Abstract
Background
In rheumatoid arthritis (RA), hand synovitis appears especially in wrist, metacarpophalangeal (MCP) and proximal interphalangeal (PIP) joints. In hand osteoarthritis (OA), potential inflammatory changes are mainly present in PIP and distal interphalangeal (DIP) joints. Joint inflammation can be visualised by fluorescence optical imaging (FOI) and musculoskeletal ultrasound (US).
Objective
Comparison of the amount and distribution of inflammatory signs in wrist and finger joints of the clinically dominant hand in patients with OA and RA by FOI and gray-scale (GSUS) and power Doppler US (PDUS).
Methods
FOI and GSUS/PDUS were performed in 1.170 joints (wrists, MCP, PIP, DIP) in 90 patients (67 RA, 23 OA). Joint inflammation was graded by a semiquantitative score (0–3) for each imaging method.
Results
GSUS/PDUS showed wrist and MCP joints mostly affected in RA. DIP joints were graded higher in OA. In FOI, RA and OA featured inflammatory changes in the respective joint groups depending on the phase of fluorescence dye flooding.
Conclusions
US and FOI detected inflammation in both RA and OA highlighting the inflammatory component in the course of OA. The different inflammatory patterns and various shapes of fluorescence enhancement in FOI may offer opportunities to distinguish and determine the inflammatory status in both diseases.
Keywords: Ultrasonography, Rheumatoid Arthritis, Osteoarthritis, Synovitis
Introduction
Novel effective therapies in rheumatology enable us to control the progressive process of chronic inflammatory joint disease1 providing new information on the topic of remission,2 which lead to new requirements for imaging methods in terms of early diagnoses, assessing therapeutic efficiency and prognosis in follow-up examinations.2 3
A distinction between rheumatoid arthritis (RA) and other arthritic and degenerative diseases needs to be drawn when a diagnosis is made.4 Osteoarthritis (OA) is currently not thought to be a primary inflammatory joint disease, although inflammatory alterations of so-called ‘activated joints’ often occur in flares5 and correlate with pain.6 7 Thus, imaging techniques are used in order to differentiate between various diagnoses,8 for example, between an activated or erosive OA and RA or psoriatic arthritis.
For the last two decades, musculoskeletal ultrasound (US) has been widely applied as an imaging modality in rheumatology, permitting the simultaneous detection of soft tissue and erosive bone lesion early in the disease course.9 Thus, synovitis and tenosynovitis as morphological features of an inflammatory process are shown in a more sensitive manner than by clinical examination.10 11 In addition, good correlations have been demonstrated between US and MRI in the detection of inflammation.11–13 Moreover, joint changes in OA, including bone abnormalities (osteophytes, erosions and cartilage damage), synovial and periarticular inflammation can be visualised via US14–16 affirming the diagnosis and informing about the inflammatory status for the treatment decision.14
Indocyanine green (ICG)-based fluorescence optical imaging (FOI) has been shown to be capable of detecting inflammatory arthritis in humans.3 17–19 Recently, Werner et al3 18 demonstrated that FOI is able to differentiate between healthy and inflammatory joints revealing a good agreement with data from clinical examinations, MRI and power Doppler US (PDUS).3
It is thereby possible that flares of inflammation could be detected in OA as well.
This study addresses the investigation of hand-OA—a previously neglected rheumatic joint disease—in comparison to RA as an important differential diagnosis made available by the use of visualised techniques (US and FOI). To this end, the amount of inflammation (grades 0–3) and the distribution of inflamed joints in both diseases were analysed, depending on the US mode (gray-scale/power Doppler) and on the phase of fluorescence dye flooding in FOI (grades 1–3, Prima Vista-Mode (PVM)).
Patients and methods
Patients
Patients with RA and OA were recruited for this study. All patients included fulfilled the European League Against Rheumatism/American College of Rheumatology criteria.20 21
Clinical and laboratory examination
In both patient groups, clinical22 and laboratory examinations were performed (see online supplementary text for detail).
