Abstract
Background:
necrotizing enterocolitis is a serious condition that affects mostly preterm infants, with high mortality rate.
Aim:
to estimate the influence of potentially contributing factors of this multifactorial disease.
Methods:
the study group included 51 necrotizing enterocolitis infants who were less than 37 week gestation who were hospitalized in NICU during a five year period. The control group consisted of 71 patients with approximately the same gestational age and birth weight. Average gestational age in the study group was 30.2 weeks (SD 3.7), average birth weight 1502g (SD 781.5). Average postnatal age in the time of the presenting NEC was 18.2 days (SD 12.8).
Results:
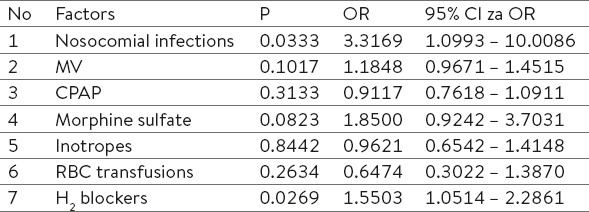
Logistic regression estimates the influence of risk factors, which in our study related to the treatment of preterm infants on the likelihood of NEC development. Our regression model consisted of seven independent variables (nosocomial infections, mechanical ventilation, nasal continuous positive pressure, morphine, inotropes, blood transfusions, and H2 blockers), which were shown to have a statistically significant impact, X2 (7, n=1222) = 49.522, p<0.0001; two independent variables (nosocomial infection and H2 blockers use) were statistically significant. Preterm infants with nosocomial infection had a three times greater chance of developing NEC, and infants who received H2 blockers had a 1.5 higher risk.
Conclusions:
Underlying pathology of very low birth weight infants and their treatment in NICU contribute to NEC development. Identifying risk factors can be crucial for the early diagnosis and outcome of disease. Awareness of risk factors should influence changes in practice to reduce the risk of NEC.
Keywords: necrotizing enterocolitis, preterm infants, contributing factors
1. INTRODUCTION
Necrotizing enterocolitis (NEC), characterized by coagulation necrosis and inflammation of the intestine, is a serious condition that usually affects preterm infants, with high mortality rate (1). The disease occurs in 1-5% of neonatal intensive care admissions, but 5-10% of very low birth weight (VLBW) infants have NEC (2). The mortality rate of VLBW preterms has continued to reduce over the time, due to better prenatal and neonatal care, antenatal corticosteroid therapy use, and noninvasive respiratory support in the neonatal intensive care units (NICU) (3, 4). Despite advances in the care of premature infants, NEC remains one of the leading causes of morbidity and mortality in this population (5).
Although the exact etiology of necrotizing enterocolitis (NEC) remains unknown, researchers suggest that it is multifactorial. Prematurity (with immature GIT and host defenses) is the primary risk factor (6); ischemia and/or reperfusion injury, exacerbated by activation of pro-inflammatory intracellular cascades may play a significant role (7).
Various studies have identified risk factors for the development of NEC, including genetic predisposition (8), alterations in the normal bacterial colonization of the gastrointestinal tract (9), and introduction and advancement of enteric feeding (10). Awareness of the risk factors for NEC changes a practice to reduce the risk, including early trophic feeding with breast milk and following the established feeding guidelines. Administration of probiotics in recent time has been shown to reduce the incidence of NEC (11).
Despite advances in management of VLBW infants, aggressive and invasive treatment is needed to achieve survival of extremely preterm infants, especially in countries in which there is a low rate of antenatal corticosteroid usage (12). However, understanding of possibly harmful effects of any management alternative is crucial in reducing the morbidity rate of and disease, including NEC; a disease in which therapeutic interventions include: red blood cell transfusions, long term antibiotic therapy for nosocomial infections, mechanical ventilation, infusion of morphine to relieve pain, reduce the stress response, and of medications including methylxanthines and H2 blockers.
