Abstract
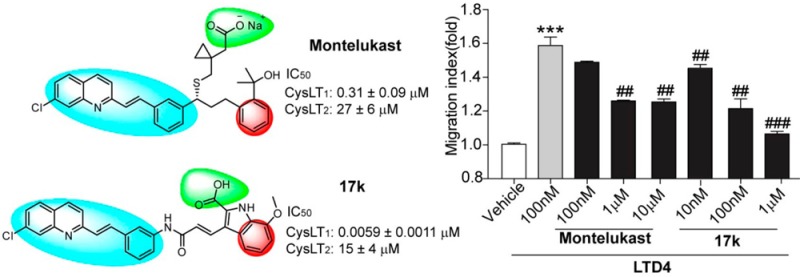
The indole derivative, 3-((E)-3-((3-((E)-2-(7-chloroquinolin-2yl)vinyl)phenyl)amino)-3-oxoprop-1-en-1-yl)-7-methoxy-1H-indole-2-carboxylic acid (17k), was identified as a novel and highly potent and selective CysLT1 antagonist with IC50 values of 0.0059 ± 0.0011 and 15 ± 4 μM for CysLT1 and CysLT2, respectively.
Keywords: Cysteinyl leukotrienes, CysLT1, CysLT2, selective antagonists, asthma
Cysteinyl-leukotrienes (CysLTs) are potent inflammatory lipid mediators synthesized from arachidonic acid.1,2 CysLTs include leukotriene C4 (LTC4), leukotriene D4 (LTD4), and leukotriene E4 (LTE4). They play established or evolving roles in asthma, allergic rhinitis, and other inflammatory conditions, such as cardiovascular diseases, cancer, and atopic dermatitis.3 CysLTs activate at least two receptors, designated as CysLT14 and CysLT2,5 which belong to the G protein-coupled receptor (GPCR) superfamily.
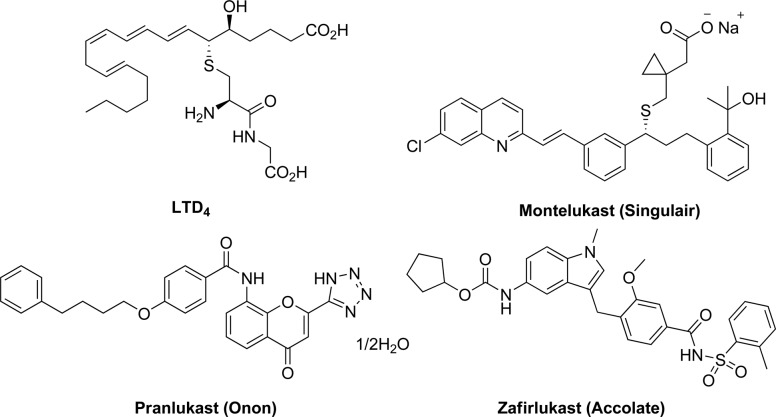
CysLT1 receptors are mainly expressed in the lung, peripheral blood leukocytes, and the spleen.4 Most of the pathophysiological effects of CysLTs in asthma are mediated by the CysLT1 receptor.6 Several CysLT1 selective antagonists have been launched for treating bronchial asthma and allergic rhinitis, such as montelukast, pranlukast, and zafirlukast (Figure 1).6
Figure 1.
LTD4 and launched drugs selectively targeting CysLT1.
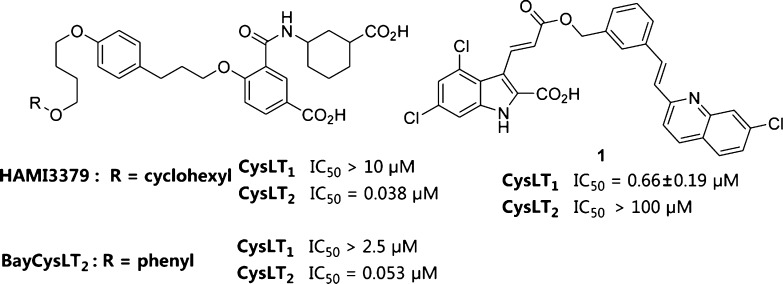
CysLT2 receptors are highly expressed in the peripheral blood leukocytes, spleen, and lymph nodes and are uniquely expressed in the heart, brain, and adrenal glands.5 Recently, Sekioka et al. reported the expression of CysLT2 receptors in asthmatic lungs and investigated their possible role in bronchoconstriction.7 However, the pharmacological roles of CysLT2 are less well-defined, and there is no specific antagonist being marketed as a therapeutic agent so far.6 Wunder et al. reported the identification of the first potent and selective CysLT2 antagonist, HAMI3379 (Figure 2), which was shown to inhibit the cardiovascular effects mainly through mediation of CysLT2 receptors.8 Meanwhile, a highly similar compound, BayCysLT2 (Figure 2), was also reported to be a potent and selective CysLT2 antagonist.9
Figure 2.
