Abstract
Surgery remains the only chance of cure for pancreatic cancer, but only 15%-25% of patients present with resectable disease at the time of primary diagnosis. Important goals in clinical research must therefore be to allow early detection with suitable diagnostic procedures, to further broaden operation techniques and to determine the most effective perioperative treatment of either chemotherapy and/or radiation therapy. More extensive operations involving extended pancreatectomy, portal vein resection and pancreatic resection in resectable pancreatic cancer with limited liver metastasis, performed in specialized centers seem to be the surgical procedures with a possible impact on survival. After many years of stagnation in pharmacological clinical research on advanced pancreatic ductal adenocarcinomas (PDAC) - since the approval of gemcitabine in 1997 - more effective cytotoxic substances (nab-paclitaxel) and combinations (FOLFIRINOX) are now available for perioperative treatment. Additionally, therapies with a broader mechanism of action are emerging (stroma depletion, immunotherapy, anti-inflammation), raising hopes for more effective adjuvant and neoadjuvant treatment concepts, especially in the context of “borderline resectability”. Only multidisciplinary approaches including radiology, surgery, medical and radiation oncology as the backbones of the treatment of potentially resectable PDAC may be able to further improve the rate of cure in the future.
Keywords: Pancreatic cancer, Perioperative treatment, Perioperative radiology, Chemotherapy
Core tip: Pancreatic cancer remains one of the most challenging tumor entities and is predicted to become the second leading cause of cancer deaths. More effective chemotherapeutic concepts in combination with early and exact imaging and more extensive surgical approaches may improve the rate of cure.
INTRODUCTION
Pancreatic ductal adenocarcinomas (PDAC) are predicted to become the second largest cause of cancer related death in the United States by 2030[1]. This is mainly due to the lack of therapeutic options, making PDAC one of the few types of cancer with a still increasing mortality rate[2]. Surgery remains the only chance of cure for this devastating disease, but only 15%-25% of patients present with resectable disease at the time of primary diagnosis[3]. Important goals in clinical research must therefore be early detection with suitable diagnostic procedures, further broadening operation techniques, and determining whether chemotherapy or radiation therapy is the most effective perioperative treatment. Only multidisciplinary approaches including surgery, radiology, medical and radiation oncology and gastroenterology may be able to further improve the rate of cure in the future[4].
This review of the treatment options of potentially resectable PDAC will focus on the 3 backbones - radiological assessment, surgical procedures and perioperative regimen (chemotherapy/radiochemotherapy) - and their potential impact on long-term survival. Current standards will be discussed as well as ongoing or recently completed clinical trials.
ROLE OF RADIOLOGY
Although explorative laparotomy is considered the gold standard for resectability assessment, radiological imaging plays a key role in planning surgical procedures. Until the recent refinement of national management guidelines for PDAC, the main concern on radiological assessment of patients with suspected PDAC was the determination of resectability[5]. In view of the high mortality rate, it is necessary to offer curative resection, as this is the only chance for long-term survival. On the other hand, this chance is rather small, with only about 20% 5-year survival rate in case of curative resection, and the procedure is not free of risk for mortality, which depends on the medical centers’ experience and ranges from 3.8% to 16.3%[6,7]. Furthermore, morbidity after resection can limit the use of adjuvant chemotherapy. Thus, the preoperative imaging must be used to determine whether a patient has a good chance of curative resection and should have explorative laparotomy or should rather undergo conservative treatment with chemotherapy without further delay. This decision is always accompanied by the double risk of either denying a patient a potentially lifesaving resection or performing unnecessary surgery in patients with unresectable tumors.
The achievement of an R0-resection is one of the most significant parameters for survival[6]. Therefore, the first goal of radiological assessment is to confirm the probability of R0-resection without the necessity of arterial reconstruction[8]. Both computed tomography (CT) and magnetic resonance imaging (MRI) have been shown to be effective to this end. The assessment accuracy of local resectability by CT was as high as 93% in a representative study conducted in a high volume center[9]. The criteria have been extensively reviewed elsewhere and are summarized in current guidelines[10-12]. Basically, arterial encasement of the celiac trunk, the common hepatic artery, the proper hepatic artery or the superior mesenteric artery is deemed unresectable, as survival remains poor even after technically successful tumor removal and arterial reconstruction (Figure 1)[8]. Similarly, survival is impaired for those patients requiring resection of the mesentericoportal venous axis; however, long-term survival can be observed in some of these cases[8]. Therefore, venous involvement of the tumor is not a criterion for unresectability if reconstruction of the mesentericoportal flow by vessel resection and reanastomosis, patch plastic or graft interposition is technically feasible (Figure 1). CT can predict clear infiltration by encasement of arteries and clear non-infiltration by non-contact to adjacent arteries (surrounding preserved fat plain) with acceptably high accuracy; however, once tumor contact to the vessels is present, the ability to decide whether the tumor can be surgically removed from the artery without resection and reconstruction of the artery decreases[13,14]. In such cases, the accuracy of prediction of local resectability by radiological imaging is limited and repeatedly results in unexpected abortion of surgical exploration. Or - vice versa - could cause preclusion of a patient from resection if no exploratory attempt was undertaken because the disease was found to be too advanced on imaging. Even though these cases are becoming rare thanks to improvements in imaging in terms of spatial resolution and speed of image acquisition, with better vessel enhancement and tumor delineation as well as minimization of image artifacts[9], it would be desirable to offer the remaining patients inside this grey zone of locally advanced tumors an approach to safely decide whether it is worth undergoing the R0-resection or continuing with conservative treatment.
Figure 1.
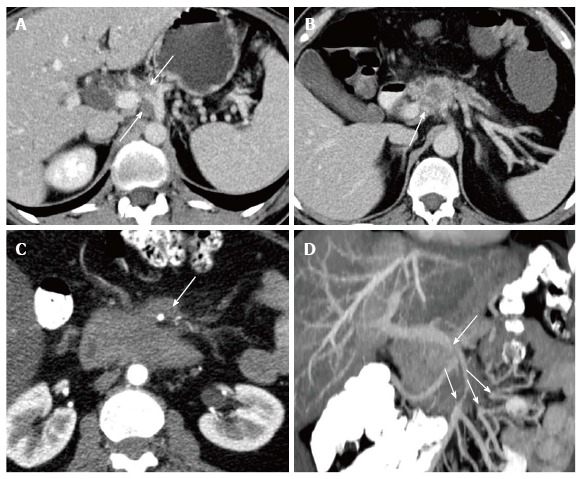
Examples of resecatability assessment in untreated pancreatic ductal adenocarcinoma by means of computed tomography. A: Unresectability due to encasement of the common hepatic artery reaching to the celiac trunc (arrows); B: Borderline respectability with infiltration of the portal vein and one-sided contact to the common hepatic artery without extension to the celiac trunc. Neoadjuvant treatment and/or pancreatic left resection with en-bloc resection of the celiac trunc (after embolization) can be considered; C: Unresectability due to encasement of the superior mesenteric artery by more than 180º; D: Infiltration of the superior mesenteric vein and venous confluence with stenosis and multiple separated mesenteric venous branches unsuitable for surgical reconstruction.
