Abstract
Transcriptionally silent heterochromatin is associated with repetitive DNA. It is poorly understood whether and how heterochromatin differs between different organisms and whether its structure can be remodelled in response to environmental signals. Here, we address this question by analysing the chromatin state associated with DNA repeats in the human fungal pathogen Candida albicans. Our analyses indicate that, contrary to model systems, each type of repetitive element is assembled into a distinct chromatin state. Classical Sir2-dependent hypoacetylated and hypomethylated chromatin is associated with the rDNA locus while telomeric regions are assembled into a weak heterochromatin that is only mildly hypoacetylated and hypomethylated. Major Repeat Sequences, a class of tandem repeats, are assembled into an intermediate chromatin state bearing features of both euchromatin and heterochromatin. Marker gene silencing assays and genome-wide RNA sequencing reveals that C. albicans heterochromatin represses expression of repeat-associated coding and non-coding RNAs. We find that telomeric heterochromatin is dynamic and remodelled upon an environmental change. Weak heterochromatin is associated with telomeres at 30 °C, while robust heterochromatin is assembled over these regions at 39 °C, a temperature mimicking moderate fever in the host. Thus in C. albicans, differential chromatin states controls gene expression and epigenetic plasticity is linked to adaptation.
Large blocks of DNA repeats are commonly clustered at rDNA loci, telomeres and centromeres and are assembled into heterochromatin. Heterochromatic regions impose a transcriptionally repressive environment that can propagate over long distances (up to 50 kb) stochastically silencing native genes as well as reporter genes inserted at these regions independently of the underlying DNA sequence1,2,3,4. Transcriptionally repressive heterochromatin is distinguishable from transcriptionally active euchromatin by several epigenetic features where heterochromatin is characterised by nucleosomes that are hypoacetylated, hypomethylated on lysine 4 of histone H3 (H3K4) and methylated on lysine 9 of histone H3 (H3K9)5,6. Histone modifiers control the transcriptionally repressive state of heterochromatin regions via chromatin modifications. For example, the conserved histone deacetylase Sir2 controls the hypoacetylated state of heterochromatic regions while the histone methyltransferase Su(var)3–9 specifically methylates H3K91,2,7,8,9,10,11. Heterochromatin has the ability to propagate thanks to specific silencing complexes. For example, in Saccharomyces cerevisiae, assembly of subtelomeric heterochromatin is mediated by the Sir silencing complex formed by Sir2, Sir3 and Sir41. Likewise, heterochromatin assembly at the rDNA locus is dependent on the RENT (REgulator of Nucleolar silencing and Telophase) complex formed by Sir2, Net1 and Cdc1412,13,14. Heterochromatin modulation could be particularly important for organisms, such as microbial pathogens, that have to adapt rapidly to different environments. This is because heterochromatin can modulate gene expression without changes in DNA sequence. However, very little is known about heterochromatin-mediated transcriptional regulation in this group of organisms. Here we address this question by investigating the chromatin states associated with DNA repeats in the most common human fungal pathogen: Candida albicans. C. albicans normally lives as a commensal in humans but it can become virulent causing systemic life-threatening infections with mortality rates of up to 50%15. Upon environmental changes, C. albicans undergoes major morphological and genomic changes that can promote adaptation and survival4,16. It is unknown whether the chromatin structure of the C. albicans genome is also plastic and capable of remodeling upon environmental changes.
The C. albicans genome has 8 diploid chromosomes and contains 3 major classes of large blocks of repetitive DNA: the rDNA locus, Major Repeat Sequences (MRS) and telomeres17,18 (Fig. S1A). The rDNA locus consists of a tandem array of a ~12 kb unit repeated 50 to 200 times on chromosome R. Each unit contains the two highly conserved 35 S and the 5 S rRNA genes that are separated by two Non-Transcribed Regions (NTS1 and NTS2), whose sequences are not conserved across eukaryotes (Fig. S1B)17,18. In other organisms, while the 35S and 5S rDNA genes are highly expressed, the NTS1 and NTS2 regions are assembled in transcriptionally silent heterochromatin12,13,14. At these locations, heterochromatin represses transcription of non coding RNAs promoting stability of the rDNA loci19,20. The MRS loci are long tracts (10–100 kb) of nested DNA repeats found on 7 of the 8 C. albicans chromosomes18,21. These repetitive domains are formed by large tandem arrays of 2.1 kb RPS unit flanked by non-repetitive HOK and RBP-2 elements (Fig. S1C). In addition, a RBP-2 element, but not an intact MRS, is found on chromosome 322. Given their highly repetitive nature, MRS repeats are expected to be ideal substrates for heterochromatin assembly. C. albicans telomeres are composed of a terminal region composed of tandemly repeating 23 bp units and subtelomeric regions (Fig. S1D)23. Due to their size and repetitive nature, the sequences of subtelomeric regions remain poorly characterised18.
DNA repeats are central to C. albicans genome plasticity and pathogenicity as they play key roles in the regulation of genome organisation and structure in the host16. However, the chromatin state of these DNA elements is unknown. The C. albicans epigenome, like the S. cerevisae epigenome, most probably lacks H3K9 methylation given that a Su(var) 3–9 orthologue cannot be identified in either C. albicans or S. cerevisiae. In contrast, the C. albicans genome encodes for two putative NAD-dependent histone deacetylases that resemble Sir2 (orf19.1992 and orf19.4762)24. While it is possible to identify orthologues for RENT complex components, components of the Sir silencing complex are not apparent. Therefore, while heterochromatin might associate with the rDNA locus, it is possible that C. albicans telomeres lack heterochromatin as it has been recently shown for the yeast Clavispora lusitaniae11.
In this study, we investigated the chromatin states associated with C. albicans repetitive elements under different environmental conditions. We found that the different types of repetitive elements are assembled in distinct chromatin states. Classical hypoacetylated heterochromatin is associated with the non-transcribed region of the rDNA locus. The histone deacetylase Sir2 (orf 19.1992) is required to maintain this repressive epigenetic state via hypoacetylation of Lysine 9 of Histone H3. Heterochromatin associated with these regions represses non-coding RNA transcription. In contrast, MRS repeats are assembled into a transcriptionally permissive chromatin state bearing both heterochromatic and euchromatic histone marks. Finally, we find that, despite the apparent absence of the Sir silencing complex, telomeric regions are assembled into a Sir2-dependent hypoacetylated and hypomethylated heterochromatin. This chromatin state silences expression of endogenous transcripts as well as inserted marker genes. Telomeric heterochromatin is plastic and affected by environmental conditions with heterochromatin being more robust at higher temperature (39 °C) than at lower temperature (30 °C). Thus, the epigenetic state associated with telomeric repeats switches in response to an environmental change that are linked to C. albicans virulence and pathogenicity.
