Abstract
The PREVENT Cancer Preclinical Drug Development Program (PREVENT) is a National Cancer Institute, Division of Cancer Prevention (NCI, DCP)-supported program whose primary goal is to bring new cancer preventive interventions (small molecules and vaccines) and biomarkers through preclinical development towards clinical trials by creating partnerships between the public sector (e.g., academia, industry) and DCP. PREVENT has a formalized structure for moving interventions forward in the prevention pipeline using a stage-gate process with go/no go decision points along the critical path for development. This review describes the structure of the program, its focus areas, and provides examples of projects currently in the pipeline.
INTRODUCTION
The PREVENT Cancer Preclinical Drug Development Program (PREVENT) (http://prevention.cancer.gov/programs-resources/programs/prevent) is a National Cancer Institute, Division of Cancer Prevention (NCI, DCP)-supported program whose aim is to provide a formalized pipeline to bring promising cancer preventive interventions (small molecules and vaccines) and biomarkers through preclinical development towards clinical trials. The Program is designed to support the best ideas in cancer prevention and facilitate their advancement to human clinical trials. DCP is a leading sponsor of cancer prevention research worldwide; as such, it provides critical resources needed by the scientific community to develop drugs to prevent cancer. The success of the PREVENT program has implications not only for the United States (US) and the developed world, where cancer has been a burden for decades, but also for developing countries, where cancer is increasingly becoming a major health problem [1, 2].
THE NEED FOR DRUGS TO PREVENT CANCER
The number of people in the US age 65 or older is expected to nearly double by 2020, to about 70 million people. Studies predict that 16% of these older Americans will have been diagnosed with cancer. This translates to 11.4 million people with cancer. Another 6.6 million younger people (<65 years of age) will also be living with the disease [3]. Even if treatment costs remain static, the NCI estimates that demographic changes alone will push the national cost for cancer care up 27% by 2020 [3]. The number of patients surviving cancers that would have killed them quickly decades ago is further driving up costs. Moreover, the cost of cancer therapies is escalating faster than other medical costs, largely due to the introduction of expensive targeted drugs. For example, the angiogenesis inhibitor bevacizumab costs from $30,000 to $62,000 per patient per treatment course, depending on the cancer [3]. More than 90% of the cancer drugs approved by the US Food and Drug Administration (FDA) in the previous few years have cost more than $20,000 for 12 weeks of treatment [3]. A dramatic illustration of this trend is the cost of Provenge®, a newer treatment approved for advanced prostate cancer. This drug was the first individualized vaccine to receive FDA approval; it comes with the enormous cost of $93,000 and extends life, on average, only four months [4].
These predictions make it clear that we must increase our efforts to prevent cancer, not only to prevent the suffering and death from this disease, but because the economic burden of not doing so will be unsustainable for the US and impossible for the rest of the world. Fortunately, the nature of the cancer process itself presents us with significant opportunities to interfere with the progression of this disease. Most common cancers (breast, colorectal, lung, prostate, pancreatic, ovarian), have a latent period of 10–20 years or more before they are clinically manifested. This allows for a long time period in which to intervene with cancer preventive drugs or vaccines with the goal of eliminating premalignant cells or stopping or slowing their growth [5, 6].
A recent study demonstrates just how cost effective preventing cancer with drugs can be [7]. In this analysis, the benefits of using the selective estrogen receptor (ER) modulator (SERM) tamoxifen to prevent breast cancer were modeled in postmenopausal women less than age 55 at the start of treatment. The model assumed women took tamoxifen for five years and were followed for a lifetime. The model also assumed that breast cancer risk reduction (ER-positive cancer) continued for 10 years after treatment cessation, as has recently been shown in long-term follow-up studies [8]. Compared to no treatment, tamoxifen use was highly cost-saving when higher risk populations were targeted (five-year risk ≥1.66%). Tamoxifen use in this population was forecast to save 85 quality-adjusted life years per 1000 postmenopausal women and provide cost savings of $47,580 compared with no treatment over a lifetime of follow-up [7]. However, the acceptance of tamoxifen to prevent breast cancer is poor because of the fear of endometrial cancer, thromboembolism, and other side effects; therefore, better agents are still needed in this niche (reviewed in [9]).
HISTORY OF THE NCI/DCP PRECLINICAL CHEMOPREVENTIVE AGENT DEVELOPMENT PROGRAM
The DCP’s chemoprevention program has been in place since the early 1980s. The preclinical development components of the program have been managed by the Chemopreventive Agent Development Research Group (CADRG). This program was designed to take agents from preclinical in vitro cell assays to animal efficacy assays to Phase 1 clinical development. To facilitate its mission, CADRG has developed an extensive portfolio of prevention-specific research tools and strategies. These tools were designed to address the demanding requirements for cancer preventive drugs to achieve regulatory approval. The program’s portfolio has been continually reevaluated and updated based on emerging scientific data. During the early years of the program, efficacy studies were carried out in rodents treated with chemical carcinogens (a few spontaneous tumor models were also employed). Genetically engineered or mutant models assumed increasing importance in the preclinical portfolio, as research in cancer genetics burgeoned. Importantly, the preclinical models used to test the efficacy of potential cancer preventives are immunocompetent animals where tumors/precancerous lesions arise in situ. These models differ significantly from chemotherapeutic models, where animals are routinely immunosuppressed in order to accept xenografts. If an agent showed significant preventive efficacy to warrant toxicity studies, a battery of standardized pharmacology/toxicology tests were conducted. Once the necessary preclinical studies and strategic planning were carried out to qualify an agent for an Investigational New Drug Application (IND), promising agents were turned over to the DCP Consortia for Early Phase Prevention Trials (Phase 0/1/2 Cancer Prevention Clinical Trials Program) for efficacy testing and biomarker validation in human intervention studies.