Preoperative treatment could serve as “problem solver” in such cases, termed “borderline resectable” cancers. They are characterized by intense tumor contact to the arterial mesenteric (up to 180° encasement) and celiac axes (up to short encasement of the common hepatic artery without extension to the celiac trunk) or infiltration of the mesentericoportal venous axis (if technically manageable) (Figure 1)[5]. This concept could help increase the rate of R0-resection in this group via tumor shrinkage and aid in selection of patients for resection by filtering out those with aggressive tumors nonresponsive to chemotherapy. Observational cohort studies of this concept have been promising, but as yet no prospective controlled studies have been performed. Arguments against this approach could be that shrinkage of initially radiologically invisible micrometastases (e.g., to the peritoneum and liver), will lead to false negative M0-assessments intraoperatively despite residual viable tumor cells in these remote lesions. In order to appropriately select patients for this new approach, the actually most accurate imaging strategy for detection of small metastases, MRI of the liver with diffusion weighted imaging (DWI) and hepatocyte specific contrast agents[15-17] would be justified.
Finding the optimal imaging strategy is also desirable for the local assessment of tumor extent. On the other hand, introduction of a third type of assessment complicates the work of the radiologists and should not be used to avoid definite statements by calling tumors borderline resectable in case of any doubt. In consequence, diagnostic imaging should consist of state of the art imaging under optimal conditions such as hardware and contrast agents used, as well as imaging protocols including dynamic high resolution contrast imaging in order to optimize delineability of the peripancreatic vasculature. One of the most important factors to be considered is how recent the imaging studies are, as it is known that regardless of the imaging quality, images older than 4 wk do not reflect the extent of tumors found on surgery[18], making repeated imaging necessary before a decision can be made.
Another problem of preoperative therapy is the necessity of histological confirmation and grading of the tumor. It therefore becomes even more important to characterize the tumor by means of radiological imaging and to exclude other tumors such as neuroendocrine tumors, sarcomas, lymphomas, as well as non-malignant diseases (e.g., mass forming pancreatitis, autoimmune related pancreatitis). This aids decision making regarding pretherapeutic biopsy strategies.
The reassessment of tumors after neoadjuvant treatment poses new challenges for diagnostic imaging. After initial reports showing results almost as good as in patients without preoperative therapy, more recent studies have increasingly shown that local tumor extent tends to be overestimated by imaging procedures after chemotherapy, and even more so after radiation[19-21]. The reliability of a preoperative radiological statement is diminished and can only be compensated for by thorough surgical exploration. This again - knowing of the “point of no return” of the resection of a pancreatic head carcinoma, beyond which R0-resection may still be found to be impossible in some cases - can be a drawback of the preoperative concept, potentially resulting in R2-resected patients. In this context new parameters are needed to identify response and shrinkage of viable tumors. DWI, perfusion measurement by MRI and CT (including dual energy CT), and positron emission tomography are currently under investigation to fill this gap with results pending. Alternative surgical strategies are also included, such as the very promising distal pancreatectomy with en-bloc resection of the celiac trunk for cases where pancreatic tail carcinoma has reached the celiac axis. This requires very precise planning based on radiologic imaging and a preoperative radiological intervention in order to train collateralization (see “surgical procedures” below)[22,23].
In conclusion, with the advent of new management options in preoperative therapy of pancreatic cancer, radiological imaging - for planning of biopsy and surgery as well as pre-surgical reassessment and preconditioning - has moved to the frontline of treatment decision making and become the basis of measures to achieve the treatment goal: Prolonged survival of the patients.
SURGICAL PROCEDURES
Much progress has been made in the field of pancreatic resection in recent years and importantly, as the quality of surgery is considered a key factor in long-term survival, consensus has been reached on a number of surgical principles. An analysis of our own patient cohorts (n = 428) suggests that postoperative complications deteriorate long-term outcome after pancreatic resection in pancreatic cancer patients. We found that severe postoperative complications had a strong negative impact on long-term survival. This effect was significant and even comparable to that of the most relevant tumor characteristics such as lymph node involvement, grading or resection margin[24]. An analysis by Birkmeyer et al[7] showed absolute differences in 5-year survival probabilities rates between low-volume hospitals (LVH) and high-volume hospitals (HVH): The absolute difference in 5-year survival in patients with pancreatic cancer between LVH and HVH was 5%. These findings underline the importance of centralization of pancreatic surgery for pancreatic cancer; in other words, patients should be transferred to specialized centers to further improve the results of surgical procedures[7].
Perioperative medical management
Several studies have suggested that blood transfusion is associated with impaired long-term survival. A recent systematic review including 23 studies with 4339 patients confirmed this assumption. Patients receiving perioperative blood transfusion had a significantly lower 5-year survival rates after pancreatic resection in 13 of 19 studies in univariate and multivariate analyses[25].
Extended pancreatectomy
The goal of any pancreatic resection for pancreatic adenocarcinoma should be the complete removal of the tumor (R0-situation). To this end, more radical or “extended” surgical techniques have been established in the last decades, whereat “extended” in this context means a more aggressive marginal clearance[26-28].
This may include total duodenopancreatectomy, resection of the portal-mesenterical axis, and extended lymph node dissection. Extended pancreatectomy is defined as standard pancreatoduodenectomy and includes the resection of the head of the pancreas and uncinate process, duodenum and first segment of the jejunum, common bile duct and gallbladder, lymphadenectomy, sometimes pylorus and/or antrum of the stomach and sometimes elements of the transverse mesocolon. Relevant vascular structures and any of the following organs involved in continuity are excluded: More than the antrum or distal half of the stomach; colon and/or mesocolon with relevant vascular structures of the transverse mesocolon (ileocolic, right, or middle colic vessels); the small intestine beyond the first segment of the jejunum; portal vein, superior mesenteric, and/or inferior mesenteric vein; hepatic artery, celiac trunk, and/or superior mesenteric artery; the inferior vena cava, right adrenal gland, right kidney and/or its vasculature; and last but not least the resection of liver and diaphragmatic crura.
The standard distal pancreatectomy is defined as the resection of the body and/or tail of the pancreas; the spleen with the splenic vessels combined with a standard lymphadenectomy; the resection of the fascia of Gerota if necessary; and potentially also elements of the transverse mesocolon apart from relevant vessels.
In contrast, an extended distal pancreatectomy refers to the standard distal pancreatectomy plus any type of gastric resection, colon and/or relevant vascular structures of the transverse mesocolon, small intestine; portal vein, superior mesenteric, and/or inferior mesenteric artery; inferior vena cava, left adrenal gland, left kidney, liver and diaphragmatic crura.
