Abstract
Background:
The increase in the incidence of methicillin-resistant Staphylococcus aureus (MRSA) infections lacking risk factors for exposure to the health care system has been associated with the recognition of new MRSA clones known as community-associated MRSA (CA-MRSA). These strains have been distinguished from health care-associated MRSA (HA-MRSA) strains by epidemiological, molecular and genetic means as well as by antibiotic susceptibility profile, tissue tropism and virulence traits.
Objective:
To assess prevalence and antibiotic susceptibility profile of CA-MRSA in Canton Sarajevo, Bosnia and Herzegovina.
Results:
Out of 1.905 positive Staphylococcus aureus isolates from various samples of outpatients collected during six months, 279 (14,64%) were MRSA isolates. Out of 279 MRSA samples, 133 (47,67%) were found in nasal swabs, from which 48 (36,09%) were in the age group <1 year and 39 (29,32 %) are in the age 1-5 year. Rate of the positive skin swabs was highest among the subject of age group <1 year (46 or 54,12 %) and 1-5 year (18 or 21,18 %). Predominantly antibiotic types among MRSA strains are resistant to penicillin and cefoxitin (36,90 %) and to penicillin, cefoxitin and erythromycin (61,35 %).
Conclusion:
Continued monitoring of epidemiology and emerging drug resistance data is critical for the effective management of these infections.
Keywords: methicillin-resistant Staphylococcus aureus, community-associated MRSA, health care-associated MRSA, drug resistance
1. INTRODUCTION
Staphylococcus aureus is an opportunistic pathogen often carried asymptomatically in the human body but it can also be the main cause of purulent infections in humans with the potential to engage all tissues and all anatomic sites. If S. aureus strains acquire the mecA gene, which is carried on a large mobile genetic element called the staphylococcal chromosomal cassette mec (SCCmec), they become methicillin-resistant S. aureus (MRSA) strains resistant to all beta-lactam antibiotics.
At the end of the nineties of the last century, a separate entity of MRSA emerged in the community – community acquired MRSA (CA-MRSA) which except the similarity with HA-MRSA (hospital acquired MRSA) had significant differences in the epidemiological, molecular and genetic sense as well as on antibiotic susceptibility, tissue tropism and virulence traits. The increase in the prevalence of CA-MRSA in many countries leads to the associated morbidity and mortality of this infection and suggests that infection with this bacteria lead to develop a new public-health problem. According to CDC case definition from 2000, there are several risk components which have defined the burden of CA-MRSA and HA-MRSA isolates, both of which circulate in the community and hospital settings. Any MRSA infection would be considered CA-MRSA if it is diagnosed among outpatients earlier than 48h after hospitalization and if the patients lack the following health care-associated MRSA risk factors: hemodialysis, surgery, residence in a health care facility or hospitalization during the previous year, the presence of an permanent indwelling catheter or a percutaneous device or previous isolation of MRSA from the patients (1). Other criteria used to define CA-MRSA or HA-MRSA infections are: antimicrobial susceptibility profiles (CA-MRSA isolates have typically been susceptible to most non-ß-lactam antimicrobial agents) (2), PCR amplification of mecA gene, DNA patterns by pulsed-field gel electrophoresis ((PFGE) (3), the allelic profiles by multi locus sequence typing (MLST), DNA sequencing of the X region of the protein A gene (spa typing) (4), SCCmec typing (5) and PCR amplification of the lukS-PV and lukF-PV genes encoding Panton-Valentine leukocidin (6). Genetic characteristics of CA-MRSA infections are associated with SCCmec type IV and V which lacks other multidrug resistance genes and more frequently associated with Panton-Valentin leukocidin (PVL toxin) which have been linked to skin and soft tissue infections and severe necrotizing pneumonia (7). However, none of these criteria are suitable for CA-MRSA screening, as each of them may be missing or found in typical HA-MRSA strains as well. Currently, there are relatively few dominant CA-MRSA lineages found worldwide. They include ST1-IV(USA400); ST8-IV (USA300); ST30-IV (Pacific/Oceania);ST59-IV and V (USA1000, Taiwan); ST80-IV (European CA-MRSA strain) (7). CA-MRSA is currently a human health problem in nearly all industrialized countries in various ranges. In the US the prevalence of these strains was recently reported as >50% while