Numerous classes of agents have been tested in the preclinical program, including steroidal and nonsteroidal anti-inflammatory drugs (NSAIDs), selective cyclooxygenase (COX)-2 inhibitors, antioxidants, SERMs, statins, histone deacetylase (HDAC) inhibitors, mammalian target of rapamycin (mTOR) inhibitors, 5′-adenosine monophosphate AMP-activated protein kinase (AMPK) activators, antagonists of the phosphatidylinositol-3-kinase (PI3K)/AKT pathway, and a few vaccines (e.g., pUMVC3-IGFBP2-HER2-IGF1R vaccine). From 2000 to 2011, DCP tested 220 new agents or combinations in various morphologic assays, i.e. in vitro and colon aberrant crypt foci (ACF) screens; 60 agents or combinations advanced to animal tumor efficacy testing, and 57 agents have been evaluated in preclinical safety toxicity and pharmacology studies. CADRG has averaged over 40 publications annually documenting agent development activity (http://prevention.cancer.gov/programs-resources/pubs).
Over the years, various types of biomarkers have assumed a prominent place in DCP’s strategic planning efforts. Appropriate risk biomarkers are continually being sought to identify cohorts for clinical studies and to identify populations that would benefit from preventive interventions. Since cancer takes many years to develop, clinical trials where cancer is the outcome are generally not feasible for applied clinical Phase 2 prevention research. Thus, identifying and validating surrogate intermediate endpoints that predict cancer risk are critical for applied prevention research. Studies carried out by the preclinical testing programs were essential to development of intermediate endpoints (e.g., patterns of proliferation and apoptosis, gene expression profiles, and use of imaging modalities), which have been used in clinical trials.
TRANSLATION OF PRECLINICAL STUDIES TO CLINICAL TRIALS
The CADRG Program has been actively involved with the majority of DCP-sponsored clinical trials. Approximately 120 agents/agent combinations have been or are being tested in DCP-funded Phase 1/2 clinical trials. CADRG has carried out preclinical efficacy studies required to qualify agents for Phase 1/2 studies (e.g., Erlotinib, myo-inositol, 9cisUAB30); additionally, the PREVENT Cancer Program has conducted specialized preclinical toxicology, pharmacology, and efficacy studies in compliance with FDA guidelines. Other translational activities include partnering with industry to repurpose approved or pre-approved drugs for cancer prevention trials (e.g., NSAIDs including COX-2 inhibitors, aromatase inhibitors, and inhaled glucocorticoids). CADRG facilitates these partnerships by collaborating to delineate scientific rationales and to define clinical development strategies.
Among the program’s early successes was the Phase 2 trial in familial adenomatous polyposis (FAP) patients conducted under a DCP-sponsored IND [10]. This work was supported by a decade of CADRG-sponsored preclinical studies showing NSAIDs and COX-2 selective agents, such as celecoxib, are among the most consistently effective preventive agents in rodent colon cancer models. Results from efficacy studies in two of these models, the azoxymethane (AOM)-treated rat and the APCMin mouse (a mouse model for FAP) [11, 12], were cited in the FDA’s approval of celecoxib to treat FAP patients and in the rationale for initiating Phase 3 studies of celecoxib. Based on these results, celecoxib was approved by the FDA under the manufacturer’s supplemental New Drug Application (sNDA) to reduce polyp burden in FAP patients as an adjunct to standard care. The follow-on 100-site Phase 3 study under the same IND (Adenoma Prevention with Celecoxib [APC] trial) demonstrated that celecoxib reduced sporadic colon adenoma incidence after three years in subjects at high risk subsequent to polypectomy at baseline [13]. This study also contributed to a meta-analysis of placebo-controlled trials with celecoxib which showed that the concomitant increase in drug-related cardiovascular events linked with COX-2 inhibitors and NSAIDs was seen only in subjects with a higher baseline risk for cardiovascular disease [14], also reviewed in [10]. CADRG’s preclinical work with combinations of NSAIDs and α-difluoromethyl-ornithine (DFMO) in colon cancer models was the basis for a DCP-sponsored Phase 2/3 clinical study of low-dose DFMO plus sulindac. This randomized, placebo-controlled double-blind study included 375 subjects with a history of resected adenomas who were at increased risk for colon cancer. The risk ratio for recurrence in the treated group relative to the placebo group was 0.30 (95% CI = 0.18–0.49; p <0.001) for one or more adenomas and 0.085 (95% CI = 0.011–0.65; p <0.001) for one or more advanced adenomas [15]. In addition, the data generated by CADRG on aromatase inhibitors predicted the significant outcomes in two phase 3 randomized human prevention trials: the Mammary Prevention 3 (MAP3) trial and the International Breast Cancer Intervention Study 2 (IBIS2). In MAP3 exemestane reduced the annual incidence of invasive breast cancer by 65% and produced a 73% reduction in ER-positive breast cancers relative to placebo in postmenopausal women at moderately increased risk for breast cancer [16]. Similarly, IBIS2 showed a 50% reduction in invasive breast cancer and a 58% reduction in ER-positive breast cancers with anastrozole compared to placebo [17]
THE NEW PREVENT CANCER PROGRAM
The new PREVENT program was created in 2011 to better meet the emerging challenges of cancer preventive drug development. It is redesigned to accelerate the cancer prevention drug pipeline by creating partnerships between the public sector (e.g., academia, industry) and DCP. Modeled on benchmarks used by the pharmaceutical industry, PREVENT cancer program has a formalized structure for moving drugs and vaccines forward in the cancer prevention pipeline using a stage-gate process with go/no go decision points along the critical path for drug development. In the past, the preclinical program successfully translated a number of preclinical studies into the clinic and allocated resources toward drug discovery. The new PREVENT cancer program takes a more pragmatic approach, optimizing available resources to translate only the most promising drugs into the clinic. Other major priorities include developing and validating methods for identifying high-risk cohorts, and evaluating response to cancer preventive drug regimens.