Musculoskeletal US
Ultrasonographic examination of the wrist (WR; midline, radial, ulnar), the metacarpophalangeal (MCP), proximal (PIP) and distal interphalangeal (DIP) joints (fingers II to V; palmar and dorsal) of the clinically dominant hand (for tenderness and/or swelling) was performed in neutral position by gray-scale US (GSUS) and PDUS following standardised procedures.23 Settings for GSUS were as follows (Mylab twice, Esaote, Genua; Italy): frequency 16 MHz and length of scanner 42 mm. The gain depended on joint regions and patients and was nearly 50%. Settings for PDUS were as follows: frequency 9.1 MHz, Pulse Repitition Frequency (PRF) 750 Hz, PD-gain depending on joint regions and patients was nearly 50%; wall filter was three. Synovitis and tenosynovitis were evaluated for their severity, graded by a semiquantitative score (0–3).10 24
Fluorescence optical imaging
The FOI System Xiralite X4 (Mivenion) was used following a standardised procedure (a detailed description is given in the online supplementary text).
To evaluate the distribution of ICG, the image sequence in the film modus and the automatically generated composite image in the PVM were analysed.3 18 For the image sequence, three phases in position to the fingertips were defined regarding development of signal intensities depending on the phase of fluorescence dye flooding and individual perfusion.18 Phase 1 (p1) includes the period between starting the investigation, application of the dye and increased signal intensities in the fingertips.18 When the dye leaves the fingertips from distal to proximal in WR direction, phase 2 (p2) begins as the period of persisting high signal intensities in the fingertips.18 It can be identified by the red colour in the fingertips. Phase 3 (p3) starts when no signal intensity can be determined in the fingertips.18
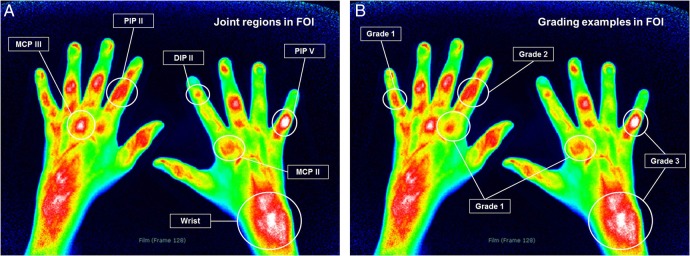
ICG distribution was assessed for the joint regions of the identical hand that had been examined by US. The evaluation of the signal intensity included colour intensity, planar size and shape of enhancement18 (figure 1). For analysing joint activity by FOI, a semiquantitative grading system ‘FOI activity score’ (FOIAS) including grades 0–3 was used3 18 (see online supplementary text).
Figure 1.
(A and B) Examples for joint regions and grades 1–3 in fluorescence optical imaging. DIP, distal interphalangeal; FOI, fluorescence optical imaging; MCP, metacarpophalangeal; PIP, proximal interphalangeal.
Statistical analyses
For statistical analyses, the percentage of frequencies of the score grades 1–3 were calculated in both cohorts for GSUS, PDUS and all phases of FOI (p1–3 and PVM) and then compared with each other (see online supplementary text for detailed description).
Results
Demographic, clinical and laboratory data
Ninety patients were recruited for this study (67 patients with RA and 23 patients with OA).
The results of the demographic, clinical and laboratory features of the study population are shown in online supplementary table S1.
Frequency distribution of inflammatory joints for US and FOI
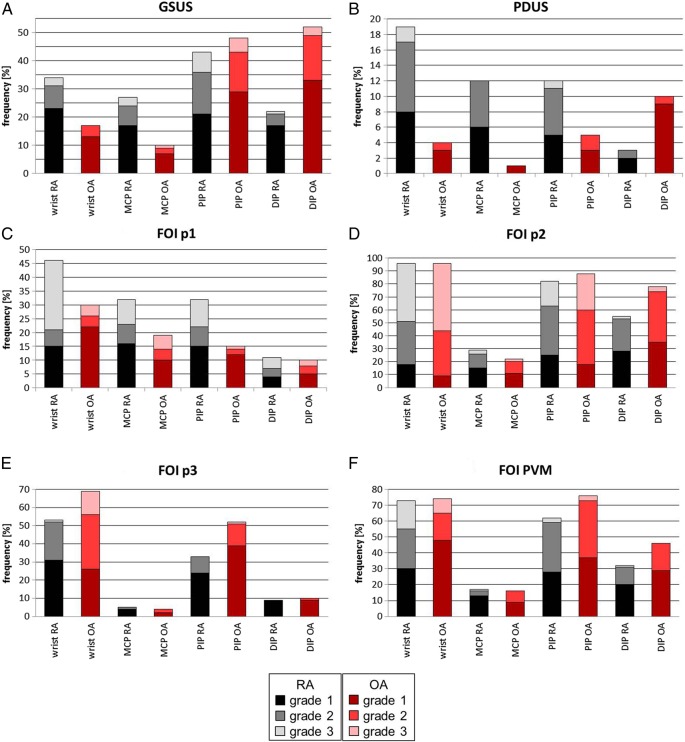
Figure 2A–F illustrates the frequencies of grades 1–3 per joint in US and FOI for the RA (black graphs) versus OA cohort (red graphs; additionally see online supplementary figures S1A–D).