The extended resection approach may have problematic consequences: Operating time and blood loss; in addition the length of stay in the intensive care unit and hospital may be increased after extended surgery. Furthermore, surgical morbidity is increased after extended pancreatectomy. Perioperative mortality seems to be similar to that of standard pancreatectomies. Data regarding survival suggested no difference between patients after standard resection compared to those who underwent extended resection[29]. However, recent long term follow-up findings suggest an inferior prognosis after extended pancreatic resection compared to those who underwent standard resection (11.7% 5-year survival vs 21% 5-year survival)[30].
Extended lymph node dissection
The resection of the regional lymph nodes around the duodenum and the pancreas plus the lymph nodes on the right side of the hepatoduodenal ligament, the right side of the superior mesenteric artery and the anterior and posterior pancreatoduodenal lymph nodes is defined as standard lymphadenectomy. Lymph node dissection beyond this area is considered extended. However, a prospective comparison of the surgical results of extended lymph node dissection and standard lymph node resection did not show any significant difference in survival between the two strategies[31]. Extended lymph node dissection is therefore not considered to improve long-term survival.
Portal vein resection
A recent publication showed that resection of the portal vein was significantly associated with the four following factors: Larger and poorly differentiated tumors, higher numbers of positive lymph nodes and positive resection margins[32]. Debate is currently ongoing as to whether routine portal vein resection during pancreatoduodenectomy for PDAC is reasonable. In this context, a French retrospective analysis has been published showing that patients (without microscopic venous tumor involvement of the portal vein) who underwent pancreatic resection combined with portal vein resection had a significantly longer overall survival than patients in a matched control group after pancreatic head resection without venous resection[33]. These data may indicate that a “per se” portal vein resection results in a more radical local tumor elimination and may improve long-term survival.
Infiltration of the celiac trunk
As a particular situation in the context of locally advanced PDAC, tumor manifestation in the pancreatic body with involvement of the celiac trunk and/or the common hepatic artery needs mentioning. Normally such a situation is considered unresectable[34]. However, a radical distal pancreatectomy with splenectomy and en-bloc resection of the celiac trunk without reconstruction of the celiac axis is a potential curative approach for these cases[35]. The weak point of this concept is the interruption of the direct arterial blood supply to the liver, bile ducts and stomach. This may lead to acute liver failure, bile duct necrosis or ischemic necrosis of the stomach. A multidisciplinary approach is therefore needed to avoid such severe complications. Preoperative digital subtraction angiography is necessary to provide information about the perfusion of the crucial vessels[23]. In cases where there is a pre-existing stenosis of the celiac trunk, spontaneous collateral blood flow from the superior mesenteric artery directly to the gastroduodenal artery via pancreaticoduodenal arcades and therefore to the hepatic artery can be seen. In such a situation, the tumor can be resected immediately including the celiac trunk. If there is no pre-existent celiac trunk stenosis, embolization of the celiac trunk is needed to enhance collateral blood flow before the operation. Important requirements for this multi-step approach are the existence of sufficient collateral arteries and no tumor invasion of the superior mesenteric artery. Sperti et al[22] reported on similar survival rates to regular R0-resections after this sophisticated intervention.
Is there a role for pancreatic resection in resectable pancreatic cancer with limited liver metastasis?
A recent retrospective analysis conducted by our institution showed that even patients with advanced pancreatic cancer (locally advanced or locally resectable with hepatic oligometastasis) seem to benefit from pancreatoduodenectomy followed by gemcitabine-based chemotherapy. Forty-five patients who had undergone palliative intended pancreatic resection followed by chemotherapy were therefore matched with 45 patients after upfront gemcitabine-based palliative chemotherapy. A subgroup of patients with locally resectable tumor and limited liver metastasis (R0/M1) showed a significant improvement in overall survival compared to patients who had not had surgery (14.4 mo vs 7.2 mo), suggesting a potential role for pancreatic resections in patients with limited liver metastasis if the primary tumor is completely removable[36].
ADJUVANT AND NEOADJUVANT CONCEPTS
Current standards
In the ongoing discussion about the role of perioperative treatment options in PDAC, ideal timing (neoadjuvant/adjuvant) and the ideal modality (chemotherapy, radiochemotherapy) remain controversial[11,37]. The most convincing contemporary data based on a high level of evidence are available for the effectiveness of adjuvant chemotherapy alone, based firstly on the results of the CONKO-001 trial. This randomized multi-center phase III trial included 368 patients and compared 6 cycles of adjuvant gemcitabine (Gemcitabine 1000 mg/m2 d1, 8, 15, q29) to observation only[38]. Median disease-free survival was significantly improved (13.4 mo for the gemcitabine group compared to 6.7 mo for the observation only group; P < 0.001) and led to a significant but numerically small advantage in overall survival (22.4 mo gemcitabine vs 20.2 mo observation only; P = 0.01). Indeed, the most important and clinically relevant result of the CONKO-001 remains that 5-year survival (an established surrogate marker for long-term survival) and rate of cure were doubled by adjuvant gemcitabine: 20.7% in the Gem group compared to 10.4% in the observation group. These data are based on long-term follow-up over more than 11 years[39] and were confirmed to a large extent by the ESPAC-3 trial. This large randomized, multicenter phase III trial included 1088 patients and compared 6 cycles of gemcitabine to 6 cycles of 5-FU (425 mg/m2 d1-5 bolus i.v., q d29) and folinic acid (20 mg/m2 d1-5 i.v., q29). Median DFS (Gem 14.3 mo vs 14.1 mo; P = 0.53) and OS (Gem 23.2 mo vs 5-FU 23.0 mo; P = 0.32) were similar in both treatment groups (and to the CONKO-001 gemcitabine group), but more relevant toxicities were observed in the 5-FU group[40]. In conclusion, both chemotherapeutic regimens are considered standard care in the adjuvant setting[41], but the better toxicity profile of gemcitabine should be taken into consideration[34]. More recently, the Japanese JASPAC-01 trial compared gemcitabine to the 5-FU prodrug S1 (80/100/120 mg/d based on body surface area, p.o., d1-28, q6w, for 4 courses). S1 is known for its specific effectiveness in Asians and is widely used, especially in the treatment of gastric cancer[42]. 385 patients were included in this randomized phase III trial which showed impressive results, with a significantly improved 2-year survival of 70% in the S1 group compared to 53% in the gemcitabine group (HR 0.56, P < 0.001). The data were presented at the annual ASCO-GI meeting in 2013[43], but full publication and a confirmatory trial, especially in non-Asian patients, must be awaited (Table 1).
Table 1.