Results
Transcriptional silencing at the C. albicans rDNA locus
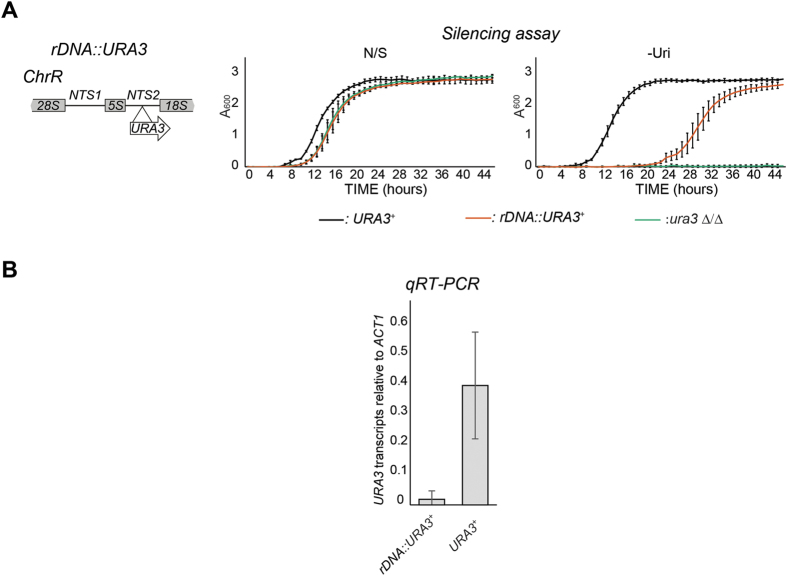
Heterochromatin assembled onto repetitive DNA represses the transcription of marker genes inserted in their proximity25,26,27,28. To assess whether transcriptionally silent chromatin exists in C. albicans, the URA3+ marker gene was integrated into the NTS2 region of the rDNA locus (rDNA::URA3+) (Fig. 1A). We investigated whether URA3+ was transcriptionally silenced when present at this locus, by growing strains in non-selective (N/S) medium and in medium lacking uridine (−Uri) in which only cells expressing sufficient Ura3 protein are able to grow. Silencing of URA3+ is expected to result in slower growth in –Uri medium compared to N/S. The rDNA::URA3+ strain displayed a reduced growth rate in selective medium and it is, therefore, silenced (Fig. 1A). Consistent with the growth assays the level of URA3 mRNA levels at the rDNA locus were significantly lower (20 fold) for rDNA::URA3+ than for a URA3+ gene expressed from its own euchromatic locus, as determined by quantitative reverse transcriptase analysis (qRT-PCR) (Fig. 1B). Therefore, in C. albicans, the non-transcribed region of the rDNA locus is assembled into a transcriptionally repressed state that is normally associated with repetitive heterochromatic regions.
Figure 1. Transcriptional silencing at the C. albicans rDNA locus.
(A) Left panel: Schematic of rDNA::URA3+ reporter strain. Right panel: silencing assay of the rDNA::URA3+ reporter strain. Ura+ (URA3/URA3) and Ura− (ura3Δ/ura3Δ) strains were included as controls. (B) qRT-PCR analyses to measure URA3+ transcript levels of the rDNA::URA3+ reporter strain relative to actin transcript levels (ACT1). Error bars in each panel: Standard deviation (SD) of three biological replicates.
Sir2-dependent heterochromatin at the rDNA locus
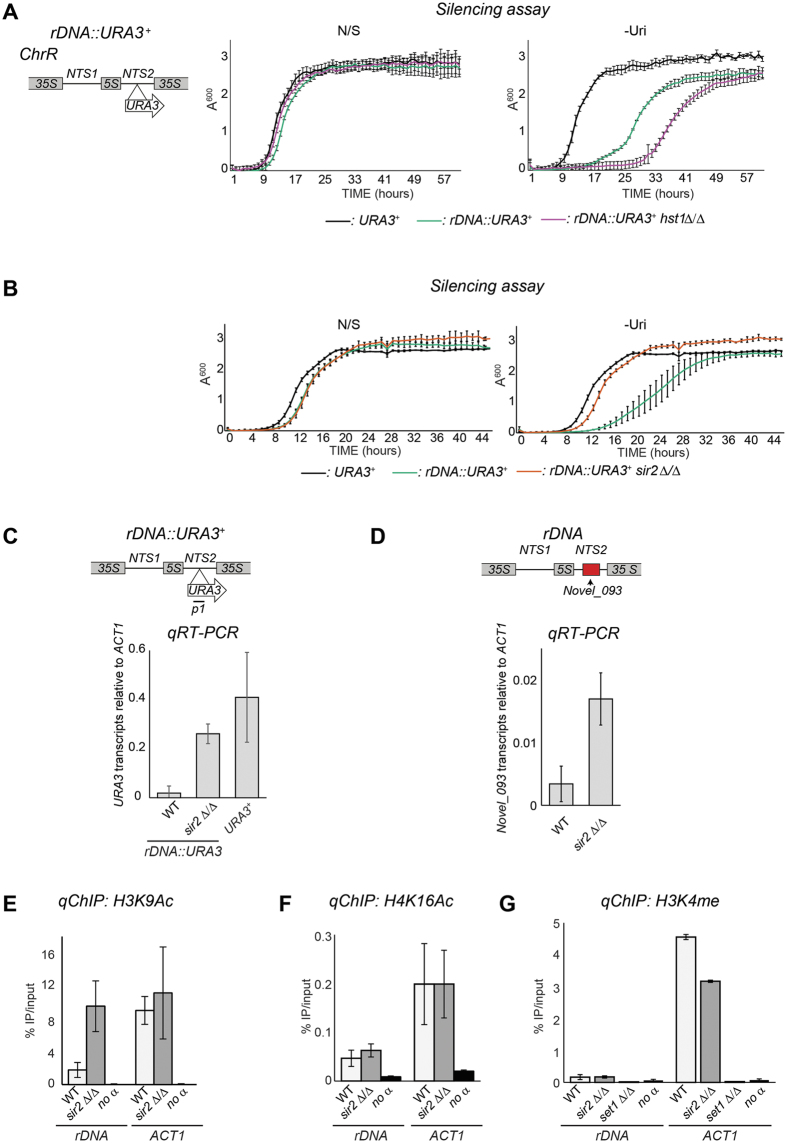
The ability of C. albicans rDNA repeats to cause transcriptional silencing suggests that the rDNA NTS regions are assembled into heterochromatin. The histone deacetylase Sir2 is a key regulator of heterochromatin in all organisms studied1,29. BLAST analyses reveal that the C. albicans genome contains 5 genes encoding proteins with homology to S. cerevisiae SIR2 (Fig. S2). Among these proteins, C. albicans Hst1 (orf19.4762) and Sir2 (orf19.1992) share the highest homology with the S. cerevisiae Sir2 (46% and 42% identity respectively) (Fig. S2A,B). Given the high homology, it is possible that C. albicans Hst1, and not Sir2, is the true ortholog of S. cerevisiae Sir2 as it has been demonstrated for the yeast Clavispora lusitaniae11. To determine whether Hst1 (orf19.4762) and/or Sir2 (orf19.1992) are required for maintaining the repressive state of the rDNA NTS region, we constructed hst1 Δ/Δ and sir2 Δ/Δ null mutants in strains carrying the rDNA::URA3+ reporter and performed marker gene silencing assay. At the NTS region of the rDNA locus, silencing was not alleviated in hst1 Δ/Δ cells (Fig. 2A) but it was strongly alleviated in sir2 Δ/Δ cells (Fig. 2B). In agreement with these results, qRT-PCR analyses revealed that URA3 mRNA levels in the rDNA::URA3+ strain were significantly higher in sir2 null cells relative to wild-type (WT) cells (Fig. 2C). Thus, the histone deacetylase Sir2, but not Hst1, is critical for the maintenance of the transcriptionally silent state associated with rDNA repeats in C. albicans. In S. cerevisiae, heterochromatin associated with the NTS region of the rDNA locus has been shown to repress transcription of a non-coding RNA20,30,31. In C. albicans, genome-wide analyses have identified an uncharacterised non-coding RNA (Novel_Ca21ChrR_093) originating from the NTS region of the rDNA locus32. To test whether C. albicans heterochromatin represses transcription of this non-coding RNA, we isolated RNA from wild-type and sir2 Δ/Δ cells and performed qRT-PCR analyses. Deletion of the SIR2 gene results in a clear upregulation of this non-coding transcript (Fig. 2D). Therefore, Sir2 is required to repress transcription of an endogenous transcript as well as inserted marker genes at the rDNA locus. To assess whether the NTS region of the rDNA locus is associated with heterochromatic pattern of histone marks (hypoaceylation of H3K9/H4K16 and hypomethylation of H3K4), we monitored the presence of these histone modifications by quantitative Chromatin ImmunoPrecipitation (qChIP)33. The rDNA locus, but not the euchromatic ACT1 locus, showed low enrichment for H3K9 acetylation, H4K16 acetylation and H3K4 methylation (Fig. 2E–G), a chromatin state typical of heterochromatic regions. Sir2 deacetylates lysine 9 on histone H3 and/or lysine 16 on histone H48,34,35. Therefore, we compared the level of histone acetylation associated with the NTS rDNA region in WT and sir2 Δ/Δ cells by performing qChIP analyses. In the sir2 null strain we detected higher levels of H3K9Ac, but not of H4K16Ac (Fig. 2E,F) demonstrating that C. albicans Sir2 is required to maintain low levels of acetylated H3K9. This is consistent with the idea that disruption of H3K9 deacetylation is critical for maintaining the transcriptionally silent state associated with heterochromatic regions. We also compared the level of H3K4 methylation associated with the NTS region of the rDNA locus in WT and sir2 Δ/Δ isolates. H3K4 methylation levels did not increase in sir2 Δ/Δ isolates compared to wt cells (Fig. 2G). Therefore hypomethylation of H3K4 is maintained independently of the histone acetylation and transcriptional state of the NTS region. Taken together these observations demonstrate that heterochromatin exists in C. albicans and it is assembled over the NTS region of the rDNA locus. Histone modification by Sir2 is critical for the maintenance of the transcriptionally silent heterochromatic state associated with this locus.
