Abstract
hTERT, a catalytic component of human telomerase, is undetectable in normal somatic cells but up-regulated in cancer and stem cells where telomere length is maintained by telomerase. Accumulated evidence indicates that hTERT may have noncanonical functions beyond telomerase by regulating the expression of particular genes. However, comprehensive identification of the genes regulated by hTERT is unavailable. In this report, we expressed WT hTERT and hTERTmut which displays dysfunctional catalytic activity, in human U2OS cancer cells and VA-13 immortalized fibroblast cells, both of which lack endogenous hTERT and hTR expression. Changes in gene expression induced by hTERT and hTERT-mut expression were determined by genome-wide RNA-seq and verified by qPCR. Our results showed that hTERT affects different genes in two cell lines, implying that the regulation of gene expression by hTERT is indirect and cell type dependent. Moreover, functional analysis identifies cell adhesion-related genes that have been changed by hTERT in both cell lines. Adhesion experiments revealed that hTERT expression significantly increases cell adhesion. Monolayer wound healing and transwell assays demonstrated increased cell migration upon hTERT expression. These results provide new evidence to support a noncanonical function for hTERT in promoting tumorigenesis.
Telomerase is a reverse transcriptase that adds tandem telomeric sequences to the end of chromosomes1,2. Telomerase is composed of two main subunits, the catalytic subunit hTERT and the RNA template hTR3,4. In most human cancers and germ/stem cells, hTERT catalyzes de novo repeat addition using hTR as a template sequence, thus preventing telomere shortening caused by the “end-replication problem” and end-processing. In human somatic cells, telomerase is absent and telomeres are progressively shortened until a critical length is reached that triggers cell senescence or apoptosis. More than 80% of tumors express telomerase3; activation of hTERT expression is a critical step in carcinogenesis.
Accumulated evidence demonstrates that hTERT has non-canonical functions beyond telomere lengthening. It has been reported that telomerase that lacks extension activity promotes tumorigenesis5. Moreover, Artandi’s group found that TERT mutations that lack catalytic activity could induce the proliferation of hair follicle stem cells in transgenic mice, possibly through transcriptional regulation of the Wnt signaling pathway6,7. TERT has also been found to play roles in apoptosis, DNA damage response, and regulation of gene expression8. Ectopic expression of hTERT was able to promote cell proliferation by either upregulating epiregulin or EGFR expression in human cells9,10,11. In cancer cells, overexpression of hTERT enhances the progression of gastric cancer by upregulating Mac-2BP12. These studies revealed that hTERT has a variety of functions aside from telomere extension. In particular, these functions involve the up- and down-regulation of some important genes. However, comprehensive understanding of genome-wide gene expression regulated by hTERT remains unclear. Although altered mRNA profiling has been reported in human and mice cells overexpressing the TERT gene, the results are complicated by the fact that increased TERT affects telomere length homeostasis that could interfere with gene transcription. To this end, it is important to study non-canonical hTERT functions in telomerase-deficient cells.
In this report, we overexpressed hTERT in human ALT cancer U2OS cells and immortalized fibroblast cells VA-13, both of which lack endogenous hTR and hTERT expression, thus preventing the influence of changes in telomere length on gene expression. We also overexpressed an hTERT with mutated amino acids that lacked catalytic activity. Comparison of gene expression profiling in cells with and without hTERT expression (or mutant hTERT) rendered the conclusion that hTERT is implicated in the regulation of cell adhesion-related genes. These experiments also demonstrated that hTERT or mutant hTERT overexpression promotes cell migration and transformation.
Results
Ectopic expression of hTERT or hTERTmut in different cells show distinct expression profiles
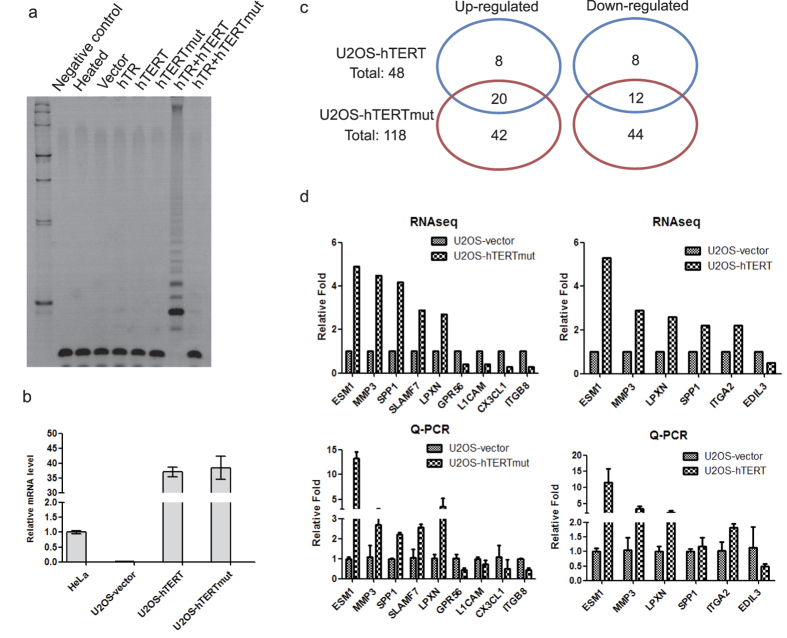
Mutant hTERT (hTERTmut) was constructed by substituting the valine and isoleucine residues at positions 710 and 711 with aspartic acid and alanine residues, respectively13. TRAP assay showed that human cancer U2OS cells have no detectable telomerase activity. Overexpression of either WT hTERT or hTR alone displayed no telomerase activity, indicating the lack of endogenous expression of hTERT and hTR (Fig. 1a). Telomerase activity was detected in cells co-expressing WT hTERT and hTR, but not in those expressing hTERTmut and hTR, demonstrating the loss of catalytic activity in the hTERTmut (Fig. 1a).