Standard adjuvant therapy
| Gemcitabine 1000 mg/m2i.v. 30 min | d1, 8, 15, q29 |
| 6 cycles | |
| 5-flourouracil 425 mg/m2 i.v. Bolus | d1-5, q29 |
| Folinic acid 20 mg/m2 i.v. Bolus | 6 cycles |
Therapeutic concepts combining chemotherapy with targeted therapy have been so far unsuccessful at further improving survival in resectable PDAC - a well-known phenomenon for unresectable PDAC as well[44]. CONKO-005, designed as a follow-up randomized phase III trial of CONKO-001, investigated the additional effect of the EGFR-tyrosine-kinase inhibitor Erlotinib (100 mg daily p.o. d1-28, q29) in combination with gemcitabine (standard dosage) for 6 cycles in 436 patients after R0 resection. No improvement could be demonstrated for the primary endpoint DFS, but a trend was described for improved long-term survival in the erlotinib + gemcitabine group[45]. Longer follow-up and ongoing molecular analyses will clarify whether it is possible to identify a subgroup which could benefit from erlotinib in the future. The parallel trial CONKO-006 for R1 resected patients investigated the concept of prolonged additive chemotherapy in this high risk patient cohort, as well as the role of the multikinase inhibitor sorafenib. All patients (n = 122) received 12 cycles of adjuvant gemcitabine and were randomized for gemcitabine alone or in combination with sorafenib (400 mg bid, p.o., d1-28, q29). No improvement in DFS or OS could be demonstrated for the overall study population by prolonging postoperative chemotherapy or using sorafenib[46].
Several trials which investigated the role of adjuvant radiochemotherapy in PDAC also failed to show a clear survival advantage in the use of intensified treatment modalities. The CAPRI trial[47] may be presented as a disappointing endpoint of this concept. In this phase III trial, 132 patients were randomized to receive either 5-FU as standard of care or an aggressive postoperative regimen of 5-FU (200 mg/m2 daily), Cisplatin (30 mg/m2 weekly) and Interferon alpha-2b (3 Mio IU 3 × weekly) in combination with radiation therapy (50.4 Gray), followed by 2 cycles of continuous 5-FU. No difference was found for the primary endpoint OS (26.5 mo vs 28.5 mo, P = 0.99) or DFS (15.2 mo vs 11.5 mo); instead a massive increase of grade 3/4 toxicities (85% vs 16%) was seen.
An overview of the completed and clinically relevant adjuvant phase III trials is given in Table 2.
Table 2.
Completed adjuvant phase III trials
| Ref. | Treatment group | Median OS (mo) | 3-yr- survival | 5-yr- survival |
| CONKO-001[38,39] | Gem | 22.8 | 37% | 21% |
| Observation | 20.2 | 20% | 9% | |
| ESPAC-3[40] | Gem | 23.6 | ||
| 5-FU/folinsäure | 23.0 | |||
| JASPAC-01[43] | S1 | 2 yr 70% | ||
| Gem | 2 yr 53% | |||
| RTOG 97-04 | RCT + Gem | 20.5 | 31% | 24% |
| (pancreatic head)[57] | RCT + 5-FU/FS | 16.9 | 22% | 11% |
| CONKO-005[45] | Gem + Erlotinib | 24.6 | 36% | 28% |
| Gem | 26.5 | 33% | 19% |
5-FU: 5-flourouracil; OS: Overall survival.
Ongoing adjuvant trials
Several adjuvant phase III studies with different concepts are ongoing, such as the combination of two or more cytostatic substances, or chemotherapy in combination with immunotherapy or radiochemotherapy (Table 3).
Table 3.
Ongoing adjuvant trials
| Trial | Registration No. | Treatment groups | Patients planned |
| ESPAC-4 | ISRCTN96397434 | Gem + capecitabine | 732 |
| Gem | |||
| APACT | NCT01964430 | Gem + nab-paclitaxel | 800 |
| Gem | |||
| ACCORD24 | NCT01526135 | FOLFIRINOX | 490 |
| Gem | |||
| RTOG-0848 | NCT01013649 | Gem + RCT (capecitabine or 5-FU) | 950 |
| Gem | |||
| Algenpantucel Immunotherapy | NCT01072981 | Gem ± RCT + Algenpantucel-I vaccine | 722 |
| Gem ± RCT |
5-FU: 5-flourouracil; RCT: Randomized controlled trial.
The ESPAC-4 trial is investigating the role of a more intense chemotherapeutic regimen combining gemcitabine (standard dosage) with capecitabine (d1-14, q22) for 6 cycles in a randomized phase III trial. Recruitment for the pancreatic cohort (732 patients) has been completed and initial results can be expected soon.
More recently, the chemotherapeutic regimens which are more effective for the palliative situation, namely FOLFIRINOX[48] and nab-paclitaxel in combination with gemcitabine[49], are also available for adjuvant treatment. The actively recruiting APACT (adjuvant therapy for pancreatic cancer trial) will provide information about whether the combination therapy of nab-paclitaxel (125 mg/m2 d1, 8, 15, q29) and gemcitabine is feasible in resected PDAC patients (n = 800) and also whether it is more effective than gemcitabine alone. The French ACCORD/PRODIGE study group will investigate a similar concept in a randomized phase III trial of 480 patients comparing 6 cycles of modified FOLFIRINOX (Irinotecan 150 mg/m2, oxaliplatin 85 mg/m2, folinic acid 400 mg/m2, 5-FU 2400 mg/m2 per 46 h d1, q 15) to gemcitabine monotherapy. As both trials are based on the current most effective and evidence-based chemotherapies for advanced PDAC with a clear advantage in comparison to gemcitabine monotherapy, they have the potential to further improve current standard therapy. The question remains as to whether these more aggressive concepts will be feasible in PDAC patients with a partially reduced performance state and after extended abdominal surgery. Accrual of the trials and (long-term) follow up data must be awaited.
With regard to radiochemotherapy, the RTOG-0848 trial is planned to investigate an additional effect of postoperative radiochemotherapy to standard chemotherapy. All patients are to receive 6 cycles of gemcitabine as standard of care and if without signs of disease recurrence will then be randomized to either receive additive radiochemotherapy or not 950 patients are planned. The protocol was amended and the initial randomization to gemcitabine ± erlotinib was terminated.
Immunotherapy is one of the most fascinating recent innovations in the treatment of oncological disease, but most clinical research to date has had disappointing results in PDAC, which is considered to be non-immunogenic[50]. Seven hundred and twenty-two patients have been recruited for a phase III study of chemotherapy and chemoradiotherapy with or without Algenpantucel-L (HyperAcute®-Pancreas), a new immunotherapeutic concept. Algenpantucel is a vaccine derived from 2 pancreatic cell lines which showed promising results in a single-arm phase II trial[51]. Results of the phase III trial are pending.
