Abstract
STUDY DESIGN
Single-blind randomized trial.
OBJECTIVES
To compare the effects of cervical and shoulder thrust manipulation (TM) and exercise on pain sensitivity, and to explore associations with clinical outcomes in patients with shoulder pain.
BACKGROUND
Experimental studies indicate that spinal TM has an influence on central pain processes, supporting its application for treatment of extremity conditions. Direct comparison of spinal and peripheral TM on pain sensitivity has not been widely examined.
METHODS
Seventy-eight participants with shoulder pain (36 female; mean ± SD age, 39.0 ± 14.5 years) were randomized to receive 3 treatments of cervical TM (n = 26), shoulder TM (n = 27), or shoulder exercise (n = 25) over 2 weeks. Twenty-five healthy participants (13 female; mean ± SD age, 35.2 ± 11.1 years) were assessed to compare pain sensitivity with that in clinical participants at baseline. Primary outcomes were changes in local (eg, shoulder) and remote (eg, tibialis anterior) pressure pain threshold and heat pain threshold occurring over 2 weeks. Secondary outcomes were shoulder pain intensity and patient-rated function at 4, 8, and 12 weeks. Analysis-of-variance models and partial-correlation analyses were conducted to examine comparative effects and the relationship between measures.
RESULTS
At baseline, clinical participants demonstrated lower local (mean difference, −1.63 kg; 95% confidence interval [CI]: −2.40, −0.86) and remote pressure pain threshold (mean difference, −1.96 kg; 95% CI: −3.09, −0.82) and heat pain threshold (mean difference, −1.15°C; 95% CI: −2.06, −0.24) compared to controls, suggesting enhanced pain sensitivity. Following intervention, there were no between-group differences in pain sensitivity or clinical outcome (P>.05). However, improvements were noted, regardless of intervention, for pressure pain threshold (range of mean differences, 0.22–0.32 kg; 95% CI: 0.03, 0.43), heat pain threshold (range of mean differences, 0.30–0.58; 95% CI: 0.06, 0.96), pain intensity (range of mean differences, −1.79 to −1.45; 95% CI: −2.34, −0.94), and function (range of mean differences, 3.15–3.82; 95% CI: 0.69, 6.20) at all time points. We did not find an association between pain sensitivity changes and clinical outcome (P>.05).
CONCLUSION
Clinical participants showed enhanced pain sensitivity, but did not respond differently to cervical or peripheral TM. In fact, in this sample, cervical TM, shoulder TM, and shoulder exercise had similar pain sensitivity and clinical effects. The lack of association between pain sensitivity and clinical pain and function outcomes suggests different (eg, nonspecific) pain pathways for clinical benefit following TM or exercise.
Keywords: manual therapy, pain mechanism, quantitative sensory testing, shoulder
Shoulder pain is a common complaint leading patients to seek health care.11,44,64 Moreover, shoulder pain is among the most expensive injury conditions to manage, along with back and knee injuries.50 Recovery from shoulder pain is problematic, with up to 40% of individuals reporting continued pain at 12 months and 50% reporting disability at 18 months.22,46,62 Conservative therapies are generally considered the first-line choice for managing shoulder pain.54 Manual therapy, including spinal thrust manipulation (TM), is one treatment option that conservative treatment programs have incorporated. Studies of manual therapy show promising findings suggesting that manual therapy may be beneficial in the treatment of some individuals with shoulder pain.12,38,47,49,65 However, the mechanisms through which these techniques act remain unknown.
Heightened pain sensitivity at local and remote areas to the shoulder has been observed in patients with unilateral shoulder pain, suggesting central sensitization.20,21,36,37,53,61 Central sensitization has been linked to the progression and maintenance of chronic pain, and disrupting this process is paramount.2,34 Thus, the changes in pain sensitivity that accompany shoulder pain provide a therapeutic target for manual therapy and a potential mechanism for its beneficial clinical effects. Bialosky et al4 have presented a mechanistic model suggesting that a manual therapy stimulus initiates a train of neurophysiological events that occur at distinct but related levels of the peripheral and central nervous system, thereby influencing the patient perception of pain. The literature clearly indicates that neurophysiological responses, such as changes in pain sensitivity, are associated with manual therapy interventions.7,19,27,28,32,48,63 However, clarification is required to better determine (1) the extent to which the specific features of the mechanical stimulus (ie, changing location of TM application) influence pain sensitivity response, and (2) the clinical relevance of the associated neurophysiological responses.
Widespread changes in pain sensitivity have been observed as an immediate response to spinal TM, indicating that spinal TM may have “general” effects on pain sensitivity.19,48 For example, Fernández-de-las-Peñas et al28 observed immediate reductions in pressure pain sensitivity at the elbow region following cervical TM. Studies using thermal temporal summation of pain have also shown an immediate effect of spinal TM for modulating central pain pathways.7–9,32 Based on this evidence, spinal TM would be the theoretically preferred option for a condition like shoulder pain, in which disrupting central pain processes is the goal. However, studies comparing the effects of spinal and peripheral TM (ie, procedures directed to the extremity) are not common. Shoulder pain is characterized by heightened pain sensitivity at regions local and remote to the shoulder.20,21,36,37,53 Effects on pain sensitivity can be tested at these regions to assess whether spinal and peripheral TM influences local or central sensitivity, or both. It is possible that spinal TM may have different effects on pain sensitivity than peripheral TM.
The primary aim of this study was to advance the current mechanistic evidence on TM by investigating the effects of the location of TM application on pain sensitivity. A secondary aim was to explore the association of changes in pain sensitivity with clinical outcome. To accomplish our aims, we first compared pain sensitivity responses between participants with unilateral shoulder pain and healthy age- and sex-matched controls to identify markers of altered pain processing. We hypothesized that participants with shoulder pain would demonstrate enhanced pain sensitivity at areas local and remote to the shoulder, and we wished to identify specific measures to target (ie, those that differed from healthy controls). Next, we randomly assigned individuals with shoulder pain to receive TM directed to the cervical spine or shoulder. We examined the extent to which cervical TM differed in its effects on local and remote pain sensitivity compared to shoulder TM or exercise (control group). Finally, we explored the association between changes in pain sensitivity and longitudinal changes in clinical outcomes. Significant relationships between changes in pain sensitivity and clinical outcomes would support pain sensitivity as a potential treatment target and mechanism by which manual therapy interventions inhibit pain.
METHODS
Study Design
This study was a single-blind randomized trial consisting of 2 experimental intervention groups and a control intervention group. The intervention portion of the randomized trial was conducted over a 2-week period. Preintervention and immediately postintervention pain sensitivity assessments were conducted at baseline, 1 week, and 2 weeks. Clinical assessments were conducted at 4, 8, and 12 weeks. A cohort of healthy participants was assessed at a single session to allow for pain sensitivity comparisons to individuals with shoulder pain.
Participants
All enrolled participants were recruited from the University of Florida campus, Shands Hospital, and the local surrounding community. Healthy participants were recruited in the same manner as participants with shoulder pain, to ensure that the groups were matched by age and sex. We targeted a clinical population of patients with a general shoulder pain complaint for 2 reasons. First, there is precedent in the literature that individuals with general shoulder pain, which commonly includes those with shoulder impingement complaints, exhibit enhanced pain sensitivity at local and remote regions, which was the primary outcome for this study.21,36,53 Second, we are not aware of evidence suggesting that patients with specific shoulder pathologies have more favorable pain sensitivity effects following manual therapy.
Eligibility Criteria for Participants With Shoulder Pain
Participants who met the following inclusion criteria were considered for enrollment: (1) aged 18 to 65 years, (2) English speaking, (3) primary complaint of unilateral shoulder pain (operationally defined as pain over the deltoid region and upper arm), (4) current episode of shoulder pain of less than 6 months, (5) current pain rated at greater than 4/10 on a numeric rating scale at rest and/or with activity, and (6) signed informed-consent form. Individuals who met the following criteria were excluded from enrollment: (1) currently being treated in physical therapy for shoulder pain; (2) current complaint of neck pain or shoulder instability; (3) history of surgery for shoulder pain or neck pain; (4) shoulder pain as a result of traumatic injury (eg, dislocation) or related to diagnosis of adhesive capsulitis or fracture; (5) past or present serious medical condition such as cancer; (6) signs of cervical nerve root involvement, such as motor weakness, hyporeflexia, or sensory disturbance; and (7) contraindications for manipulation.
Eligibility Criteria for Healthy Participants
Participants had to be 18 to 65 years of age and have no current complaints of shoulder pain or neck pain. Individuals were excluded from enrollment if they (1) had any neurological impairments of the upper extremity, such as motor weakness, hyporeflexia, or sensory disturbance; (2) were currently taking pain medication; or (3) had a previous history of shoulder surgery.
Demographics
All participants completed a standard intake questionnaire to report demographic information, such as age, sex, ethnicity, race, employment, marital status, education, and income. Additionally, participants with shoulder pain reported health-related information, such as painful side and duration of shoulder pain.