2. PATIENTS AND METHODS
This retrospective study was performed on all NEC preterm infants (<37 weeks gestation at birth) admitted in the NICU of our institution over a period of five years, from 2008 to 2012. Gestational age was routinely determined from that last menstrual period, early ultrasound investigation or using the New Ballard score, and recorded as completed weeks (13).
Diagnosis of NEC was made based on the presence of clinical, radiological and/or histopathological evidence that fulfilled the criteria of Bell’s (14) as well as Walsh’s modification of these criteria (15).
Definition of nosocomial infection (NI): NI infection is defined as an infection that occurs after 48 hours of hospitalization, resulting in a positive blood, cerebrospinal fluid (CSF), or urine culture with clinical manifestations such as hospital-acquired bloodstream infections, nosocomial pneumonia, sepsis, urinary tract infection and meningitis.
Medical NEC was defined as the presence of radiological signs of intestinal pneumatosis and when the disease is treated with antibiotics for more than two days. Surgical NEC was defined as any surgical treatment. The infants’ medical records were reviewed daily for medical course information until hospital discharge or death of infant. A standardized format was used for data collection.
Statistical analysis
Statistical analysis was performed using SPSS 16.0 (SPSS Inc, Chicago, IL, USA). The number of infants with each investigated factors (nosocomial infections, MV, NCPAP, morphine sulfate, inotropes, RBC transfusions, H2 blockers) was compared between groups with and without NEC. Categorical variables were compared using the χ2 test. The means of continuous variables were compared using Student’s t test, and the data are presented as mean (SD). The influence of relevant confounding variables, identified by univariate analysis, was assessed using multivariate logistic regression analysis. Confidence intervals presented for odds ratios are adjusted for the clustering of infants within participating nurseries. Statistical level of 95% (p<0.05) was considered as significant for all performed tests.
3. RESULTS
During the study period, 830 preterm infants were admitted in the NICU; 51 (6.1%) got NEC. Control group consisted of 71 randomly selected preterm infants that were not significantly different in BW and GA from premature infants with NEC. In the group of patients with NEC, based on the diagnostic criteria (12, 13), presence of the medical NEC established in 30 patients (58.8%) while the surgical NEC found in 21 patients (41.2%).
Average gestational age of preterm infants with NEC was 30.2 GW (SD 3.7), average birth weight 1502.75 g (SD 781.5). Postnatal age in time of appearance of NEC was 18.2 days (SD 12.8) (2-57 days); 49% infants were older than 2 weeks. In one patient NEC developed before starting of enteral feeding. The most common gastrointestinal symptoms in the study group were: abdominal distension in 89% cases, macroscopic or microscopic blood in the stool in 56.9% and increasing gastric residuum in 46% cases.
Table 2.
Risk factors related to the hospitalization and treatment of VLBW infants

29/51 (56.9%) of premature infants with NEC had at least one or more of NI prior to NEC diagnosis. In the control group of patients, NI’s were present in 23.9% (17/71). There was statistically significant difference regarding infections between groups χ2 (1, N = 122) = 12.328, p = 0.0004.
37/51 (72.5%) infants in NEC group received morphine sulfate during the hospitalization, (average 2.7 days), in control group 14/71 (19.7%), average 0.37 days. Statistically significant difference was found related to morphine sulfate treatment between groups χ2 (1, n=122) = 31.914, p=0.0001.
33/51 (64.7%) infants in NEC group were treated with inotropes an average of 2 days, compared to the control group 12.7% (9/71). Statistically significant difference is noted between group of NEC and control group.χ2 (1, n=122) = 33.325, p=0.0001.
Statistically significant difference was found in number of days of mechanical ventilation in NEC group (Md=8, n=43) compared to control group (Md=3, n=22), U=262.00, z=2.955, p=0.0031.
There was statistical difference between average number of days on NCPAP in NEC group (Md=5 n=25) and control group (Md=3 n=39). U=313.50, z=2.413; p=0.0158.
Table 3.
Model for logistic regression – factors of treatments
20/51 (39.2%) infants in NEC group received H2 blockers (an average of 2.2 days), in control group 6/71 (8.5%) (an average of 0.25 days), which is statistically significant χ2 (1, n=122) = 14.967, p=0.0001.