Figure 2. Sir2-dependent heterochromatin at the rDNA locus.
(A) Left panel: Schematic of rDNA::URA3+ reporter strain. Right panel: Silencing assay of the rDNA::URA3+ reporter strain in WT and hst1 Δ/Δ isolates. A URA+ (URA3+) strain was included as a control. (B) Silencing assay of the rDNA::URA3+ reporter strain WT and sir2 Δ/Δ isolates. A URA+ (URA3+) strain was included as a control. (C) qRT-PCR analyses to measure URA3+ transcript levels relative to ACT1 transcript levels in rDNA::URA3+ WT and sir2 Δ/Δ isolates. A URA3+ strain is included as a control (D) qRT-PCR analyses to measure levels of the rDNA non-coding RNA (Novel_Chr3_093) relative to ACT1 in WT and sir2 Δ/Δ isolates. (E,F) qChIP to detect H3K9Ac, H4K16Ac levels associated with the rDNA locus and ACT1 in WT and sir2 Δ/Δ isolates and (G) H3K4me2 levels associated with the rDNA locus and ACT1 in WT, sir2 Δ/Δ and set1 Δ/Δ isolates. Error bars in each panel: Standard deviation (SD) of three biological replicates.
MRS repeats are assembled into transcriptionally permissive chromatin bearing euchromatic and heterochromatic histone modifications
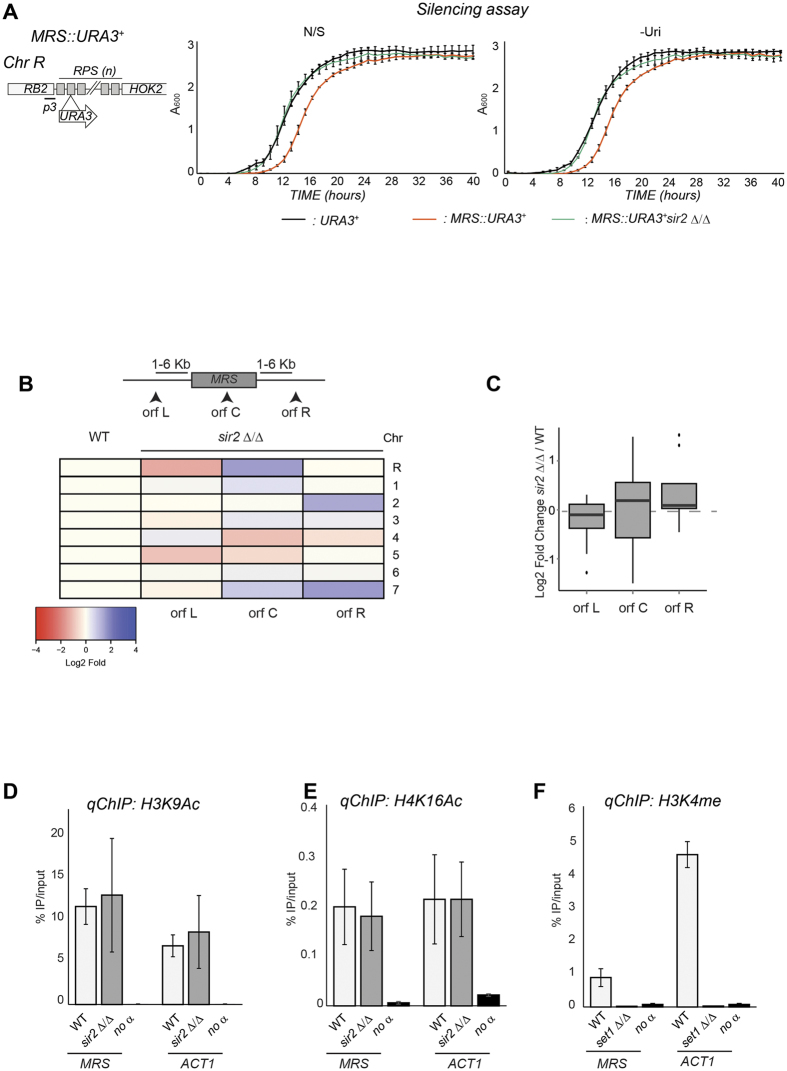
Having established that transcriptionally silent heterochromatin exists in C. albicans and is associated with the NTS region of the rDNA locus, we analysed the chromatin state associated with the MRS repeats by assessing silencing of a marker gene inserted into the tandem RPS repeats (MRS::URA3+) (Fig. 3A). As shown in Fig 3A, the MRS::URA3+ is not silenced (Fig. 3A). If the MRS repeats are associated with Sir2-dependent heterochromatin, the genes close to these regions should be upregulated in sir2 Δ/Δ isolates compared to WT cells. To address this question, we isolated RNA from WT and sir2 Δ/Δ cells and performed RNA-seq analyses. FPKM (fragments per kilobase of exons per million mapped reads) were determined for all the genes proximal to MRS repeats and compared in sir2 Δ/Δ and WT strains. Upon deletion of the SIR2 gene, we did not observe any clear effect on expression of MRS associated (orf C) and proximal genes (orf L and R) as only 3 out of 24 genes were expressed at more than 2 fold of WT in sir2 Δ/Δ isolates (Fig. 3B and Table S5). Averaging the log2 fold change of the MRS-associated genes confirms these results (average log2 fold change = 0) (Fig. 3C). These data demonstrate that MRS repeats do not impose a Sir2-dependent transcriptionally repressive state.
Figure 3. MRS repeats are assembled into transcriptionally-permissive chromatin.
(A) Top panel: Schematic of MRS::URA3+ reporter strain. Bottom panel: Silencing assay of the MRS::URA3+ reporter strain in WT and sir2 Δ/Δ isolates. A URA+ (URA3+) strain was included as a control. (B,C) RNA deep-sequencing of sir2 Δ/Δ and WT isolates. (B) Normalised read counts (FPKM) of MRS associated (MRS-C) and proximal (MRS-L and MRS-R) genes were calculated from RNA-seq data for WT and sir2 Δ/Δ isolates. The heat-map depicts the log2 fold ratio of FPKM data between sir2 Δ/Δ and WT isolates. (C) Boxplot showing log2 fold changes in transcriptional expression for MRS-internal (MRS-C) and adjacent (MRS-L and MRS-R) genes between sir2 Δ/Δ and WT isolates. (D,E) qChIP to detect H3K9Ac, H4K16Ac levels associated with the MRS repeats and ACT1 in WT and sir2 Δ/Δ isolates and (F) H3K4me2 levels associated with the MRS repeats and ACT1 in WT and set1 Δ/Δ isolates. Error bars in each panel: Standard deviation (SD) of three biological replicates.