in France in 2000–2003 CA-MRSA were isolated from 1–3% of all skin and soft tissue infections (8). The risk factors for acquisition of CA-MRSA are: history of colonization/infection with CA-MRSA, close contact with a person colonized/infected with CA-MRSA, adults older than 65 years; children younger than 2 years, neonates, indigenous people and special communities (contact sports, injection drug use, living in poor conditions, military personnel, men who have sex with men etc) (8,9). While HA-MRSA cases reflect the age distribution of hospitalized elder patients, CA-MRSA predominantly affects otherwise healthy people and usually it peaks in children and adolescents and 30-40 year old persons. As the predominant route of transmission, close contact likely reflects transmission from children to their parents. It should, however, be noted that when CA-MRSA strains are introduced into hospitals, it results in clinical pictures that are indistinguishable from those caused by HA-MRSA strains. Since 2000, there have been many reported neonatal CA-MRSA outbreaks in neonatal intensive care units and other settings. These strains have been associated with visiting fathers, maternal mastitis, expressed breast milk, peripartum maternal infections and colonized or infected health care workers (10). CA-MRSA strains are especially aggressive, causing skin and soft tissue infections, fasciitis, necrotizing pneumonia, and blood stream infections (11). CA-MRSA also has the ability to survive and spread in the community, leading to an increasing number of colonized persons in the general population. Carriage rates among the general population are still low in most places including Europe (<1%). In the US, the latest NHANES survey in 2003-4 showed 1,5% positive. In Mexico and Taiwan there have been far larger carriage rates of MRSA - as high as 10% (12). That leads to the increased introduction of CA-MRSA into hospitals by persons without any known risk factors and CA-MRSA becomes the cause of healthcare-associated infections (HAI) with significant levels in some countries. 25% of HA-MRSA infections are caused by the ST80-IV strain in Greece (13) whereas in the US, CA-MRSA strains, especially ST8-IV (USA300), are now very frequent in many US hospitals where, to some degree, they have displaced HA-MRSA strains. In Taiwan ST59-VT causes 13% of HA-MRSA and 47% of MRSA infections in persons with community-onset infections with healthcare-associated risk factors (HACO-MRSA) (14). CA-MRSA isolates have typically been susceptible to most non-beta-lactam antimicrobial drugs including several oral agents (clindamycin, ciprofloxacin). This enables clinicians to have a number of options when selecting empiric treatments for putative CA-MRSA infections (15). This study will provide increased options for empirical therapy of infections caused by these strains. Also these data can be used for the effective management of these infections.
2. MATERIAL AND METHOD
During six months (from September 2014 to February 2015) there were 1.905 positive Staphylococcus aureus isolates from various samples of outpatients. All laboratory testing was performed in the microbiological laboratory of Institute for Public Health of Canton Sarajevo (Bosnia and Herzegovina). All samples were cultured and identified using internal protocols, approved for routine application in general bacteriological procedures in the microbiological laboratory. For the identification of MRSA strains routine phenotypic method of disk diffusion with cefoxitin as a representative beta-lactam antibiotic was used, which showed certain advantages in numerous studies in relation to oxacillin. The strains were tested on the antimicrobial sensitivity of the following antibiotics: penicillin (PEN), cefoxitin (FOX), erythromycin (ERY), clindamycin (CD), trimethoprim sulfamethoxazole (TSX), fucidic acid (FA), chloramphenicol (CHL), ciprofloxacin (CIP), gentamycin (GEN), rifampicin (RD) and tetracycline (TE) using disk diffusion method according to EUCAST (European Committee on antimicrobial susceptibility testing). Confirmation of MRSA strains was done with selective chromogenic medium, Chromatic MRSA, Liofilchem.
3. RESULTS
Out of 1.905 positive Staphylococcus aureus isolates from various samples of outpatients in Canton Sarajevo collected during six months, 279 (14,64 %) were MRSA isolates.
Table 1. presents sources of MRSA strains related to age divided into groups containing the consecutive ten years period, except group <1 which is specifically set aside because of great epidemiological importance for this survey.