PREVENT can provide all the necessary support, documentation, and strategic planning to move cancer preventive drugs, vaccines, and biomarkers (efficacy, toxicity) from preclinical drug development up to (but not including) Phase 1 clinical studies. Examples of resources PREVENT cancer program can provide are outlined in Figure 1. Projects can enter the pipeline at any point along the drug development continuum, but must show a clear translational potential to be considered for funding. NCI does not seek intellectual property from projects developed through the PREVENT cancer program, which are implemented via contracts outside of DCP (see http://prevention.cancer.gov/programs-resources/programs/prevent).
Figure 1.
Examples of Resources PREVENT Can Provide
Governance Structure
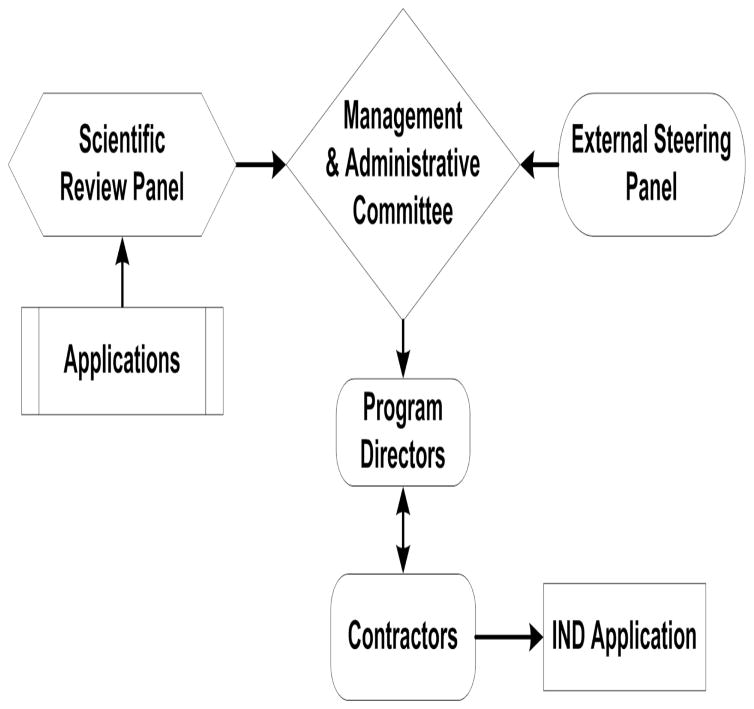
Three groups of experts are key components of PREVENT cancer program’s governance structure (see Fig. 2). The External Steering Panel, comprised of leaders in drug development from academia and the private sector; they advise on areas of scientific focus, help set program priorities, and develop strategic plans to maximize available resources. The Scientific Review Panel is comprised of external chemoprevention and drug development experts who review and prioritize applications submitted to PREVENT cancer program. The Management and Administration Committee consists of senior DCP and non-DCP NIH scientists; they allocate available resources based on overall program needs such as addressing underserved disease sites and expanding efforts to new mechanistic drug classes; monitor the progress of projects in the pipeline; and make go/no-go decisions on projects at each development stage.
Figure 2.