There was statistical difference in RBC transfusions in NEC group (Mean 1.53 n=31) and control (mean 0.55 n=21).χ2 (1, n=122) = 10.578, p=0.0011.
Logistic regression is done to estimate the influence of factors related to treatment of sick infants on possibility to develop NEC. Model consists of 7 independent variables (nosocomial infections, MV, NCPAP, morphine sulfate, inotropes, RBC transfusions, H2 blockers).
Whole model with all predictors was statistically significant, χ2 (7, n=122) = 49.522, p<0.0001, which indicates that model can recognize infants who may develop NEC later. Two independent variables (nosocomial infections and H2 blockers use gave statistically significant attribution to the model. Logistic regression analysis showed that there was a statistically significant association in the number of nosocomial infections prior NEC diagnosis with the development of NEC (p<0.05). Based on the result of logistic regression analysis, it can be concluded that each additional infection increased the odds of developing NEC by 3 times, and use of H2 blockers 1.5 times.
4. DISCUSSION
Incidence of NEC in our study was 6.1% (51/830) consequently admitted preterm infants in NICU. The incidence generally varies from neonatal units, regions and countries, as can be determined by different definition of NEC (2). Although in clinical practices predominant definition is suggested by Bell et al. (14) and modified by Walsh et al. (15), in literature still exists mismatch of uniform recognition and classification of suspected NEC in VLBW and ELBW infants (2). To determine potential risk factors and predictors for NEC development (clinical, radiological and laboratory), we tried to achieve the most homogeneous study group of infants with NEC and control group; there was no statistical difference between groups in gestational age and birth weight. Average gestational age in patients with NEC was 29.96 ± 3.67, BW 1461.08 ± 781.47 g, which is in agreement with similar studies (16, 17).
Investigations related to nosocomial infection and developing of NEC indicate that increasing number of infections before clinical manifestation is associated with increasing risk for NEC (18). Although there is no exact identification of reasons for this kind of connection in the literature, it is assumed that can be in relationship with duration of parenteral nutrition. Parenteral nutrition has been shown to have immunosuppressive effect with decreasing the grade of phagocytosis and neutralization coagulase negative staphylococci. (19). Results of our study showed that the number of nosocomial infections before clinical manifestation of NEC was significantly higher than in control group (p=0.0004).
Respiratory insufficiency of preterm infants is relatively frequent, including inability to maintain normal gas exchange. That is why respiratory support routinely carried out especially in VLBW and ELBW infants, using mechanical ventilation (MV) or continuous positive pressure ventilation through nasal prongs (NCPAP). NCPAP as non-invasive procedure used today as important alternative, has reducing the incidence of chronic lung disease, retinopathy, intraventricular bleeding and incidence of neurodevelopmental disorders. Meyer et al. (20) concluded that VLBW and modality of respiratory support can be a risk factor for NEC development, so it can serve as a predictor for development and severity of the disease. Possible explanations can be in cognition that VLBW infants have higher need for respiratory support before gastrointestinal symptoms, compared to infants of the same birth weight without NEC. Dolgin et al. (21) in their study found that preterm infants with surgical NEC have higher need for respiratory support–MV or NCPAP compared to preterm infants with medical NEC. Results of our study showed that duration of mechanical ventilation in days prior gastrointestinal symptoms in infants with NEC was statistically significantly higher compared to control group (p=0.0031).
Although anemia of prematurity is the usual in this group of age, caused by incomplete placental transport of Fe, leak of complete fetal erythropoiesis, iatrogenic blood loss, low level of erythropoietin in plasma and increasing needs due to fast body growth, frequent red blood cell transfusions were common clinical practices in NICUs. This practice is now revised and becomes more restrictive. Reasons for this are in the cognition of relationship between RBC, acute intestinal injury and serious gastrointestinal reaction, especially in ELBW and extremely preterm infants (22, 23).