Acetylated H3K9 and H4K16 qChIP analyses demonstrated that MRSs are assembled into highly acetylated chromatin where H3K9 and H4K16 are acetylated to a level similar to the active and euchromatic locus actin 1 (ACT1) (Fig. 3D,E). Histone acetylation level associated with these regions are similar in WT and sir2 Δ/Δ isolates (Fig. 3D,E). In contrast, levels of H3K4 methylation associated with MRS repeats are strikingly low compared to the euchromatic ACT1 locus (Fig. 3F). Therefore, MRS repeats are not assembled into classical transcriptionally silent heterochromatin but they are associated with an intermediate chromatin state bearing features of euchromatin (high histone acetylation) and heterochromatin (H3K4 hypomethylation).
Heterochromatin at C. albicans telomeric regions
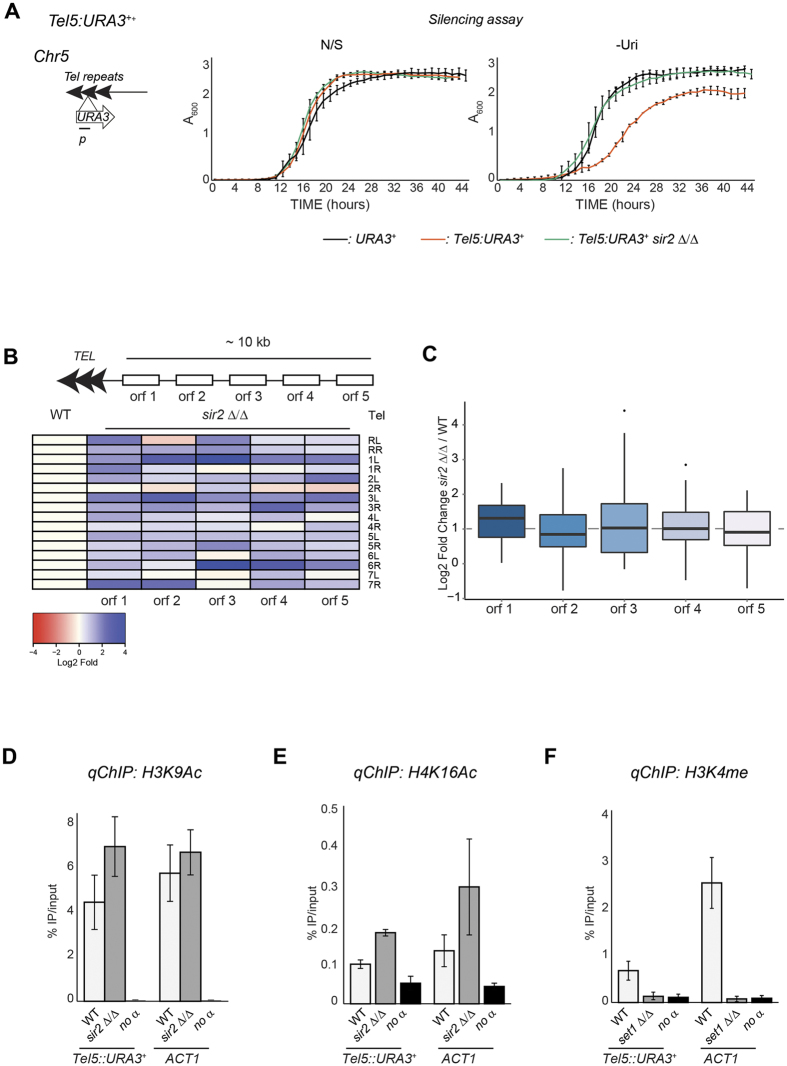
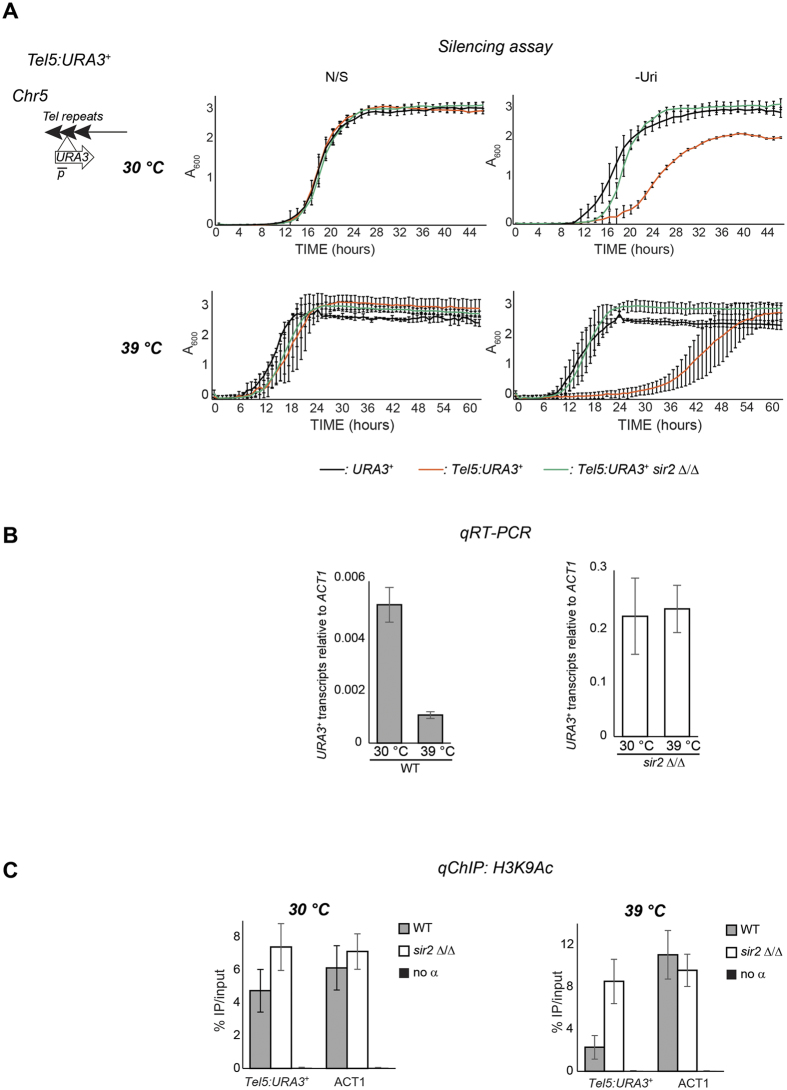
In many organisms, heterochromatin assembly over telomeric regions is dependent not only on Sir2 but also on the Sir silencing-complex1,2. With the exception of Sir2, BLAST analyses fail to identify orthologues of telomeric silencing proteins in C. albicans. It is therefore possible that telomeres are not assembled into heterochromatin. To test this hypothesis, we analysed silencing of a URA3+ marker gene integrated into a telomeric region (Tel5::URA3+). The Tel5::URA3+ marker gene displayed a small but reproducible reduction in growth rate on -Uri media compared to N/S media, indicative of weak silencing (Fig. 4A). Sir2 is required to maintain this transcriptionally repressive state as silencing is alleviated in sir2 Δ/Δ cells (Fig. 4A). Consistent with the growth assays, the levels of URA3+ mRNA levels are low in WT cells and dramatically increased in sir2 Δ/Δ isolates (Fig. S3).
