of monocytes to heart macrophages in steady-state and after myocardial infarction. Circ Res 2014;115:284–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Epelman S, Lavine KJ, Beaudin AE, Sojka DK, Carrero JA, Calderon B, Brija T, Gautier EL, Ivanov S, Satpathy AT, Schilling JD, Schwendener R, Sergin I, Razani B, Forsberg EC, Yokoyama WM, Unanue ER, Colonna M, Randolph GJ, Mann DL. Embryonic and adult-derived resident cardiac macrophages are maintained through distinct mechanisms at steady state and during inflammation. Immunity 2014;40:91–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhu SN, Chen M, Jongstra-Bilen J, Cybulsky MI. GM-CSF regulates intimal cell proliferation in nascent atherosclerotic lesions. J Exp Med 2009;206:2141–2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Choi JH, Do Y, Cheong C, Koh H, Boscardin SB, Oh YS, Bozzacco L, Trumpfheller C, Park CG, Steinman RM. Identification of antigen-presenting dendritic cells in mouse aorta and cardiac valves. J Exp Med 2009;206:497–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ginhoux F, Jung S. Monocytes and macrophages: developmental pathways and tissue homeostasis. Nat Rev Immunol 2014;14:392–404. [DOI] [PubMed] [Google Scholar]
- 17. Gomez Perdiguero E, Klapproth K, Schulz C, Busch K, Azzoni E, Crozet L, Garner H, Trouillet C, de Bruijn MF, Geissmann F, Rodewald HR. Tissue-resident macrophages originate from yolk-sac-derived erythro-myeloid progenitors. Nature 2015;518:547–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tchaikovski V, Tchaikovski S, Olieslagers S, Waltenberger J. Monocyte dysfunction as a previously unrecognized pathophysiological mechanism in ApoE-/- mice contributing to impaired arteriogenesis. Int J Cardiol 2015;190:214–216. [DOI] [PubMed] [Google Scholar]
- 19. Ruparelia N, Godec J, Lee R, Chai JT, Dall'Armellina E, McAndrew D, Digby JE, Forfar JC, Prendergast BD, Kharbanda RK, Banning AP, Neubauer S, Lygate CA, Channon KM, Nicholas Haining W, Choudhury RP. Acute myocardial infarction activates distinct inflammation and proliferation pathways in circulating monocytes, prior to recruitment, and identified through conserved transcriptional responses in mice and humans. Eur Heart J 2015;36:1923–1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nahrendorf M, Swirski FK. Monocyte and macrophage heterogeneity in the heart. Circ Res 2013;112:1624–1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hoeffel G, Chen J, Lavin Y, Low D, Almeida FF, See P, Beaudin AE, Lum J, Low I, Forsberg EC, Poidinger M, Zolezzi F, Larbi A, Ng LG, Chan JK, Greter M, Becher B, Samokhvalov IM, Merad M, Ginhoux F. C-myb(+) erythro-myeloid progenitor-derived fetal monocytes give rise to adult tissue-resident macrophages. Immunity 2015;42:665–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ginhoux F, Greter M, Leboeuf M, Nandi S, See P, Gokhan S, Mehler MF, Conway SJ, Ng LG, Stanley ER, Samokhvalov IM, Merad M. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science 2010;330:841–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Molawi K, Wolf Y, Kandalla PK, Favret J, Hagemeyer N, Frenzel K, Pinto AR, Klapproth K, Henri S, Malissen B, Rodewald HR, Rosenthal NA, Bajenoff M, Prinz M, Jung S, Sieweke MH. Progressive replacement of embryo-derived cardiac macrophages with age. J Exp Med 2014;211:2151–2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Robbins CS, Chudnovskiy A, Rauch PJ, Figueiredo JL, Iwamoto Y, Gorbatov R, Etzrodt M, Weber GF, Ueno T, van Rooijen N, Mulligan-Kehoe MJ, Libby P, Nahrendorf M, Pittet MJ, Weissleder R, Swirski FK. Extramedullary hematopoiesis generates Ly-6C(high) monocytes that infiltrate atherosclerotic