Neoadjuvant concepts
Chemotherapies with response rates (in terms of tumor shrinkage) of about 30% - compared to formerly < 10% for gemcitabine monotherapy - have become available[52,53] in the treatment of PDAC for the first time due to the introduction of FOLFIRINOX and gemcitabine/nab-paclitaxel. This is of special relevance for the further development of neoadjuvant and perioperative treatment strategies, as pancreatic cancer must be considered a systemic disease. Many patients experience early recurrence postoperatively, which in the majority of cases may in fact be metastatic spread which was undetected at the time of primary diagnosis. Analogue to the ongoing discussion on the role of induction chemotherapy in locally advanced unresectable PDAC[11], the question as to whether patients with resectable PDAC and disease progression under neoadjuvant therapies would really profit from primary resection must be posed (Table 4).
Table 4.
Ongoing neoadjuvant/borderline resectable trials
| Trial | Registration No. | Treatment groups | Primary endpoint | Patients planned |
| NEONAX | NCT02047513 | 2 cycles Gem + nab-paclitaxel neoadjuvant | DFS | 166 |
| 4 cycles Gem + nab-paclitaxel adjuvant | ||||
| 6 cycles Gem + nab-paclitaxel adjuvant | ||||
| Perioperative mFOLFIRINOX | NCT02047474 | 3 cycles FOLFORINOX | DFS | 46 |
| Neoadjuvant + adjuvant | ||||
| NEOLAP | NCT02125136 | 2 cycles Gem + nab-paclitaxel | Conversion rate | 168 |
| + 2 × FOLFIRINOX | (including locally advanced PDAC) | |||
| + adjuvant 3 × Gem + nab-paclitaxel | ||||
| 4 cycles Gem + nab-paclitaxel | ||||
| + adjuvant 3 × Gem + nab-paclitaxel |
DFS: Disease-free survival; PDAC: Pancreatic ductal adenocarcinomas.
Two trials are ongoing to investigate these questions in clearly defined study protocols: The German NEONAX is investigating the role of perioperative gemcitabine/nab-paclitaxel and perioperative modified FOLFIRINOX is being investigated in the United States in a phase II trial by the University of Yale.
Treatment strategies for borderline resectable PDAC
In addition to the aim of improving the rate of cure in terms of hindering recurrence of disease after curatively intended surgery, a further aim must be to render more patients resectable after intensified induction treatment. In this context, the term “borderline resectable” PDAC is relevant and relatively recent but different definitions are currently in use[4] (Table 4).
Katz et al[54] presented data at the Annual ASCO meeting in 2015 of a small phase II trial which may be the starting point for a new era in borderline resectable PDAC. Twenty-two patients with ECOG 0 and 1 were included and prospectively analyzed. Participants were centrally reviewed to assess borderline criteria [tumor-vessel interface (TVI) with superior mesenteric/portal vein (SMV) ≥ 180°, TVI with superior mesenteric artery (SMA) < 180°, TVI with any degree of hepatic artery]. All patients were intended to be treated with neoadjuvant modified FOLFIRINOX (oxaliplatin 85 mg/m2, irinotecan 180 mg/m2, leucovorin 400 mg/m2 on day 1 followed by 5-FU 2400 mg/m2 × 48 h for 4 cycles) and CRT (50.4 Gray in 28 fractions) with capecitabine (825 mg/m2 BID) prior to pancreatectomy and postoperative gemcitabine (1000 mg/m2 d1, 8, 15 for 2 cycles). Of the 15 (68%) patients who underwent pancreatectomy, 14 (93%) of the operations were R0, suggesting efficacy for this therapeutic concept.
The German NEOLAP trial includes borderline resectable and non-resectable PDAC and focuses in particular on the fact that the effectiveness of neoadjuvant treatment may not be reflected by a radiologically measurable response[21]. After an induction treatment with gemcitabine + nab-paclitaxel for 2 cycles, participants will be randomized for 2 further cycles of gemcitabine + nab-paclitaxel or 2 cycles of FOLFIRINOX. In the case of stable disease an obligatory explorative laparotomy/laparoscopy will be performed to answer the question of a possible discrepancy between radiological assessment and intraoperative evaluation of resectability.
CONCLUSION
Although the progress in the last decades in pancreatic surgery has been clinically relevant, as demonstrated by a clinically relevant decrease of mortality in specialized centers, more extensive operations will be needed to further improve long-term survival. Extended pancreatectomy, portal vein resection and pancreatic resection in resectable pancreatic cancer with limited liver metastasis seem to be the surgical procedures with the highest impact in this field.
In this context, the concept of borderline resectability appears very promising. Although this concept has already been adopted to some extent in some national guidelines, it is still evolving. Criteria for imaging are defined quite strictly and pragmatically, as they do not only reflect technical resectability but must be considered prognostic parameters indicating more advanced extent of tumors. Furthermore, variability of the vasculature of the individual patient is not reflected by the criteria, leaving the individual decision dependent on the experience of the treating medical institution. This includes alternative surgical strategies such as the promising distal pancreatectomy with en-bloc resection of the celiac trunk in case of pancreatic tail carcinoma reaching the celiac axis, which requires very precise planning based on radiologic imaging and a preoperative radiological intervention in order to train collateralization. The timing of imaging studies is another important factor, as it is known that regardless of imaging quality, images older than 4 wk rarely represent the tumor spread found upon surgery. In such cases imaging has to be repeated before the decision is made.
Adjuvant chemotherapy for PDAC can double the rate of cure, as demonstrated by the clinical trials outlined in this review. Although this is comparable to results for other cancers, further improvements are urgently needed, as the starting point is catastrophically unfavorable, with rates of recurrence of disease of about 90% without perioperative treatment. Since the approval of gemcitabine in 1997 more effective cytotoxic substances (nab-paclitaxel) and combinations (FOLFIRINOX) are now available for advanced PDAC, raising hopes for more effective adjuvant and neoadjuvant treatment concepts for potentially resectable tumours. The fact that therapies with a broader mechanism of action will become available for research projects in the near future is equally important. In addition to the omnipresent immunotherapy, stromal depletion, e.g., with hyaluronidase[55] and anti-inflammatory concepts[56], appear most promising.
The only hope for improving long-term survival in this still challenging disease is a multidisciplinary approach, ideally with the close collaboration of radiologists, surgeons and oncologists in specialized tumor centers.