Figure 1. Dysregulated genes in U2OS-hTERT and U2OS-hTERTmut cells.
(a) Telomerase activity was detected by TRAP assay. Only U2OS cells transfected with both hTERT and hTR showed telomerase activity. (b) hTERT and hTERTmut stably overexpressed in U2OS with empty vector as a control. hTERT mRNA levels were quantified by qPCR and normalized to HeLa cells. (c) Venn diagrams depicting the genes that were up- and down-regulated by hTERT and hTERTmut in U2OS. The gene expression profiles were determined by RNA-seq as described in the Materials and methods section. (d) RNA-seq results were confirmed by qPCR. Several differentially expressed genes were randomly selected in the indicated stable cell lines. RNA-seq and qPCR results are showed in the upper and lower panels, respectively.
Wild-type hTERT or hTERTmut was stably expressed in U2OS cells. The empty pBabe vector was used as a control (Fig. 1b). To explore changes in gene expression due to hTERT or hTERTmut expression, whole genome gene expression profiles of U2OS-hTERT, U2OS-hTERTmut, and U2OS-vector cell lines were determined by RNA-seq using next-generation sequencing. Differentially expressed genes were sorted with the DESeq package14, where transcripts with adjusted p-value < 0.05 (padj) were considered as valid candidates. The results showed that expression of wild-type hTERT resulted in 48 changes compared with the empty vector, including 28 up-regulated and 20 down-regulated genes. The expression of hTERTmut led to 118 changes compared with the vector control, including 62 up-regulated and 56 down-regulated genes (Supplementary Table S1). Thirty two common changes were found, including 20 up-regulated and 12 down-regulated genes (Fig. 1c).
To validate the RNA-seq data, randomly selected genes were subjected to quantitative RT-PCR to determine the change in expression level when hTERT or hTERTmut was expressed (Fig. 1d and Fig. S1). Our results showed that these genes displayed changes in their expression levels similar to the results obtained by RNA-seq, demonstrating that the RNA-seq data were reliable.
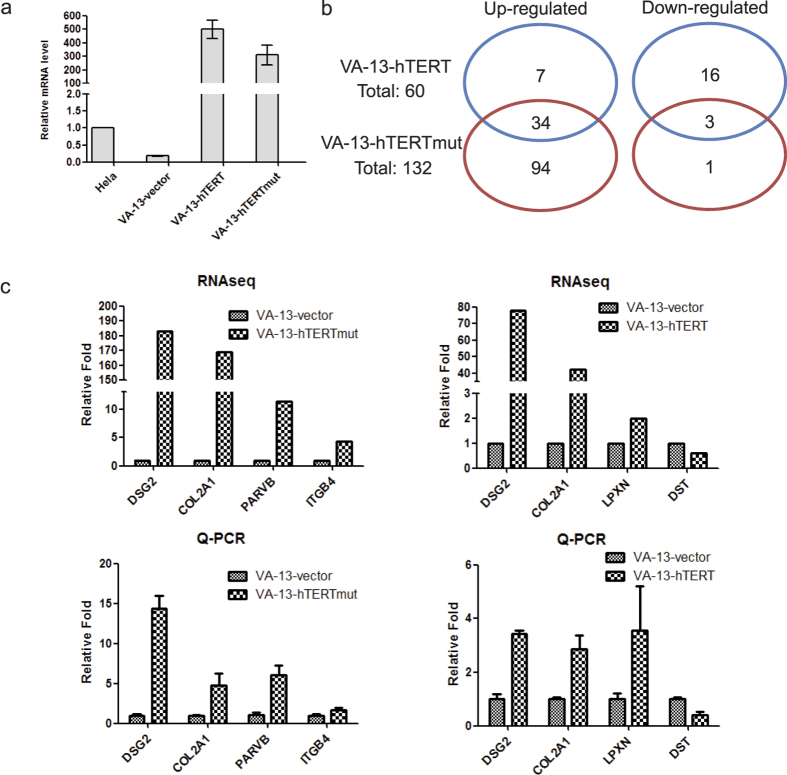
We then tested whether hTERT or hTERTmut affected gene expression in another cell line, VA-13, an immortalized human fibroblast cell line with no detectable expression of either hTERT or hTR. Wild-type hTERT, hTERTmut, and empty vector were expressed in VA-13 cells and gene expression profiles were obtained by RNA-seq (Fig. 2). Data analysis showed that hTERT expression up-regulated 41 genes and down-regulated 19 genes, while expression of hTERTmut up-regulated 128 genes and down-regulated 4 genes (Supplementary Table S2). There were 37 commonly changed genes on both lists, including 34 up-regulated and 3 down-regulated genes (Fig. 2b). Differently expressed genes identified by RNA-seq were also validated by individual quantitative RT-PCR. The consistent results were obtained that demonstrated the reliability of RNA-seq (Fig. 2c and Fig. S2). Interestingly, only one gene, KRT17, was changed in both U2OS and VA-13 cells. Possible reasons for the lack of overlap between the two cell lines may be that hTERT regulates gene transcription by associating with other modulating factors such as BRG1 and p6515,16. Different associating factors may be present in U2OS and VA-13 cells that determine which gene hTERT targets.