Primary Outcomes
Pain sensitivity measures included static mechanical pain sensitivity (pressure pain threshold [PPT]), static thermal pain sensitivity (heat pain threshold [HPT]), and dynamic thermal pain sensitivity (temporal summation of pain). Multimodal stimulus assessment was conducted to provide a comprehensive assessment of pain sensitivity.40,52 Furthermore, static and dynamic pain sensitivity measures were included, because these measures reflect different aspects of pain processing. Static measures are believed to reflect the basal state of the somatosensory system (ie, loss or gain in sensory threshold), and dynamic measures are believed to reflect central modulation of pain.3,33
Mechanical Pain Sensitivity
Pressure pain thresholds were assessed by a trained examiner using a handheld pressure algometer (Pain Diagnostics and Thermography, Inc, Great Neck, NY). A mechanical force was applied to the participant’s skin at a target rate of 1 kg/s, until the participant reported the first moment of pain. The amount of pressure (kg) was recorded. Pressure pain threshold measurements were taken at 2 anatomical sites: the tip of the acromion on the affected side (the dominant-side acromion in healthy participants) (PPT-Acr) and bilateral tibialis anterior muscle belly (PPT-TA). The PPT-Acr was deemed local pressure pain sensitivity, whereas the PPT-TA was deemed remote pressure pain sensitivity. A total of 3 measurements were collected at each site, rotating between each side to avoid habituation or summation. The measurement average at each site was used in these analyses.
Heat Pain Sensitivity: Heat Pain Threshold
Heat pain threshold was assessed at the anterior forearm using a 30 × 30-mm thermode connected to a PATHWAY model ATS (Medoc Ltd, Ramat Yishai, Israel). A standard heat stimulus with a steady temperature increase was initiated and participants were instructed to report the first moment of pain. Once indicated, the temperature (°C) associated with HPT was recorded. A total of 2 measurements were collected at each side, rotating between sides to avoid habituation or summation. The measurement average was used in these analyses.
Heat Pain Sensitivity: Temporal Summation of Pain
Temporal summation of pain was assessed at the anterior forearm using a contact thermode with 2.5-cm2 surface areas connected to a PATHWAY model CHEPS (Medoc Ltd). Five consecutive heat pulses with a peak temperature of 48°C were applied at a rate of 30°C/s, with an interstimulus interval of 2.5 seconds. Participants were instructed to rate the pain intensity of the second pain associated with each heat pulse on a 101-point numeric rating scale, with 0 as “no pain” and 100 as the “worst pain imaginable.” The temporal summation protocol was conducted once per side, and the average pain intensity ratings of the first heat pulse, fifth heat pulse, and temporal summation (fifth pulse minus first pulse) were used in these analyses.
Secondary Outcomes
Shoulder Pain Intensity
Shoulder pain intensity was measured using the Brief Pain Inventory.16,17,59 The Brief Pain Inventory rates pain on an 11-point numeric rating scale for pain intensity, with 0 as “no pain” and 10 as “pain as bad as you can imagine.” Participants rated their shoulder pain intensity over 3 conditions: worst pain in the last 24 hours, least pain in the last 24 hours, and pain right now. An average shoulder pain intensity rating from the 3 responses was used in these analyses. The standard error of measurement (SEM) and minimum detectable change with 90% confidence level (MDC90) for the 11-point numeric rating scale are 1.5 and 3.5 points, respectively.57
Shoulder Function
Shoulder function was measured using the Penn Shoulder Score function subscale.43 The Penn Shoulder Score function subscale is a 20-item questionnaire measuring the level of difficulty in performing specific activities. Individual responses to each item are reported on a 4-point Likert scale, with 0 as “can’t do at all,” 1 as “much difficulty,” 2 as “with some difficulty,” and 3 as “no difficulty.” There is also an option for selecting “X” or “did not do before injury.” All 20 responses are summed for a total score of 60 points. Modification of the total score is made with any “X” responses, and computations for handling these responses are reported elsewhere.43 The Penn Shoulder Score function subscale is a reliable measure (intraclass correlation coefficient = 0.93) with established validity.43 The SEM and MDC90 for the Penn Shoulder Score function subscale are 6.1 and 8.6 points, respectively.43
Procedures
All procedures were conducted in accordance with the University of Florida Institutional Review Board policy and approval for human subjects research. After providing written informed consent, study participants completed a baseline assessment, including standardized questionnaires and pain sensitivity measurement. Participants with shoulder pain were randomized to 1 of 3 intervention groups (cervical TM, shoulder TM, home exercise program [HEP]) using concealed opaque envelopes. The randomization sequence was computer generated by an individual not responsible for determining study eligibility, outcome assessment, or intervention. The intervention provider prescreened each participant for any abnormal symptoms with neck movement that would preclude the use of cervical TM. Interventions were delivered in accordance with randomization and the study protocol. Afterward, repeat assessment was conducted. A total of 3 intervention sessions over a 2-week period were completed for the primary purpose of assessing the effects on mechanistic outcome.5,6 Preintervention and immediate postintervention pain sensitivity assessments were conducted at baseline and at 1- and 2-week follow-ups. Clinical assessments for shoulder pain intensity and function were conducted at baseline and after 4, 8, and 12 weeks. All outcome assessments were conducted by an assessor blinded to intervention status.
Intervention
The primary experimental interventions included cervical and shoulder TM. The control intervention included a standard HEP for the shoulder region. All interventions were administered by a licensed physical therapist (n = 4) with specialty training in manual therapy or a licensed chiropractor (n = 1). The cumulative time of clinical experience for all intervention providers was 71 years (range, 6–25 years).
Cervical Manipulation
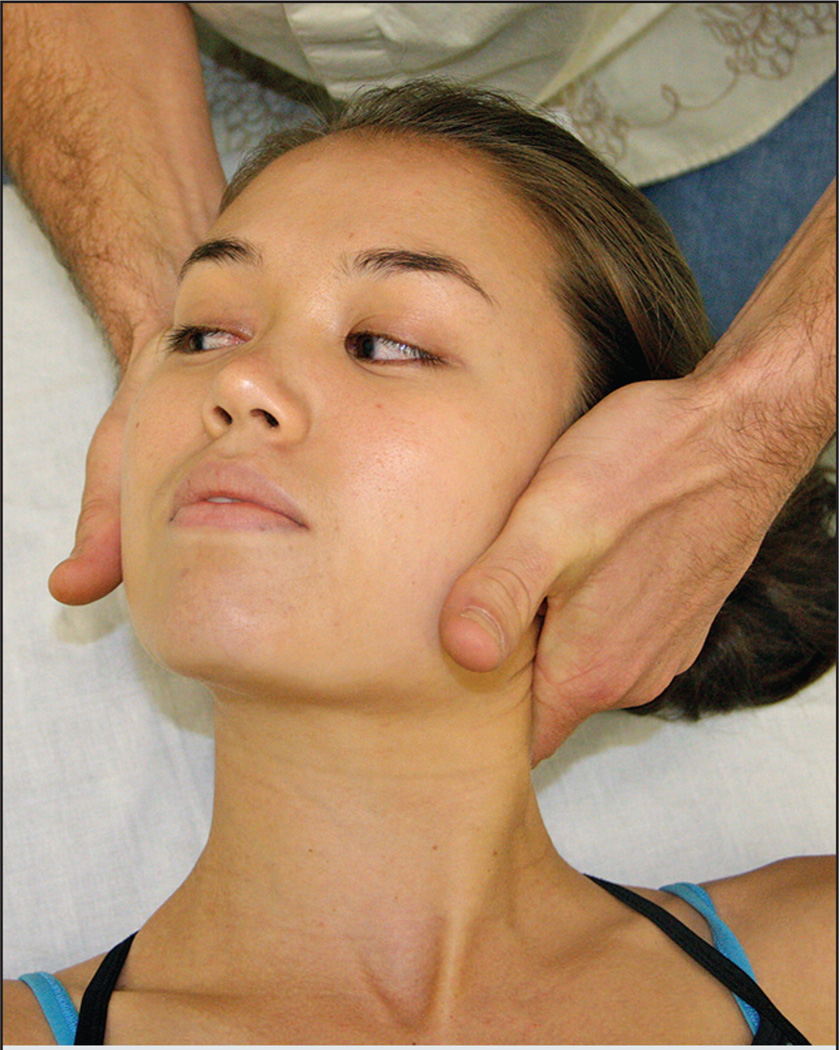
Cervical TM is a commonly used technique in physical therapy and has been incorporated in previous pain sensitivity trials.24,27,28 Cervical TM involved the intervention provider prepositioning the participant’s head in a side-flexed and contralateral rotated position, while the participant lay supine (FIGURE 1). The provider’s hands cradled the head, with the TM hand in contact with the mid cervical region (eg, C5 vertebral level). The technique was performed as a high-velocity, low-amplitude force in a rotation direction on the side of shoulder pain. The delivery of the TM procedure was consistent with the protocols of published trials, in which up to 2 TMs per side were allowed unless cavitation was achieved.7,8,14,29,32 The same procedures for cervical TM application were followed at subsequent sessions.
FIGURE 1.
Cervical thrust manipulation technique.