Table 1.
Sources of MRSA strains according to age groups. *p=0,604, **p<0,0001
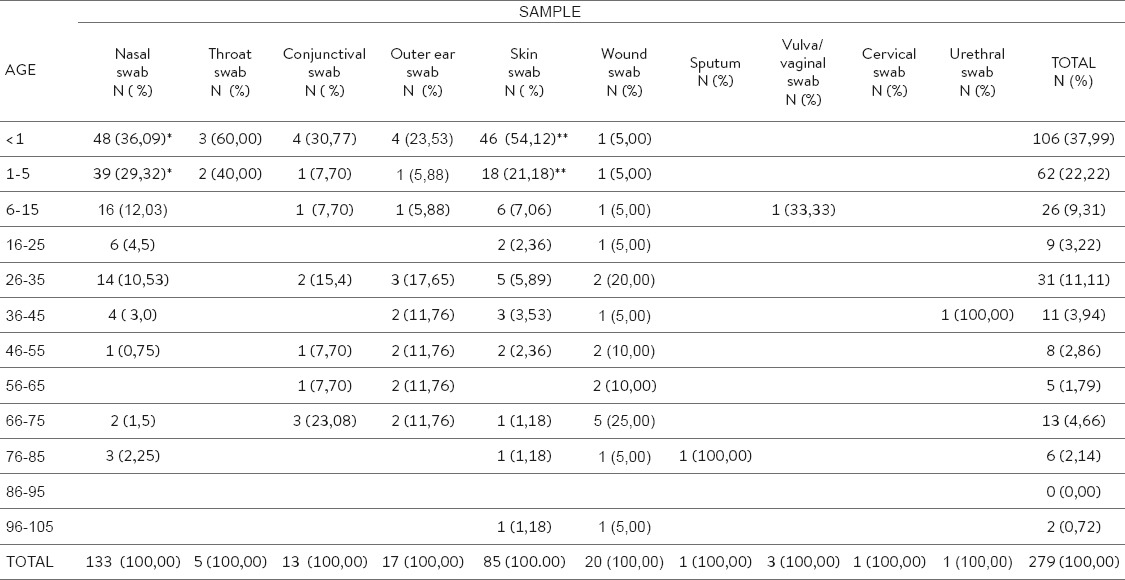
Out of total MRSA strains, 133 (47,670%) were found in nasal swabs, out of there were 48 (36,09%) in the age group <1 year and 39 (29,32%) are in the age 1-5 year which is not significant (p=0,604) to relate the other ages.
Also, MRSA isolates were isolated with higher frequency from skin swabs so that out of total MRSA strains there were 85 (30,46 %) positive. Rate of the positive isolates was highest among the subjects of age group <1 year (46 or 54,12 %) and 1-5 years (18 or 21,18 %) which is significant (p<0,0001) in relation the other ages. Certain area of the skin (perineum, axillae, gluteal and region of mammae) were mostly the places of suppurative skin infections especially in newborns. In Table 1. can also be seen that CA-MRSA strains tend to occur in the older age group (> 26 year) as a cause of conjunctivitis or infection of outer ear and, particularly, wound infections.
All isolates of Staphylococcus aureus were tested by disc diffusion technique to 11 antimicrobial agents: penicillin, cefoxitin, erythromycin, clindamycin, sulfamethoxazole-trimethoprim, fucidic acid, chloramphenicol, ciprofloxacin, gentamycin, rifampicin and tetracycline.
As it can be seen in the Table 2, MSSA strains showed lower percentage of sensitivity to penicillin (7,00 %) while the predominant antibiotic phenotype among MSSA cells is characterized by resistance to penicillin (84,6 %) or to penicillin and associated with most common inducible type of resistance of erythromycin to clindamycin (7,40 %) or to penicillin with associated resistance to gentamycin (1,00 %).
Table 2.