lesions. Circulation 2012;125:364–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Robbins CS, Hilgendorf I, Weber GF, Theurl I, Iwamoto Y, Figueiredo JL, Gorbatov R, Sukhova GK, Gerhardt LM, Smyth D, Zavitz CC, Shikatani EA, Parsons M, van Rooijen N, Lin HY, Husain M, Libby P, Nahrendorf M, Weissleder R, Swirski FK. Local proliferation dominates lesional macrophage accumulation in atherosclerosis. Nat Med 2013;19:1166–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Shankman LS, Gomez D, Cherepanova OA, Salmon M, Alencar GF, Haskins RM, Swiatlowska P, Newman AA, Greene ES, Straub AC, Isakson B, Randolph GJ, Owens GK. KLF4-dependent phenotypic modulation of smooth muscle cells has a key role in atherosclerotic plaque pathogenesis. Nat Med 2015;21:628–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Swirski FK, Libby P, Aikawa E, Alcaide P, Luscinskas FW, Weissleder R, Pittet MJ. Ly-6Chi monocytes dominate hypercholesterolemia-associated monocytosis and give rise to macrophages in atheromata. J Clin Invest 2007;117:195–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yvan-Charvet L, Pagler T, Gautier EL, Avagyan S, Siry RL, Han S, Welch CL, Wang N, Randolph GJ, Snoeck HW, Tall AR. ATP-binding cassette transporters and HDL suppress hematopoietic stem cell proliferation. Science 2010;328:1689–1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Leuschner F, Rauch PJ, Ueno T, Gorbatov R, Marinelli B, Lee WW, Dutta P, Wei Y, Robbins C, Iwamoto Y, Sena B, Chudnovskiy A, Panizzi P, Keliher E, Higgins JM, Libby P, Moskowitz MA, Pittet MJ, Swirski FK, Weissleder R, Nahrendorf M. Rapid monocyte kinetics in acute myocardial infarction are sustained by extramedullary monocytopoiesis. J Exp Med 2012;209:123–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hilgendorf I, Gerhardt LM, Tan TC, Winter C, Holderried TA, Chousterman BG, Iwamoto Y, Liao R, Zirlik A, Scherer-Crosbie M, Hedrick CC, Libby P, Nahrendorf M, Weissleder R, Swirski FK. Ly-6Chigh monocytes depend on Nr4a1 to balance both inflammatory and reparative phases in the infarcted myocardium. Circ Res 2014;114:1611–1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nahrendorf M, Swirski FK, Aikawa E, Stangenberg L, Wurdinger T, Figueiredo JL, Libby P, Weissleder R, Pittet MJ. The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J Exp Med 2007;204:3037–3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Frangogiannis NG. The inflammatory response in myocardial injury, repair, and remodelling. Nat Rev Cardiol 2014;11:255–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lambert JM, Lopez EF, Lindsey ML. Macrophage roles following myocardial infarction. Int J Cardiol 2008;130:147–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Massa M, Rosti V, Ferrario M, Campanelli R, Ramajoli I, Rosso R, De Ferrari GM, Ferlini M, Goffredo L, Bertoletti A, Klersy C, Pecci A, Moratti R, Tavazzi L. Increased circulating hematopoietic and endothelial progenitor cells in the early phase of acute myocardial infarction. Blood 2005;105:199–206. [DOI] [PubMed] [Google Scholar]
- 35. Assmus B, Iwasaki M, Schachinger V, Roexe T, Koyanagi M, Iekushi K, Xu Q, Tonn T, Seifried E, Liebner S, Kranert WT, Grunwald F, Dimmeler S, Zeiher AM. Acute myocardial infarction activates progenitor cells and increases Wnt signalling in the bone marrow. Eur Heart J 2012;33:1911–1919. [DOI] [PubMed] [Google Scholar]
- 36. Swirski FK, Nahrendorf M. Leukocyte behavior in atherosclerosis, myocardial infarction, and heart failure. Science 2013;339:161–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ernst E, Hammerschmidt DE, Bagge U, Matrai A, Dormandy JA. Leukocytes and the risk of ischemic diseases. JAMA 1987;257:2318–2324. [PubMed] [Google Scholar]