Figure 2.
(A–F) Score frequencies for grade 1–3 per joint in total for synovitis and tenosynovitis, dorsal and palmar side in ultrasound and fluorescence optical imaging for rheumatoid arthritis (RA) vs osteoarthritis (OA) cohort. DIP, distal interphalangeal; FOI, fluorescence optical imaging; GSUS, gray-scale US; MCP, metacarpophalangeal; p1, phase 1; p2, phase 2; p3, phase 3; PDUS, power Doppler US; PIP, proximal interphalangeal; PVM, Prima Vista-Mode.
Gray-scale US
A comparison of the two cohorts with regard to frequency distributions of grades 1–3 in GSUS showed that the PIP and DIP joints of the patients with OA revealed score degrees of 1–3 more often compared with the respective joints in the RA cohort. In contrast, more WR and MCP joints of patients with RA showed inflammatory changes in comparison with those of patients with OA (figure 2A).
Power Doppler US
In PDUS, score degrees of 1–3 were generally less present in comparison with GSUS (figure 2B). Patients with RA featured more inflammatory changes in terms of synovitis and tenosynovitis in PDUS (figure 3B) when compared with patients with OA, except for DIP joints in OA. In OA cohort, grade 2 was most commonly found in the PIP joints, while none of the joint groups had a value of grade 3 (figure 3D).
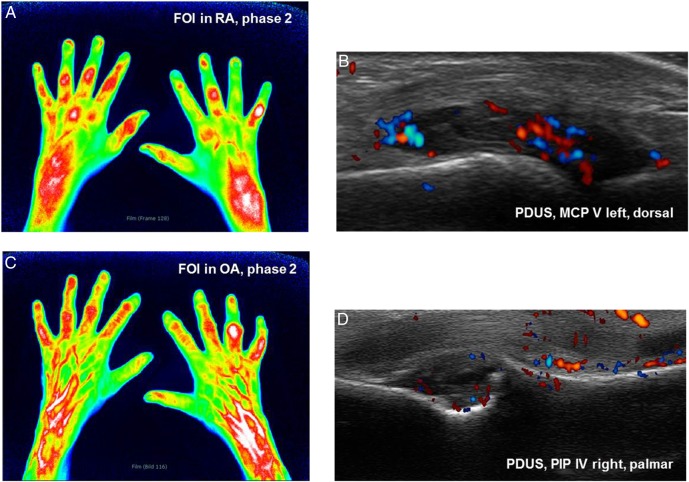
Figure 3.
(A and B) Phase 2 in fluorescence optical imaging (FOI) and the corresponding power Doppler US (PDUS) image of metacarpophalangeal (MCP) V left dorsal in patient with rheumatoid arthritis (RA). (A) Phase 2 shows planar signals in proximal interphalangeal (PIP), MCP and wrists in both hands. (B) PDUS activity grade 2 as a sign for active synovitis in left MCP V. (C and D) Phase 2 in FOI and corresponding PDUS of PIP IV right palmar in patient with osteoarthritis (OA). (C) Streaky FOI signals in DIP and PIP joints as degenerative signs as well as planar signal in PIP IV in token of an active inflammatory joint. FOI signals of higher level in right wrist compared to the left side. (D) PDUS of same right PIP joint shows synovitis and PDUS activity as well as osteophyte.
Fluorescence optical imaging
The highest frequencies of grades 1–3 in all joints were demonstrated in phase 2 in both cohorts.