Figure 2. Dysregulated genes in the VA-13-hTERT and VA-13-hTERTmut cells.
(a–c) Same as (b–d) in Fig. 1 with VA-13 cells instead of U2OS cells.
Characterization of the functional changes due to hTERT or hTERTmut expression
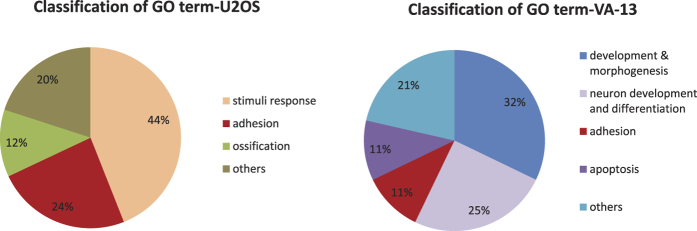
Although hTERT regulates different genes in U2OS and VA-13 cells, functional similarities between these genes may exist. Thus, we performed gene functional analysis using Gene Ontology (GO)17. The biological processes enriched in both hTERT and hTERTmut expressed cells are shown in Table 1 and Table 2 (U2OS and VA-13 cell lines, respectively). The GO terms can be classified into different categories according to their functions (Fig. 3). In U2OS cells, the commonly altered GO terms due to hTERT and hTERTmut expression are involved in stimuli response (44%), adhesion (24%), ossification (12%), and others (20%) (Table 1 and Fig. 3). Stimuli response- and ossification-related genes are consistent with the identity of U2OS as osteosarcoma cells. In VA-13 cells, the GO terms are related to development & morphogenesis (32%), neuron development & differentiation (25%), adhesion (11%), apoptosis (11%), and others (Table 2 and Fig. 3). More than 50% (32% + 25%) of the GO terms are related to development, reflecting the nature of VA-13 cells, which originate from WI38 fibroblasts with stem-like features and have the potential to be reprogrammed into stem cells or directly converted into neuronal cells18,19,20.
Table 1. hTERT and hTERTmut co-related biology process in U2OS.
| Biology Process | U2OS-hTERT | U2OS-hTERTmut |
|---|---|---|
| PValue | PValue | |
| GO:0010033~response to organic substance | 0.0004 | 0.0029 |
| GO:0030155~regulation of cell adhesion | 0.0004 | 0.0003 |
| GO:0007584~response to nutrient | 0.0004 | 0.0003 |
| GO:0033273~response to vitamin | 0.0006 | 0.0010 |
| GO:0031667~response to nutrient levels | 0.0016 | 0.0020 |
| GO:0032963~collagen metabolic process | 0.0024 | 0.0008 |
| GO:0009991~response to extracellular stimulus | 0.0024 | 0.0035 |
| GO:0044259~multicellular organismal macromolecule metabolic process | 0.0029 | 0.0011 |
| GO:0009719~response to endogenous stimulus | 0.0036 | 0.0055 |
| GO:0044236~multicellular organismal metabolic process | 0.0041 | 0.0019 |
| GO:0001649~osteoblast differentiation | 0.0052 | 0.0000 |
| GO:0010810~regulation of cell-substrate adhesion | 0.0063 | 0.0375 |
| GO:0007155~cell adhesion | 0.0084 | 0.0001 |
| GO:0022610~biological adhesion | 0.0085 | 0.0001 |
| GO:0009612~response to mechanical stimulus | 0.0092 | 0.0061 |
| GO:0045785~positive regulation of cell adhesion | 0.0105 | 0.0074 |
| GO:0048729~tissue morphogenesis | 0.0111 | 0.0320 |
| GO:0040012~regulation of locomotion | 0.0132 | 0.0391 |
| GO:0009725~response to hormone stimulus | 0.0144 | 0.0111 |
| GO:0009628~response to abiotic stimulus | 0.0145 | 0.0359 |
| GO:0006952~defense response | 0.0200 | 0.0007 |
| GO:0060341~regulation of cellular localization | 0.0259 | 0.0248 |
| GO:0001503~ossification | 0.0355 | 0.0001 |
| GO:0014070~response to organic cyclic substance | 0.0390 | 0.0468 |
| GO:0060348~bone development | 0.0401 | 0.0002 |
Table 2. hTERT and hTERTmut co-related biology process in VA-13.