Figure 4. Heterochromatin at C. albicans telomeric regions.
Left panel: Schematic of Tel5::URA3+ reporter strain. Right panels: Silencing assay of the Tel5::URA3+ reporter strain in WT and sir2 Δ/Δ. (A) URA+ (URA3+) strain was included as a control. (B,C) RNA deep-sequencing of sir2 Δ/Δ and WT isolates. (B) Normalised read counts (FPKM) of subtelomeric genes were calculated from RNA-seq data for WT and sir2 Δ/Δ isolates. The heat-map depicts the log2 fold ratio of FPKM data between sir2 Δ/Δ and WT isolates. (C) Boxplot showing log2 fold changes in transcriptional expression of subtelomeric genes between sir2 Δ/Δ and WT isolates (D–F) qChIP to detect H3K9Ac and H4K16ac levels associated with Tel5::URA3+ and ACT1 in WT and sir2 Δ/Δ strains. (C) H3K4me2 levels associated with Tel5::URA3+ and ACT1 in WT and set1 Δ/Δ isolates. Error bars in each panel: Standard deviation (SD) of three biological replicates.
Telomeric heterochromatin has been shown to repress gene expression of proximal genes in a Sir2-dependent manner36,37,38,39. To assess whether the weak marker gene silencing associated with telomere 5 is a general property of all C. albicans telomeres and if it extends over subtelomeric regions, we analysed the transcriptional profile of coding and non-coding subtelomeric transcripts in WT and sir2 Δ/Δ isolates. This analysis reveals that deletion of SIR2 results in transcriptional upregulation of many subtelomeric genes (Fig. 4B and Table S6). Although not all the subtelomeric genes are upregulated to the same extent, on average deletion of Sir2 results in a 2 fold upregulation of telomeric-proximal genes compared to WT (Fig. 4C). We used qRT-PCR analyses with primers specific for each subtelomeric gene to validate the difference in gene expression between WT and sir2 Δ/Δ isolates. All the genes tested were more highly expressed in sir2 Δ/Δ compared to WT cells (Fig. S4). Therefore, telomeric heterochromatin silences expression of genes located in proximity (~10/15 kb) of telomeres. Telomeres are composed of a 23 bp unit tandemly repeated23. Their repetitive nature makes the design of suitable primers for qChIP analysis particularly challenging. Thus, we assessed the telomeric chromatin state by performing qChIP analyses with primers specific for the Tel5: URA3+ marker gene. We found that telomeric chromatin is only mildly hypoacetylated on H3K9 and H4K16 and that histone acetylation levels are increased in sir2 Δ/Δ null mutant compared to WT cells (Fig. 4D,E). In contrast, a low level of H3K4 methylation is associated with telomeric regions (Fig. 4F).
Taken together these observations demonstrate that telomeric repeats are assembled into weak heterochromatin. This chromatin state is dependent on Sir2 and is able to silence embedded marker genes as well as native proximal genes.
Telomeric heterochromatin is plastic and remodelled upon environmental changes
C. albicans is characterised by remarkable genomic and phenotypic plasticity that allow rapid adaptation to different environmental niches4,16. Therefore, we tested whether the chromatin state of C. albicans repetitive elements is also plastic and remodelled upon environmental changes. Remodelling could lead to dynamic chromatin structure where DNA repeats are assembled into robust transcriptionally repressive heterochromatin under specific environmental conditions and into weak heterochromatin under different environmental conditions. To test this hypothesis, we asked if physiologically-relevant stress conditions that C. albicans regularly encounters in the host or stress caused by antifungal agents affects the transcriptional states associated with the NTS region of the rDNA locus, telomeric repeats and MRS repeats. Silencing assays revealed that treatment with hydrogen peroxide (H202), mimicking the production of reactive oxygen species by the host’s immune cells, or Fluconazole, the most widely used antifungal drug, does not change the transcriptional state associated with any of the loci tested (Figs S5, S6 and S7). Likewise we found that expression of the URA3+ marker gene inserted at the rDNA locus and MRS repeats was not affected by high temperature (39 °C, mimicking moderate fever in the host) (Figs S5 and S6). In contrast, telomere-associated silencing was much stronger at 39 °C than 30 °C (Fig. 5A) indicating the telomeric chromatin is plastic. Growing cells at 39 °C did not strongly induce hyphal formation in the time frame of the experiment (Fig. S8). Therefore, the observed stronger silencing is not a consequence of a different morphology. To assess whether temperature-dependent silencing is a general feature of all C. albicans telomeres, we analysed silencing of a second reporter strain with a URA3+ gene integrated at the telomeric repeats of chromosome 7 left arm (Tel7::URA3+). As with the telomere repeat tract on Chr5, silencing of telomeric repeats on Chr7 was much stronger at 39 °C compared to 30 °C (Fig. S8). Importantly, silencing was dependent on Sir2 as silencing growth rate in –Uri was increased in sir2 Δ/Δ cells (Fig. 5A). These results were confirmed by analyses of URA3+ mRNA levels by qRT-PCR: in WT, but not in sir2 Δ/Δ cells, Tel5: URA3+ RNA levels were lower at 39 °C compared to 30 °C (Fig. 5B). Consistent with the silencing assays, qChIP of the TEL5::URA3+ marker revealed lower levels of H3K9 acetylation at 39 °C compared to 30 °C (Fig. 5C). Thus telomere repeats are silenced to a greater degree at febrile temperature than at 30 °C, a temperature considered relevant for growth of C. albicans on the skin. These results indicate that telomeric heterochromatin is dynamic and can be remodelled in a Sir2-dependent manner in response to an environmental change.
Figure 5. Telomeric heterochromatin is plastic.
(A) Left panel: Schematic of Tel5::URA3+ reporter strain. Right panels: Silencing assay assessing transcriptional silencing of the Tel5::URA3+ reporter strain in WT and sir2 Δ/Δ isolates at 30 °C, in the presence of 1 mM H2O2, 200 ng/μl fluconazole and at 39 °C. A URA+ (URA3+) strain was included as a control. (B) qRT-PCR analyses to measure Tel5::URA3+ transcript levels relative to ACT1 at 30 °C and 39 °C in WT (left panel) and sir2 Δ/Δ (right panel) strains. (C) qChIP to detect H3K9Ac levels associated with Tel5::URA3+ and ACT1 in WT and sir2 Δ/Δ isolats at 30 °C (Left panel) and 39 °C (Right panel). Error bars in each panel: Standard deviation (SD) of three biological replicates.
Discussion
This study provides the first comprehensive analysis of the chromatin state of C. albicans DNA repeats. Our data demonstrate that, in C. albicans, differential chromatin states control gene expression and epigenetic plasticity is linked to adaptation to a specific environmental niche.
We find that the NTS region of the rDNA locus maintains a bona fide silent heterochromatin state that represses coding and non-coding transcription and is marked by the histone modification pattern typical of heterochromatic regions: hypoacetylation of histone H3K9 and histone H4K16 together with hypomethylation of H3K4 (Fig. 1). We identify Sir2 (orf19.1992) as one of the key enzymes necessary to maintain this chromatin state (Fig. 2). In S. cerevisiae, Sir2 targeting to the rDNA locus is dependent on the RENT complex13,14,40. BLAST analyses identify orthologues of the RENT complex components Net1 (orf19.267) and Cdc14 (orf 19.4192). Therefore, it is very likely that, as observed in S. cerevisiae, a conserved RENT complex targets heterochromatin at the C. albicans rDNA locus. However, the mode of action of Sir2 in C. albicans differs from S. cerevisiae: while at S. cerevisiae rDNA locus, Sir2 specifically deacetylates K16 on histone H4, C. albicans Sir2, reduces H3K9 acetylation. Therefore, heterochromatin at the non-transcribed regions of the rDNA array seems ubiquitous but it can differ in its structure.