Phase 1
In phase 1 (figure 2C), grades 1–3 were present more frequently in the RA cohort in comparison with the OA group. Moreover, the WR was the leading joint in both cohorts.
In the OA cohort, grade 1 as the major grade level in phase 1 was evident mostly in the WRs.
Phase 2
Viewing phase 2 in figure 2D, the leading joint group in both cohorts was the group of the WRs with the same total amount in both groups. The joints with the lowest percentage rates were the MCP joints; here, there was a higher percentage in the RA cohort than in the OA cohort (figure 3A). In comparison with patients with RA, the frequencies of grades 1–3 in DIP joints of patients with OA reached a higher proportion (figure 3C).
Phase 3
The WR and PIP joints were in the lead in phase 3 with higher levels in the OA cohort (figure 2E). Among these, the WR was the joint with the highest percentage.
It should be noted that the PIP joints of patients with OA attained the highest level of grade 1 for all joints in both cohorts in this phase.
PVM
PVM revealed practically the same pattern of the total percentage rates among the joints as was described for phase 2 (figure 2F). Leading joint groups in this FOI mode were the WRs and PIP joints. Higher percentage rates in PIP and DIP could be detected in patients with OA.
Discussion
Up to now, FOI has been shown to be capable of detecting inflammatory changes in human arthritic joints17 in good agreement with clinical examination, MRI and PDUS.3 18
To our knowledge, this study is the first one using FOI (phases 1–3, PVM) for the examination of potential inflammation in OA. On that account, we compared the distribution of inflammation in WR and finger joints of OA with RA patients by FOI and US (GSUS/PDUS).
Regarding the frequency distributions of inflammation in the individual joints, the inflammatory patterns of affected joints in both US modes confirmed our expectations. Thus, higher percentage rates of RA joints in PDUS as a sign of active inflammation were found. Considering FOI results, inflammatory changes can be visualised in patients with RA and also clearly in patients with OA.
As far as we know, increased blood volumes as well as the formation of new microvessels as seen by dysregulated microcirculation and angiogenic activity in the early course of RA25 26 cause an enrichment of ICG in the inflammatory tissue.17 A transition of ICG into the interstitial space and interactions with endothelial components has been considered.17 27 Phase 1 appears to symbolise the flooding in of the dye, phase 2 may visualise the distribution and persistence of ICG and phase 3 may show the washing out of the dye, as well as those ICG molecules remaining within the inflamed tissue. Especially the flooding in and the washing out of the dye ICG may depend on an increased and dysregulated microcirculation leading to the assumption that phase 1 visualises active inflammation and phase 3 reveals capillary leakage (see online supplementary figure S2 and S3).18 In reference to our results, the frequency rates showed higher grading levels in phase 1 for RA, connoting active inflammation. Interestingly, the grading of signal intensities in WRs and PIP joints in phase 3 in the OA cohort attained higher percentage levels. Therefore, FOI may underline the findings of previous studies that inflammation plays an important role in the disease course of OA.5–7 14 16 Confirming the inflammatory component in the pathogenesis of hand OA, synovitis in GSUS and activity in PDUS have recently been shown to predict radiographic progression of hand OA.28
In FOI, score frequency patterns for phase 2 (WR and PIP joints) and PVM (WR and MCP joints) were similar for both diseases. Werner et al18 had already stated that phase 2 may reflect potential subclinical inflammation.
During the course of evaluating signal intensities, we have recognised various shapes and manifestations of the signals detected, with the consequence that upon further analysis of these, various diagnoses can be made (figure 3C). This is a very new and interesting aspect; however, future investigation must follow to confirm this hypothesis.
In summary, US as well as FOI were able to detect active inflammation in OA. Thus, an inflammatory component in the course of OA should not be underestimated. Moreover, this could lead to the clinical usage of FOI in patients with OA in the future to visualise inflammation, make a therapeutic decision, be of help in clinical trials and make use of the opportunity for follow-up investigations.
Supplementary Material
Acknowledgments
We thank Gabriela Schmittat for technical assistance. Furthermore, we thank Dr Bernd Schicke for assistance with the statistical calculation. One of the technical devices (FOI) was provided via an unrestricted educational grant by Pfizer Company, Berlin, Germany.