Footnotes
Conflict-of-interest statement: Marianne Sinn received travel support and honoraria for scientific presentations by Bayer Healthcare, Celgene, Roche, Leo Pharma. Marcus Bahra received travel support by Celgene. Timm Denecke received travel support and honoraria for scientific presentations by Bayer Healthcare, Siemens Medical Solutions, Toshiba, Novartis. Sue Travis has no conflict of interests to declare. Uwe Pelzer received travel support and honoraria for scientific presentations from Celgene, Amgen, BBraun. Hanno Riess received fees for serving as a speaker for Aspen Europa, Bayer Health Care AG, Bristol Myers Squipp, Boehringer Ingelheim, Daiichi-Sankyo, Leo Pharma, Novartis Pharma, Pfizer Pharma and Sanofi-Aventis.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: July 2, 2015
First decision: August 25, 2015
Article in press: December 21, 2015
P- Reviewer: Giovannetti E, Ho KM, Sommariva A S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
References
- 1.Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74:2913–2921. doi: 10.1158/0008-5472.CAN-14-0155. [DOI] [PubMed] [Google Scholar]
- 2.Malvezzi M, Bertuccio P, Levi F, La Vecchia C, Negri E. European cancer mortality predictions for the year 2014. Ann Oncol. 2014;25:1650–1656. doi: 10.1093/annonc/mdu138. [DOI] [PubMed] [Google Scholar]
- 3.Vincent A, Herman J, Schulick R, Hruban RH, Goggins M. Pancreatic cancer. Lancet. 2011;378:607–620. doi: 10.1016/S0140-6736(10)62307-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ryan DP, Hong TS, Bardeesy N. Pancreatic adenocarcinoma. N Engl J Med. 2014;371:1039–1049. doi: 10.1056/NEJMra1404198. [DOI] [PubMed] [Google Scholar]
- 5.Seufferlein T, Adler G. [The S3 guideline exocrine pancreatic cancer] Med Klin (Munich) 2009;104:869–874. doi: 10.1007/s00063-009-1183-7. [DOI] [PubMed] [Google Scholar]
- 6.Barugola G, Partelli S, Marcucci S, Sartori N, Capelli P, Bassi C, Pederzoli P, Falconi M. Resectable pancreatic cancer: who really benefits from resection? Ann Surg Oncol. 2009;16:3316–3322. doi: 10.1245/s10434-009-0670-7. [DOI] [PubMed] [Google Scholar]
- 7.Birkmeyer JD, Sun Y, Wong SL, Stukel TA. Hospital volume and late survival after cancer surgery. Ann Surg. 2007;245:777–783. doi: 10.1097/01.sla.0000252402.33814.dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boggi U, Del Chiaro M, Croce C, Vistoli F, Signori S, Moretto C, Amorese G, Mazzeo S, Cappelli C, Campani D, et al. Prognostic implications of tumor invasion or adhesion to peripancreatic vessels in resected pancreatic cancer. Surgery. 2009;146:869–881. doi: 10.1016/j.surg.2009.04.029. [DOI] [PubMed] [Google Scholar]
- 9.Grieser C, Steffen IG, Grajewski L, Stelter L, Streitparth F, Schnapauff D, Glanemann M, Langrehr J, Andreou A, Neuhaus P, et al. Preoperative multidetector row computed tomography for evaluation and assessment of resection criteria in patients with pancreatic masses. Acta Radiol. 2010;51:1067–1077. doi: 10.3109/02841851.2010.520023. [DOI] [PubMed] [Google Scholar]
- 10.Denecke T, Grieser C, Neuhaus P, Bahra M. Radiologic resectability assessment in pancreatic cancer. Rofo. 2014;186:23–29. doi: 10.1055/s-0033-1350190. [DOI] [PubMed] [Google Scholar]
- 11.Heestand GM, Murphy JD, Lowy AM. Approach to patients with pancreatic cancer without detectable metastases. J Clin Oncol. 2015;33:1770–1778. doi: 10.1200/JCO.2014.59.7930. [DOI] [PubMed] [Google Scholar]
- 12.Appel BL, Tolat P, Evans DB, Tsai S. Current staging systems for pancreatic cancer. Cancer J. 2012;18:539–549. doi: 10.1097/PPO.0b013e318278c5b5. [DOI] [PubMed] [Google Scholar]
- 13.Lu DS, Reber HA, Krasny RM, Kadell BM, Sayre J. Local staging of pancreatic cancer: criteria for unresectability of major vessels as revealed by pancreatic-phase, thin-section helical CT. AJR Am J Roentgenol. 1997;168:1439–1443. doi: 10.2214/ajr.168.6.9168704. [DOI] [PubMed] [Google Scholar]
- 14.Loyer EM, David CL, Dubrow RA, Evans DB, Charnsangavej C. Vascular involvement in pancreatic adenocarcinoma: reassessment by thin-section CT. Abdom Imaging. 1996;21:202–206. doi: 10.1007/s002619900046. [DOI] [PubMed] [Google Scholar]
- 15.Zech CJ, Korpraphong P, Huppertz A, Denecke T, Kim MJ, Tanomkiat W, Jonas E, Ba-Ssalamah A. Randomized multicentre trial of gadoxetic acid-enhanced MRI versus conventional MRI or CT in the staging of colorectal cancer liver metastases. Br J Surg. 2014;101:613–621. doi: 10.1002/bjs.9465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Niekel MC, Bipat S, Stoker J. Diagnostic imaging of colorectal liver metastases with CT, MR imaging, FDG PET, and/or FDG PET/CT: a meta-analysis of prospective studies including patients who have not previously undergone treatment. Radiology. 2010;257:674–684. doi: 10.1148/radiol.10100729. [DOI] [PubMed] [Google Scholar]
- 17.Löwenthal D, Zeile M, Lim WY, Wybranski C, Fischbach F, Wieners G, Pech M, Kropf S, Ricke J, Dudeck O. Detection and characterisation of focal liver lesions in colorectal carcinoma patients: comparison of diffusion-weighted and Gd-EOB-DTPA enhanced MR imaging. Eur Radiol. 2011;21:832–840. doi: 10.1007/s00330-010-1977-2. [DOI] [PubMed] [Google Scholar]
- 18.Glant JA, Waters JA, House MG, Zyromski NJ, Nakeeb A, Pitt HA, Lillemoe KD, Schmidt CM. Does the interval from imaging to operation affect the rate of unanticipated metastasis encountered during operation for pancreatic adenocarcinoma? Surgery. 2011;150:607–616. doi: 10.1016/j.surg.2011.07.048. [DOI] [PubMed] [Google Scholar]
- 19.Donahue TR, Isacoff WH, Hines OJ, Tomlinson JS, Farrell JJ, Bhat YM, Garon E, Clerkin B, Reber HA. Downstaging chemotherapy and alteration in the classic computed tomography/magnetic resonance imaging signs of vascular involvement in patients with pancreaticobiliary malignant tumors: influence on patient selection for surgery. Arch Surg. 2011;146:836–843. doi: 10.1001/archsurg.2011.152. [DOI] [PubMed] [Google Scholar]