| Biology Process | VA-13-hTERT | VA-13-hTERTmut |
|---|---|---|
| PValue | PValue | |
| GO:0030182~neuron differentiation | 0.0003 | 0.0000 |
| GO:0048666~neuron development | 0.0005 | 0.0001 |
| GO:0030198~extracellular matrix organization | 0.0038 | 0.0460 |
| GO:0007155~cell adhesion | 0.0047 | 0.0894 |
| GO:0022610~biological adhesion | 0.0047 | 0.0900 |
| GO:0022604~regulation of cell morphogenesis | 0.0072 | 0.0005 |
| GO:0031175~neuron projection development | 0.0073 | 0.0036 |
| GO:0042981~regulation of apoptosis | 0.0098 | 0.0446 |
| GO:0043067~regulation of programmed cell death | 0.0103 | 0.0473 |
| GO:0010941~regulation of cell death | 0.0105 | 0.0483 |
| GO:0030705~cytoskeleton-dependent intracellular transport | 0.0108 | 0.0074 |
| GO:0008360~regulation of cell shape | 0.0116 | 0.0082 |
| GO:0006928~cell motion | 0.0138 | 0.0299 |
| GO:0001501~skeletal system development | 0.0155 | 0.0032 |
| GO:0060351~cartilage development involved in endochondral bone morphogenesis | 0.0181 | 0.0453 |
| GO:0007409~axonogenesis | 0.0205 | 0.0166 |
| GO:0030030~cell projection organization | 0.0247 | 0.0074 |
| GO:0048667~cell morphogenesis involved in neuron differentiation | 0.0252 | 0.0055 |
| GO:0048812~neuron projection morphogenesis | 0.0265 | 0.0242 |
| GO:0042127~regulation of cell proliferation | 0.0300 | 0.0010 |
| GO:0008284~positive regulation of cell proliferation | 0.0359 | 0.0013 |
| GO:0035107~appendage morphogenesis | 0.0361 | 0.0000 |
| GO:0035108~limb morphogenesis | 0.0361 | 0.0000 |
| GO:0000904~cell morphogenesis involved in differentiation | 0.0374 | 0.0113 |
| GO:0048858~cell projection morphogenesis | 0.0378 | 0.0406 |
| GO:0048736~appendage development | 0.0388 | 0.0000 |
| GO:0060173~limb development | 0.0388 | 0.0000 |
| GO:0001944~vasculature development | 0.0401 | 0.0442 |
| GO:0043627~response to estrogen stimulus | 0.0402 | 0.0087 |
| GO:0007411~axon guidance | 0.0416 | 0.0093 |
| GO:0032990~cell part morphogenesis | 0.0421 | 0.0474 |
Figure 3. Categorization of commonly regulated hTERT and hTERTmut GO terms.
The biological processes commonly enriched in U2OS-hTERT and U2OS-hTERTmut cells were classified into different group according to the related functions.
hTERT regulates cell adhesion independent of its telomerase activity
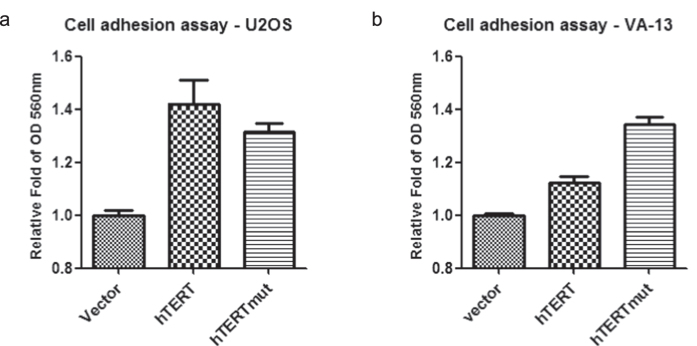
Strikingly, cell adhesion-related genes are present in the catalogues from both the U2OS and VA-13 cells expressing hTERT or hTERTmut (Fig. 3, Tables S3 & S4). This result encouraged us to propose that hTERT may function in regulating cell adhesion independent of its role in telomerase activity. To test this hypothesis, we performed cell adhesion assays using fibronectin as a substrate. The results showed that hTERT or hTERTmut expressing cells displayed increased adhesion to the extracellular matrix (ECM) (Fig. 4).
Figure 4. hTERT and hTERTmut expression promoted cell adhesion to the ECM.
(a) U2OS-vector, U2OS-hTERT, and U2OS-hTERTmut cells were cultured on fibronectin-coated plates for 1 hour and the adherent cells were detected by crystal violet staining. (b) VA-13-vector, VA-13-hTERT, and VA-13-hTERTmut cells were cultured on fibronectin-coated plates for 1 hour and adherent cells were detected by crystal violet staining.