Selective CysLT receptor antagonists.
Although the marketed CysLT1 selective antagonists are effective therapeutics for the general treatment of mild to moderate bronchial asthma, it is known that the current CysLT1 antagonists do not have sufficient effects for some nonresponsive patients. A recent report demonstrated that CysLT1 may also play a role in multiple sclerosis; blocking this receptor protects the integrity of the blood–brain barrier and reduces infiltration of pathogenic T cells into the central nervous system.10 More recently, Ludwig et al. reported that antiasthmatic drug montelukast, which antagonizes CysLT1, reduces neuroinflammation, promotes hippocampal neurogenesis, and improves learning and memory in old animals.11 Therefore, the development of new CysLT antagonists is still of great interest.
A high-throughput screening (HTS) campaign of our compound library yielded indole derivative 1, which showed micromolar CysLT1 antagonist activity with an IC50 value of 0.66 ± 0.19 μM and exhibited no CysLT2 antagonist activity (Figure 2). Compound 1 shares same hydrophobic (E)-3-(2-(7-chloroquinolin-2-yl)vinyl)phenyl group as montelukast, but it has a unique and essential indole-2-carboxylic acid moiety, which is different from the corresponding counterparts of known CysLT1 antagonists. The structural novelty of compound 1 encouraged us to further optimize and develop more potent CysLT1 antagonists.
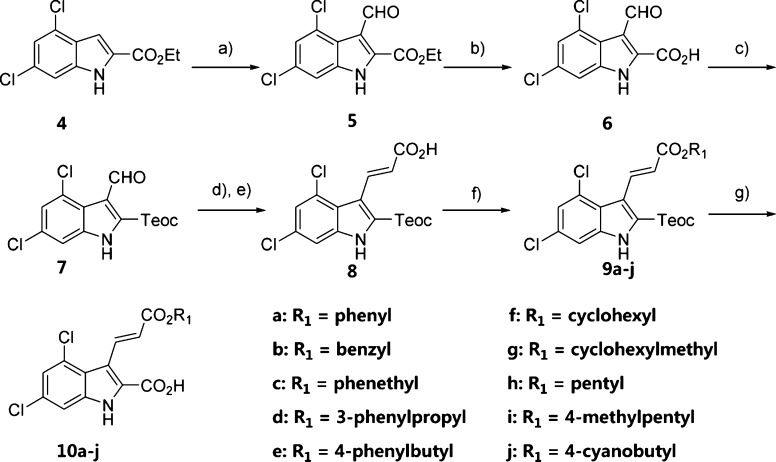
First, we investigated the effects of the ester groups and designed compounds 10a–10j. The syntheses of compounds 10a–10j are described in Scheme 1. Ethyl-4,6-dichloro-1H-indole-2-carboxylate 4 was prepared from 3,5-dichloroaniline in a two-step synthetic procedure using a Japp–Klingemann condensation followed by a Fischer indole (aza-Cope) ring closure reaction. Vilsmeier–Haack formylation of 4 afforded aldehyde 5.12 Subsequent ester hydrolysis of 5 afforded carboxylic acid 6, which upon esterification afforded 7. Compound 7 was reacted with tert-butyl(triphenylphosphoranylidene)acetate in a Horner–Wadsworth–Emmons reaction followed by the chemoselective deprotection of the tert-butyl ester group, which yielded the α,β-unsaturated acid intermediate 8.13 Esterification of 8 generated compounds 9a–9j, which were then converted to compounds 10a–10j using TBAF in THF.
Scheme 1. Syntheses of Compounds 10a–10j.
Reagents and conditions: (a) DMF, POCl3, DCE, reflux, 7 h; (b) EtOH, LiOH·H2O, 50 °C, 2 h; (c) 2-(trimethylsilyl)ethan-1-ol, EDCI, DMAP, DCE, rt, overnight; (d) tert-butyl (triphenylphosphoranylidene)acetate, toluene, reflux, overnight; (e) 98% HCOOH, rt, overnight; (f) R1Br, Cs2CO3, DMF or R1OH, DCC/EDCI, DMAP, DMF; (g) TBAF, THF, rt, 2 h.