We demonstrated that the MRS repeats are assembled into chromatin hypomethylated on H3K4, as observed in heterochromatic regions, but highly acetylated, as in euchromatic regions. Low levels of H3K4 methylation are not sufficient to create a transcriptionally repressive environment as a marker gene inserted at MRS repeats is not silenced. In addition, expression of MRS-proximal genes is not regulated by Sir2 (Fig. 3).
In many organisms, insertion of artificial DNA array is sufficient to seed heterochromatin41. Therefore, it is surprising that MRSs, being composed of very long tracts of nested repeats, are not assembled into classical heterochromatin. Why are MRSs not associated with heterochromatin? One primary function of heterochromatin is to inhibit recombination promoting genome stability. It is possible that hypomethylation on H3K4 is sufficient to block recombination or that an alternative mechanism promote genome stability at the MRSs.
Alternatively, genome instability at the MRSs could be beneficial for C. albicans, an organism lacking a canonical sexual cycle and meiosis42. Lack of heterochromatin at these loci could ensure high level of mitotic recombination, a key event to generate genomic diversity. In support of this hypothesis, analyses of clinical isolates suggest that MRSs might act as recombination hotspots as they can expand and contract and are known sites of translocations21,43,44. However it has been shown that, under standard laboratory growth conditions, recombination rate at the MRS repeats is not higher compared to a non-repetitive locus even though MRSs might have an effect on chromosome disjunction45,46 Our understanding of the biology and the function of the MRS loci remains limited, making it very difficult to assess whether this epigenetic signature controls MRS function. Further studies will reveal whether and how the epigenetic state associated with these repetitive elements contributes to C. albicans biology.
Traditionally, telomeric heterochromatin has been described as a repressive chromatin structure that silences expression of subtelomeric genes2. Recent studies have challenged this hypothesis and highlighted the diversity of structure and functions of telomeric chromatin across organisms. For example, in S. cerevisiae the Sir silencing complex is responsible for the assembly of hypoacetylated telomeric heterochromatin1. However, this chromatin state has a limited ability to repress transcription of subtelomeric genes47. The yeast C. lusitaniae appears to lack telomeric heterochromatin11. In the fungal pathogen Cryptococcus neoformans telomeric chromatin is methylated on K27 of histone H3 and silences expression of genes located in a 40 kb subtelomeric region48. We find that, C. albicans telomeres have also a specialised chromatin structure. Despite the apparent absence of Sir silencing proteins, C. albicans telomeres are associated with transcriptionally repressive heterochromatin (Fig. 5). Sir2 (orf19.1992) controls heterochromatin assembly at telomeres by deacetylation of histones. We hypothesise that an as yet unidentified protein complex targets Sir2 to telomeres. We demonstrate that telomeric heterochromatin transcriptionally silences subtelomeric genes as deletion of the SIR2 gene causes their upregulation. This modulation is likely to have major impacts on the biology and the pathogenicity of C. albicans as many subtelomeric genes have key regulatory functions. For example, the subtelomeric TLO genes encode proteins with similarity to Med2, a component of the Mediator complex that regulates transcription by RNA polymerase II49 and the subtelomeric gene Nag4 encodes for a putative transporter50.
We find that telomeric heterochromatin is dynamic: the ability of telomere terminal repeats to repress the expression of an embedded URA3 gene is affected by a physiologically-relevant stress condition with silencing much stronger at a temperature (39 °C) mimicking fever in the host than at lower temperatures (30 °C). This enhanced silencing is linked to changes in chromatin structure, as telomeres at 39 °C had lower levels of H3K9 acetylation and Sir2 was required for the increased silencing. This effect is specific for telomeric regions and for temperature shift as other loci are not affected by temperature changes and other physiologically relevant stresses do not lead to chromatin remodelling. Therefore, in C. albicans, adaptation to higher temperature is linked to chromatin remodelling at telomeric regions.
Dynamic heterochromatin is seen at telomeres in many species: For example, in S. cerevisiae, transcriptional silencing at higher temperature is enhanced at telomeres and weaker at the rDNA locus51. This could be due to a temperature-sensitive protein important for telomere function52 or to changes in the levels of heat shock factors at different temperatures53. The dynamics of heterochromatin also affects virulence and pathogenesis in at least some microbial pathogens. For example, telomeric heterochromatin regulates the expression of the antigenic variation gene in parasites38. The biological consequences of telomere chromatin plasticity in C. albicans remain to be determined. It is possible that changes in telomeric heterochromatin correlate with changes in expression of subtelomeric genes. Alternatively, chromatin remodelling at telomeres could regulate genomic stability of these loci.
In conclusion, this study highlights the diversity and plasticity of chromatin states associated with DNA repeats in Candida albicans, the most common human fungal pathogen. We show that in C. albicans differential heterochromatin states control gene expression independently of the underlying DNA sequence and remodelling of heterochromatin is linked to adaptation in a stress condition.
Methods
Growth conditions
Yeast cells were cultured in rich medium (YPAD) containing extra adenine (0.1 mg/ml) and extra uridine (0.08 mg/ml), complete SC medium (FormediumTM) or SC Drop-Out media (FormediumTM). Cells were grown at 30 °C or 39 °C as indicated.
Yeast strain construction
Strains are listed in Supplementary Table S1. Integration and deletion of genes were performed as previously described54. Oligonucleotides and plasmids used for strain constructions are listed in Supplementary Table S2 and Supplementary Table S4, respectively. Transformation was performed by electroporation (Gene PulserTM, Bio-Rad) using the protocol described in55. Correct integration events were checked by PCR and/or Southern blotting using primers listed in Supplementary Table S2 (Fig. S9)
Silencing assay
Growth analyses with rDNA::URA3+, Tel5::URA3+ and MRS::URA3+ strains were performed using a plate reader (SpectrostarNano, BMG labtech) in 24 well or 96 well plate format at 30 °C. When indicated Silencing assays were performed in the presence of 200 ng/μl of fluconazole (Sigma), 1 mM H202 (Sigma) and 39 °C. For each silencing assay in a 24 well plate format, 1 ml of a starting culture was inoculated in SC or SC-Uri media to reach a concentration of 60 cells/μl. Growth was assessed by measuring A600, using the following conditions: OD600 nm, 3600 s cycle time, 30 flashes per well, 400 rpm shaking frequency, double orbital shaking mode, 850 s additional shaking time after each cycle, 0.5 s post delay, for 44 to 60 hours at 30 °C. For each silencing assay in 96 well plate format, 1:100 dilution of an starting culture was inoculated in a final volume of 95 μl of SC or SC-Uri media to reach a concentration of 60 cells/μl. Growth was assessed by measuring A600, using the following conditions: OD600 nm, 616 cycle time, 3 flashes per well, 700 rpm shaking frequency, orbital shaking mode, 545 s additional shaking time after each cycle 0.5 s post delay, for 44 hours.
Graphs represent data from three biological replicates. Error bars: standard deviations of three biological replicates. Data was processed using SpectrostarNano MARS software and Microsoft Excel.
RNA extraction and cDNA synthesis
All strains were grown in YPAD rich media. RNA extraction was performed using a yeast RNA extraction kit (E.Z.N.A.® Isolation Kit RNA Yeast, Omega Bio-Tek) following the manufacturer’s instructions. RNA quality was checked by electrophoresis under denaturing conditions in 1% agarose, 1× HEPES, 6% Formaldehyde (Sigma). RNA concentration was measured using a NanoDrop ND-1000 Spectrophotometer. cDNA synthesis was performed using iScript™ Reverse Transcription Supermix for RT-qPCR (Bio-Rad) following manufacturer’s instructions and a Bio-Rad CFXConnectTM Real-Time System.