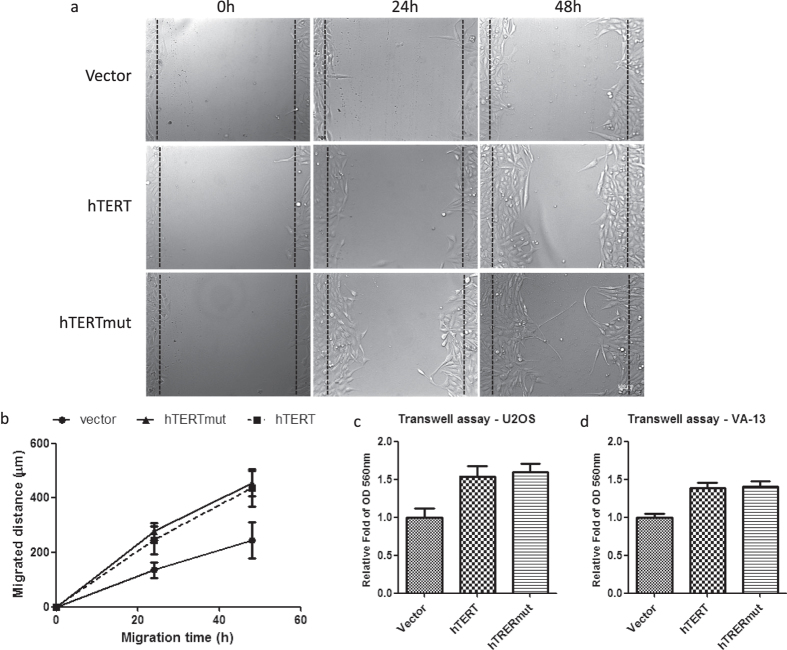
Cell adhesion is particularly important in tumor progression and metastasis. During metastasis, cell adhesion is down-regulated to detach cells from the primary carcinoma and then up-regulated to attach the same cells to distant tissues21. In this context, increased cell adhesion may enhance cell migration. We carried out monolayer wound healing and transwell assays to explore cell migration abilities. Wound closure speeds were significantly increased in both U2OS-hTERT and U2OS-hTERTmut expressed cells compared with control cells (Fig. 5a,b). Consistently, hTERT or hTERTmut expressing cells displayed an elevated ability to traverse a membrane from the serum-free to serum side (10% FBS) in the transwell assay (Fig. 5c,d).
Figure 5. hTERT and hTERTmut expression promoted cell migration.
(a) hTERT and hTERTmut expression promoted U2OS cell monolayer wound healing. Cells were cultured in a 6-well plate. After scratching a wound, cells were cultured in serum-free medium and photographed at 0, 24, and 48 h. (b) Statistical data from the cell migration distances in (a). (c,d) hTERT and hTERTmut expression promoted U2OS and VA-13 cell migration in a transwell assay.
Discussion
Noncanonical functions of TERT have been previously reported. For example, TERT protein has been shown to be involved in regulating gene expression of the Wnt pathway, thereby promoting hair growth in mice. Moreover, it has also been found that hTERT binds to an NF-κB subunit and directly regulates a subset of NF-κB targeted genes, such as IL-6, TNF-α, and MMPs15,22. Furthermore, TERT has been implicated in production of small interfering RNAs in a Dicer (also known as DICER1)-dependent manner. Despite the significance of these findings, it is challenging to extend these conclusions to different species or cell types23. Our data demonstrate that hTERT regulates different genes in different cells. Similar results have been reported previously6,9,10,11,12. For example, it was found that 172 genes displayed changes in expression in hTERT-immortalized BJ fibroblasts10. In contrast, in telomerase-immortalized bovine adrenocortical cell, 284 genes displayed changes in expression11. However, overlapping genes that exhibited changes in their expression between the two cell lines were very rare. These results and ours lead to the conclusion that hTERT regulates gene transcription in a cell type-dependent manner. A possible explanation is that regulation of gene expression by hTERT is indirect and mediated by co-factors that might be different in different cell types. In support of this hypothesis, hTERT has been reported to associate with BRG1, p65, and RMRP to modulate the transcription of genes related to the Wnt, NF-κB, and siRNA pathways, respectively15,16,22,24. Given that these genes might be differentially expressed in different cell lines, the downstream genes regulated by hTERT might also be different.
Even though hTERT expression mostly affected different genes in U2OS and VA-13 cells, a similar change in function was indeed found. Gene functional analysis identified a significant number of genes that are potentially involved in cell adhesion and migration. Of them many genes have been previously demonstrated to affect cell motion, EMT, cell invasion and cancer metastasis through regulating cell adhesion. For example, PDPN, SPP1, BARX2 and MMPs genes are reported to promote cell adhesion to ECM25,26,27,28,29; DSG2 facilitates the formation of structure that mediates cell-cell adhesion30; IL-8 enhances colon cancer cell migration by activating the expression of a disintegrin and metalloprotease (ADAM), a enzyme required for cell motion31; and ARHGDIB induces EMT, thus promoting cell invasion and cancer metastasis32,33.
Besides up-regulated genes, we also found that several genes are down-regulated by hTERT or hTERTmut. For instance, COL3A1 and GPR56 are reported to suppress the migration of neuron cells34; their down-regulation is expected to promote cell migration. Moreover, KRT17 is the only gene identified that is upregulated by expression of hTERT or hTERTmut in both U2OS and VA-13. It has been found that KRT17 promotes cell adhesion by activating AKT/PKB signaling pathway35.
Because hTERTmut and WT hTERT have the same phenotype, we concluded that the function of hTERT in promoting adhesion and cell migration was independent of telomerase activity. It was previously reported that TERT transgenic mice were more susceptible to develop skin tumors upon chemical carcinogenesis compared with normal mice36. Consistently, both wild-type hTERT and mutant hTERT without catalytic activity were reported to promote tumorigenesis in human cells5, and knockdown of endogenous hTERT could inhibit tumorigenicity in human HCT116 cells37. The mechanism for enhanced tumorigenesis by hTERT is complicated and37 might involve multiple events, including cell proliferation, anti-apoptosis, energy metabolism, tolerance to chromosomal instabilities, etc38,39,40,41,42,43. Many of these events might be related to telomere length homeostasis. In our experiments, U2OS are typical cancer cells that use the alternative lengthening of telomeres (ALT) pathway to maintain their telomere length, while VA-13 are immortalized cells that stem from human normal fibroblast cells. Our results indicated that the promotion of cell adhesion and migration may be a common function of hTERT in human cells. Our results also showed that although the genes are different in the two cells, some of them are involved in the formation of extracellular matrix (ECM) and matrix metalloproteinases (MMPs), a group of proteases responsible for ECM proteolysis. This provides a clue for further study regarding the regulatory mechanism underlying the increased cell adhesion and migration by hTERT.