Antibiophenotypes of isolated MSSA and MRSA strains
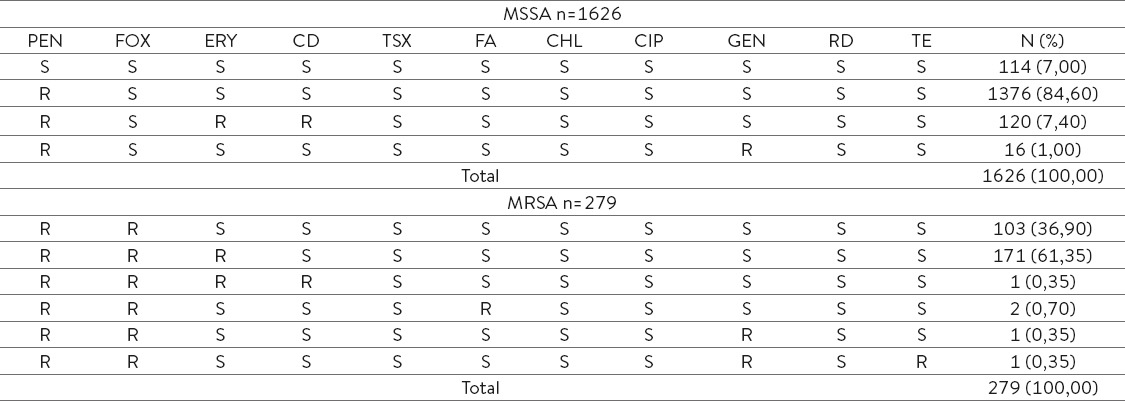
Predominant antibiotic phenotypes among MRSA strains are resistant to penicillin and cefoxitin (36,90 %) and to penicillin, cefoxitin and erythromycin (61,35 %). Also, some strains show resistance to other antibiotic groups such as fucidic acid, gentamycin and tetracycline.
4. DISCUSSION
Results of our study indicate a growing trend of identification of MRSA strains among positive cultures from various samples of outpatients in Canton Sarajevo. To our knowledge, this is not the first report of routine surveillance for this issue in Bosnia and Herzegovina. Prior studies of MRSA have been reported from hospital and community condition (16, 17,18, 19, 20, 21). The following trends indicate the severity of this issue: the increase of MRSA isolates in the hospital and the community, the increase of staphylococcal infections resistant to methicillin in relation to the methicillin-sensitive staphylococcus infection in the community and the growth of MRSA infections in previously healthy individuals. The emergence and spread of CA-MRSA in the hospital environment caused the alarm among officials and public health clinicians (22). Recent studies provide the evidence of infiltration of CA-MRSA strains in the hospitals. The ratio of the isolates in the hospital with IV SCCmec increased from <20% to> 50% between 1999-2004 in the US hospitals. Another study conducted in intensive care units showed the increase of MRSA incidence from 35.9% to 64.4% between 1992-2003, but the number of MRSA isolates that were resistant to gentamycin, tetracycline and sulfamethoxazole-trimethoprim reduced which indicated that it was the word about community acquired strains of MRSA (23). Although we have no molecular or genetic characteristics of isolated strains, we can assume with high probability based on epidemiological and clinical features, antibiotic susceptibility and tissue tropism that agents analyzed in this study are CA MRSA strains. The majority of identified MRSA strains originated from nasal carriers and skin and soft tissue infections (p<0,0001) in newborns delivered in the local maternity hospitals and in children of pediatric age (1-5 year). Generally, infections caused by CA-MRSA are resistant only to beta-lactams and can carry the gene for synthesis of Panton- Valentine leukocidin (PVL) that is responsible for infection of the soft tissue of the skin (24). In this study, the largest number of isolates had a resistance only to beta-lactams or to beta-lactams associated with macrolide but the fact that MRSA strains in the community appear to be resistant to other groups of antimicrobial agents such as fucidic acid, tetracycline and gentamicin should be concerned. That may lead to the emergence of multi-resistant CA-MRSA cells in the future.
5. CONCLUSION
The lack of data on the prevalence and incidence of CA-MRSA infection should establish a system of surveillance of this appearance in the hospital and community settings in Canton Sarajevo. Data related to the adaptability of this agent in new environments as well as the comparative advantages to the nosocomial pathogens suggest that it is possible to expect complete domination of CA MRSA in the hospital and community environment. This can lead to changes in certain epidemiological parameters of nosocomial infections due to very expressed virulence of this pathogen so that both healthy and sick people are vulnerable to infection and because of the possibility of acquiring multi-resistant genes which can cause serious problems related to the treatment.