High-throughput RNA sequencing
Strand-specific cDNA Illumina Barcoded Libraries were generated from 1 μg of total RNA extracted from WT and sir2 Δ/Δ and sequenced with an Illumina iSeq2000 platform. Illumina Library and Deep-sequencing was performed by the Genomics Core Facility at EMBL (Heidelberg, Germany). Raw reads were analysed following the RNA deep sequencing analysis pipeline described56 using Galaxy (https://usegalaxy.org/) and Linux platform. Heatmaps and boxplot graphs were generated with R (http://www.r-project.org/). RNA sequencing data are deposited into ArrayExpress (accession number E-MTAB-4488).
Quantitative Chromatin ImmunoPrecipitation (qChIP)
qChIP was performed as described33 with the following modifications: 5 ml of an overnight culture grown in YPAD with extra uridine (0.08 mg/ml), diluted into fresh YPAD with extra uridine (0.08 mg/ml) and grown until OD600 nm of 1.4. Cells (50 ml/sample) were fixed with 1% Paraformaldehyde (Sigma) for 15 min at room temperature. Cells were lysed using acid-washed glass beads (Sigma) and a Disruptor genieTM (Scientific Industries) for 30 min at 4 °C. Chromatin was sheared to 500–1000 bp using a Bioruptor (Diagenode) for a total of 20 min (30 s ON and OFF cycle) at 4 °C. Immunoprecipitation was performed overnight at 4 °C using 2 μL of antibody anti-H3K4me2 (Active Motif- Cat Number: 39141), anti-H3K9ac (Active Motif- Cat Number: 39137), and anti-H4K16ac (Active Motif- Cat Number: 39167) and 25 μl of Protein G magnetic beads (Dynal - InVitrogen). DNA was eluted with a 10% slurry of Chelex 100-resin (Bio-Rad) using the manufacturer’s instructions.
qPCR reactions
Primers used are listed in Supplementary Table S3. Real-time qPCR and RT-qPCR was performed in the presence of SYBR Green (Bio-Rad) on a Bio-Rad CFXConnectTM Real-Time System. Data were analysed with Bio-Rad CFX Manager 3.1 software and Microsoft Excel. Enrichments were calculated as the percentage ratio of specific IP over input for qChIP analysis and as enrichment over actin for RT-qPCR. Histograms represent data from three biological replicates. Error bars: standard deviation of three biological replicates.
Southern blot
Genomic DNA was extracted using glass acid beads (Sigma), phenol: cholorform: isoamyl alcohol (25:24:1) (Sigma) and RNAse A treated (Fisher). Following centrifugation, pellets were precipitated with 0.05 mM Sodium Acetate (Sigma) and Ethanol (Fisher) at −20 °C during 30 minutes and resuspended in water.Genomic DNA was then digested with corresponding enzymes and run in 1% agarose gel. DNA was transferred to a nylon membrane (Zeta probe membranes, Bio-Rad), probed with DIG probes (Roche) and hybridized as described57.
Microscopy
Microscopy was carried out using an Olympus IX81 inverted microscope. Images were captured with a Hamamatsu photonics C4742 digital camera, with light excitation from an Olympus MT20 illumination system. Olympus CellR imaging software was used to control the apparatus.
Additional Information
How to cite this article: Freire-Benéitez, V. et al. Candida albicans repetitive elements display epigenetic diversity and plasticity. Sci. Rep. 6, 22989; doi: 10.1038/srep22989 (2016).
Supplementary Material
Acknowledgments
We thank R. Allshire, A. Mitchell and A. Brown for reagents, strains and materials and A. Pidoux for critical reading of the manuscript. We thank the Gene Core Facility at EMBL (Heidelberg-Germany) for RNA Sequencing and M. Wass for support with the RNA Seq analyses. This work was supported by BBSRC (BB/L008041/1 to A.B and D.T), MRC (MR/M019713/1 to A.B. and R.J.P.), a Royal Society International Exchange Scheme (IE131376) grant (to A.B. and J.B.), a Royal Society Research Grant (RG130149) (to A.B.) and the NIAID R01 AI075096 (to J.B.), the People Programme (Marie Curie Actions) and the European Union’s Seventh Framework Programme (FP7/2007-2013) under REA grant agreement number 303635 and an ERC Advanced Award, number 340087, RAPLODAPT (to J.B.).
Footnotes
Author Contributions A.B., J.B. and V.F.B. conceived and designed the experiments. V.F.B., R.J.P. and D.T. conducted the experiments. A.B., V.F.B., R.J.P. and D.T. analysed the results. A.B. and J.B. wrote the manuscript.
References
- Rusche L. N., Kirchmaier A. L. & Rine J. The establishment, inheritance, and function of silenced chromatin in Saccharomyces cerevisiae. Annu. Rev. Biochem. 72, 481–516 (2003). [DOI] [PubMed] [Google Scholar]
- Bühler M. & Gasser S. M. Silent chromatin at the middle and ends: lessons from yeasts. EMBO J. 28, 2149–61 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saksouk N., Simboeck E. & Déjardin J. Constitutive heterochromatin formation and transcription in mammals. Epigenetics Chromatin 8, 3 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadosh D. Shaping Up for Battle: Morphological Control Mechanisms in Human Fungal Pathogens. PLoS Pathog. 9, 1–4 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouzarides T. Chromatin Modifications and Their Function. Cell 128, 693–705 (2007). [DOI] [PubMed] [Google Scholar]
- Strahl B. D. & Allis C. D. The language of covalent histone modifications. Nature 403, 41–45 (2000). [DOI] [PubMed] [Google Scholar]
- Kueng S., Oppikofer M. & Gasser S. M. SIR proteins and the assembly of silent chromatin in budding yeast. Annu. Rev. Genet. 47, 275–306 (2013). [DOI] [PubMed] [Google Scholar]
- Shankaranarayana G. D. et al. Sir2 Regulates Histone H3 Lysine 9 Methylation and Heterochromatin Assembly in Fission Yeast. Curr. Biol. 13, 1240–1246 (2003). [DOI] [PubMed] [Google Scholar]
- Nakayama J., Rice J. C., Strahl B. D., Allis C. D. & Grewal S. I. Role of histone H3 lysine 9 methylation in epigenetic control of heterochromatin assembly. Science 292, 110–3 (2001). [DOI] [PubMed] [Google Scholar]
- Rea S. et al. Regulation of chromatin structure by site-specific histone H3 methyltransferases. Nature 406, 593–9 (2000). [DOI] [PubMed] [Google Scholar]
- Froyd C. a., Kapoor S., Dietrich F. & Rusche L. N. The deacetylase Sir2 from the yeast Clavispora lusitaniae lacks the evolutionarily conserved capacity to generate subtelomeric heterochromatin. PLoS Genet. 9, e1003935 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J. & Moazed D. Association of the RENT complex with nontranscribed and coding regions of rDNA and a regional requirement for the replication fork block protein Fob1 in rDNA silencing. Genes Dev. 17, 2162–76 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straight a. F. et al. Net1, a Sir2-associated nucleolar protein required for rDNA silencing and nucleolar integrity. Cell 97, 245–56 (1999). [DOI] [PubMed] [Google Scholar]
- Shou W. et al. Exit from mitosis is triggered by Tem1-dependent release of the protein phosphatase Cdc14 from nucleolar RENT complex. Cell 97, 233–44 (1999). [DOI] [PubMed] [Google Scholar]
- Pfaller M. A. & Diekema D. J. Epidemiology of invasive mycoses in North America. Crit. Rev. Microbiol. 36, 1–53 (2010). [DOI] [PubMed] [Google Scholar]
- Selmecki A., Forche A. & Berman J. Genomic plasticity of the human fungal pathogen Candida albicans. Eukaryot. Cell 9, 991–1008 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones T. et al. The diploid genome sequence of Candida albicans. Proc. Natl. Acad. Sci. USA 101, 7329–34 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van het Hoog M. et al. Assembly of the Candida albicans genome into sixteen supercontigs aligned on the eight chromosomes. Genome Biol. 8, R52 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi T. & Ganley A. R. D. Recombination regulation by transcription-induced cohesin dissociation in rDNA repeats. Science 309, 1581–4 (2005). [DOI] [PubMed] [Google Scholar]