Governance Structure of the New PREVENT Program
Application Process and Evaluation
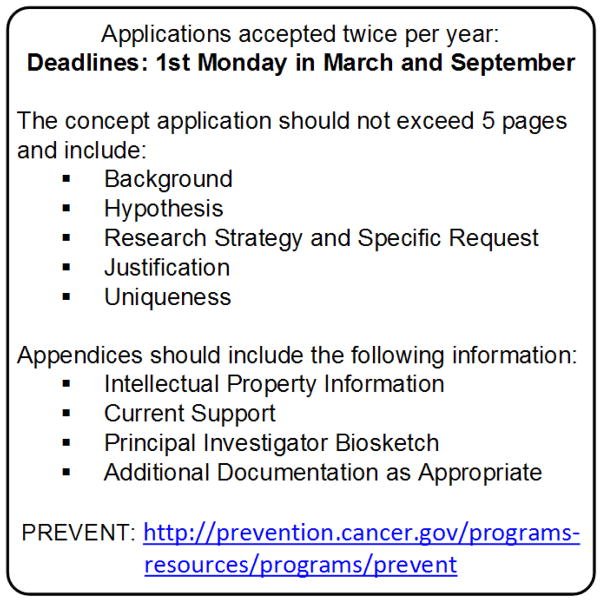
PREVENT cancer program solicits applications from academia, small businesses, and the pharmaceutical industry twice per year (March and September) via a web-based application process (see Fig. 3). Successful applications do not directly lead to a grant or contract award; rather, NCI allocates various contract resources and expertise towards implementation of approved projects. To date, PREVENT cancer program has supported approximately 30% of submitted applications through contract Task Orders. Applications undergo several rounds of review. The first review by the Scientific Review Panel addresses scientific merit, experimental design, etc. Top scoring applications undergo secondary review by the Management and Administration Committee for relevance to the program’s mission, resources and needs. Prioritized projects are then implemented as Task Orders resulting from contract Requests for Proposals (RFPs) issued to the prime contract pools. These prime contract pools have expertise in conducting animal experiments relevant to cancer prevention, efficacy/intermediate endpoint biomarker studies, and toxicology/pharmacology studies. Thus, applicants have access to laboratories with extensive experience in conducting needed prevention experiments. Results, in the form of data or products, are returned to the applicant and may also be used by NCI to make decisions regarding further clinical development. Examples could include vialed products produced under cGMP conditions or toxicology reports to support filing of INDAs with the Food and Drug Administration.
Figure 3.
Schematic of the Current PREVENT Application Process
PREVENT CANCER PROGRAM FOCUS AREAS
Strategies that target inflammation continue to be a major focal point of the PREVENT Cancer Program. Though inflammation is generally a normal host response to tissue damage, if not curtailed, inadequate resolution leads to chronic low-grade inflammation that predisposes to various maladies including cancer. As such, chronic inflammation is considered a hallmark risk factor for the development of cancer, estimated to account for approximately 20% of all cancers (Ryan and Faupel-Badger, this volume) [18]. Though many of the underlying molecular mechanisms linking inflammation and cancer remain to be fully delineated, evidence from literature indicates that excessive cytokines, growth factors, and reactive oxygen and nitrogen species (ROS/NOS) released from inflammatory cells could lead to genetic alterations and instability in the epithelium and subsequent cancer initiation. Chronic inflammation in cells, mediated by ROS, also promotes tumor development by enhancing apoptosis resistance in damaged tissue. Other signaling molecules often dysregulated by inflammation include growth factor-stimulated protein kinases and transcription factors implicated in tumor growth and progression. Among these are the Janus-activated kinases (JAKs), PI3K/AKT, mitogen-activated protein kinases (MAPKs), and transcription factors, including members of the signal transducer and activator of transcription (STAT) family, nuclear-factor kappa B (NF-κB), activation protein-1 (AP-1), and hypoxia inducible factor-1α (HIF-1α). Therefore, novel small molecules and/or vaccines targeting these pathways to hinder inflammation-driven processes continue to be actively sought by the program.
Prophylactic vaccines have had remarkable success in preventing cancers of viral origin, such as hepatitis B and human papillomavirus (HPV). Development of immunoprevention vaccines targeting cancers of non-viral origin is another core PREVENT cancer program’s objective. This strategy holds great promise for halting cancer at its earliest stages in healthy individuals who are at risk, but otherwise have an intact immune system, as opposed to patients with late-stage cancers who have notoriously poor immune systems. One advantage of cancer immunoprevention strategies is that they will require only a limited number of vaccinations to produce long-lasting efficacy, i.e. immunoprotection, hopefully resulting in lowered toxicity and increased acceptance by the public. Such vaccines may be suited for preventing the onset of multiple cancer types in high-risk groups, or as a means for delaying onset or recurrence in patients suffering from the disease. The rationale for testing vaccines at the very earliest stages of cancer progression is based on the limited effectiveness of many cancer therapeutic vaccines, owing to the inception of tumor-associated immunosuppression once frank tumors are formed [19]. One hypothesis being tested in ongoing vaccine projects is whether prophylactic vaccination with tumor antigens, prior to cancer onset, can surmount this limitation and generate immunologic memory leading to eradication of early lesions in animal cancer prevention models. Combinations of drugs and vaccines are also being explored as a tactic to further improve efficacy.
The use of agent combinations and intermittent dosing/scheduling regimens to decrease systemic exposure and reduce toxicity for cancer preventive interventions continues to be a top program priority. Agent combinations allow the use of lower doses of individual drugs, and should minimize drug-associated toxicities. Similarly, intermittent dosing can decrease systemic drug exposure and lower drug-related adverse events. Regional, including topical delivery of chemopreventive agents, also represents a potential strategy to reduce systemic toxicity. All of these methods should increase the benefit/risk ratio, something that is exceptionally important for FDA approval of cancer chemopreventives and acceptance of preventive interventions by both the medical community and patients [9].
Another high PREVENT cancer program priority includes projects that will elucidate the genomics of early pre-cancerous lesions and facilitate the identification of high-risk populations likely to benefit from cancer preventive interventions. The ability to detect intraepithelial neoplasia and associated genomic changes at the earliest stages is critical for improving cancer preventive interventions that reduce the onset of carcinogenesis and ultimately cancer incidence. Additional studies to discover the underlying genetic alterations of pre-cancerous lesions are imperative for identifying at-risk populations and specific subgroups most likely to benefit from small molecule approaches and/or immunoprevention vaccine strategies.