Shoulder Manipulation
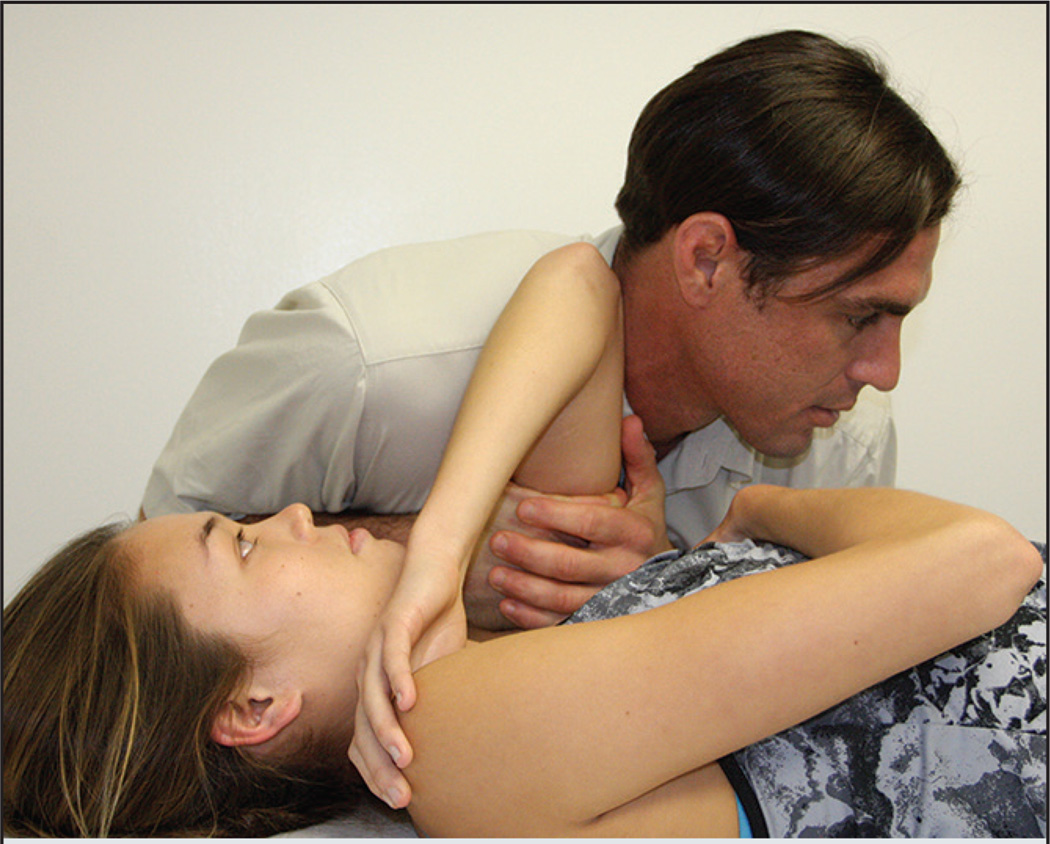
The shoulder TM technique is a thrust technique directed to the extremity region rather than to the spine. This technique is a variant of the common distraction mobilization for the shoulder region. The shoulder TM was performed with the participant in supine and the shoulder placed in approximately 90° of flexion, with internal rotation (FIGURE 2). The provider’s hands were in contact with the inner arm to be manipulated. The direction of the manipulation was lateral distraction. The delivery of the TM procedure was consistent with the protocols of published trials, in which up to 2 TMs were provided to the affected side unless cavitation was achieved.7,8,14,29,32 The same procedures for shoulder TM application were followed at subsequent sessions.
FIGURE 2.
Shoulder thrust manipulation technique.
Home Exercise Program
The HEP used in this study included standard range-of-motion and isometric strengthening exercises designed to address general flexibility and strength impairments of the painful shoulder region. The range-of-motion exercises included self-generated movements for shoulder flexion, abduction, and internal and external rotation. Each range-of-motion exercise was held for 30 seconds and performed twice within each exercise bout. The isometric strengthening exercises focused on resisted internal and external rotation. Each isometric exercise was held for 10 seconds and performed 5 times within each exercise bout. The HEP group received formal training and supervision of the exercise program during the initial intervention session and a handout with details of the HEP for performance at home twice a day. Home exercise program participants were directed to perform each exercise at subsequent sessions. Home exercise program compliance was encouraged during each intervention session but not formally monitored. The same HEP handout was given to the cervical and shoulder TM groups for these participants to perform at home, but participants in the TM groups did not perform exercises within sessions.
Sample-Size Determination
Sample size was determined a priori for detecting differences in pain sensitivity (effect size, 0.3), with an alpha level of .05 and power of 0.80. Effect-size determination was based on previously published research using similar pain sensitivity outcomes in response to manual therapy.19,32 The minimum total sample size was 22 participants per group (66 total), with an additional 22 healthy participants included for comparisons to determine sensitization state. To account for potential dropouts, we targeted recruitment of an additional 20% of the entire sample, for a total of 108 participants.
Data Analysis
We used IBM SPSS Statistics for Windows Version 21.0 (IBM Corporation, Armonk, NY) and Microsoft Excel 2010 (Microsoft Corporation, Redmond, WA) for all analyses. The alpha level for statistical significance was set a priori at .05. All continuous data were assessed for normality through observation of plots and with Kolmogorov-Smirnov and Shapiro-Wilk tests. In cases where normality assumptions were not met, assumption-free tests were conducted. All categorical data were assessed with Pearson chi-square or Fisher exact tests.
Participant data were analyzed based on the intention-to-treat principle. In brief, participants were analyzed in the group to which they were randomized, regardless of whether this intervention was provided at follow-up sessions or whether participants adhered to group instructions. Missing data were handled using a standard method of data imputation. Imputation methods allow for use of data from the entire sample and reduce bias from participant attrition. We used the last-observation-carried-forward method, in which the data from the observed previous session were carried forward to the final time point.58 The last-observation-carried-forward method was selected as a conservative approach for dealing with missing data and is appropriate for conditions that do not show progressive decline.60 We also performed a sensitivity analysis that included only complete cases, without imputation for any missing values.
Baseline comparisons were made between clinical and healthy participants for demographic and pain sensitivity measures. Differences in pain sensitivity between groups were used to indicate altered pain processing in clinical participants. Only pain sensitivity variables showing differences between groups were used as the primary mechanistic outcome in the intervention analyses. Baseline comparisons were made between randomized groups on key demographic and clinical variables to ensure randomization balance. We used the Levene test to assess homogeneity of variance prior to performing analysis of variance (ANOVA).
We assessed differences in pain sensitivity effects over the 2-week treatment between randomized groups in 2 ways. First, we examined the immediate effects of intervention on pain sensitivity at the initial session using a 2-way, mixed-model ANOVA with time (preintervention, postintervention) as the within-subject factor and randomized group as the between-subject factor. Second, we examined the average of effects of intervention on pain sensitivity across all sessions using a 1-way ANOVA. For this analysis, the dependent variable was the average of the simple change score (eg, posttreatment minus pretreatment) in pain sensitivity across all sessions. Clinical effects following intervention were assessed with a mixed-model ANOVA with time (baseline, 4 weeks, 8 weeks, 12 weeks) as the within-subject factor and intervention group as the between-subject factor for pain intensity and function. We also examined proportion differences in individuals exceeding the MDC90 for pain intensity and function from baseline to each follow-up time point. Associations between immediate and average pain sensitivity effects and clinical outcome at each time point and as a linear clinical change over time were assessed using partial correlations, accounting for baseline clinical outcome scores.
RESULTS
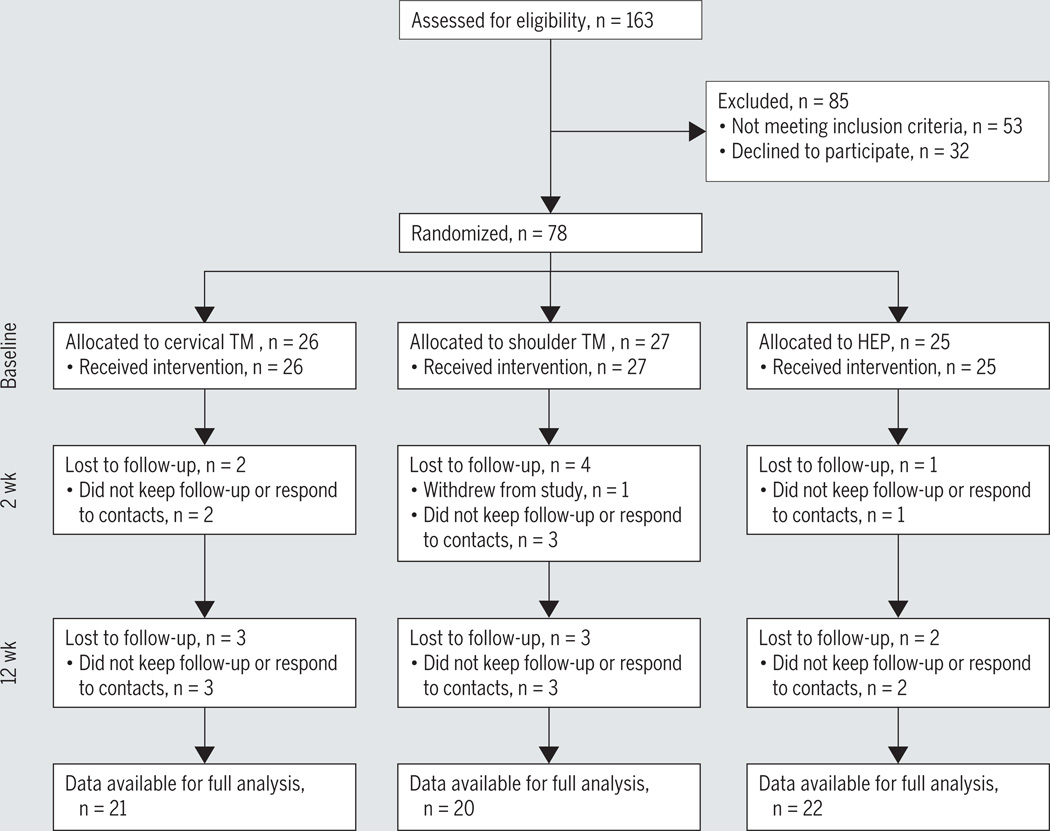
Seventy-eight participants with shoulder pain and 25 healthy participants were enrolled in this study from September 2012 to February 2014 (FIGURE 3). TABLE 1 includes information on demographics for each participant group. Postrandomization comparisons between intervention groups indicated no differences for demographic, pain sensitivity, or clinical variables (P>.05) (TABLE 2). FIGURE 3 depicts the follow-up rates for each group at the conclusion of the 2-week intervention (91.0% total follow-up) and 12-week clinical assessment (80.8% total follow-up). No differences in loss to follow-up proportions were observed at 2 weeks or 12 weeks (P>.05). Results presented are for the intention-to-treat analysis. Sensitivity analyses using only complete cases resulted in similar findings (data not shown).