However, mechanism of which RBC causes injury on GI tract of preterm infants is not researched enough. Simmonds et al. (24) offered some explanations as decreased capacity of nitric oxide storage in packed RBC, excessive intestinal immunological response and alteration of mesenteries blood flow after RBC transfusion result in intestinal hypoxia and intestinal mucosal injury. All of that suggest that RBC transfusions can cause alteration gastrointestinal microcirculation in the supply of oxygen during this vulnerable period and significantly contribute to NEC developing.
Our study showed that preterm infants with NEC had significantly more RBC transfusions before clinical signs of NEC compared to control group (p=0.0005). This result is in concordance with similar studies that determined positive correlation between RBC transfusions and appearance of NEC (22, 23).
Morphine sulfate is used to be common praxis in ventilated infants in NICUs due better of synchronization with ventilator, pain relief and reduction of stress response. Its use has been decreasing steadily because of its adverse effects including hypotension, bradycardia, delay in beginning of enteral feeding and reduction of gastrointestinal motility. (25). General acceptability of morphine administration in preterm infants in era of non-invasive ventilation and high antenatal steroid use is now questionable (25).
Hällström et al. (26) first noticed that duration of morphine sulfate administration have significant influence on NEC development. Additional explanation is that reduction of gastrointestinal motility allows prolonged contact GI bacteria with feeding substrates and gut wall, bacterial translocation with increasing possibility for development of disease. Our results showed that infants with NEC had statistically significant higher number of days of morphine administration compared to control group (p<0.0001).
Use of inotropes (dubutamine, dopamine) is common in presence of shock or hypotension, to achieve cardiovascular stabilization. Action of dobutamine is based on its action on β receptors, resulting in improving of heart contractibility, vasodilatation and mild tachycardia. Bedside this action, Hentschel et al. (27) using doppler ultrasonography on mesenteric artery, determined increased intestinal perfusion and concluded that is no influence on NEC development, although pathophysiological mechanism of this connections still is not clear.
Use of inhibitors gastric acid secretion can cause insufficient elimination of ingested pathogens and increase risk of nosocomial infections, due to alkalization of gastric content which normally presents the main non-immune mechanism of defense against infection (28). HistaminH2 receptors blockers and proton pump inhibitors (ranitidin, famotin, and cimetidin) increase the risk of infection and NEC in neonatal period (28). Administration of those medicaments reduces proteolytic activity of gastric secretion, allowing gastric colonization with gram negative strains, and consecutive pneumonia and gram negative sepsis. (28) Use of Histamine H2 blockers in NICUs is empiric, especially in infants with proven GI bleeding and GI reflux. Although in these cases administration can protect mucosa from extensive production of gastric acid and prevent stress ulcers, in the same time it can neutralize natural defense against overgrowth propagation (28). In study in Terin et al (28) in infants receiving H2 blockers number of infection was 4 times higher. Our investigation showed significant association between H2 blockers and NEC development (p=0.0001).
Logistic regression of 7 independent variables (nosocomial infections, MV, NCPAP, morphine sulfate, inotropes, RBC transfusions, H2 blockers) was statistically significant, χ2 (7, n=122) = 49.522, p<0.0001, which indicates that model can recognize infants who may develop NEC later. Two independent variables (nosocomial infections and H2 blockers use gave statistically significant attribution to the model.
Based on the result of logistic regression analysis, it can be concluded that each additional infection increased the odds of developing NEC by 3 times, and administration of H2 blockers 1.5 times.
5. CONCLUSION
Underlying pathology of VLBW infants and their treatment in NICU contribute to NEC development. Identifying risk factors can be crucial for the early diagnosis and outcome of disease. Awareness of those risk factors changes practices to reduce the risk of NEC.
Footnotes
• Author’s contribution: All authors in this paper have contributed in all phases in it’s preparing. First author made final proof reading.
• Conflict of interest: none declared.