Materials and Methods
Cell culture, vectors, and transfections
U2OS and VA-13 cells were cultivated in Dulbecco’s Modified Eagle Medium with 10% fetal bovine serum, 100 U/ml penicillin, and 100 μg/ml streptomycin. To generate hTERT or hTERTmut stably overexpressing cell lines, 293 T cells were transfected with pBabe-hTERT, pBabe-hTERTmut, or empty pBabe plasmids and the retroviral packaging plasmids pCMV-VSV.G and pCMV-Gag-Pol (Addgene) using calcium phosphate precipitation. The viral supernatants were collected 72 h after transfection, ultracentrifugated at 40,000 rpm for 2 h at 4 °C, and then used to infect U2OS and VA-13 cells. Forty-eight hours later, cells were selected with 2 μg/ml puromycin for 3 days, and then the retained cells were cultured in 1 μg/ml puromycin to produce a polyclonal cell population.
Cell adhesion assay
A 96-well plate was coated with 2.5 μg/ml human fibronectin (Millipore, CA) for 2 h at room temperature. Cells were seeded into a 96-well plate at a density of 4 × 104 cells/well and cultured for 1 h at 37 °C in a CO2 incubator. The cells were then rinsed three times with 10% formalin and stained with crystal violet for 5 min at room temperature. Then, the cells were washed three times with ddH2O and dissolved with 100 μl 33% acetic acid. The absorbance was detected at 560 nm on a Synergy H1 Multi-Mode Reader (BioTek).
Cell migration assay
Cell migration was measured by the transwell method. Briefly, cells were serum-deprived for 4 h and then seeded into Transwell Permeable Supports (Corning, NY), which were pre-equilibrated with serum-free DMEM for 1 h. For each group, 1 × 105 cells/insert were seeded into the upper compartment of the transwell in 100 μl serum-free DMEM. The insert was put into a 24-well plate that contained 600 μl of DMEM with 10% FBS. After 24 h of culture, the upper surface of the filter was erased with a cotton swab. Then, 10% formalin was added to the lower chamber for 10 min and subsequently stained with crystal violet for 5 min at room temperature. The insert was then washed three times with ddH2O and the crystal violet stained cells dissolved with 500 μl of 33% acetic acid. The absorbance was detected at 560 nm on a Synergy H1 Multi-Mode Reader (BioTek).
Monolayer wound healing assay
Cells were seeded into a 6-well plate at a density of 7 × 105 cells/well and cultured until confluent. A yellow pipette tip was used to make a straight scratch, which simulated a wound, and then the media was replaced with serum-free DMEM. Images were taken at 0, 24, and 48 h after scratching using a 10x objective.
Sample preparation and sequencing
Total RNA was extracted using the Trizol reagent (Takara). A total of 3 μg RNA per sample was used as input material for the RNA sample preparations. Sequencing libraries were generated using a NEBNext Ultra RNA Library Prep Kit for Illumina (NEB, USA) according to the manufacturer’s recommendations. Index codes were added to attribute sequences to each sample. Briefly, mRNA was purified from total RNA using poly-T oligo-attached magnetic beads. Fragmentation was carried out using divalent cations under elevated temperature in NEBNext First Strand Synthesis Reaction Buffer (5X). First strand cDNA was synthesized using random hexamer primers and M-MuLV Reverse Transcriptase (RNase H−). Second strand cDNA synthesis was subsequently performed using DNA polymerase I and RNase H. The remaining overhangs were converted into blunt ends with exonuclease/polymerase activities. After adenylation of the DNA fragment 3′ ends, NEBNext Adaptors with hairpin loop structures were ligated to prepare for hybridization. The library fragments were purified with an AMPure XP system (Beckman Coulter, Beverly, USA) to select cDNA fragments that were preferentially 150–200 bp in length. Then, 3 μl USER Enzyme (NEB, USA) was added to the size-selected, adaptor-ligated cDNAs at 37 °C for 15 min followed by 5 min at 95 °C before PCR. PCR was performed with Phusion High-Fidelity DNA polymerase, Universal PCR primers, and index (X) primers. Finally, PCR products were purified (AMPure XP system) and the library quality was assessed on an Agilent Bioanalyzer 2100 system. The clustering of the index-coded samples was performed on a cBot Cluster Generation System using a TruSeq SR Cluster Kit v3-cBot-HS (Illumia) according to the manufacturer’s instructions. Following cluster generation, the library preparations were sequenced on an Illumina Hiseq 2000 platform and 100 bp paired reads were generated.