- 20.Tamm EP, Loyer EM, Faria S, Raut CP, Evans DB, Wolff RA, Crane CH, Dubrow RA, Charnsangavej C. Staging of pancreatic cancer with multidetector CT in the setting of preoperative chemoradiation therapy. Abdom Imaging. 2006;31:568–574. doi: 10.1007/s00261-005-0194-y. [DOI] [PubMed] [Google Scholar]
- 21.Katz MH, Fleming JB, Bhosale P, Varadhachary G, Lee JE, Wolff R, Wang H, Abbruzzese J, Pisters PW, Vauthey JN, et al. Response of borderline resectable pancreatic cancer to neoadjuvant therapy is not reflected by radiographic indicators. Cancer. 2012;118:5749–5756. doi: 10.1002/cncr.27636. [DOI] [PubMed] [Google Scholar]
- 22.Sperti C, Berselli M, Pedrazzoli S. Distal pancreatectomy for body-tail pancreatic cancer: is there a role for celiac axis resection? Pancreatology. 2010;10:491–498. doi: 10.1159/000276984. [DOI] [PubMed] [Google Scholar]
- 23.Denecke T, Andreou A, Podrabsky P, Grieser C, Warnick P, Bahra M, Klein F, Hamm B, Neuhaus P, Glanemann M. Distal pancreatectomy with en bloc resection of the celiac trunk for extended pancreatic tumor disease: an interdisciplinary approach. Cardiovasc Intervent Radiol. 2011;34:1058–1064. doi: 10.1007/s00270-010-9997-5. [DOI] [PubMed] [Google Scholar]
- 24.Kamphues C, Bova R, Schricke D, Hippler-Benscheidt M, Klauschen F, Stenzinger A, Seehofer D, Glanemann M, Neuhaus P, Bahra M. Postoperative complications deteriorate long-term outcome in pancreatic cancer patients. Ann Surg Oncol. 2012;19:856–863. doi: 10.1245/s10434-011-2041-4. [DOI] [PubMed] [Google Scholar]
- 25.Mavros MN, Xu L, Maqsood H, Gani F, Ejaz A, Spolverato G, Al-Refaie WB, Frank SM, Pawlik TM. Perioperative Blood Transfusion and the Prognosis of Pancreatic Cancer Surgery: Systematic Review and Meta-analysis. Ann Surg Oncol. 2015;22:4382–4391. doi: 10.1245/s10434-015-4823-6. [DOI] [PubMed] [Google Scholar]
- 26.Kulemann B, Hoeppner J, Wittel U, Glatz T, Keck T, Wellner UF, Bronsert P, Sick O, Hopt UT, Makowiec F, et al. Perioperative and long-term outcome after standard pancreaticoduodenectomy, additional portal vein and multivisceral resection for pancreatic head cancer. J Gastrointest Surg. 2015;19:438–444. doi: 10.1007/s11605-014-2725-8. [DOI] [PubMed] [Google Scholar]
- 27.Reddy SK, Tyler DS, Pappas TN, Clary BM. Extended resection for pancreatic adenocarcinoma. Oncologist. 2007;12:654–663. doi: 10.1634/theoncologist.12-6-654. [DOI] [PubMed] [Google Scholar]
- 28.Bahra M, Neumann U. Surgical techniques for resectable pancreatic cancer. Recent Results Cancer Res. 2008;177:29–38. doi: 10.1007/978-3-540-71279-4_4. [DOI] [PubMed] [Google Scholar]
- 29.Hartwig W, Hackert T, Hinz U, Hassenpflug M, Strobel O, Büchler MW, Werner J. Multivisceral resection for pancreatic malignancies: risk-analysis and long-term outcome. Ann Surg. 2009;250:81–87. doi: 10.1097/SLA.0b013e3181ad657b. [DOI] [PubMed] [Google Scholar]
- 30.Hartwig W, Hackert T, Hinz U, Gluth A, Bergmann F, Strobel O, Büchler MW, Werner J. Pancreatic cancer surgery in the new millennium: better prediction of outcome. Ann Surg. 2011;254:311–319. doi: 10.1097/SLA.0b013e31821fd334. [DOI] [PubMed] [Google Scholar]
- 31.Pedrazzoli S, DiCarlo V, Dionigi R, Mosca F, Pederzoli P, Pasquali C, Klöppel G, Dhaene K, Michelassi F. Standard versus extended lymphadenectomy associated with pancreatoduodenectomy in the surgical treatment of adenocarcinoma of the head of the pancreas: a multicenter, prospective, randomized study. Lymphadenectomy Study Group. Ann Surg. 1998;228:508–517. doi: 10.1097/00000658-199810000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Delpero JR, Boher JM, Sauvanet A, Le Treut YP, Sa-Cunha A, Mabrut JY, Chiche L, Turrini O, Bachellier P, Paye F. Pancreatic adenocarcinoma with venous involvement: is up-front synchronous portal-superior mesenteric vein resection still justified? A survey of the Association Française de Chirurgie. Ann Surg Oncol. 2015;22:1874–1883. doi: 10.1245/s10434-014-4304-3. [DOI] [PubMed] [Google Scholar]
- 33.Turrini O, Ewald J, Barbier L, Mokart D, Blache JL, Delpero JR. Should the portal vein be routinely resected during pancreaticoduodenectomy for adenocarcinoma? Ann Surg. 2013;257:726–730. doi: 10.1097/SLA.0b013e318269d23c. [DOI] [PubMed] [Google Scholar]
- 34.Seufferlein T, Porzner M, Becker T, Budach V, Ceyhan G, Esposito I, Fietkau R, Follmann M, Friess H, Galle P, et al. [S3-guideline exocrine pancreatic cancer] Z Gastroenterol. 2013;51:1395–1440. doi: 10.1055/s-0033-1356220. [DOI] [PubMed] [Google Scholar]
- 35.Grützmann R, Distler M, Weitz J. [Appleby operation for locally advanced tumour of the pancreatic body and tail--a video demonstration] Zentralbl Chir. 2015;140:151–154. doi: 10.1055/s-0035-1545837. [DOI] [PubMed] [Google Scholar]
- 36.Bahra M, Pratschke J, Klein F, Neuhaus P, Boas-Knoop S, Puhl G, Denecke T, Pullankavumkal JR, Sinn M, Riess H, et al. Cytoreductive Surgery for Pancreatic Cancer Improves Overall Outcome of Gemcitabine-Based Chemotherapy. Pancreas. 2015;44:930–936. doi: 10.1097/MPA.0000000000000365. [DOI] [PubMed] [Google Scholar]
- 37.Sultana A, Cox T, Ghaneh P, Neoptolemos JP. Adjuvant therapy for pancreatic cancer. Recent Results Cancer Res. 2012;196:65–88. doi: 10.1007/978-3-642-31629-6_5. [DOI] [PubMed] [Google Scholar]
- 38.Oettle H, Post S, Neuhaus P, Gellert K, Langrehr J, Ridwelski K, Schramm H, Fahlke J, Zuelke C, Burkart C, et al. Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer: a randomized controlled trial. JAMA. 2007;297:267–277. doi: 10.1001/jama.297.3.267. [DOI] [PubMed] [Google Scholar]