FIGURE 3.
Summary of recruitment, enrollment, randomization, follow-up, and analysis for study. Abbreviations: HEP, home exercise program; TM, thrust manipulation.
TABLE 1.
Baseline Demographic and Pain Sensitivity Information for Clinical and Healthy Participants*
| Variable | Clinical (n = 78) | Healthy (n = 25) | P Value |
|---|---|---|---|
| Age, y† | 39.0 ± 14.5 | 35.2 ± 11.1 | .30 |
| Sex (female) | 46.2 | 52.0 | .61 |
| Ethnicity (Hispanic or Latino) | 5.1 | 12.0 | .36 |
| Race | .11 | ||
| White | 62.3 | 44.0 | |
| Black or African American | 33.8 | 40.0 | |
| Asian | 2.6 | 12.0 | |
| Other | 1.3 | 4.0 | |
| Employment | .25 | ||
| Full time | 25.6 | 40.0 | |
| Part time | 24.4 | 32.0 | |
| Unemployed | 44.9 | 28.0 | |
| Retired | 5.1 | 0.0 | |
| Marital status | .68 | ||
| Single | 52.6 | 48.0 | |
| Married | 21.8 | 36.0 | |
| Living with significant other | 5.1 | 4.0 | |
| Divorced | 19.2 | 12.0 | |
| Widowed | 1.3 | 0.0 | |
| Education | .88 | ||
| Less than high school | 6.4 | 0.0 | |
| Graduated high school | 19.2 | 16.0 | |
| Some college | 28.2 | 36.0 | |
| Graduated college | 15.4 | 20.0 | |
| Some postgraduate college | 12.8 | 12.0 | |
| Completed postgraduate degree | 17.9 | 16.0 | |
| Income | .32 | ||
| Less than $20 000 | 51.9 | 36.0 | |
| $20 000 to $35 000 | 19.5 | 32.0 | |
| $35 000 to $50 000 | 9.1 | 8.0 | |
| $50 000 to $75 000 | 3.9 | 12.0 | |
| More than $75 000 | 15.6 | 12.0 | |
| Pressure pain sensitivity† | |||
| PPT acromion, kg | 3.1 ± 1.6 | 4.7 ± 1.9 | <.05 |
| PPT TA, kg | 5.2 ± 2.4 | 7.1 ± 2.6 | <.05 |
| Heat pain sensitivity† | |||
| HPT, °C | 42.6 ± 1.9 | 43.7 ± 2.3 | <.05 |
| First heat pulse (0–100) | 63.0 ± 26.6 | 59.0 ± 30.6 | .62 |
| Fifth heat pulse (0–100) | 54.6 ± 30.0 | 52.7 ± 27.6 | .74 |
| Temporal summation | −8.4 ± 22.5 | −6.4 ± 17.4 | .96 |
Abbreviations: HPT, heat pain threshold; PPT, pressure pain threshold; TA, tibialis anterior.
Values are percent unless otherwise indicated.
Values are mean ± SD.
TABLE 2.
Baseline Demographic, Pain Sensitivity, and Clinical Information for Randomized Groups*
| Variable | Cervical TM (n = 26) |
Shoulder TM (n = 27) |
HEP (n = 25) | P Value |
|---|---|---|---|---|
| Demographics | ||||
| Age, y | 36.7 ± 16.0 | 39.4 ± 13.6 | 41.0 ± 14.1 | .57 |
| Sex (female), % | 50.0 | 48.1 | 40.0 | .75 |
| Pain duration, wk | 19.1 ± 31.9 | 21.5 ± 48.8 | 21.5 ± 23.3 | .96 |
| Affected side (right), % | 73.1 | 85.2 | 84.0 | .50 |
| Pain sensitivity | ||||
| PPT acromion, kg | 2.6 ± 1.3 | 3.4 ± 1.4 | 3.2 ± 2.0 | .15 |
| PPT TA, kg | 4.7 ± 2.2 | 5.4 ± 2.6 | 5.5 ± 2.6 | .52 |
| HPT, °C | 42.3 ± 1.8 | 42.6 ± 2.1 | 42.7 ± 1.7 | .73 |
| Clinical | ||||
| Pain intensity (0–10) | 5.0 ± 1.9 | 4.1 ± 1.6 | 4.2 ± 2.1 | .42 |
| Shoulder function (0–60) | 43.8 ± 8.1 | 46.2 ± 7.5 | 42.4 ± 9.3 | .28 |
Abbreviations: HEP, home exercise program; HPT, heat pain threshold; PPT, pressure pain threshold; TA, tibialis anterior; TM, thrust manipulation.
Values are mean ± SD unless otherwise indicated.
Comparison of Pain Sensitivity Between Clinical and Healthy Participants
There was a difference between cohorts for PPT-Acr (U = 429.5, P<.05), PPT-TA (U = 548.5, P<.05), and HPT (U = 651.0, P<.05), but not for temporal summation responses (P>.05) (TABLE 1). Participants with shoulder pain demonstrated enhanced pressure sensitivity (lower PPTs) at local (mean difference, −1.63 kg; 95% confidence interval [CI]: −2.40, −0.86) and remote (mean difference, −1.96 kg; 95% CI: −3.09, −0.82) sites and enhanced heat sensitivity (lower HPT) at a remote site (mean difference, −1.15°C; 95% CI: −2.06°C, −0.24°C). Thus, PPT-Acr, PPTTA, and HPT were used as markers of change in altered pain processing in subsequent analyses.
Effects of Intervention on Pain Sensitivity
TABLE 3 includes information on the effects of intervention on pain sensitivity. No interaction effect was noted for the immediate within-session change in PPT-Acr (F2,75 = 1.02, P>.05), PPT-TA (F2,75 = 0.60, P>.05), or HPT (F2,73 = 0.02, P>.05). However, a small time effect was noted for PPT-Acr (F1,75 = 7.24, P<.05, r = 0.30), PPT-TA (F1,75 = 5.48, P<.05, r = 0.26), and HPT (F1,73 = 9.28, P<.05, r = 0.34), with higher PPT-Acr (mean difference, 0.22 kg; 95% CI: 0.06, 0.38), PPT-TA (mean difference, 0.23 kg; 95% CI: 0.03, 0.43), and HPT (mean difference, 0.58°C; 95% CI: 0.20°C, 0.96°C) observed immediately after intervention. No difference between groups was observed for the average within-session effects over the 2-week intervention period for PPT-Acr (F2,75 = 1.95, P>.05), PPT-TA (F2,75 = 1.28, P>.05), and HPT (F2,73 = 0.75, P>.05). The average within-session effect (mean difference) was 0.32 kg (95% CI: 0.21, 0.43) for PPT-Acr, 0.24 kg (95% CI: 0.13, 0.36) for PPT-TA, and 0.30°C (95% CI: 0.06°C, 0.54°C) for HPT.
TABLE 3.
Comparison of Immediate and Average Within-Session Pain Sensitivity Effects Following Intervention*
| Preintervention | Postintervention | Immediate Effect (post – pre) |
P Value† | Average Effect‡ | P Value | |
|---|---|---|---|---|---|---|
| PPT acromion, kg | .37 | .15 | ||||
| Cervical TM | 2.6 (1.9, 3.2) | 2.9 (2.3, 3.6) | 0.37 (0.10, 0.65) | 0.45 (0.24, 0.66) | ||
| Shoulder TM | 3.4 (2.8, 4.0) | 3.6 (3.0, 4.2) | 0.19 (−0.10, 0.45) | 0.32 (0.11, 0.52) | ||
| HEP | 3.2 (2.6, 3.8) | 3.3 (2.7, 3.9) | 0.10 (−0.19, 0.38) | 0.18 (0.02, 0.35) | ||
| All groups | 0.22 (0.06, 0.38)§ | 0.32 (0.21, 0.43) | ||||
| PPT TA, kg | .55 | .28 | ||||
| Cervical TM | 4.7 (3.8, 5.7) | 5.1 (4.0, 6.2) | 0.35 (0.01, 0.69) | 0.36 (0.12, 0.59) | ||
| Shoulder TM | 5.4 (4.4, 6.3) | 5.5 (4.4, 6.5) | 0.09 (−0.24, 0.42) | 0.13 (−0.04, 0.31) | ||
| HEP | 5.5 (4.5, 6.4) | 5.7 (4.6, 6.8) | 0.25 (−0.96, 0.60) | 0.25 (0.05, 0.45) | ||
| All groups | 0.23 (0.03, 0.43)§ | 0.24 (0.13, 0.36) | ||||
| HPT, °C | .98 | .47 | ||||
| Cervical TM | 42.3 (41.6, 43.1) | 42.9 (42.1, 43.8) | 0.62 (−0.04, 1.3) | 0.51 (0.08, 0.94) | ||
| Shoulder TM | 42.6 (41.8, 43.3) | 43.2 (42.4, 44.0) | 0.57 (−0.06, 1.2) | 0.18 (−0.31, 0.68) | ||
| HEP | 42.7 (42.0, 43.5) | 43.3 (42.4, 44.1) | 0.54 (−0.13, 1.2) | 0.20 (−0.15, 0.55) | ||
| All groups | 0.58 (0.20, 0.96)§ | 0.30 (0.06, 0.54) |
Abbreviations: HEP, home exercise program; HPT, heat pain threshold; PPT, pressure pain threshold; TA, tibialis anterior; TM, thrust manipulation.