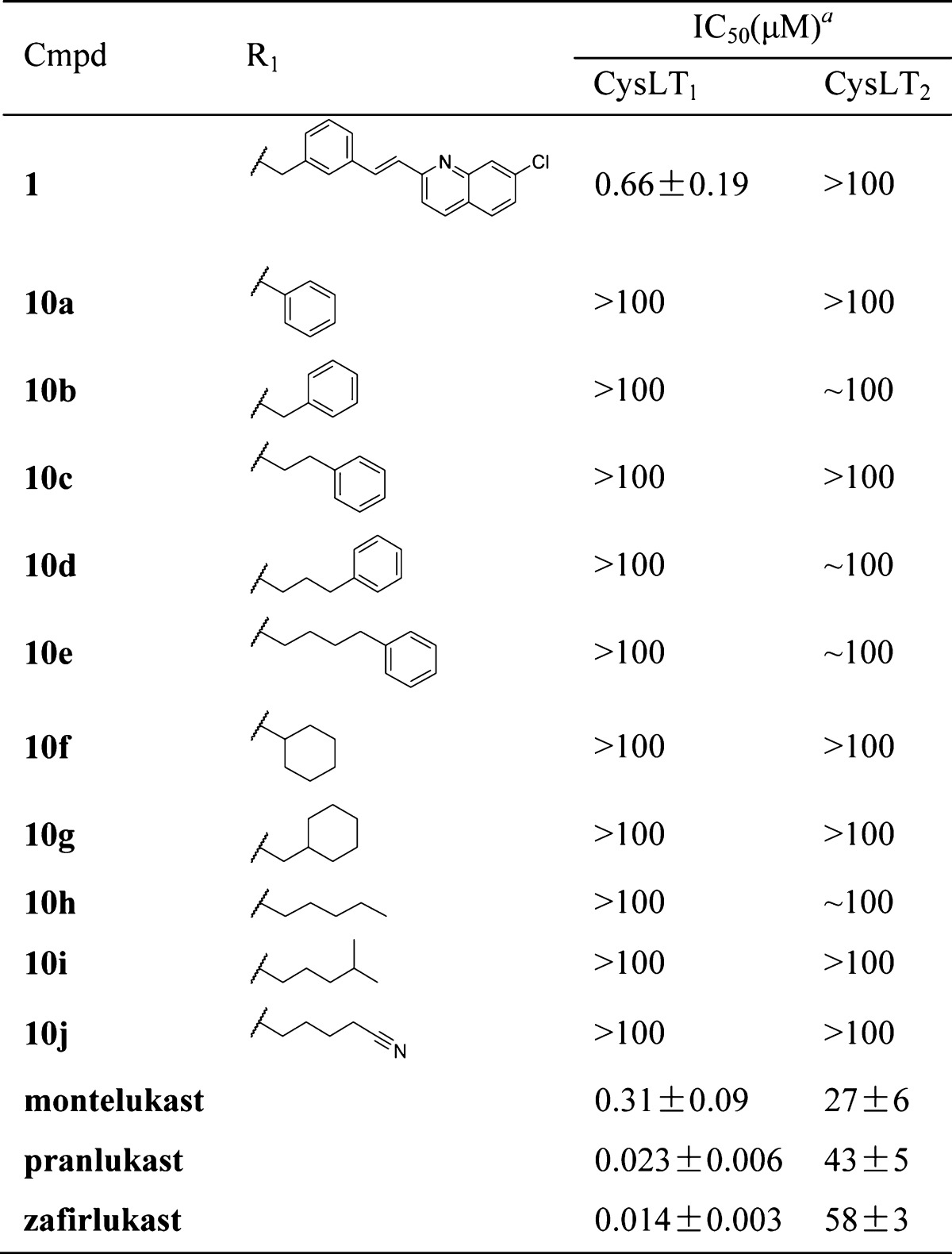
As shown in Table 1, the results revealed that the (E)-3-(2-(7-chloroquinolin-2-yl)vinyl)phenyl group was necessary for the antagonist activity of the compounds; replacing it with other ester groups eliminated antagonist activity against both CysLT1 and CysLT2. Therefore, we attempted to modify the functional groups at other positions of hit compound 1 while retaining the (E)-3-(2-(7-chloroquinolin-2-yl)vinyl)phenyl group to increase its antagonist activity against CysLT1.
Table 1. Effects of Ester Groups on Activity Profiles.
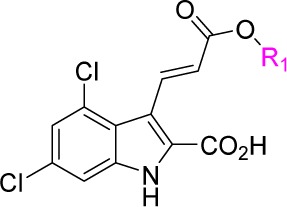
Assay protocols are provided in the Supporting Information. IC50 values were obtained from one experiment with three replicates.