REFERENCES
- 1.Caplan MS. Neonatal necrotizing enterocolitis. Introduction. Semin Perinatol. 2008;32(2):69. doi: 10.1053/j.semperi.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 2.Holman RC, Stoll BJ, Curns AT, Yorita KL, Steiner CA, Schonberger LB. Necrotizing enterocolitis hospitalizations among neonates in the United States. Paediatr Perinat Epidemiol. 2006;20(6):498–506. doi: 10.1111/j.1365-3016.2006.00756.x. [DOI] [PubMed] [Google Scholar]
- 3.Neu J, Mshvildadze M, Mai V. A roadmap for understanding and preventing necrotizing enterocolitis. Curr Gastroenterol Rep. 2008;10(5):450–7. doi: 10.1007/s11894-008-0084-x. [DOI] [PubMed] [Google Scholar]
- 4.Kafetzis DA, Skevaki C, Costalos C. Neonatal necrotizing enterocolitis:an overview. Curr Opin Infect Dis. 2003;16(4):349–55. doi: 10.1097/00001432-200308000-00007. [DOI] [PubMed] [Google Scholar]
- 5.Lin PW, Stoll BJ. Necrotising enterocolitis. Lancet. 2006;368(9543):1271–83. doi: 10.1016/S0140-6736(06)69525-1. [DOI] [PubMed] [Google Scholar]
- 6.Moss RL, Kalish LA, Duggan C, Johnston P, Brandt ML, Dunn JC, et al. Clinical parameters do not adequately predict outcome in necrotizing enterocolitis:a multi-institutional study. J Perinatol. 2008;28(10):665–74. doi: 10.1038/jp.2008.119. [DOI] [PubMed] [Google Scholar]
- 7.Lin PW, Nasr TR, Stoll BJ. Necrotizing enterocolitis:recent scientific advances in pathophysiology and prevention. Semin Perinatol. 2008;32(2):70–82. doi: 10.1053/j.semperi.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 8.Sampath V, Le M, Lane L, Patel AL, Cohen JD, Simpson PM, Garland JS, Hines RN. The NFKB1 (g.-24519delATTG) variant is associated with necrotizing enterocolitis (NEC) in premature infants. J Surg Res. 2011;169(1):e51–57. doi: 10.1016/j.jss.2011.03.017. [DOI] [PubMed] [Google Scholar]
- 9.Torrazza RM, Neu J. The altered gut microbiome and necrotizing enterocolitis. Clin Perinatol. 2013;40(1):93–108. doi: 10.1016/j.clp.2012.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kliegman RM. The relationship of neonatal feeding practices and the pathogenesis and prevention of necrotizing enterocolitis. Pediatrics. 2003;111(3):671–2. doi: 10.1542/peds.111.3.671. [DOI] [PubMed] [Google Scholar]
- 11.Frost BL, Caplan MS. Probiotics and prevention of neonatal necrotizing enterocolitis. Curr Opin Pediatr. 2011;23(2):151–5. doi: 10.1097/MOP.0b013e328343d65f. [DOI] [PubMed] [Google Scholar]
- 12.Güran Ö, Bülbül A, Uslu S, Dursun M, Zubarioğlu U, Nuhoğlu A. Mortality and morbidity in very low birth weight infants. Turk Arch Ped. 2013:102–9. [Google Scholar]
- 13.Ballard JL, Khoury JC, Wedig K, Wang L, Eilers-Walsman BL, Lipp R. New Ballard Score, expanded to include extremely premature infants. J Pediatr. 1991;119(3):417–23. doi: 10.1016/s0022-3476(05)82056-6. [DOI] [PubMed] [Google Scholar]
- 14.Bell MJ, Ternberg JL, Feigin RD, Keating JP, Marshall R, Barton L, Brotherton T. Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Ann Surg. 1978;187:1–7. doi: 10.1097/00000658-197801000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Walsh MC, Kliegman RM. Necrotizing enterocolitis:treatment based on staging criteria. Pediatr Clin North Am. 1986;33:179–201. doi: 10.1016/S0031-3955(16)34975-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin PW, Nasr TR, Stoll BJ. Necrotizing enterocolitis:recent scientific advances in pathophysiology and prevention. Semin Perinatol. 2008;32(2):70–82. doi: 10.1053/j.semperi.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 17.Thompson AM, Bizzarro MJ. Necrotizing enterocolitis in newborns:pathogenesis, prevention and management. Drugs. 2008;68(9):1227–38. doi: 10.2165/00003495-200868090-00004. [DOI] [PubMed] [Google Scholar]