OVERVIEW OF PREVENT CANCER PROGRAM PROJECTS AND SPECIFIC EXAMPLES
Since its inception in 2011, the PREVENT Cancer Program has reviewed 125 application submissions spanning 10 distinct organ systems. Of these, approximately 40 projects have been selected for funding, including 20 studies focused on evaluating the cancer preventive efficacy of small molecules, 10 focused on immunoprevention, and 10 projects centered on biomarker discovery (see Table 1). Among the group of agent-based projects are studies aimed at development of new small molecules and proof-of-principle testing, studies exploring the repurposing of approved drugs for cancer prevention indications, and projects involving drug reformulation and toxicology support. Furthermore, some studies are focused on the identification and validation of pathway-related molecular biomarkers to correlate animal efficacy data with molecular target data in clinical samples. Other projects will examine novel drug combinations and alternate dosing schedules to determine whether these approaches are capable of reducing toxicity while maintaining efficacy to ultimately augment the benefit/risk ratio. Also new to the program are 10 cancer preventive vaccine development projects focused on harnessing the immune system as a means of eradicating and/or thwarting the progression of early premalignant lesions to invasive cancers. Many of the immunoprevention vaccines under study are directed against tumor-specific antigens or other regulatory proteins aberrantly expressed throughout the cancer progression continuum (i.e., tumor-associated antigens). Goals of these vaccine projects are to assess immune response and perform efficacy evaluations in cancer prevention models of breast, colon, lung, ovary, and pancreas. Several vaccines will also be tested in combination with a drug.
Table 1.
Summary of Awarded PREVENT Projects
| Biomarkers | Small Molecules | Vaccine | Total | ||
|---|---|---|---|---|---|
| Agent/Vaccine Production | Cervix | 1 | 1 | ||
| Mammary | 1 | 1 | |||
| Animal Efficacy | Colon | 2 | 4 | 1 | 7 |
| Lung | 3 | 4 | 3 | 10 | |
| Mammary | 1 | 4 | 2 | 7 | |
| Oral Cavity | 1 | 1 | |||
| Ovarian | 1 | 1 | |||
| Pancreas | 3 | 1 | 4 | ||
| Prostate | 2 | 2 | |||
| Uterus, Liver | 1 | 1 | |||
| Biomarker, Human | Colon | 2 | 2 | ||
| Breast | 1 | 1 | |||
| Non-clinical Toxicology | Cervix | 1 | 1 | 2 | |
| Total | 10 | 20 | 10 | 40 |
Several of the awarded projects target molecular pathways associated with inflammation. Examples include efficacy studies with nonsteroidal anti-inflammatories and dual microsomal prostaglandin E2 (PGE2) synthase-1 (mPGES-1)/5-lipoxygenase (5-LOX) inhibitors that target both arachidonic acid-derived prostaglandin and leukotriene inflammatory mediators. Unlike conventional COX-2 inhibitors, no cardiovascular toxicity has been reported from concomitantly blocking the COX/LOX pathways. Other molecular targets under investigation include STAT3 inhibitors, small molecule blockers of inducible nitric-oxide synthase (iNOS), and agonists of ERβ, all of which are directly or indirectly linked to inflammation-driven signaling conduits. Antagonists of the P2X7 receptor are also being tested in a transgenic pancreatic cancer model (p48Cre/+-LSL-KrasG12D/+); these receptors are upregulated in the inflammasome during pancreatic tumor development. Efficacy and modulation of inflammasome signaling pathway biomarkers, applicable to early-phase clinical studies, will also be determined utilizing transcriptome and miRNA analysis. Finally, a biomarker study to identify a blood-based metabolomic signature indicative of obesity-induced inflammatory lesions found in human breast tissue is in progress. The next sections provide a more detailed description of awarded projects by organ type.
Breast Cancer
Over 200,000 new cases of breast cancer are diagnosed in the US each year [20], underscoring the need to develop novel, safe and effective drugs to prevent malignant transformation of breast tissue and early onset of the disease. One project is evaluating the potential of GLG-302, a small-molecule antagonist of the JAK2/STAT3 signaling pathway, to prevent breast cancer. GLG-302 was identified by structure-based virtual screening of the National Cancer Institute’s chemical libraries. Strong evidence suggests that aberrant constitutive activation of STAT3 promotes the initiation and progression of human breast and other cancers (reviewed in [21]). Moreover, in preliminary work, GLG-302 was shown to block STAT3 signaling and inhibit growth of ER-negative MDA-MB-231 breast tumors. GLG-302 also effectively inhibited mammary cancer growth in MMTV-Neu transgenic mice by blocking Stat3:Stat3 dimer formation, DNA-binding, transcriptional activation, and inducing apoptosis [22]. The goal of this project is to determine whether long-term oral exposure to GLG-302 can prevent malignant transformation of breast epithelium and formation of ER-positive mammary tumors in methylnitrosourea (MNU)-treated female rats and ER-negative mammary tumors in MMTV-Neu mice. Initial safety studies indicate GLG-302 is well tolerated in mice, rats, and dogs.