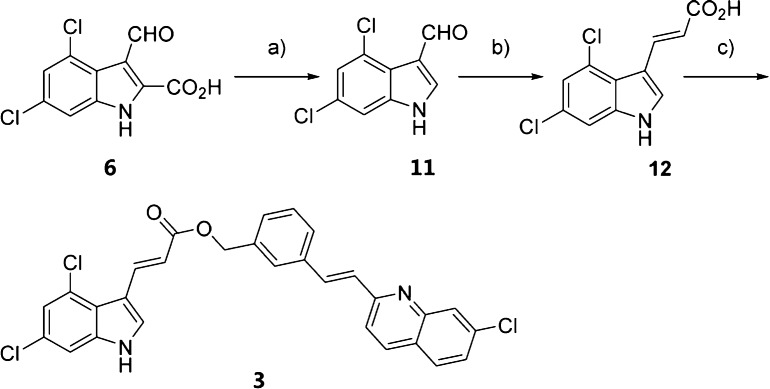
The chlorine atoms in the indole ring of compound 1 were removed, and proposed compound 2 (Table 2) was synthesized following the previously described method. We removed the carboxylic acid group of compound 1 and yielded compound 3, which was synthesized following the method shown in Scheme 2. Decarboxylation of 6 provided aldehyde 11,14 which was subsequently converted to 12 using a Doebner–Knoevenagel modification reaction;15 finally, esterification of 12 provided 3.
Table 2. Activity Profiles of Indole Derivatives.
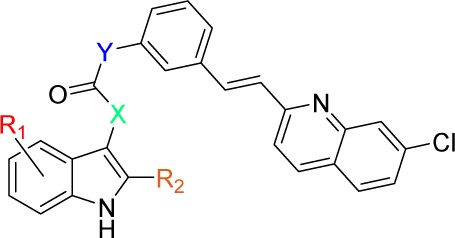
| IC50 (μM)a |
||||||
|---|---|---|---|---|---|---|
| Cmpd | R1 | R2 | X | Y | CysLT1 | CysLT2 |
| 1 | 4,6-diCl | –COOH | –CH=CH– | –OCH2– | 0.66 ± 0.19 | >100 |
| 2 | H | –COOH | –CH=CH– | –OCH2– | 0.12 ± 0.02 | >100 |
| 3 | 4,6-diCl | H | –CH=CH– | –OCH2– | 31 ± 13 | >100 |
| 17a | 4,6-diCl | –COOH | –CH=CH– | –NH– | 0.29 ± 0.14 | >100 |
| 17b | H | –COOH | –CH=CH– | –NH– | 0.0090 ± 0.0043 | 58 ± 26 |
| 19b | H | –COOH | –NH– | 0.035 ± 0.005 | 23 ± 1 | |
| 21b | H | –COOH | –CH2CH2– | –NH– | 0.058 ± 0.014 | 50 ± 18 |
Assay protocols are provided in the Supporting Information. IC50 values were obtained from one experiment with three replicates.
Scheme 2. Synthesis of 3.
Reagents and conditions: (a) CuCl, quinoline, microwave, 200 °C, 10 min; (b) malonic acid, pyridine, piperidine, 50 °C, 12 h; (c) (E)-2-(3-(bromomethyl)styryl)-7-chloroquinoline, Cs2CO3, DMF, rt, overnight.
In order to replace the ester bond of compound 1 with an amide bond, we intended to synthesize (E)-3-(2-(7-chloroquinolin-2-yl)vinyl)benzylamine; unfortunately, we failed to obtain the intermediate after several synthetic attempt. Considering the easy availability of (E)-3-(2-(7-chloroquinolin-2-yl)vinyl)aniline, compound 17a was prepared (Scheme 3). As shown in Table 2, compounds 2 and 17a exhibited slightly improved antagonist activities against CysLT1 compared to hit compound 1, which suggested that derivatives with no substituents in the indole ring were better than derivatives with chlorine atoms and that amide bonds were superior to ester bonds in the internal chain. However, compound 3, which lacked the indole-2-carboxylic acid moiety, was approximately 47-fold less potent than hit compound 1; this demonstrated that the carboxylic acid group at position 2 of the indole ring was necessary. These results further supported the common features of CysLT1 antagonists, namely, they all contained a lipophilic region, which incorporates into the lipophilic pocket of the CysLT1 receptor and an acidic moiety modeling the C1-carboxylic acid of LTD4.16−18 Furthermore, these results were in accordance with the essential structural elements for CysLT1 receptor ligands.16,17,19 The activity results of compounds in Table 1 and compound 3 demonstrated that the indole ring, the carboxylic acid function, and the (E)-3-(2-(7-chloroquinolin-2-yl)vinyl)phenyl group were the three necessary pharmocophores for the novel series, the activity results of compounds 2 and 17a revealed that removal of the chlorine atoms in the indole ring and replacement of the ester bond with the amide function were favorable for improvement of the potency; therefore, compound 17b (Scheme 3) was suggested based on the structure–activity relationships (SARs) in the present study. Satisfactorily, 17b demonstrated significantly better antagonist activity against CysLT1 than hit compound 1, and its antagonist activity against CysLT2 remained very low. Subsequently, to explore the effects of the α, β-unsaturated double bond at position 3 of the indole ring of 17b on activity profiles, 17b was modified leading to 19b and 21b (Scheme 3). As shown in Table 2, 19b and 21b were approximately 4- and 6-fold less potent against CysLT1 than 17b, and they demonstrated stronger antagonist activities against CysLT2 than 17b, which revealed that the importance of the α, β-unsaturated double bond.