- 18.Zvizdic Z, Heljic S, Firdus A, Jonuzi A, Zvizdic D. Relationship of nosocomial infections with the development of necrotizing enterocolitis in preterm infants. Mater Sociomed. 2014;26(1):4–6. doi: 10.5455/msm.2014.26.4-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Flidel-Rimon O, Friedman S, Lev E, Juster-Reicher A, Amitay M, Shinwell ES. Early enteral feeding and nosocomial sepsis in very low birthweight infants. Arch Dis Child Fetal Neonatal. (Ed) 2004;89(4):F289–292. doi: 10.1136/adc.2002.021923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meyer M, Mildenhall L, Wong M. Outcomes for infants weighing less than 1000 grams cared for with a nasal continuous positive airway pressure-based strategy. J Paediatr Child Health. 2004;40(1-2):38–41. doi: 10.1111/j.1440-1754.2004.00287.x. [DOI] [PubMed] [Google Scholar]
- 21.Dolgin SE, Shlasko E, Levitt MA, Hong AR, Brillhart S, Rynkowski M, Holzman I. Alterations in respiratory status:early signs of severe necrotizing enterocolitis. J Pediatr Surg. 1998;33(6):856–8. doi: 10.1016/s0022-3468(98)90659-5. [DOI] [PubMed] [Google Scholar]
- 22.Blau J, Calo JM, Dozor D, Sutton M, Alpan G, La Gamma EF. Transfusion-related acute gut injury:necrotizing enterocolitis in very low birth weight neonates after packed red blood cell transfusion. J Pediatr. 2011;158(3):403–9. doi: 10.1016/j.jpeds.2010.09.015. [DOI] [PubMed] [Google Scholar]
- 23.Paul DA, Mackley A, Novitsky A, Zhao Y, Brooks A, Locke RG. Increased odds of necrotizing enterocolitis after transfusion of red blood cells in premature infants. Pediatrics. 2011;127(4):635–41. doi: 10.1542/peds.2010-3178. [DOI] [PubMed] [Google Scholar]
- 24.Simmonds A, LaGamma Ef. Addressing the ”new” NEC, part I:rediscovering the basics. Indian J Pediatr. 2006;73(11):1011–8. doi: 10.1007/BF02758310. [DOI] [PubMed] [Google Scholar]
- 25.Cignacco E, Hamers JP, van Lingen RA, Zimmermann LJ, Müller R, Gessler P, et al. Pain relief in ventilated preterms during endotracheal suctioning:a randomized controlled trial. Swiss Med Wkly. 2008;138(43-44):635–45. doi: 10.4414/smw.2008.12288. [DOI] [PubMed] [Google Scholar]
- 26.Hällström M, Koivisto AM, Janas M, Tammela O. Frequency of and risk factors for necrotizing enterocolitis in infants born before 33 weeks of gestation. Acta Paediatr. 2003;92(1):111–3. doi: 10.1111/j.1651-2227.2003.tb00479.x. [DOI] [PubMed] [Google Scholar]
- 27.Hentschel R, Hensel D, Brune T, Rabe H, Jorch G. Impact on blood pressure and intestinal perfusion of dobutamine or dopamine in hypotensive preterm infants. Biol Neonate. 1995;68(5):318–24. doi: 10.1159/000244252. [DOI] [PubMed] [Google Scholar]
- 28.Terrin G, Passariello A, De Curtis M, Manguso F, Salvia G, Lega L, et al. Ranitidine is associated with infections, necrotizing enterocolitis, and fatal outcome in newborns. Pediatrics. 2012;129(1):e40–45. doi: 10.1542/peds.2011-0796. [DOI] [PubMed] [Google Scholar]