Lung Cancer
An urgent unmet need exists for effective preventive interventions to block or thwart the development of lung cancer. The discovery of novel, effective lung cancer therapies has been a major focus area of the pharmaceutical industry. Unfortunately, since 1998 only 10 drugs have been approved by the FDA to treat lung cancer, whereas 167 other drugs failed in clinical trials. These novel treatments are applicable to only a subset of lung cancer patients. Lung cancer is an insidious and incurable malady, especially once it metastasizes to other organs; hence, development of preventive strategies for this cancer continues to be a priority for public health.
One PREVENT cancer program’s repurposing project is evaluating the FDA-approved glucocorticoid and anti-asthmatic drug ciclesonide for the prevention of lung cancer in which chronic inflammation is known to play a prominent role [23]. As potent anti-inflammatories, inhaled glucocorticoids, widely used to treat asthma and chronic obstructive pulmonary disease (COPD), have been shown in retrospective cohort studies to lower lung cancer incidence in smokers with COPD [24, 25]. Similarly, inhaled corticosteroids have demonstrated excellent lung cancer preventive activity in rodent models [26–28]. However, in trials of high-risk individuals with computed tomography (CT)–detected peripheral solid lung nodules, neither budesonide or fluticasone has proven effective [29–31]. The low penetration of budesonide to the peripheral lung was suggested as a possible factor [30]. Nevertheless, a subgroup analysis from this study revealed that nonsolid and partially solid ground-glass nodules with areas of opacity tended to regress in subjects treated with budesonide for one year [30].
Ciclesonide is a pro-drug with an improved therapeutic index, minimal systemic bioavailability, and higher deposition rate to the peripheral lung in humans compared with budesonide, fluticasone, and other inhaled corticosteroids [32, 33]. Ciclesonide is inactive until converted to its active metabolite desisobutyryl-ciclesonide by endogenous esterases in lung airways where it exerts a wide range of antiinflammatory actions in lung. For example, it inhibited tumor necrosis factor (TNFα), and interleukin-2 (IL-2), IL-4, IL-5, IL-8, and IL-12, and reduced expression of intracellular adhesion molecule-1, granulocyte-macrophage colony stimulating factor, and monocyte chemoattractant protein 1 in clinical and nonclinical studies [34, 35]. Aims of this project are to evaluate the toxicity and efficacy of ciclesonide delivered intratracheally against benzo[a]pyrene (B[a]P)-induced lung adenomas in A/J mice. Since cell proliferation inhibition is a mechanism of glucocorticoids, modulation of inflammatory biomarker expression in the lung will be monitored to confirm inhibitory activity. Results from this study could lead to a Phase 2 trial.
Another study is testing the peptide-based vaccine known as GV1001 for the prevention of both non-small cell lung cancer (NSCLC) and squamous cell carcinomas (SCCs) of the lung. This vaccine targets human telomerase (hTERT), which is widely expressed across all histological subtypes of lung cancer, and is considered a key molecular driver of lung carcinogenesis [36]. Enhanced TERT expression occurs early in premalignant lung lesions and increases as lesions progress in both humans and mouse tumor models [37], thus it appears to be a suitable target for lung cancer prevention. This project will determine if early vaccination with a TERT-directed vaccine produces an immunological response and prevents lung tumor formation in two well-characterized preclinical lung tumor models—the B[a]P-induced A/J x p53+/− mouse model of NSCLC, which gauges both adenoma and adenocarcinoma development, and the N-nitroso-tris-chloroethylurea (NTCU) Swiss mouse model of lung SCC. The immunological response induced by TERT peptide vaccination will be monitored and compared with tumor multiplicity and tumor load. GV1001 has been extensively tested in Phase 1 and 2 trials in NSCLC patients and has a positive safety profile, with no serious adverse effects [38]. A Phase 3 trial of GV1001 in combination with chemotherapy against pancreatic cancer failed to demonstrate a therapeutic effect [39]. As noted above, this may be partially explained by the immunocompromised status of the patients. Therefore, vaccinating at early precancerous stages, when patients are not immunocompromised, may prove to be more effective.
Another approach being explored is the use of intermittent dosing to reduce toxicity of molecularly targeted drugs for lung cancer prevention. Knowledge about molecular drivers underlying lung tumor formation and progression has increased in recent years and has led to targeted agents which are partially to strongly effective in cancer therapeutic settings. However, the toxicity of many newly developed targeted drugs precludes their use for cancer prevention purposes. Among the most promising agents are epidermal growth factor receptor (EGFR) inhibitory agents (e.g., gefitinib), the MEK1/2 inhibitors (e.g., AZD6244), which block downstream of the K-ras oncogene in NSCLCs, and the PI3K pathway inhibitors (e.g., XL147), which may have utility against SCCs. All of these classes of agents have toxic side effects. Emerging preclinical and clinical data demonstrate that lowering the dosing schedule from daily to weekly dosing may be one promising strategy to reduce toxicity of erlotinib, and possibly other agents, without compromising efficacy [40–42]. The hypothesis to be tested is whether weekly dosing of these three agents will retain their preventive efficacy and produce less toxicity in mouse models of NSCLC and SCC. If positive, this approach may be clinically applicable for prevention of lung cancer (secondary or primary) or lung cancer recurrence in high-risk groups.