Scheme 3. Syntheses of 17a–17k, 19b, and 21b.
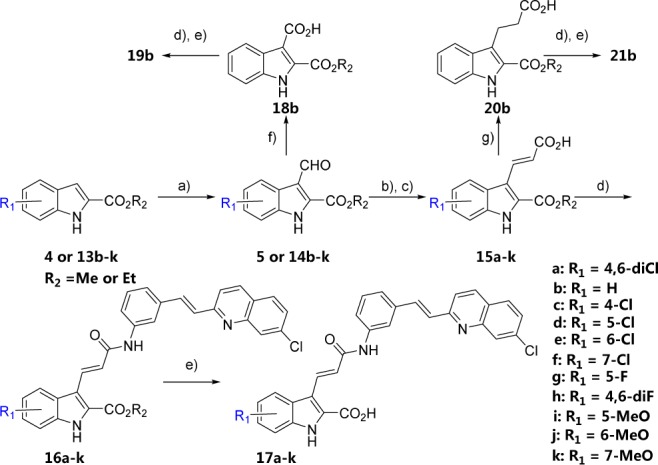
Reagents and conditions: (a) DMF, POCl3, DCE, reflux, 7 h; (b) tert-butyl (triphenylphosphoranylidene)acetate, toluene, reflux, overnight; (c) TFA, DCM, rt, 3 h; (d) (E)-3-(2-(7-chloroquinolin-2-yl)vinyl)aniline, HATU, DIPEA, DMF, rt, overnight; (e) NaOH aq, EtOH, 50 °C, overnight; (f) 30% H2O2, NaClO2, NaH2PO4·2H2O, CH3CN, rt, overnight; (g) Pd/C, H2, EtOH-THF, rt, 7 h.
Finally, we investigated the effects of the substituents of the indole ring on the activity profiles with compounds 17c–17k. The syntheses of 17a–17k, 19b, and 21b are described in Scheme 3. Vilsmeier–Haack formylation of 4 and 13b–13k afforded aldehydes 5 and 14b–14k. The Wittig-type olefination of the latter compounds and the subsequent deprotection of the tert-butyl ester group with TFA afforded compounds 15a–15k. Oxidation of 14b afforded 18b. Reduction of 15b by catalytic hydrogenation afforded 20b. Condensation of 15a–15k, 18b, and 20b with (E)-3-(2-(7-chloroquinolin-2-yl)vinyl)aniline and subsequent hydrolysis provided compounds 17a–17k, 19b, and 21b, respectively.
As shown in Table 3, the fluorine substituted derivatives were more potent than the chlorine substituted derivatives (17d vs 17g and 17h vs 17a (Table 2)); 17c, 17d, 17e, and 17f demonstrated that substitution at position 4 of the indole ring was the least favorable. Moreover, the methoxy group substituted derivatives (17i, 17j, and 17k) exhibited substitution at position 7 of the indole ring was the most favorable. Among these derivatives, compounds 17b, 17g, and 17k showed comparable low nanomolar level potencies against CysLT1, while some derivatives exhibited weak (17b, 17g, and 17k) to no (17j) CysLT2 antagonist activities.
Table 3. Effects of the Substituents of the Indole Ring on Activity Profiles.