Footnotes
Contributors: All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. No medical writer was involved in the preparation of the manuscript. A-MG, SGW, SO and MB had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study conception and design: A-MG, SGW, GRB, MB and SO. Acquisition of data: A-MG, SGW, MB and SO. Analysis and interpretation of data: A-MG, SGW, GRB, MB and SO. Manuscript preparation: A-MG, SGW, GRB, MB and SO. Statistical analysis: A-MG, SGW, MB, SO and Schicke (see acknowledgements).
Funding: This work was supported by the BMBF project ‘ArthroMark’, subproject no. 7 ‘Clinical study on Biomarkers and Imaging’.
Competing interests: None declared.
Patient consent: Obtained.
Ethics approval: The approval of the study was granted by the ethics committee of the Charité—Universitätsmedizin Berlin, Germany.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Combe B, Landewe R, Lukas C, et al. EULAR recommendations for the management of early arthritis: report of a task force of the European Standing Committee for International Clinical Studies Including Therapeutics (ESCISIT). Ann Rheum Dis 2007;66:34–45. 10.1136/ard.2005.044354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smolen JS, Landewe R, Breedveld FC, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs. Ann Rheum Dis 2010;69:964–75. 10.1136/ard.2009.126532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Werner SG, Langer HE, Ohrndorf S, et al. Inflammation assessment in patients with arthritis using a novel in vivo fluorescence optical imaging technology. Ann Rheum Dis 2012;71:504–10. 10.1136/annrheumdis-2010-148288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Szkudlarek M, Wakefield RJ, Backhaus M, et al. The discriminatory capacity of ultrasound in rheumatoid arthritis: active vs inactive, early vs advanced, and more. Rheumatology 2012;51(Suppl 7):vii6–9. 10.1093/rheumatology/kes334 [DOI] [PubMed] [Google Scholar]
- 5.Iagnocco A. Ultrasound in osteoarthritis. Clin Exp Rheumatol 2014;32(1 Suppl 80):S48–52. [PubMed] [Google Scholar]
- 6.Keen HI, Wakefield RJ, Grainger AJ, et al. An ultrasonographic study of osteoarthritis of the hand: synovitis and its relationship to structural pathology and symptoms. Arthritis Rheum 2008;59:1756–63. 10.1002/art.24312 [DOI] [PubMed] [Google Scholar]
- 7.Kortekaas MC, Kwok WY, Reijnierse M, et al. Pain in hand osteoarthritis is associated with inflammation: the value of ultrasound. Ann Rheum Dis 2010;69:1367–9. 10.1136/ard.2009.124875 [DOI] [PubMed] [Google Scholar]
- 8.Kellner H. Arthrosonographie: Von der Aussenseitermethode zum standardisierten und integrierten Bestandteil der bildgebenden Diagnostik in der Rheumatologie. Z Rheumatol 2001;60:137–8. 10.1007/s003930170061 [DOI] [PubMed] [Google Scholar]
- 9.Ohrndorf S, Backhaus M. Advances in sonographic scoring of rheumatoid arthritis. Ann Rheum Dis 2013;72(Suppl 2):ii69–75. 10.1136/annrheumdis-2012-202197 [DOI] [PubMed] [Google Scholar]
- 10.Szkudlarek M, Court-Payen M, Jacobsen S, et al. Interobserver agreement in ultrasonography of the finger and toe joints in rheumatoid arthritis. Arthritis Rheum 2003;48:955–62. 10.1002/art.10877 [DOI] [PubMed] [Google Scholar]
- 11.Backhaus M, Kamradt T, Sandrock D, et al. Arthritis of the finger joints: a comprehensive approach comparing conventional radiography, scintigraphy, ultrasound, and contrast-enhanced magnetic resonance imaging. Arthritis Rheum 1999;42:1232–45. [DOI] [PubMed] [Google Scholar]
- 12.Scheel AK, Hermann KG, Ohrndorf S, et al. Prospective 7 year follow up imaging study comparing radiography, ultrasonography, and magnetic resonance imaging in rheumatoid arthritis finger joints. Ann Rheum Dis 2006;65:595–600. 10.1136/ard.2005.041814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wakefield RJ, Gibbon WW, Conaghan PG, et al. The value of sonography in the detection of bone erosions in patients with rheumatoid arthritis: a comparison with conventional radiography. Arthritis Rheum 2000;43:2762–70. [DOI] [PubMed] [Google Scholar]