The utilization of predictive biomarkers of cancer preventive efficacy for Phase 2 clinical trials is an important goal of the program. One such project will determine genes modulated by myo-inositol, budesonide, and pioglitazone during progression of NSCLC and small-cell lung cancer (SCLC) in mice; animal gene expression signature data will then be compared with corresponding human genetic data from prevention trials to identify biomarkers that predict efficacy. Biomarker data will be generated from chemoprevention studies conducted with myo-inositol, budesonide, and pioglitazone in mouse lung models of NSCLC and SCC. These agents were chosen because they have chemopreventive activity in at least one of the test mouse models and either human biomarker data are available from prevention studies (myo-inositol) or data should become available in the near future (budesonide and pioglitazone). Microarray and RNA sequencing assays will be used to determine genes significantly over- or under-expressed in high-risk lung mouse tissue (histologically normal from carcinogen-treated mice) and in early lung lesions. Based on the results of gene expression patterns, molecular pathways that are dysregulated during the early stages of lung tumor development will be identified. These studies could yield promising genetic biomarkers with strong predictive values for specific interventions in human lung cancer prevention trials.
Colon Cancer
Colon cancer is the second leading cause of cancer-related mortality in the US [20]. The estimated timeline for malignant transformation and progression of normal colorectal mucosa to pre-invasive adenomas and colon adenocarcinoma is estimated to take 5–15 years. Thus, this timeframe provides an ample window of opportunity for preventive interventions that target high-risk patient populations.
One project is evaluating a multi-antigen vaccine approach in combination with the colon cancer preventive agent celecoxib to deter colon cancer development. The vaccine being tested is a multi-antigen vaccine comprised of epitopes derived from three proteins (CDC25B, COX-2, and PRL-3) overexpressed in human colorectal adenomas and carcinomas, and in colon tumors in ApcMin mice. Importantly, all three proteins have been shown to elicit an immunological response in individuals exposed to the antigens during colon cancer development. Aims are to determine the effects of immunization with the multiantigen vaccine singly and in combination with celecoxib on colon tumor formation in Apc Min mice and in AkrMin (AKR/J x B6-Min F1) mice. As discussed further below, AkrMin mice develop both adenomas and adenocarcinomas throughout the small intestine and colon and live substantially longer than ApcMin mice, thus better recapitulating colon cancer progression in humans [43]. In both models, immunization will be initiated relatively late, at 8 weeks of age in ApcMin mice and 16 weeks of age in AkrMin mice. In preliminary work by the investigator, immunizing these mice with the PRL-3, CDC25B, or COX-2 peptide was found to decrease adenoma formation by 33–55%. This study will determine whether a vaccine targeting all three antigens is more efficacious, and if combining the vaccine with celecoxib further augments efficacy in reducing adenoma progression. Whether vaccination with the multiantigen vaccine has superior T-cell and immunological activity compared with single-targeted protein vaccines in both mouse models will also be examined. If effective, this strategy could have important implications for colon cancer prevention.
Another approach to preventing colon cancer is examining the combined effects of aspirin and omeprazole on colon cancer development. Ample epidemiological and clinical trial data support the use of NSAIDs for reducing colorectal cancer risk. Among the NSAIDs, the cancer chemopreventive effects of aspirin are the most well-established against colorectal and other cancers [44]; however, gastrointestinal toxicity associated with long-term use may limit its utility for cancer prevention purposes. Additionally, omeprazole has been shown to reduce carcinogen-induced colon cancer in mice and rats via potential antiinflammatory, antioxidative, and antimutagenic effects [45–47]. This drug combination is already used worldwide to reduce cardiovascular risk. The hypothesis being tested is whether the combination of aspirin and omeprazole provides a safer approach and can improve the efficacy of aspirin.
Another colorectal cancer prevention project aims to repurpose eldecalcitol (ED-71), a novel vitamin D3 analog used for the treatment of post-menopausal osteoporosis in Japan since 2011. The approval of this new analog was based on results of a three-year randomized, double-blind Phase 3 active comparator study of oral eldecalcitol (0.75 μg/day) versus alfacalcidol (1.0 μg/day) in vitamin D3 sufficient osteoporotic patients. Eldecalcitol showed superior effects in increasing bone mineral density, reducing bone resorption markers, and lowering vertebral fraction incidence compared with alfacalcidol [48]. Furthermore, the overall safety profile of eldecalcitol was favorable, with no sustained hypercalcemia or unexpected drug accumulation after multiple dosing. Several characteristics of eldecalcitol differentiate it from other vitamin D analogs. For example, eldecalcitol is rapidly absorbed and has high affinity for serum vitamin D binding protein, which contribute to its unique pharmacokinetics and long plasma half-life of 53 hours in healthy male volunteers. Therefore, eldecalcitol achieves a sustainable exposure level that enhances its biological activity. Unlike other vitamin D analogs, eldecalcitol is not a substrate for CYP3A4, and its systemic clearance is via liver enzymes and not CYP24A1 produced from the kidney. Additionally, the unique 3-hydroxypropoxy group of eldecalcitol stabilizes the eldecalcitol–vitamin D receptor complex, prolonging residence time, thus providing superior efficacy. Finally, eldecalcitol possesses minimal suppression of parathyroid hormone. Because of these distinct characteristics, one could presume eldecalcitol may possess additional pharmacodynamic effects in extra-skeletal tissues. The PREVENT Cancer Program is currently assessing the efficacy of eldecalcitol in colorectal cancer prevention in the ApcMin mouse model, prior to testing in individuals at high-risk for colorectal cancer.