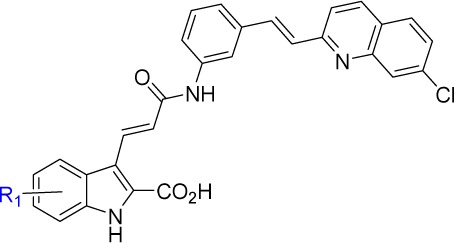
| IC50 (μM)a |
|||
|---|---|---|---|
| Cmpd | R1 | CysLT1 | CysLT2 |
| 17b | H | 0.0090 ± 0.0043 | 58 ± 26 |
| 17c | 4-Cl | 0.067 ± 0.022 | 36 ± 13 |
| 17d | 5-Cl | 0.017 ± 0.002 | >100 |
| 17e | 6-Cl | 0.022 ± 0.002 | 87 ± 17 |
| 17f | 7-Cl | 0.016 ± 0.004 | >100 |
| 17g | 5-F | 0.0078 ± 0.0013 | 46 ± 20 |
| 17h | 4,6-diF | 0.038 ± 0.001 | 76 ± 14 |
| 17i | 5-MeO | 0.025 ± 0.007 | 46 ± 7 |
| 17j | 6-MeO | 0.012 ± 0.007 | >100 |
| 17k | 7-MeO | 0.0059 ± 0.0011 | 15 ± 4 |
Assay protocols are provided in the Supporting Information. IC50 values were obtained from one experiment with three replicates.
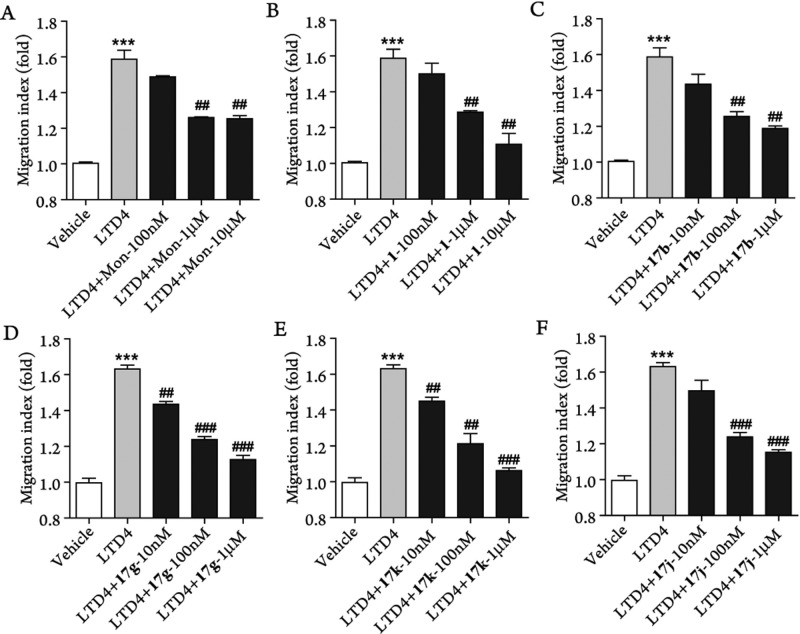
CysLTs have been reported to induce chemotactic activity in eosinophils20 and monocytes.21 We previously found that LTD4 could also induce chemotaxis of splenocytes isolated from mice with experimental autoimmune encephalomyelitis (EAE), an animal model of multiple sclerosis,10 and this effect could be blocked by montelukast.10 We then tested compounds 1, 17b, 17g, 17j, and 17k with the chemotaxis assay. We found that these compounds could also block LTD4-induced chemotaxis of leukocytes isolated from the spleen of EAE-mice in a dose-dependent manner (Figure 3). Compound 1 exhibited similar activity as montelukast (Figure 3A,B), and both compounds displayed ∼50% inhibition of chemotaxis at concentrations of 1 μM. Compounds 17b, 17g, 17k, and 17j showed much better inhibitory effects, and all these compounds displayed ≥50% inhibition at concentrations of 100 nM (Figure 3C–F). In particular, compounds 17g and 17k, which displayed best antagonist activity in calcium assay (Table 3) showed significant inhibition of chemotaxis at 10 nM concentration (Figure 3D,E).
Figure 3.
Selective CysLT1 antagonists inhibit leukocyte chemotaxis induced by LTD4. Data are from three independent experiments (means ± SEM). ***p < 0.001, versus vehicle control; ##p < 0.01, ###p < 0.001 versus LTD4 (100 nM) treatment group (Student t test).
In summary, we have discovered a novel class of selective CysLT1 antagonists. Our results indicated that it is essential that such antagonists possess (E)-3-(2-(7-chloroquinolin-2-yl)vinyl)phenyl and indole-2-carboxylic acid moieties for effective CysLT1 antagonist activity. Additionally, α, β-unsaturated amide moieties at position 3 of the indole rings were also important factors. The most potent compound (17k, IC50 value of 0.0059 ± 0.0011 μM (CysLT1)) demonstrated significantly more potent CysLT1 antagonist activity than the known selective CysLT1 antagonist, montelukast, both in calcium mobilization assay and chemotaxis assay. The further optimization and development including the pharmacokinetic profile of the most potent antagonists and their pharmacological effects evaluated in relevant animal models are in progress in our lab.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsmedchemlett.5b00482.