Data analysis
All of the RNA-Seq reads were first checked with FastQC (http://www.bioinformatics.babraham.ac.uk/projects/fastqc/). Using homemade Java and GNUBash scripts, unpaired reads and those containing low PHRED score (<Q20) bases, Ns, or no adaptors were removed from the raw reads. The remaining high-quality 100 bp paired-end reads were then mapped to the UCSC build hg19 human genome with Tophat2 (Version 2.0.2)44 in paired-end mode. Reads that uniquely aligned were used for further analysis. The Htseq-count script (Version 0.6.0)3 was employed to assign the uniquely mapped reads to the genes. To obtain the expression level of each gene in different samples, fragments per kilobase per million sequenced reads (FPKM) was manually calculated per Trapnell C et al.45. Differential expression analysis was performed using the DESeq (version 1.20) package14 and genes with adjusted p-values smaller than 0.05 were considered to be differentially expressed.
Additional Information
Accession codes: The raw data of RNAseq have been submitted to GEO database. The GEO accession number is GSE73277.
How to cite this article: Liu, H. et al. hTERT promotes cell adhesion and migration independent of telomerase activity. Sci. Rep. 6, 22886; doi: 10.1038/srep22886 (2016).
Supplementary Material
Acknowledgments
This work was supported by the National Natural Science Foundation of China Grants [31301110; 31271472; 31322033], the Fundamental Research Funds for the Central Universities [131gpy60], the National Basic Research Program of China [2014CB964703]; the Guangdong Province Higher Vocational Colleges & Schools Pearl River Scholar Funded Scheme (2013); Foundation of Key Laboratory of Gene Engineering of the Ministry of Education.
Footnotes
Author Contributions H.L. prepared Figure 3, Figure 5, Table 1 and Table 2; Q.L. prepared Figure 1c,d and Figure 2b,c; Y.G. prepared Figure 1a,b and Figure 2a; Q.Z. performed the RNAseq data analysis; X.Z. prepared Figure 4. All authors reviewed the manuscript; Y.Z. and H.L. analyzed data and wrote the manuscript.
References
- Greider C. W. & Blackburn E. H. The telomere terminal transferase of Tetrahymena is a ribonucleoprotein enzyme with two kinds of primer specificity. Cell 51, 887–898 (1987). [DOI] [PubMed] [Google Scholar]
- Greider C. W. & Blackburn E. H. Identification of a specific telomere terminal transferase activity in Tetrahymena extracts. Cell 43, 405–413 (1985). [DOI] [PubMed] [Google Scholar]
- Meyerson M. et al. hEST2, the putative human telomerase catalytic subunit gene, is up-regulated in tumor cells and during immortalization. Cell 90, 785–795 (1997). [DOI] [PubMed] [Google Scholar]
- Feng J. et al. The RNA component of human telomerase. Science 269, 1236–1241 (1995). [DOI] [PubMed] [Google Scholar]
- Stewart S. A. et al. Telomerase contributes to tumorigenesis by a telomere length-independent mechanism. Proc Natl Acad Sci USA 99, 12606–12611 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J. et al. TERT promotes epithelial proliferation through transcriptional control of a Myc- and Wnt-related developmental program. PLoS Genet 4, e10 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarin K. Y. et al. Conditional telomerase induction causes proliferation of hair follicle stem cells. Nature 436, 1048–1052 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cong Y. & Shay J. W. Actions of human telomerase beyond telomeres. Cell Res 18, 725–732 (2008). [DOI] [PubMed] [Google Scholar]
- Smith L. L., Coller H. A. & Roberts J. M. Telomerase modulates expression of growth-controlling genes and enhances cell proliferation. Nat Cell Biol 5, 474–479 (2003). [DOI] [PubMed] [Google Scholar]
- Lindvall C. et al. Molecular characterization of human telomerase reverse transcriptase-immortalized human fibroblasts by gene expression profiling: activation of the epiregulin gene. Cancer Res 63, 1743–1747 (2003). [PubMed] [Google Scholar]
- Perrault S. D., Hornsby P. J. & Betts D. H. Global gene expression response to telomerase in bovine adrenocortical cells. Biochem Biophys Res Commun 335, 925–936 (2005). [DOI] [PubMed] [Google Scholar]
- Park Y. P. et al. Up-regulation of Mac-2 binding protein by hTERT in gastric cancer. Int J Cancer 120, 813–820 (2007). [DOI] [PubMed] [Google Scholar]
- Hahn W. C. et al. Inhibition of telomerase limits the growth of human cancer cells. Nat Med 5, 1164–1170 (1999). [DOI] [PubMed] [Google Scholar]
- Anders S. & Huber W. Differential expression analysis for sequence count data. Genome Biol 11, R106 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh A. et al. Telomerase directly regulates NF-kappaB-dependent transcription. Nat Cell Biol 14, 1270–1281 (2012). [DOI] [PubMed] [Google Scholar]
- Park J. I. et al. Telomerase modulates Wnt signalling by association with target gene chromatin. Nature 460, 66–72 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis G. Jr. et al. DAVID: Database for Annotation, Visualization, and Integrated Discovery. Genome Biol 4, P3 (2003). [PubMed] [Google Scholar]
- Takahashi K. et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131, 861–872 (2007). [DOI] [PubMed] [Google Scholar]
- Takahashi K. & Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126, 663–676 (2006). [DOI] [PubMed] [Google Scholar]