Values are mean (95% confidence interval) unless otherwise indicated.
Analysis of variance represents group-by-time interaction.
Average within-session change over the 2-week intervention period.
Significant time effect at P<.05.
Effects of Intervention on Clinical Outcome and Associations With Pain Sensitivity
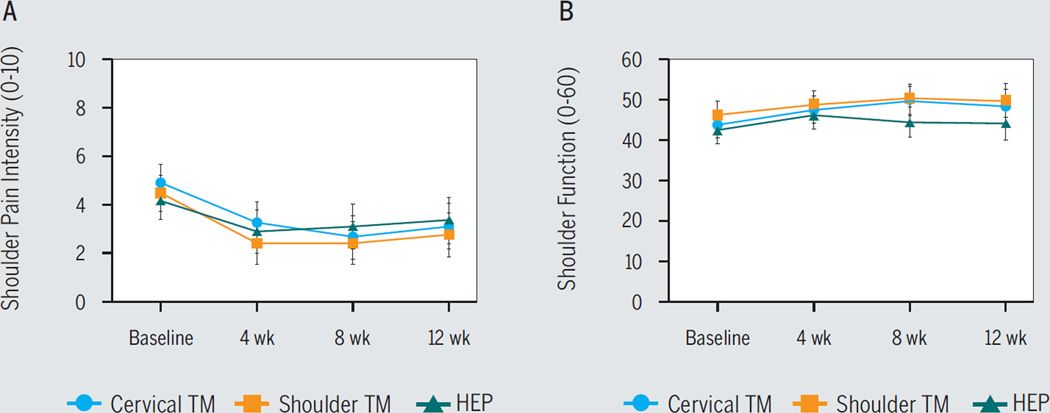
No interaction effect was noted for shoulder pain intensity (F6,225 = 1.83, P>.05) (FIGURE 4A) or function (F6,216 = 1.372, P>.05) (FIGURE 4B). However, a time effect was observed for shoulder pain intensity (F3,225 = 41.382, P<.05): compared to baseline, lower pain ratings were observed at 4 weeks (mean difference, −1.67; 95% CI: −2.21, −1.13), 8 weeks (mean difference, −1.79; 95% CI: −2.34, −1.24), and 12 weeks (mean difference, −1.45; 95% CI: −1.96, −0.94), indicating decreased pain intensity independent of intervention group. Similarly, a time effect was observed for shoulder function (F3,216 = 10.43, P<.05): compared to baseline, higher function ratings were observed at 4 weeks (mean difference, 3.23; 95% CI: 1.24, 5.23), 8 weeks (mean difference, 3.82; 95% CI: 1.44, 6.20), and 12 weeks (mean difference, 3.15; 95% CI: 0.69, 5.61), regardless of intervention group. TABLE 4 depicts the proportion of individuals exceeding the MDC90 for the clinical outcomes at each time point. No proportion differences between groups were noted (P>.05). TABLE 5 includes correlation values for the relationship between immediate and average within-session pain sensitivity effects and clinical outcome. Overall, there were no associations between changes in pain sensitivity and clinical pain intensity or function (P>.05).
FIGURE 4.
Influence of intervention group status on (A) shoulder pain intensity and (B) function. Error bars are 95% confidence interval. Higher shoulder function scores indicate greater shoulder function. Abbreviations: HEP, home exercise program; TM, thrust manipulation.
TABLE 4.
Proportion of Individuals Exceeding the Error for Shoulder Pain Intensity and Function From Baseline to Each Follow-up Time Point*
| Shoulder Pain Intensity | Shoulder Function | |||||
|---|---|---|---|---|---|---|
| 4 wk | 8 wk | 12 wk | 4 wk | 8 wk | 12 wk | |
| Cervical TM | 15.4 (7.4, 23.4) | 26.9 (17.1, 36.7) | 11.5 (4.4, 18.6) | 20.0 (10.9, 29.1) | 40.0 (28.9, 51.1) | 24.0 (14.3, 33.7) |
| Shoulder TM | 18.5 (9.9, 27.1) | 18.5 (9.9, 27.1) | 11.1 (4.1, 18.1) | 12.0 (4.6, 19.4) | 24.0 (14.3, 33.7) | 20.0 (10.9, 29.1) |
| HEP | 12.0 (4.8, 19.2) | 8.0 (2.0, 14.0) | 8.0 (2.0, 14.0) | 20.0 (10.9, 29.1) | 12.0 (4.6, 19.4) | 12.0 (4.6, 19.4) |
| All groups | 15.4 (7.4, 23.4) | 17.9 (9.4, 26.4) | 10.3 (3.5, 17.1) | 17.3 (8.7, 25.9) | 25.3 (15.5, 35.1) | 18.7 (9.9, 27.5) |
Abbreviations: HEP, home exercise program; TM, thrust manipulation.
Values are percent (95% confidence interval). The minimum detectable change at the 90% confidence level was 3.5 for shoulder pain intensity and 8.6 for function.
TABLE 5.
Association Between Immediate and Average Within-Session Pain Sensitivity Changes and Clinical Outcome*
| Shoulder Pain Intensity | Shoulder Function | |||||||
|---|---|---|---|---|---|---|---|---|
| 4 wk | 8 wk | 12 wk | Linear† | 4 wk | 8 wk | 12 wk | Linear† | |
| Immediate effect | ||||||||
| PPT acromion | 0.07 | 0.01 | −0.12 | −0.08 | 0.05 | −0.03 | 0.17 | 0.19 |
| PPT TA | −0.01 | −0.15 | 0.01 | 0.11 | 0.07 | 0.12 | 0.13 | 0.08 |
| HPT | −0.10 | −0.11 | 0.05 | 0.08 | −0.03 | −0.03 | 0.07 | <0.01 |
| Average effect | ||||||||
| PPT acromion | 0.12 | −0.01 | 0.06 | 0.06 | 0.02 | −0.10 | 0.07 | 0.04 |
| PPT TA | 0.07 | −0.06 | 0.01 | 0.06 | 0.07 | 0.09 | 0.17 | 0.06 |
| HPT | −0.08 | −0.06 | −0.06 | −0.04 | <0.01 | 0.03 | 0.14 | 0.02 |
Abbreviations: HPT, heat pain threshold; PPT, pressure pain threshold; TA, tibialis anterior.
Values are partial correlation coefficients and account for baseline clinical outcome scores.
Linear association is the correlation between change in pain sensitivity and an estimate of the weekly change in clinical outcome across the 12-week period.
DISCUSSION
We used a shoulder pain model to examine the comparative effects of spinal and peripheral TM on pain sensitivity. Overall, we found similar pain-modulating effects when a TM stimulus was applied to the painful extremity or to the nonpainful cervical spine. Furthermore, these effects did not differ from a standard exercise program and suggest that similar effects on pain sensitivity may be gained from an active exercise approach. Collectively, the findings reported in this study suggest similar pain-modulating mechanisms on central sensitivity for the interventions studied. While noteworthy, our exploratory analyses of the relationship to clinical outcomes showed no association between pain sensitivity response and positive benefits of longer-term clinical outcomes for pain intensity and shoulder function.
Treatment Effects on Primary Outcome
The mechanistic model of manual therapy presented by Bialosky et al4 suggests that general neurophysiological responses occur following application of a manual therapy stimulus. The findings of the current study expand on the mechanistic model by positing that similar neurophysiological responses occur when a manual therapy stimulus is applied to differing locations. Studies of similar intent have used low back or neck pain models, and the majority show similar effects on self-reported pain intensity or pain sensitivity.1,15,25,26,41,42,45,55,56 In a study of 148 patients with chronic low back pain, de Oliveira et al25 studied the effects of changing location of TM (thoracic versus lumbar) on pain intensity and pressure pain sensitivity. The authors found no difference in pain intensity or local and remote pressure pain sensitivity between TM techniques. Similarly, Martínez-Segura et al45 found no difference in local or remote pressure pain sensitivity in patients with chronic neck pain following cervical or thoracic TM. In contrast, Fernández-Carnero et al26 found differences in pressure pain sensitivity at the painful and nonpainful elbows in a small sample of patients with lateral epicondylalgia, depending on whether cervical or thoracic TM was applied. The findings of the present study agree with the majority of this evidence, with the distinction that the present study compared peripheral and spinal TM techniques, the former being directed to the painful region and the latter to a more central, nonpainful region.
There are potential explanations for why we did not observe differences between intervention groups. The lack of treatment-dependent differences could suggest that the interventions in this study share mechanisms,23 because the effects of pain sensitivity were similar. In a meta-analysis by Gay et al,30 muscle-biased manual therapy (ie, massage) was found to have a similar favorable effect on pain sensitivity when compared to other forms of active intervention, such as exercise or joint-biased manual therapy. Naugle et al51 examined the acute effects of exercise on pain sensitivity and found a consistent pain-modulating effect following exercise in healthy and clinical participants. In a study by Burrows et al,13 the authors found that an acute bout of upper-body exercise resulted in a reduction in pressure pain sensitivity in the knee of patients with knee osteoarthritis. Alternatively, the lack of treatment-dependent differences could indicate that all 3 interventions operate under a more general, nonspecific effect. Active approaches may include similar nonspecific components, such as expectation of receiving treatment, placebo, and patient-provider interaction effects, factors for which the design of the current study could not directly account.