Synthetic procedures, analytic data, procedures for the biological assays, and NMR spectras of the key compounds (PDF)
Author Contributions
§ These authors (H.C. and H.Y.) contributed equally to this work.
This work was supported by grants from the Ministry of Science and Technology of China (2015CB964503) and the National Natural Science Foundation of China (81425024).
The authors declare no competing financial interest.
The name of compound 17k was corrected in the abstract text in the version published ASAP January 27, 2016; the corrected version was published ASAP February 12, 2016. The version published ASAP on February 12, 2016 had an error in Table 1; the corrected version was published ASAP on February 15, 2016.
Supplementary Material
References
- Bäck M. Functional characteristics of cysteinyl-leukotriene receptor subtypes. Life Sci. 2002, 71, 611–622. 10.1016/S0024-3205(02)01733-2. [DOI] [PubMed] [Google Scholar]
- Capra V.; Thompson M. D.; Sala A.; Cole D. E.; Folco G.; Rovati G. E. Cysteinyl-leukotrienes and their receptors in asthma and other inflammatory diseases: critical update and emerging trends. Med. Res. Rev. 2007, 27, 469–572. 10.1002/med.20071. [DOI] [PubMed] [Google Scholar]
- Peters-Golden M.; William R.; Henderson J. Mechanisms of disease Leukotrienes. N. Engl. J. Med. 2007, 357, 1841–1852. 10.1056/NEJMra071371. [DOI] [PubMed] [Google Scholar]
- Lynch K. R.; O’Neill G. P.; Liu Q.; Im D. S.; Sawyer N.; Metters K. M.; Coulombe N.; Abramovitz M.; Figueroa D. J.; Zeng Z.; Conolly B. M.; Bai C.; Austin C. P.; Chateauneuf A.; Stocco R.; Greig G. M.; Kargman S.; Hooks S. B.; Hosfield E.; Williams D. L. Jr.; Ford-Hutchinson A. W.; Caskey C. T.; Evans J. F. Characterization of the human cysteinyl leukotriene CysLT1 receptor. Nature 1999, 399, 789–793. 10.1038/21658. [DOI] [PubMed] [Google Scholar]
- Heise C. E.; O’Dowd B. F.; Figueroa D. J.; Sawyer N.; Nguyen T.; Im D.-S.; Stocco R.; Bellefeuille J. N.; Abramovitz M.; Cheng R.; Williams D. L. Jr.; Zeng Z.; Liu Q.; Ma L.; Clements M. K.; Coulombe N.; Liu Y.; Austin C. P.; George S. R.; O’Neill G. P.; Metters K. M.; Lynch K. R.; Evans J. F. Characterization of the human cysteinyl leukotriene 2 receptor. J. Biol. Chem. 2000, 275, 30531–30536. 10.1074/jbc.M003490200. [DOI] [PubMed] [Google Scholar]
- Singh R. K.; Tandon R.; Dastidar S. G.; Ray A. A review on leukotrienes and their receptors with reference to asthma. J. Asthma 2013, 50, 922–931. 10.3109/02770903.2013.823447. [DOI] [PubMed] [Google Scholar]
- Sekioka T.; Kadode M.; Fujii M.; Kawabata K.; Abe T.; Horiba M.; Kohno S.; Nabe T. Expression of CysLT2 receptors in asthma lung and their possible role in bronchoconstriction. Allergol. Int. 2015, 64, 351–358. 10.1016/j.alit.2015.04.008. [DOI] [PubMed] [Google Scholar]
- Wunder F.; Tinel H.; Kast R.; Geerts A.; Becker E. M.; Kolkhof P.; Hütter J.; Ergüden J.; Härter M. Pharmacological characterization of the first potent and selective antagonist at the cysteinyl leukotriene 2 (CysLT2) receptor. Br. J. Pharmacol. 2010, 160, 399–409. 10.1111/j.1476-5381.2010.00730.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni N. C.; Yan D.; Ballantyne L. L.; Barajas-Espinosa A.; Amand T. St.; Pratt D. A.; Funk C. D. A selective cysteinyl leukotriene receptor 2 antagonist blocks myocardial ischemia/reperfusion injury and vascular permeability in mice. J. Pharmacol. Exp. Ther. 2011, 339, 768–778. 10.1124/jpet.111.186031. [DOI] [PubMed] [Google Scholar]