- 39.Oettle H, Neuhaus P, Hochhaus A, Hartmann JT, Gellert K, Ridwelski K, Niedergethmann M, Zülke C, Fahlke J, Arning MB, et al. Adjuvant chemotherapy with gemcitabine and long-term outcomes among patients with resected pancreatic cancer: the CONKO-001 randomized trial. JAMA. 2013;310:1473–1481. doi: 10.1001/jama.2013.279201. [DOI] [PubMed] [Google Scholar]
- 40.Neoptolemos JP, Stocken DD, Bassi C, Ghaneh P, Cunningham D, Goldstein D, Padbury R, Moore MJ, Gallinger S, Mariette C, et al. Adjuvant chemotherapy with fluorouracil plus folinic acid vs gemcitabine following pancreatic cancer resection: a randomized controlled trial. JAMA. 2010;304:1073–1081. doi: 10.1001/jama.2010.1275. [DOI] [PubMed] [Google Scholar]
- 41.Seufferlein T, Bachet JB, Van Cutsem E, Rougier P. Pancreatic adenocarcinoma: ESMO-ESDO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012;23 Suppl 7:vii33–vii40. doi: 10.1093/annonc/mds224. [DOI] [PubMed] [Google Scholar]
- 42.Pasini F, Fraccon AP, DE Manzoni G. The role of chemotherapy in metastatic gastric cancer. Anticancer Res. 2011;31:3543–3554. [PubMed] [Google Scholar]
- 43.Fukutomi A, Uesaka K, Boku M, Kanemoto H, Konishi M, Matsumoto I, Kaneoka Y, Shimizu Y, Nakamori S, Sakamoto H, et al. JASPAC 01: Randomized phase III trial of adjuvant chemotherapy with gemcitabine versus S-1 for patients with resected pancreatic cancer. 2013 ASCO Annual Meeting Abstracts. J Clin Oncol. 2013;31 suppl:abstr 4008. [Google Scholar]
- 44.Vaccaro V, Sperduti I, Vari S, Bria E, Melisi D, Garufi C, Nuzzo C, Scarpa A, Tortora G, Cognetti F, et al. Metastatic pancreatic cancer: Is there a light at the end of the tunnel? World J Gastroenterol. 2015;21:4788–4801. doi: 10.3748/wjg.v21.i16.4788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sinn M, Liersch T, Gellert K, Messmann H, Bechstein WO, Waldschmidt D, Jacobasch L, Wilhelm M, Rau BM, Grützmann R, et al. CONKO-005: Adjuvant therapy in R0 resected pancreatic cancer patients with gemcitabine plus erlotinib versus gemcitabine for 24 weeks-A prospective randomized phase III study. J Clin Oncol. 2015;33 suppl:abstr 4007. doi: 10.1200/JCO.2017.72.6463. [DOI] [PubMed] [Google Scholar]
- 46.Sinn M, Liersch T, Riess H, Stübs P, Waldschmidt DT, Pelzer U, Stieler J, Striefler JK, Bahra M, Dörken B, et al. CONKO-006, A randomized double-blinded phase IIb-study of adjuvant therapy with gemcitabine sorafenib/placebo for patients with R1-resection of pancreatic cancer. Ann Oncol. 2014;25(suppl 4):1–41. [Google Scholar]
- 47.Schmidt J, Abel U, Debus J, Harig S, Hoffmann K, Herrmann T, Bartsch D, Klein J, Mansmann U, Jäger D, et al. Open-label, multicenter, randomized phase III trial of adjuvant chemoradiation plus interferon Alfa-2b versus fluorouracil and folinic acid for patients with resected pancreatic adenocarcinoma. J Clin Oncol. 2012;30:4077–4083. doi: 10.1200/JCO.2011.38.2960. [DOI] [PubMed] [Google Scholar]
- 48.Conroy T, Desseigne F, Ychou M, Bouché O, Guimbaud R, Bécouarn Y, Adenis A, Raoul JL, Gourgou-Bourgade S, de la Fouchardière C, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817–1825. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 49.Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, Seay T, Tjulandin SA, Ma WW, Saleh MN, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369:1691–1703. doi: 10.1056/NEJMoa1304369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang J, Reiss KA, Khatri R, Jaffee E, Laheru D. Immune Therapy in GI Malignancies: A Review. J Clin Oncol. 2015;33:1745–1753. doi: 10.1200/JCO.2015.60.7879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hardacre JM, Mulcahy M, Small W, Talamonti M, Obel J, Krishnamurthi S, Rocha-Lima CS, Safran H, Lenz HJ, Chiorean EG. Addition of algenpantucel-L immunotherapy to standard adjuvant therapy for pancreatic cancer: a phase 2 study. J Gastrointest Surg. 2013;17:94–100; discussion 100-101. doi: 10.1007/s11605-012-2064-6. [DOI] [PubMed] [Google Scholar]
- 52.Ko AH. Progress in the treatment of metastatic pancreatic cancer and the search for next opportunities. J Clin Oncol. 2015;33:1779–1786. doi: 10.1200/JCO.2014.59.7625. [DOI] [PubMed] [Google Scholar]
- 53.Hartlapp I, Müller J, Kenn W, Steger U, Isbert C, Scheurlen M, Germer CT, Einsele H, Kunzmann V. Complete pathological remission of locally advanced, unresectable pancreatic cancer (LAPC) after intensified neoadjuvant chemotherapy. Onkologie. 2013;36:123–125. doi: 10.1159/000348527. [DOI] [PubMed] [Google Scholar]
- 54.Katz MH, Shi S, Ahmad SA, Herman JM, de Wilton Marsh R. Preoperative modified FOLFIRINOX (mFOLFIRINOX) followed by chemoradiation (CRT) for borderline resectable (BLR) pancreatic cancer (PDAC): Initial results from Alliance Trial A021101. J Clin Oncol. 2015;33 suppl:abstr 4008. [Google Scholar]
- 55.Hingorani SR, Harris WP, Hednifar AE, Bullcok AJ, Wu XW, Huang Y, Jiang P. High response rate and PFS with PEGPH20 added to nab-paclitaxel/gemcitabine in stage IV previously untreated pancreatic cancer patients with high-HA tumors: Interim results of a randomized phase II study. J Clin Oncol. 2015;33 suppl:abstr 4006. [Google Scholar]
- 56.Hurwitz H, Uppal N, Wagner SA. A randomized double-blind phase 2 study of ruxolitinib (RUX) or placebo (PBO) with capecitabine (CAPE) as second-line therapy in patients (pts) with metastatic pancreatic cancer (mPC) J Clin Oncol. 2014;32 suppl:5s, abstr 4000. doi: 10.1200/JCO.2015.61.4578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Regine WF, Winter KA, Abrams RA, Safran H, Hoffman JP, Konski A, Benson AB, Macdonald JS, Kudrimoti MR, Fromm ML, et al. Fluorouracil vs gemcitabine chemotherapy before and after fluorouracil-based chemoradiation following resection of pancreatic adenocarcinoma: a randomized controlled trial. JAMA. 2008;299:1019–1026. doi: 10.1001/jama.299.9.1019. [DOI] [PubMed] [Google Scholar]