Footnotes
• Author’s contribution: Amina Obradovic and Dunja Hodzic - collecting data from primary care and microbiological laboratory and processing to Excel. Mufida Aljicevic and Fatima Numanovic - collection of literature on antibiotic resistance and correspondence with the author about design of the article. Lutvo Sporišević - collecting medical history and send samples to the laboratory.
• Declaration of Interest: There is no conflict of interest.
REFERENCES
- 1.David MZ, Daum RS. Community-associated methicillin-resistant S. aureus:Epidemiology and Clinical Consequences of an Emerging Epidemic. Clin Microbiol Rev. 2010;23(3):631. doi: 10.1128/CMR.00081-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Naimi TS, LeDell KH, Como-Sabetti K, Borchardt SM, Boxrud DJ, Etienne J, Johnson SK, Vadenesch F, Fridkin S, O'Boyle C, Danila RN, Lynfield R. Comparison of community - and health care-associated methicillin-resistant Staphylococcus aureus infection. JAMA. 2003;290:2976–84. doi: 10.1001/jama.290.22.2976. [DOI] [PubMed] [Google Scholar]
- 3.McDougal LK, Steward CD, Killgore GE, Chaitram JM, McAllister SK, Tenover FC. Pulsed-field gel electrophoresis typing of oxacillin-resistant Staphylococcus aureus isolates from the United States:establishing a national database. J Clin Microbiol. 2003;41:5113–20. doi: 10.1128/JCM.41.11.5113-5120.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Strommenger B, Braulke C, Heuck D, Schmidt C, Pasemann B, Nübel U, Witte W. Spa typing of Staphylococcus aureus as a frontline tool in epidemiological typing. J Clin Microbiol. 2007;46:574–81. doi: 10.1128/JCM.01599-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.International Working Group on the Classification of Staphylococcal Cassette Chromosome Elements (IWG-SCC). Classification of staphylococcal cassette chromosome mec (SCCmec):guidelines for reporting novel SCCmec elements. Antimicrob Agents Chemother. 2009;53:4961–67. doi: 10.1128/AAC.00579-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mongkolrattanothai K, Boyle S, Kahana MD, Daum RS. Severe Staphylococcus aureus infections caused by clonally related community-acquired methicillin-susceptible and methicillin-resistant isolates. Clin Infect Dis. 2003;37:1050–8. doi: 10.1086/378277. [DOI] [PubMed] [Google Scholar]
- 7.DeLeo FR, Otto M, Kreiswirth BN, Chambers HF. Community-associated meticillin-resistant Staphylococcus aureus. Lancet. 2010;375:1557–68. doi: 10.1016/S0140-6736(09)61999-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gillet Y, Issartel B, Vanhems P, Fournet JC, Lina G, Bes M, et al. Association between Staphylococcus aureus strains carrying gene for Panton-Valentine leukocidin and highly lethal necrotising pneumonia in young immunocompetent patients. Lancet. 2002;359:753–9. doi: 10.1016/S0140-6736(02)07877-7. [DOI] [PubMed] [Google Scholar]
- 9.Ho PL, Cheung C, Mak GC, Tse CW, Ng TK, Cheung CH, et al. Molecular epidemiology and household transmission of community-associated methicillin-resistant Staphylococcus aureus in Hong Kong. Diagn Microbiol Infect Dis. 2006;57:145–51. doi: 10.1016/j.diagmicrobio.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 10.Roberts JR, McCawley L, Laxton M, Trumbo H. Genital community associated methicillin-resistant Staphylococcus aureus infection can be a sexually transmitted disease. Ann Emerg Med. 2007;50:93–4. doi: 10.1016/j.annemergmed.2007.02.027. [DOI] [PubMed] [Google Scholar]