Association Across Primary and Secondary Outcomes
In our exploratory analyses, we did not find associations between changes in pain sensitivity and longer-term clinical outcome. These findings are in agreement with 2 recent systematic reviews on the topic.35,39 In a meta-analysis by Hübscher et al,39 the authors found weak association between static and dynamic pain sensitivity measures and clinical pain and disability in individuals with spine pain. Grosen et al35 also found limited evidence for pain sensitivity to predict treatment response in experimental and clinical paradigms. Both reviews focused on baseline pain sensitivity status on clinical outcome. The current study examined changes in pain sensitivity as a predictor of future clinical outcome change and did not find a significant relationship. These findings, however, should be viewed cautiously, as these were secondary and an exploratory aspect of the current study.
Recent work by Gay et al31 has indicated that a complex interaction between pain sensitivity and psychological status (eg, pain-related fear) may influence the relationship between pain intensity and disability in patients with low back pain. A potential implication of this work is that reducing pain sensitivity in patients with elevated pain-related fear may influence clinical outcome, suggesting the need to consider psychological status. Additionally, the presence of pain sensitivity subgroups could impact the association between pain sensitivity change and clinical outcome. Pain sensitivity subgroups have been identified in previous studies of individuals with musculoskeletal pain complaints, including shoulder pain.10,18,21,53 It is possible that the relationship between changes in pain sensitivity and outcomes would be stronger in individuals who are centrally sensitized, but further investigation of this hypothesis is warranted.
Limitations
There are limitations in this study to consider. We did not enroll a sample of patients with a specific shoulder pain diagnosis. We ruled out common shoulder diagnoses or complaints such as adhesive capsulitis, fracture, instability/dislocation, and postoperative shoulder pain, and thus we suspect that the enrolled participants were predominantly individuals with impingement and/or rotator cuff involvement. Currently, there is limited evidence of differential pain sensitivity effects following manual therapy for specific shoulder pain pathologies. However, it is possible that shoulder pain subgroups may respond more favorably to manual therapy, and this was not considered in this study.49 We included comparison groups that contained active treatment, and thus our interpretation of outcome cannot account for natural history or placebo/nonspecific effects. Regardless, we found that a treatment group that did not receive targeted intervention to the painful shoulder yielded similar within-session pain-processing effects. We were unable to blind participants from the intervention received and cannot control for participant bias of receiving or not receiving hands-on therapy. We directed our TM intervention at random (not based on physical examination) to patients with shoulder pain and did not measure shoulder or neck stiffness or other tissue characteristics, patient response to movement, or patient preference for TM. We also did not incorporate psychological measures within the primary aims of this study. These factors could be useful in future randomized studies to determine whether individuals respond favorably if certain factors are present.
Clinical Implications
This study was focused and designed with a mechanistic intent, but it does have preliminary clinical implications. Our results did not support the superiority of spinal or peripheral TM on our primary outcome of pain processing or secondary clinical outcomes of pain intensity and function. The specificity of TM does not appear influential in its effect on pain sensitivity and, potentially, clinical outcomes. Manual therapy directed to the spine and periphery is advocated for managing extremity pain conditions such as shoulder pain. Clinicians choosing techniques for musculoskeletal pain may consider the use of spinal TM, based on the preliminary evidence from this study, to be of similar benefit to techniques directly targeting the region of the painful extremity. This knowledge may be clinically useful in cases where an individual is hesitant to receive TM due to pain in the painful region. Furthermore, the apparent benefit received from either form of TM was not different from the benefit received from active shoulder exercises, as all individuals improved over the long term. Therefore, active exercise may be warranted if a decrease in pain sensitivity is the treatment goal. Clinicians should be aware, however, that the magnitudes of the clinical effects following interventions were small and may reflect the need for longer treatment duration, greater dosage, or inclusion of additional therapeutic strategies. Additionally, these effects might have been greater in a sample of patients seeking health care for their primary complaint.
CONCLUSION
The results of this study suggest that cervical TM and shoulder-directed intervention (shoulder TM or exercise) result in similar pain-processing and clinical effects. The lack of association between pain-processing effects and clinical outcome may suggest other underlying pain pathways and/or other mechanisms involved in producing clinical benefit.
KEY POINTS.
FINDINGS
Cervical manipulation, shoulder manipulation, and shoulder exercise all had similar pain sensitivity and clinical effects. There was a lack of association between pain sensitivity effects and clinical outcomes.
IMPLICATIONS
Clinicians can expect similar pain sensitivity and clinical effects following cervical and shoulder manipulation and exercise involving isometric resistance when managing shoulder pain. The lack of association between pain sensitivity and clinical outcome may suggest nonspecific pathways of clinical benefit.
CAUTION
No rest or placebo control group was included in this study. Small pain sensitivity and clinical effects were observed that could be attributed to short duration, low dosage, or restriction of additional therapeutic strategies.
Acknowledgments
The authors acknowledge assistance from Lauren Mackie (data collection), Dr Charles Gay, DC (intervention, manuscript review), Dr Corey Simon, PT, DPT, FAAOMPT (intervention, manuscript review), Danielle Coronado, MEd, MPA (randomization sequence), and Health Street (recruitment).
This study was supported by a research grant from the Orthopaedic Section of the American Physical Therapy Association. Dr Coronado received support from the National Institutes of Health (NIH) T32 Interdisciplinary Training in Rehabilitation and Neuromuscular Plasticity grant (5T32HD043730) while at the University of Florida. Dr Bialosky received support from the Rehabilitation Research Career Development program (5K12HD055929-02). Dr George received support from the NIH/National Institute of Arthritis and Musculoskeletal and Skin Diseases (AR055899). The study protocol was reviewed and approved by the Institutional Review Board of the University of Florida.
Footnotes
The authors certify that they have no affiliations with or financial involvement in any organization or entity with a direct financial interest in the subject matter or materials discussed in the article.
REFERENCES
- 1.Aquino RL, Caires PM, Furtado FC, Loureiro AV, Ferreira PH, Ferreira ML. Applying joint mobilization at different cervical vertebral levels does not influence immediate pain reduction in patients with chronic neck pain: a randomized clinical trial. J Man Manip Ther. 2009;17:95–100. doi: 10.1179/106698109790824686. http://dx.doi.org/10.1179/106698109790824686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arendt-Nielsen L, Graven-Nielsen T. Translational musculoskeletal pain research. Best Pract Res Clin Rheumatol. 2011;25:209–226. doi: 10.1016/j.berh.2010.01.013. http://dx.doi.org/10.1016/j.berh.2010.01.013. [DOI] [PubMed] [Google Scholar]