- Wang L.; Du C.; Lv J.; Wei W.; Cui Y.; Xie X. Antiasthmatic drugs targeting the cysteinyl leukotriene receptor 1 alleviate central nervous system inflammatory cell infiltration and pathogenesis of experimental autoimmune encephalomyelitis. J. Immunol. 2011, 187, 2336–2345. 10.4049/jimmunol.1100333. [DOI] [PubMed] [Google Scholar]
- Marschallinger J.; Schäffner I.; Klein B.; Gelfert R.; Rivera F. J.; Illes S.; Grassner L.; Janssen M.; Rotheneichner P.; Schmuckermair C.; Coras R.; Boccazzi M.; Chishty M.; Lagler F. B.; Renic M.; Bauer H.-C.; Singewald N.; Blümcke I.; Bogdahn U.; Couillard-Despres S.; Lie D. C.; Abbracchio M. P.; Aigner L. Structural and functional rejuvenation of the aged brain by an approved anti-asthmatic drug. Nat. Commun. 2015, 6, 8466. 10.1038/ncomms9466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kose M.; Ritter K.; Thiemke K.; Gillard M.; Kostenis E.; Muller C. E. Development of [3H]2-Carboxy-4,6-dichloro-1H-indole-3-propionic Acid ([3H]PSB-12150): A Useful Tool for Studying GPR17. ACS Med. Chem. Lett. 2014, 5, 326–30. 10.1021/ml400399f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Fabio R.; Capelli A. M.; Conti N.; Cugola A.; Donati D.; Feriani A.; Gastaldi P.; Gaviraghi G.; Hewkin C. T.; Micheli F.; Missio A.; Mugnaini M.; Pecunioso A.; Quaglia A. M.; Ratti E.; Rossi L.; Tedesco G.; Trist D. G.; Reggiani A. Substituted indole-2-carboxylates as in vivo potent antagonists acting as the strychnine-insensitive glycine binding site. J. Med. Chem. 1997, 40, 841–850. 10.1021/jm960644a. [DOI] [PubMed] [Google Scholar]
- Jones G. B.; Chapman B. J. Decarboxylation of indole-2-carboxylic acids: improved procedures. J. Org. Chem. 1993, 58, 5558–5559. 10.1021/jo00072a052. [DOI] [Google Scholar]
- Xu Y. Y.; Li S. N.; Yu G. J.; Hu Q. H.; Li H. Q. Discovery of novel 4-anilinoquinazoline derivatives as potent inhibitors of epidermal growth factor receptor with antitumor activity. Bioorg. Med. Chem. 2013, 21, 6084–6091. 10.1016/j.bmc.2013.06.070. [DOI] [PubMed] [Google Scholar]
- Zwaagstra M. l. E.; Schoenmakers S. H. H. F.; Nederkoorn P. H. J.; Gelens E.; Timmerman H.; Zhang M.-Q. Development of a three-dimensional CysLT1 (LTD4) antagonist model with an incorporated amino acid residue from the receptor. J. Med. Chem. 1998, 41, 1439–1445. 10.1021/jm970180w. [DOI] [PubMed] [Google Scholar]
- von Sprecher A.; Beck A.; Gerspacher M.; Sallmann A.; Anderson G. P.; Subramanian N.; Niederhauser U.; Bray M. A. Strategies in the design of peptidoleukotriene antagonists. J. Lipid Mediators. 1993, 6, 265–273. [PubMed] [Google Scholar]
- Dong X.; Wang L.; Huang X.; Liu T.; Wei E.; Du L.; Yang B.; Hu Y. Pharmacophore identification, synthesis, and biological evaluation of carboxylated chalcone derivatives as CysLT1 antagonists. Bioorg. Med. Chem. 2010, 18, 5519–5527. 10.1016/j.bmc.2010.06.047. [DOI] [PubMed] [Google Scholar]
- Zhang M.-Q.; Zwaagstra M. E. Structural requirements for leukotriene CysLT1 receptor ligands. Curr. Med. Chem. 1997, 4, 229–246. [Google Scholar]
- Fregonese L.; Silvestri M.; Sabatini F.; Rossi G. A. Cysteinyl leukotrienes induce human eosinophil locomotion and adhesion molecule expression via a CysLT1 receptor-mediated mechanism. Clin. Exp. Allergy 2002, 32, 745–750. 10.1046/j.1365-2222.2002.01384.x. [DOI] [PubMed] [Google Scholar]
- Woszczek G.; Chen L.-Y.; Nagineni S.; Kern S.; Barb J.; Munson P. J.; Logun C.; Danner R. L.; Shelhamer J. H. Leukotriene D4 induces gene expression in human monocytes through cysteinyl leukotriene type I receptor. J. Allergy Clin. Immunol. 2008, 121, 215–221. 10.1016/j.jaci.2007.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.