- Li C., Mueller J. E. & Bryk M. Sir2 repressess endogenous polymerase II transcription units in the ribosomal DNA nontranscribed spacer Mol Biol Cell. 17, 3848–3859 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chibana H. et al. Diversity of tandemly repetitive sequences due to short periodic repetitions in the chromosomes of Candida albicans. J. Bacteriol. 176, 3851–8 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chibana H. & Magee P. T. The enigma of the major repeat sequence of Candida albicans. Future Microbiol. 4, 171–9 (2009). [DOI] [PubMed] [Google Scholar]
- McEachern M. J. & Hicks J. B. Unusually large telomeric repeats in the yeast Candida albicans. Mol. Cell. Biol. 13, 551–60 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Martín J., Uría J. & Johnson A. D. Phenotypic switching in Candida albicans is controlled by a SIR2 gene. EMBO J. 18, 2580–92 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henikoff S. & Dreesen T. D. Trans-inactivation of the Drosophila brown gene: evidence for transcriptional repression and somatic pairing dependence. Proc. Natl. Acad. Sci. USA 86, 6704–8 (1989). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottschling D. E., Aparicio O. M., Billington B. L. & Zakian V. A. Position effect at S. cerevisiae telomeres: reversible repression of Pol II transcription. Cell 63, 751–62 (1990). [DOI] [PubMed] [Google Scholar]
- Bryk M. et al. Transcriptional silencing of Ty1 elements in the RDN1 locus of yeast. Genes Dev. 11, 255–69 (1997). [DOI] [PubMed] [Google Scholar]
- Smith J. S. & Boeke J. D. An unusual form of transcriptional silencing in yeast ribosomal DNA. Genes Dev. 11, 241–254 (1997). [DOI] [PubMed] [Google Scholar]
- Greiss S. & Gartner A. Sirtuin/Sir2 phylogeny, evolutionary considerations and structural conservation. Mol. Cells 28, 407–15 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasiljeva L., Kim M., Terzi N., Soares L. M. & Buratowski S. Transcription Termination and RNA Degradation Contribute to Silencing of RNA Polymerase II Transcription within Heterochromatin. Mol. Cell 29, 313–323 (2008). [DOI] [PubMed] [Google Scholar]
- Kobayashi T. A new role of the rDNA and nucleolus in the nucleus - RDNA instability maintains genome integrity. BioEssays 30, 267–272 (2008). [DOI] [PubMed] [Google Scholar]
- Bruno V. M. et al. Comprehensive annotation of the transcriptome of the human fungal pathogen Candida albicans using RNA-seq. Genome Res. 20, 1451–1458 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pidoux A., Mellone B. & Allshire R. Analysis of chromatin in fission yeast. Methods 33, 252–259 (2004). [DOI] [PubMed] [Google Scholar]
- Imai S., Armstrong C. M., Kaeberlein M. & Guarente L. Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone deacetylase. Nature 403, 795–800 (2000). [DOI] [PubMed] [Google Scholar]
- Tanny J. C. & Moazed D. Coupling of histone deacetylation to NAD breakdown by the yeast silencing protein Sir2: Evidence for acetyl transfer from substrate to an NAD breakdown product. Proc. Natl. Acad. Sci. USA 98, 415–420 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen K. R., Ibarra P. T. & Thon G. Evolutionary-conserved telomere-linked helicase genes of fission yeast are repressed by silencing factors, RNAi components and the telomere-binding protein Taz1. Nucleic Acids Res. 34, 78–88 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freitas-Junior L. H. et al. Telomeric heterochromatin propagation and histone acetylation control mutually exclusive expression of antigenic variation genes in malaria parasites. Cell 121, 25–36 (2005). [DOI] [PubMed] [Google Scholar]
- Merrick C. J. & Duraisingh M. T. Heterochromatin-mediated control of virulence gene expression. Mol. Microbiol. 62, 612–20 (2006). [DOI] [PubMed] [Google Scholar]
- Kaur R., Domergue R., Zupancic M. L. & Cormack B. P. A yeast by any other name: Candida glabrata and its interaction with the host. Curr. Opin. Microbiol. 8, 378–384 (2005). [DOI] [PubMed] [Google Scholar]
- Huang J., et al. Inhibition of homologous recombination by a cohesin-associated clamp complex recruited to the rDNA recombination enhancer. Genes Dev. 20, 2887–2901 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubarry M., Loïodice I., Chen C. L., Thermes C. & Taddei A. Tight protein-DNA interactions favor gene silencing. Genes Dev. 25, 1365–70 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett R. J. The parasexual lifestyle of Candida albicans. Curr. Opin. Microbiol. 28, 10–17 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu W. S., Magee B. B. & Magee P. T. Construction of an SfiI macrorestriction map of the Candida albicans genome. J. Bacteriol. 175, 6637–51 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwaguchi S. I. et al. Extensive chromosome translocation in a clinical isolate showing the distinctive carbohydrate assimilation profile from a candidiasis patient. Yeast 18, 1035–46 (2001). [DOI] [PubMed] [Google Scholar]
- Lephart P. R., Chibana H. & Magee P. T. Effect of the Major Repeat Sequence on Chromosome Loss in Candida albicans. Eukaryot Cell. 4, 733–41 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lephart P. R. & Magee P. T. Effect of the major repeat sequence on mitotic recombination in Candida albicans. Genetics 174, 1737–44 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellahi A., Thurtle D. M. & Rine J. The Chromatin and Transcriptional Landscape of Native Saccharomyces cerevisiae Telomeres and Subtelomeric Domains. Genetics 200, 505–21 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumesic P. A. et al. Product binding enforces the genomic specificity of a yeast polycomb repressive complex. Cell 160, 204–18 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haran J. et al. Telomeric ORFs (TLOs) in Candida spp. Encode Mediator Subunits That Regulate Distinct Virulence Traits. PLoS Genet. 10, e1004658 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendland J., Schaub Y. & Walther A. N-acetylglucosamine utilization by Saccharomyces cerevisiae based on expression of Candida albicans NAG genes. Appl. Environ. Microbiol. 75, 5840–5 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi X., Yu Q., Sandmeier J. J. & Elizondo S. Regulation of transcriptional silencing in yeast by growth temperature. J. Mol. Biol. 344, 893–905 (2004). [DOI] [PubMed] [Google Scholar]
- Paschini M. et al. A naturally thermolabile activity compromises genetic analysis of telomere function in Saccharomyces cerevisiae. Genetics 191, 79–93 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morano K. a., Grant C. M. & Moye-Rowley W. S. The response to heat shock and oxidative stress in Saccharomyces cerevisiae. Genetics 190, 1157–1195 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson R. B., Davis D. & Mitchell A. P. Rapid Hypothesis Testing with Candida albicans through Gene Disruption with Short Homology Regions. J. Bacteriol 181, 1868–74 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Backer M. D. et al. Transformation of Candida albicans by electroporation. Yeast 15, 1609–18 (1999). [DOI] [PubMed] [Google Scholar]
- Trapnell C. et al. Differential analysis of gene regulation at transcript resolution with RNA-seq. Nat. Biotechnol. 31, 46–53 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketel C. et al. Neocentromeres form efficiently at multiple possible loci in Candida albicans. PLoS Genet. 5, e1000400 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.