- 14.Haugen IK, Hammer HB. Role of modern imaging techniques in hand osteoarthritis research and clinical practice. Curr Rheumatol Rep 2014;16:399 10.1007/s11926-013-0399-y [DOI] [PubMed] [Google Scholar]
- 15.Iagnocco A, Naredo E. Osteoarthritis: research update and clinical applications. Rheumatology 2012;51(Suppl 7):vii2–5. [DOI] [PubMed] [Google Scholar]
- 16.Vlychou M, Koutroumpas A, Malizos K, et al. Ultrasonographic evidence of inflammation is frequent in hands of patients with erosive osteoarthritis. Osteoarthritis Cartilage 2009;17:1283–7. 10.1016/j.joca.2009.04.020 [DOI] [PubMed] [Google Scholar]
- 17.Fischer T, Ebert B, Voigt J, et al. Detection of Rheumatoid Arthritis Using Non-Specific Contrast Enhanced Fluorescence Imaging. Acad Radiol 2010;17:375–81. 10.1016/j.acra.2009.09.016 [DOI] [PubMed] [Google Scholar]
- 18.Werner SG, Langer HE, Schott P, et al. Indocyanine green-enhanced fluorescence optical imaging in patients with early and very early arthritis: a comparative study with magnetic resonance imaging. Arthritis Rheum 2013;65:3036–44. 10.1002/art.38175 [DOI] [PubMed] [Google Scholar]
- 19.Meier R, Thurmel K, Moog P, et al. Detection of synovitis in the hands of patients with rheumatologic disorders: diagnostic performance of optical imaging in comparison with magnetic resonance imaging. Arthritis Rheum 2012;64:2489–98. 10.1002/art.34467 [DOI] [PubMed] [Google Scholar]
- 20.Aletaha D, Neogi T, Silman AJ, et al. 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann Rheum Dis 2010;69:1580–8. 10.1136/ard.2010.138461 [DOI] [PubMed] [Google Scholar]
- 21.Altman R, Alarcon G, Appelrouth D, et al. The American College of Rheumatology criteria for the classification and reporting of osteoarthritis of the hand. Arthritis Rheum 1990;33:1601–10. 10.1002/art.1780331101 [DOI] [PubMed] [Google Scholar]
- 22.Prevoo ML, van't Hof MA, Kuper HH, et al. Modified disease activity scores that include twenty-eight-joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum 1995;38:44–8. 10.1002/art.1780380107 [DOI] [PubMed] [Google Scholar]
- 23.Backhaus M, Burmester GR, Gerber T, et al. Guidelines for musculoskeletal ultrasound in rheumatology. Ann Rheum Dis 2001;60:641–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scheel AK, Hermann KG, Kahler E, et al. A novel ultrasonographic synovitis scoring system suitable for analyzing finger joint inflammation in rheumatoid arthritis. Arthritis Rheum 2005;52:733–43. 10.1002/art.20939 [DOI] [PubMed] [Google Scholar]
- 25.Kennedy A, Ng CT, Biniecka M, et al. Angiogenesis and blood vessel stability in inflammatory arthritis. Arthritis Rheum 2010;62:711–21. 10.1002/art.27287 [DOI] [PubMed] [Google Scholar]
- 26.Andersson SE, Johansson A, Lexmuller K, et al. Physiological characterization of mBSA antigen induced arthritis in the rat. II. Joint blood flow, glucose metabolism, and cell proliferation. J Rheumatol 1998;25:1778–84. [PubMed] [Google Scholar]
- 27.Mordon S, Devoisselle JM, Soulie-Begu S, et al. Indocyanine green: physicochemical factors affecting its fluorescence in vivo. Microvasc Res 1998;55:146–52. 10.1006/mvre.1998.2068 [DOI] [PubMed] [Google Scholar]
- 28.Mathiessen A, Slatkowsky-Christensen B, Kvien TK, et al. Ultrasound-detected inflammation predicts radiographic progression in hand osteoarthritis after 5 years. Ann Rheum Dis 2015. Published Online First: 1 Apr 2015. 10.1136/annrheumdis-2015-207241 10.1136/annrheumdis-2015-207241 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.