- 11.Young LM, Price CS. Community acquired methicillin-resistant Staphylococcus aureus emerging as an important cause of necrotizing fasciitis. Surgical Infections. 2008;9:469–74. doi: 10.1089/sur.2007.052. [DOI] [PubMed] [Google Scholar]
- 12.Hamdan-Partida A, Teresita Sainz-Espun T, Bustos-Martínez J. Characterization and Persistence of Staphylococcus aureus strains isolated from the anterior nares and throats of healthy carriers in a Mexican community. J Clin Microbiol. 2010;48(5):1701–5. doi: 10.1128/JCM.01929-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chini V, Petinaki E, Foka A, Paratiras S, Dimitracopoulos G, Spiliopoulou I. Spread of Staphylococcus aureus clinical isolates carrying Panton-Valentine leukocidin genes during a 3-year period in Greece. Clin Microbiol Infect. 2006;12:29–34. doi: 10.1111/j.1469-0691.2005.01295.x. [DOI] [PubMed] [Google Scholar]
- 14.Wang J, Liao C, Fang C, Chie WC, Lai MS, Lauderdale T, et al. Prevalence of and risk factors for colonization by methicillin-resistant Staphylococcus aureus among adults in community settings in Taiwan. J Clin Microbiol. 2009;47:2957–63. doi: 10.1128/JCM.00853-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Naimi TS, LeDell KH, Como-Sabetti K, Borchardt SM, Boxrud DJ, Etienne J. et al. Comparison of community- and health care-associated methicillin-resistant Staphylococcus aureus infection. JAMA. 2003;290:2976–84. doi: 10.1001/jama.290.22.2976. [DOI] [PubMed] [Google Scholar]
- 16.Šiširak M, Zvizdić A, Hukić M. Methicillin-resistant Staphylococcus aureus (MRSA) as a cause of nosocomial wound infections. Bosnian Journal of Basic Medical Sciences. 2010;10(1):34–7. doi: 10.17305/bjbms.2010.2733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nurkić M. Causes of infection surgical wound. The fourth Symposium of Hospital Infection Control of Bosnia and Herzegovina, Tuzla, Bosnia and Herzegovina, June 14th-16th. Proceedings. 2006:9–19. [Google Scholar]
- 18.Bešlagić E, Bektaš S, Aljičević M, Balta S, Hamzić S. Frequency and Antibiotic Susceptibility of Methicillin-Resistant Staphylococcus Aureus (MRSA) in Canton Sarajevo, Bosnia and Herzegovina. Folia Medica. 2011;46(1):35–40. [Google Scholar]
- 19.Rebic V, Bektas S, Aljicevic M, Beslagic E, Mulabdic V, Hamzic S. Phenotypic methods for detection of methicillin-resistant Staphylococcus aureus. Mater Sociomed. 2012;24(Suppl 1):20–31. [Google Scholar]
- 20.Ostojić M. Epidemiologic Genotyping of Methicillin-Resistant Staphylococcus aureus (MRSA) by Pulsed-Field Gel Electrophoresis (PFGE) Bosnian Journal of Basic Medical Sciences. 2008;8(3):259–65. doi: 10.17305/bjbms.2008.2930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Uzunović-Kamberović S, Sivić S. Methicillin-resistant Staphlococcus aureus (MRSA) in the community – laboratory based study. Acta Medica Academica. 2007;36(1):3–9. [Google Scholar]
- 22.Zetola N, Francis JS, Nuermberger EL, Bishai WR. Community acquired methicillin-resistant Staphylococcus aureus:an emerging threat. Lancet Infect Dis. 2005;5:275–86. doi: 10.1016/S1473-3099(05)70112-2. [DOI] [PubMed] [Google Scholar]
- 23.Maree CL, Daum RS, Boyle-Vavra S, Matayoshi K, Miller LG. Community associated methicillin-resistant Staphylococcus aureus isolates causing healthcare-associated infections. Emerg Infect Dis. 2007;13:236–42. doi: 10.3201/eid1302.060781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klevens RM, Morrison MA, Nadle J, Petit S, Gershman K, Ray S, et al. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. J Am Med Assoc. 2007;298(15):1763–71. doi: 10.1001/jama.298.15.1763. [DOI] [PubMed] [Google Scholar]