- 3.Arendt-Nielsen L, Yarnitsky D. Experimental and clinical applications of quantitative sensory testing applied to skin, muscles and viscera. J Pain. 2009;10:556–572. doi: 10.1016/j.jpain.2009.02.002. http://dx.doi.org/10.1016/j.jpain.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 4.Bialosky JE, Bishop MD, Price DD, Robinson ME, George SZ. The mechanisms of manual therapy in the treatment of musculoskeletal pain: a comprehensive model. Man Ther. 2009;14:531–538. doi: 10.1016/j.math.2008.09.001. http://dx.doi.org/10.1016/j.math.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bialosky JE, Bishop MD, Price DD, Robinson ME, Vincent KR, George SZ. A randomized sham-controlled trial of a neurodynamic technique in the treatment of carpal tunnel syndrome. J Orthop Sports Phys Ther. 2009;39:709–723. doi: 10.2519/jospt.2009.3117. http://dx.doi.org/10.2519/jospt.2009.3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bialosky JE, Bishop MD, Robinson ME, Price DD, George SZ. Heightened pain sensitivity in individuals with signs and symptoms of carpal tunnel syndrome and the relationship to clinical outcomes following a manual therapy intervention. Man Ther. 2011;16:602–608. doi: 10.1016/j.math.2011.06.003. http://dx.doi.org/10.1016/j.math.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bialosky JE, Bishop MD, Robinson ME, Zeppieri G, Jr, George SZ. Spinal manipulative therapy has an immediate effect on thermal pain sensitivity in people with low back pain: a randomized controlled trial. Phys Ther. 2009;89:1292–1303. doi: 10.2522/ptj.20090058. http://dx.doi.org/10.2522/ptj.20090058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bialosky JE, George SZ, Horn ME, Price DD, Staud R, Robinson ME. Spinal manipulative therapy–specific changes in pain sensitivity in individuals with low back pain ( NCT01168999) J Pain. 2014;15:136–148. doi: 10.1016/j.jpain.2013.10.005. http://dx.doi.org/10.1016/j.jpain.2013.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bishop MD, Beneciuk JM, George SZ. Immediate reduction in temporal sensory summation after thoracic spinal manipulation. Spine J. 2011;11:440–446. doi: 10.1016/j.spinee.2011.03.001. http://dx.doi.org/10.1016/j.spinee.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Börsbo B, Liedberg GM, Wallin M, Gerdle B. Subgroups based on thermal and pressure pain thresholds in women with chronic whiplash display differences in clinical presentation – an explorative study. J Pain Res. 2012;5:511–521. doi: 10.2147/JPR.S37062. http://dx.doi.org/10.2147/JPR.S37062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bot SD, van der Waal JM, Terwee CB, et al. Incidence and prevalence of complaints of the neck and upper extremity in general practice. Ann Rheum Dis. 2005;64:118–123. doi: 10.1136/ard.2003.019349. http://dx.doi.org/10.1136/ard.2003.019349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brantingham JW, Cassa TK, Bonnefin D, et al. Manipulative therapy for shoulder pain and disorders: expansion of a systematic review. J Manipulative Physiol Ther. 2011;34:314–346. doi: 10.1016/j.jmpt.2011.04.002. http://dx.doi.org/10.1016/j.jmpt.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 13.Burrows NJ, Booth J, Sturnieks DL, Barry BK. Acute resistance exercise and pressure pain sensitivity in knee osteoarthritis: a randomised crossover trial. Osteoarthritis Cartilage. 2014;22:407–414. doi: 10.1016/j.joca.2013.12.023. http://dx.doi.org/10.1016/j.joca.2013.12.023. [DOI] [PubMed] [Google Scholar]
- 14.Childs JD, Fritz JM, Flynn TW, et al. A clinical prediction rule to identify patients with low back pain most likely to benefit from spinal manipulation: a validation study. Ann Intern Med. 2004;141:920–928. doi: 10.7326/0003-4819-141-12-200412210-00008. [DOI] [PubMed] [Google Scholar]
- 15.Chiradejnant A, Maher CG, Latimer J, Stepkovitch N. Efficacy of “therapist-selected” versus “randomly selected” mobilisation techniques for the treatment of low back pain: a randomised controlled trial. Aust J Physiother. 2003;49:233–241. doi: 10.1016/s0004-9514(14)60139-2. [DOI] [PubMed] [Google Scholar]
- 16.Cleeland CS, Nakamura Y, Mendoza TR, Edwards KR, Douglas J, Serlin RC. Dimensions of the impact of cancer pain in a four country sample: new information from multidimensional scaling. Pain. 1996;67:267–273. doi: 10.1016/0304-3959(96)03131-4. [DOI] [PubMed] [Google Scholar]
- 17.Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singapore. 1994;23:129–138. [PubMed] [Google Scholar]
- 18.Coronado RA, Bialosky JE, Robinson ME, George SZ. Pain sensitivity subgroups in individuals with spine pain: potential relevance to short-term clinical outcome. Phys Ther. 2014;94:1111–1122. doi: 10.2522/ptj.20130372. http://dx.doi.org/10.2522/ptj.20130372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coronado RA, Gay CW, Bialosky JE, Carnaby GD, Bishop MD, George SZ. Changes in pain sensitivity following spinal manipulation: a systematic review and meta-analysis. J Electromyogr Kinesiol. 2012;22:752–767. doi: 10.1016/j.jelekin.2011.12.013. http://dx.doi.org/10.1016/j.jelekin.2011.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coronado RA, Kindler LL, Valencia C, George SZ. Thermal and pressure pain sensitivity in patients with unilateral shoulder pain: comparison of involved and uninvolved sides. J Orthop Sports Phys Ther. 2011;41:165–173. doi: 10.2519/jospt.2011.3416. http://dx.doi.org/10.2519/jospt.2011.3416. [DOI] [PubMed] [Google Scholar]
- 21.Coronado RA, Simon CB, Valencia C, George SZ. Experimental pain responses support peripheral and central sensitization in patients with unilateral shoulder pain. Clin J Pain. 2014;30:143–151. doi: 10.1097/AJP.0b013e318287a2a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Croft P, Pope D, Silman A. The clinical course of shoulder pain: prospective cohort study in primary care. Primary Care Rheumatology Society Shoulder Study Group. BMJ. 1996;313:601. doi: 10.1136/bmj.313.7057.601. http://dx.doi.org/10.1136/bmj.313.7057.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Day MA, Thorn BE. Using theoretical models to clarify shared and unique mechanisms in psychosocial pain treatments: a commentary on McCracken and Morley’s theoretical paper. J Pain. 2014;15:237–238. doi: 10.1016/j.jpain.2014.01.486. http://dx.doi.org/10.1016/j.jpain.2014.01.486. [DOI] [PubMed] [Google Scholar]
- 24.de Camargo VM, Alburquerque-Sendín F, Bérzin F, Stefanelli VC, de Souza DP, Fernández-delas-Peñas C. Immediate effects on electromyographic activity and pressure pain thresholds after a cervical manipulation in mechanical neck pain: a randomized controlled trial. J Manipulative Physiol Ther. 2011;34:211–220. doi: 10.1016/j.jmpt.2011.02.002. http://dx.doi.org/10.1016/j.jmpt.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 25.de Oliveira RF, Liebano RE, da Cunha Menezes Costa L, Rissato LL, Costa LO. Immediate effects of region-specific and non–region-specific spinal manipulative therapy in patients with chronic low back pain: a randomized controlled trial. Phys Ther. 2013;93:748–756. doi: 10.2522/ptj.20120256. http://dx.doi.org/10.2522/ptj.20120256. [DOI] [PubMed] [Google Scholar]
- 26.Fernández-Carnero J, Cleland JA, Arbizu RL. Examination of motor and hypoalgesic effects of cervical vs thoracic spine manipulation in patients with lateral epicondylalgia: a clinical trial. J Manipulative Physiol Ther. 2011;34:432–440. doi: 10.1016/j.jmpt.2011.05.019. http://dx.doi.org/10.1016/j.jmpt.2011.05.019. [DOI] [PubMed] [Google Scholar]
- 27.Fernández-Carnero J, Fernández-de-las-Peñas C, Cleland JA. Immediate hypoalgesic and motor effects after a single cervical spine manipulation in subjects with lateral epicondylalgia. J Manipulative Physiol Ther. 2008;31:675–681. doi: 10.1016/j.jmpt.2008.10.005. http://dx.doi.org/10.1016/j.jmpt.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 28.Fernández-de-las-Peñas C, Pérez-de-Heredia M, Brea-Rivero M, Miangolarra-Page JC. Immediate effects on pressure pain threshold following a single cervical spine manipulation in healthy subjects. J Orthop Sports Phys Ther. 2007;37:325–329. doi: 10.2519/jospt.2007.2542. http://dx.doi.org/10.2519/jospt.2007.2542. [DOI] [PubMed] [Google Scholar]
- 29.Flynn T, Fritz J, Whitman J, et al. A clinical prediction rule for classifying patients with low back pain who demonstrate short-term improvement with spinal manipulation. Spine (Phila Pa 1976) 2002;27:2835–2843. doi: 10.1097/00007632-200212150-00021. [DOI] [PubMed] [Google Scholar]
- 30.Gay CW, Alappattu MJ, Coronado RA, Horn ME, Bishop MD. Effect of a single session of muscle-biased therapy on pain sensitivity: a systematic review and meta-analysis of randomized controlled trials. J Pain Res. 2013;6:7–22. doi: 10.2147/JPR.S37272. http://dx.doi.org/10.2147/JPR.S37272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gay CW, Horn ME, Bishop MD, Robinson ME, Bialosky JE. Investigating dynamic pain sensitivity in the context of the fear-avoidance model. Eur J Pain. 2015;19:48–58. doi: 10.1002/ejp.519. http://dx.doi.org/10.1002/ejp.519. [DOI] [PubMed] [Google Scholar]
- 32.George SZ, Bishop MD, Bialosky JE, Zeppieri G, Jr, Robinson ME. Immediate effects of spinal manipulation on thermal pain sensitivity: an experimental study. BMC Musculoskelet Disord. 2006;7:68. doi: 10.1186/1471-2474-7-68. http://dx.doi.org/10.1186/1471-2474-7-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Granovsky Y, Yarnitsky D. Personalized pain medicine: the clinical value of psychophysical assessment of pain modulation profile. Rambam Maimonides Med J. 2013;4:e0024. doi: 10.5041/RMMJ.10131. http://dx.doi.org/10.5041/RMMJ.10131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Graven-Nielsen T, Arendt-Nielsen L. Assessment of mechanisms in localized and widespread musculoskeletal pain. Nat Rev Rheumatol. 2010;6:599–606. doi: 10.1038/nrrheum.2010.107. http://dx.doi.org/10.1038/nrrheum.2010.107. [DOI] [PubMed] [Google Scholar]
- 35.Grosen K, Fischer IW, Olesen AE, Drewes AM. Can quantitative sensory testing predict responses to analgesic treatment? Eur J Pain. 2013;17:1267–1280. doi: 10.1002/j.1532-2149.2013.00330.x. http://dx.doi.org/10.1002/j.1532-2149.2013.00330.x. [DOI] [PubMed] [Google Scholar]