- Sun C. K. et al. Senescence impairs direct conversion of human somatic cells to neurons. Nat Commun 5, 4112 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Paschos K. A., Canovas D. & Bird N. C. The role of cell adhesion molecules in the progression of colorectal cancer and the development of liver metastasis. Cell Signal 21, 665–674 (2009). [DOI] [PubMed] [Google Scholar]
- Ding D., Xi P., Zhou J., Wang M. & Cong Y. S. Human telomerase reverse transcriptase regulates MMP expression independently of telomerase activity via NF-kappaB-dependent transcription. FASEB J 27, 4375–4383 (2013). [DOI] [PubMed] [Google Scholar]
- Listerman I., Gazzaniga F. S. & Blackburn E. H. An investigation of the effects of the core protein telomerase reverse transcriptase on Wnt signaling in breast cancer cells. Mol Cell Biol 34, 280–289 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maida Y. et al. An RNA-dependent RNA polymerase formed by TERT and the RMRP RNA. Nature 461, 230–235 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J. et al. Osteopontin (OPN/SPP1) isoforms collectively enhance tumor cell invasion and dissemination in esophageal adenocarcinoma. Oncotarget 6, 22239–22257 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson G. A., Burghardt R. C. & Bazer F. W. Osteopontin: a leading candidate adhesion molecule for implantation in pigs and sheep. J Anim Sci Biotechnol 5, 56 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang Q., Liu J., Li J. & Sun Y. Podoplanin: a novel regulator of tumor invasion and metastasis. Med Oncol 31, 24 (2014). [DOI] [PubMed] [Google Scholar]
- Tsuneki M., Yamazaki M., Maruyama S., Cheng J. & Saku T. Podoplanin-mediated cell adhesion through extracellular matrix in oral squamous cell carcinoma. Lab Invest 93, 921–932 (2013). [DOI] [PubMed] [Google Scholar]
- Tsau C. et al. Barx2 and Fgf10 regulate ocular glands branching morphogenesis by controlling extracellular matrix remodeling. Development 138, 3307–3317 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta A. et al. Cell cycle- and cancer-associated gene networks activated by Dsg2: evidence of cystatin a deregulation and a potential role in cell-cell adhesion. PLoS One 10, e0120091 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh Y. et al. IL-8 promotes cell proliferation and migration through metalloproteinase-cleavage proHB-EGF in human colon carcinoma cells. Cytokine 29, 275–282 (2005). [DOI] [PubMed] [Google Scholar]
- Cho H. J. et al. RhoGDI2 promotes epithelial-mesenchymal transition via induction of Snail in gastric cancer cells. Oncotarget 5, 1554–1564 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaulfuss S. et al. Leupaxin, a novel coactivator of the androgen receptor, is expressed in prostate cancer and plays a role in adhesion and invasion of prostate carcinoma cells. Mol Endocrinol 22, 1606–1621 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo R. et al. G protein-coupled receptor 56 and collagen III, a receptor-ligand pair, regulates cortical development and lamination. Proc Natl Acad Sci USA 108, 12925–12930 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankar S. et al. A novel role for keratin 17 in coordinating oncogenic transformation and cellular adhesion in Ewing sarcoma. Mol Cell Biol 33, 4448–4460 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Suarez E. et al. Increased epidermal tumors and increased skin wound healing in transgenic mice overexpressing the catalytic subunit of telomerase, mTERT, in basal keratinocytes. EMBO J 20, 2619–2630 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y., Zhang Y. W., Zhang Z. X., Miao Z. H. & Ding J. hTERT-targeted RNA interference inhibits tumorigenicity and motility of HCT116 cells. Cancer Biol Ther 7, 228–236 (2008). [DOI] [PubMed] [Google Scholar]
- Fleisig H. B. et al. Telomerase reverse transcriptase expression protects transformed human cells against DNA-damaging agents, and increases tolerance to chromosomal instability. Oncogene 10.1038/onc.2015.75. (2015). [DOI] [PubMed] [Google Scholar]
- Zhou L., Zheng D., Wang M. & Cong Y. S. Telomerase reverse transcriptase activates the expression of vascular endothelial growth factor independent of telomerase activity. Biochem Biophys Res Commun 386, 739–743 (2009). [DOI] [PubMed] [Google Scholar]
- Massard C. et al. hTERT: a novel endogenous inhibitor of the mitochondrial cell death pathway. Oncogene 25, 4505–4514 (2006). [DOI] [PubMed] [Google Scholar]
- Rahman R., Latonen L. & Wiman K. G. hTERT antagonizes p53-induced apoptosis independently of telomerase activity. Oncogene 24, 1320–1327 (2005). [DOI] [PubMed] [Google Scholar]
- Xiang H. et al. Human telomerase accelerates growth of lens epithelial cells through regulation of the genes mediating RB/E2F pathway. Oncogene 21, 3784–3791 (2002). [DOI] [PubMed] [Google Scholar]
- Bagheri S. et al. Genes and pathways downstream of telomerase in melanoma metastasis. Proc Natl Acad Sci USA 103, 11306–11311 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D. et al. TopHat2: accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol 14, R36 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. et al. Accurate quantification of transcriptome from RNA-Seq data by effective length normalization. Nucleic Acids Res 39, e9 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.