- 38. Engstrom G, Melander O, Hedblad B. Leukocyte count and incidence of hospitalizations due to heart failure. Circ Heart Fail 2009;2:217–222. [DOI] [PubMed] [Google Scholar]
- 39. Maekawa Y, Anzai T, Yoshikawa T, Asakura Y, Takahashi T, Ishikawa S, Mitamura H, Ogawa S. Prognostic significance of peripheral monocytosis after reperfused acute myocardial infarction: a possible role for left ventricular remodeling. J Am Coll Cardiol 2002;39:241–246. [DOI] [PubMed] [Google Scholar]
- 40. Tsujioka H, Imanishi T, Ikejima H, Kuroi A, Takarada S, Tanimoto T, Kitabata H, Okochi K, Arita Y, Ishibashi K, Komukai K, Kataiwa H, Nakamura N, Hirata K, Tanaka A, Akasaka T. Impact of heterogeneity of human peripheral blood monocyte subsets on myocardial salvage in patients with primary acute myocardial infarction. J Am Coll Cardiol 2009;54:130–138. [DOI] [PubMed] [Google Scholar]
- 41. Morrison SJ, Scadden DT. The bone marrow niche for haematopoietic stem cells. Nature 2014;505:327–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mendelson A, Frenette PS. Hematopoietic stem cell niche maintenance during homeostasis and regeneration. Nat Med 2014;20:833–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ehninger A, Trumpp A. The bone marrow stem cell niche grows up: mesenchymal stem cells and macrophages move in. J Exp Med 2011;208:421–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Nagai Y, Garrett KP, Ohta S, Bahrun U, Kouro T, Akira S, Takatsu K, Kincade PW. Toll-like receptors on hematopoietic progenitor cells stimulate innate immune system replenishment. Immunity 2006;24:801–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kiel MJ, Yilmaz OH, Iwashita T, Yilmaz OH, Terhorst C, Morrison SJ. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell 2005;121:1109–1121. [DOI] [PubMed] [Google Scholar]
- 46. Dutta P, Sager HB, Stengel KR, Naxerova K, Courties G, Saez B, Silberstein L, Heidt T, Sebas M, Sun Y, Wojtkiewicz G, Feruglio PF, King K, Baker JN, van der Laan AM, Borodovsky A, Fitzgerald K, Hulsmans M, Hoyer F, Iwamoto Y, Vinegoni C, Brown D, Di Carli M, Libby P, Hiebert SW, Scadden DT, Swirski FK, Weissleder R, Nahrendorf M. Myocardial infarction activates CCR2(+) hematopoietic stem and progenitor cells. Cell Stem Cell 2015;16:477–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Geissmann F, Manz MG, Jung S, Sieweke MH, Merad M, Ley K. Development of monocytes, macrophages, and dendritic cells. Science 2010;327:656–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Shi C, Jia T, Mendez-Ferrer S, Hohl TM, Serbina NV, Lipuma L, Leiner I, Li MO, Frenette PS, Pamer EG. Bone marrow mesenchymal stem and progenitor cells induce monocyte emigration in response to circulating toll-like receptor ligands. Immunity 2011;34:590–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zouggari Y, Ait-Oufella H, Bonnin P, Simon T, Sage AP, Guerin C, Vilar J, Caligiuri G, Tsiantoulas D, Laurans L, Dumeau E, Kotti S, Bruneval P, Charo IF, Binder CJ, Danchin N, Tedgui A, Tedder TF, Silvestre JS, Mallat Z. B lymphocytes trigger monocyte mobilization and impair heart function after acute myocardial infarction. Nat Med 2013;19:1273–1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Swirski FK, Nahrendorf M, Etzrodt M, Wildgruber M, Cortez-Retamozo V, Panizzi P, Figueiredo JL, Kohler RH, Chudnovskiy A, Waterman P, Aikawa E, Mempel TR, Libby P, Weissleder R, Pittet MJ. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science 2009;325:612–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Leuschner F, Panizzi P, Chico-Calero I, Lee WW, Ueno T, Cortez-Retamozo V, Waterman P, Gorbatov R, Marinelli B, Iwamoto Y, Chudnovskiy A, Figueiredo JL, Sosnovik DE, Pittet MJ, Swirski FK, Weissleder R, Nahrendorf M. Angiotensin-converting enzyme inhibition prevents the release of monocytes from their splenic reservoir in mice with myocardial infarction. Circ Res 2010;107:1364–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Dutta P, Hoyer FF, Grigoryeva LS, Sager HB, Leuschner F, Courties G, Borodovsky A, Novobrantseva T, Ruda VM, Fitzgerald K, Iwamoto Y, Wojtkiewicz G, Sun Y, Da Silva N, Libby P, Anderson DG, Swirski FK, Weissleder R, Nahrendorf M. Macrophages retain hematopoietic stem cells in the spleen via VCAM-1. J Exp Med 2015;212:497–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Yusuf S, Hawken S, Ounpuu S, Dans T, Avezum A, Lanas F, McQueen M, Budaj A, Pais P, Varigos J, Lisheng L. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case–control study. Lancet 2004;364:937–952. [DOI] [PubMed] [Google Scholar]
- 54. Nahrendorf M, Swirski FK. Lifestyle effects on hematopoiesis and atherosclerosis. Circ Res 2015;116:884–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Heidt T, Sager HB, Courties G, Dutta P, Iwamoto Y, Zaltsman A, von Zur Muhlen C, Bode C, Fricchione GL, Denninger J, Lin CP, Vinegoni C, Libby P, Swirski FK, Weissleder R, Nahrendorf M. Chronic variable stress activates hematopoietic stem cells. Nat Med 2014;20:754–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Powell ND, Sloan EK, Bailey MT, Arevalo JM, Miller GE, Chen E, Kobor MS, Reader BF, Sheridan JF, Cole SW. Social stress up-regulates inflammatory gene expression in the leukocyte transcriptome via beta-adrenergic induction of myelopoiesis. Proc Natl Acad Sci USA 2013;110:16574–16579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Nagareddy PR, Kraakman M, Masters SL, Stirzaker RA, Gorman DJ, Grant RW, Dragoljevic D, Hong ES, Abdel-Latif A, Smyth SS, Choi SH, Korner J, Bornfeldt KE, Fisher EA, Dixit VD, Tall AR, Goldberg IJ, Murphy AJ. Adipose tissue macrophages promote myelopoiesis and monocytosis in obesity. Cell Metab 2014;19:821–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Nagareddy PR, Murphy AJ, Stirzaker RA, Hu Y, Yu S, Miller RG, Ramkhelawon B, Distel E, Westerterp M, Huang LS, Schmidt AM, Orchard TJ, Fisher EA, Tall AR, Goldberg IJ. Hyperglycemia promotes myelopoiesis and impairs the resolution of atherosclerosis. Cell Metab 2013;17:695–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Kim EJ, Kim S, Kang DO, Seo HS. Metabolic activity of the spleen and bone marrow in patients with acute myocardial infarction evaluated by 18f-fluorodeoxyglucose positron emission tomograpic imaging. Circ Cardiovasc Imaging 2014;7:454–460. [DOI] [PubMed] [Google Scholar]
- 60. Emami H, Singh P, MacNabb M, Vucic E, Lavender Z, Rudd JH, Fayad ZA, Lehrer-Graiwer J, Korsgren M, Figueroa AL, Fredrickson J, Rubin B, Hoffmann U, Truong QA, Min JK, Baruch A, Nasir K, Nahrendorf M, Tawakol A. Splenic metabolic activity predicts risk of future cardiovascular events: demonstration of a cardiosplenic axis in humans. JACC Cardiovasc Imaging 2015;8:121–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Wollenweber T, Roentgen P, Schafer A, Schatka I, Zwadlo C, Brunkhorst T, Berding G, Bauersachs J, Bengel FM. Characterizing the inflammatory tissue response to acute myocardial infarction by clinical multimodality noninvasive imaging. Circ Cardiovasc Imaging 2014;7:811–818. [DOI] [PubMed] [Google Scholar]
- 62. Han Y, Jing J, Tu S, Tian F, Xue H, Chen W, Chen J, Reiber JH, Chen Y. ST elevation acute myocardial infarction accelerates non-culprit coronary lesion atherosclerosis. Int J Cardiovasc Imaging 2014;30:253–261. [DOI] [PubMed] [Google Scholar]