- 36.Gwilym SE, Oag HC, Tracey I, Carr AJ. Evidence that central sensitisation is present in patients with shoulder impingement syndrome and influences the outcome after surgery. J Bone Joint Surg Br. 2011;93:498–502. doi: 10.1302/0301-620X.93B4.25054. http://dx.doi.org/10.1302/0301-620X.93B4.25054. [DOI] [PubMed] [Google Scholar]
- 37.Hidalgo-Lozano A, Fernández-de-las-Peñas C, Alonso-Blanco C, Ge HY, Arendt-Nielsen L, Arroyo-Morales M. Muscle trigger points and pressure pain hyperalgesia in the shoulder muscles in patients with unilateral shoulder impingement: a blinded, controlled study. Exp Brain Res. 2010;202:915–925. doi: 10.1007/s00221-010-2196-4. http://dx.doi.org/10.1007/s00221-010-2196-4. [DOI] [PubMed] [Google Scholar]
- 38.Ho CY, Sole G, Munn J. The effectiveness of manual therapy in the management of musculoskeletal disorders of the shoulder: a systematic review. Man Ther. 2009;14:463–474. doi: 10.1016/j.math.2009.03.008. http://dx.doi.org/10.1016/j.math.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 39.Hübscher M, Moloney N, Leaver A, Rebbeck T, McAuley JH, Refshauge KM. Relationship between quantitative sensory testing and pain or disability in people with spinal pain—a systematic review and meta-analysis. Pain. 2013;154:1497–1504. doi: 10.1016/j.pain.2013.05.031. http://dx.doi.org/10.1016/j.pain.2013.05.031. [DOI] [PubMed] [Google Scholar]
- 40.Janal MN, Glusman M, Kuhl JP, Clark WC. On the absence of correlation between responses to noxious heat, cold, electrical and ischemic stimulation. Pain. 1994;58:403–411. doi: 10.1016/0304-3959(94)90135-X. [DOI] [PubMed] [Google Scholar]
- 41.Kanlayanaphotporn R, Chiradejnant A, Vachalathiti R. Immediate effects of the central posteroanterior mobilization technique on pain and range of motion in patients with mechanical neck pain. Disabil Rehabil. 2010;32:622–628. doi: 10.3109/09638280903204716. http://dx.doi.org/10.3109/09638280903204716. [DOI] [PubMed] [Google Scholar]
- 42.Kanlayanaphotporn R, Chiradejnant A, Vachalathiti R. The immediate effects of mobilization technique on pain and range of motion in patients presenting with unilateral neck pain: a randomized controlled trial. Arch Phys Med Rehabil. 2009;90:187–192. doi: 10.1016/j.apmr.2008.07.017. http://dx.doi.org/10.1016/j.apmr.2008.07.017. [DOI] [PubMed] [Google Scholar]
- 43.Leggin BG, Michener LA, Shaffer MA, Brenneman SK, Iannotti JP, Williams GR., Jr The Penn Shoulder Score: reliability and validity. J Orthop Sports Phys Ther. 2006;36:138–151. doi: 10.2519/jospt.2006.36.3.138. http://dx.doi.org/10.2519/jospt.2006.36.3.138. [DOI] [PubMed] [Google Scholar]
- 44.Luime JJ, Koes BW, Hendriksen IJ, et al. Prevalence and incidence of shoulder pain in the general population; a systematic review. Scand J Rheumatol. 2004;33:73–81. doi: 10.1080/03009740310004667. [DOI] [PubMed] [Google Scholar]
- 45.Martínez-Segura R, De-la-Llave-Rincón AI, Ortega-Santiago R, Cleland JA, Fernández-delas-Peñas C. Immediate changes in widespread pressure pain sensitivity, neck pain, and cervical range of motion after cervical or thoracic thrust manipulation in patients with bilateral chronic mechanical neck pain: a randomized clinical trial. J Orthop Sports Phys Ther. 2012;42:806–814. doi: 10.2519/jospt.2012.4151. http://dx.doi.org/10.2519/jospt.2012.4151. [DOI] [PubMed] [Google Scholar]
- 46.McBeth J, Jones K. Epidemiology of chronic musculoskeletal pain. Best Pract Res Clin Rheumatol. 2007;21:403–425. doi: 10.1016/j.berh.2007.03.003. http://dx.doi.org/10.1016/j.berh.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 47.McClatchie L, Laprade J, Martin S, Jaglal SB, Richardson D, Agur A. Mobilizations of the asymptomatic cervical spine can reduce signs of shoulder dysfunction in adults. Man Ther. 2009;14:369–374. doi: 10.1016/j.math.2008.05.006. http://dx.doi.org/10.1016/j.math.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 48.Millan M, Leboeuf-Yde C, Budgell B, Amorim MA. The effect of spinal manipulative therapy on experimentally induced pain: a systematic literature review. Chiropr Man Therap. 2012;20:26. doi: 10.1186/2045-709X-20-26. http://dx.doi.org/10.1186/2045-709X-20-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mintken PE, Cleland JA, Carpenter KJ, Bieniek ML, Keirns M, Whitman JM. Some factors predict successful short-term outcomes in individuals with shoulder pain receiving cervicothoracic manipulation: a single-arm trial. Phys Ther. 2010;90:26–42. doi: 10.2522/ptj.20090095. http://dx.doi.org/10.2522/ptj.20090095. [DOI] [PubMed] [Google Scholar]
- 50.Mroz TM, Carlini AR, Archer KR, et al. Frequency and cost of claims by injury type from a state workers’ compensation fund from 1998 through 2008. Arch Phys Med Rehabil. 2014;95:1048–1054. e6. doi: 10.1016/j.apmr.2013.11.025. http://dx.doi.org/10.1016/j.apmr.2013.11.025. [DOI] [PubMed] [Google Scholar]
- 51.Naugle KM, Fillingim RB, Riley JL., 3rd A meta-analytic review of the hypoalgesic effects of exercise. J Pain. 2012;13:1139–1150. doi: 10.1016/j.jpain.2012.09.006. http://dx.doi.org/10.1016/j.jpain.2012.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Neziri AY, Curatolo M, Nuesch E, et al. Factor analysis of responses to thermal, electrical, and mechanical painful stimuli supports the importance of multi-modal pain assessment. Pain. 2011;152:1146–1155. doi: 10.1016/j.pain.2011.01.047. http://dx.doi.org/10.1016/j.pain.2011.01.047. [DOI] [PubMed] [Google Scholar]
- 53.Paul TM, Soo Hoo J, Chae J, Wilson RD. Central hypersensitivity in patients with subacromial impingement syndrome. Arch Phys Med Rehabil. 2012;93:2206–2209. doi: 10.1016/j.apmr.2012.06.026. http://dx.doi.org/10.1016/j.apmr.2012.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pedowitz RA, Yamaguchi K, Ahmad CS, et al. American Academy of Orthopaedic Surgeons Clinical Practice Guideline on: optimizing the management of rotator cuff problems. J Bone Joint Surg Am. 2012;94:163–167. [PubMed] [Google Scholar]
- 55.Puentedura EJ, Landers MR, Cleland JA, Mintken PE, Huijbregts P, Fernández-de-las-Peñas C. Thoracic spine thrust manipulation versus cervical spine thrust manipulation in patients with acute neck pain: a randomized clinical trial. J Orthop Sports Phys Ther. 2011;41:208–220. doi: 10.2519/jospt.2011.3640. http://dx.doi.org/10.2519/jospt.2011.3640. [DOI] [PubMed] [Google Scholar]
- 56.Schomacher J. The effect of an analgesic mobilization technique when applied at symptomatic or asymptomatic levels of the cervical spine in subjects with neck pain: a randomized controlled trial. J Man Manip Ther. 2009;17:101–108. doi: 10.1179/106698109790824758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Spadoni GF, Stratford PW, Solomon PE, Wishart LR. The evaluation of change in pain intensity: a comparison of the P4 and single-item numeric pain rating scales. J Orthop Sports Phys Ther. 2004;34:187–193. doi: 10.2519/jospt.2004.34.4.187. http://dx.doi.org/10.2519/jospt.2004.34.4.187. [DOI] [PubMed] [Google Scholar]
- 58.Streiner D, Geddes J. Intention to treat analysis in clinical trials when there are missing data. Evid Based Ment Health. 2001;4:70–71. doi: 10.1136/ebmh.4.3.70. [DOI] [PubMed] [Google Scholar]
- 59.Tan G, Jensen MP, Thornby JI, Shanti BF. Validation of the Brief Pain Inventory for chronic non-malignant pain. J Pain. 2004;5:133–137. doi: 10.1016/j.jpain.2003.12.005. http://dx.doi.org/10.1016/j.jpain.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 60.Unnebrink K, Windeler J. Intention-to-treat: methods for dealing with missing values in clinical trials of progressively deteriorating diseases. Stat Med. 2001;20:3931–3946. doi: 10.1002/sim.1149. http://dx.doi.org/10.1002/sim.1149. [DOI] [PubMed] [Google Scholar]
- 61.Valencia C, Kindler LL, Fillingim RB, George SZ. Investigation of central pain processing in shoulder pain: converging results from 2 musculoskeletal pain models. J Pain. 2012;13:81–89. doi: 10.1016/j.jpain.2011.10.006. http://dx.doi.org/10.1016/j.jpain.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.van der Windt DA, Koes BW, de Jong BA, Bouter LM. Shoulder disorders in general practice: incidence, patient characteristics, and management. Ann Rheum Dis. 1995;54:959–964. doi: 10.1136/ard.54.12.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Voogt L, de Vries J, Meeus M, Struyf F, Meuffels D, Nijs J. Analgesic effects of manual therapy in patients with musculoskeletal pain: a systematic review. Man Ther. doi: 10.1016/j.math.2014.09.001. In press. http://dx.doi.org/10.1016/j.math.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 64.Walker-Bone K, Palmer KT, Reading I. Cog-musculoskeletal disorders of the upper limb in the general population. Arthritis Rheum. 2004;51:642–651. doi: 10.1002/art.20535. http://dx.doi.org/10.1002/art.20535. [DOI] [PubMed] [Google Scholar]
- 65.Walser RF, Meserve BB, Boucher TR. The effectiveness of thoracic spine manipulation for the management of musculoskeletal conditions: a systematic review and meta-analysis of randomized clinical trials. J Man Manip Ther. 2009;17:237–246. doi: 10.1179/106698109791352085. http://dx.doi.org/10.1179/106698109791352085. [DOI] [PMC free article] [PubMed] [Google Scholar]