Ovarian Cancer
The development of immunoprevention strategies to impede the progression of ovarian cancer is an important unmet clinical need. An estimated 21,980 new cases of ovarian cancer were expected in the US in 2014; moreover, most women are diagnosed with stage III or IV disease, when survival rate is extremely low (<30%) [49]. BRCA1/2 mutations confer a 20–40% increased risk for developing ovarian cancer [49]. Likewise, women with hereditary non-polyposis colorectal cancer have a 10% enhanced risk of developing the disease; hence, both groups would be prime candidates for an ovarian immunoprevention vaccine. One study is testing a vaccine directed against the tumor-associated protein mesothelin. As a highly immunogenic protein overexpressed in ovarian and other cancers, but not normal tissues, mesothelin is a prime candidate for cancer therapeutic and immunoprevention interventions [50, 51]. Goals of this project are to develop a protein-based immunoprevention vaccine containing mesothelin and cyclic di-nucleotide (CDN) and evaluate efficacy against ovarian cancer in a double knock-out (DKO) genetically engineered mouse model (see below). The addition of CDN to the vaccine is expected to enhance production of antigen-specific cytotoxic CD8+ T cells capable of infiltrating and destroying tumor cells, thus potentiating cell-mediated immune responses. As in other vaccine projects, tumor growth, histology, and immune response will be monitored.
NEW ANIMAL MODELS
Table 2 lists some of the new genetically engineered animal models undergoing efficacy evaluations in either agent- or vaccine-based investigations for the prevention of cancers of the colon, lung, mammary gland, pancreas, and ovary. Examples include the AkrMin mouse, a hybrid produced by cross breeding ApcMin mice with Akr/J mice (see above) [43]. Unlike standard ApcMin that develop adenomas only in the small intestine, AKR/Min mice develop adenomas and invasive adenocarcinomas in both the small intestine and the colon. Therefore, this model will allow the testing of preventive interventions throughout the course of adenoma progression to overt colon cancer.
Table 2.
Examples of Genetically Engineered Animal Models
| Organ Site | Species | Genetic Modification | Endpoint Measured | Reference |
|---|---|---|---|---|
| Colon | Mouse | Min (AKR mutant) | Adenoma, adenocarcinoma | [43] |
| Lung | Mouse | EGFR(L858R) | Adenocarcinoma | [52] |
| Mouse | p53(Ala135Val), p16+/−, Kras+/− | Adenocarcinoma | Unpublished Data | |
| Mammary | Mouse | BRCA1 and TP53 mutant | Tumor | [53] |
| Ovary | Mouse | Pax8-Cre Brca, Tp53, Pten mutated | Serous carcinoma | [54] |
| Pancreas | Mouse | hMuc1(insertion) and KrasG12D/+ | Adenocarcinoma | [55] |
| Mouse | KrasG12D/+ | Adenocarcinoma | [56] |
Other new transgenic models include mutant A/J mice containing genetic alterations and activating mutations in KRAS, p53, and p16, commonly found in human lung tumors. These models will be used to assess intermittent dosing of targeted drugs for lung cancer prevention. Transgenic mice bearing the EGFRL858R mutation, also found in human lung adenocarcinomas, will also be evaluated.
New transgenic pancreatic cancer models (e.g., p48Cre/+-LSL-KrasG12D/+) incorporating genetic mutations in the KRAS and MUC1 genes, also found in human pancreatic cancers, are being explored for vaccine and small molecule strategies to block the progression and possible onset of pancreatic tumorigenesis. MUC1 is overexpressed in early pancreatic intraepithelial neoplasia (PanIN) and pancreatic ductal adenocarcinoma (PDAC) in transgenic KRAS PDAC models [57]. Strategies to prevent or blunt ovarian cancer have been hindered by the lack of suitable experimental models. This is especially true for high-grade serous carcinoma, which is the most common and deadly histological ovarian cancer subtype due to its ability to spread to the peritoneum and reproductive tract [58, 59]. Though multiple transgenic approaches have been attempted, none recapitulates all aspects of human disease. Defining the cell of origin and pathogenesis of serous ovarian cancer has been the greatest challenge, with most efforts focused on the ovary epithelium as the site of tumor initiation. However, emerging clinical data suggest that ovarian high-grade serous carcinomas arise from precursor lesions that originate in the secretory cells of the fallopian tube epithelium and not the ovary [59, 60]. These early precursor lesions, denoted serous tubal intraepithelial carcinomas (STIC), have been detected in BRCA1/2 mutation carriers who are at high risk for developing serous carcinomas and in women with disseminated disease [61, 62]. Recently, a genetically engineered mouse model has been developed that mirrors precursor STIC lesions and genetic mutations originating in fallopian tube secretory cells characteristic of human high-grade serous carcinoma [54]. The model specifically targets fallopian tube secretory cells by driving expression of the Cre recombinase from a Pax8 promoter essential for development of the fallopian tube but not the ovaries. The model entails the generation of Pax8-Cre mice harboring mutated BRCA1/BRCA2, TP53, and Pten genes known to be altered in human secretory epithelium within the fallopian tubes. These mice will be employed to investigate whether immunization with a vaccine directed against mesothelin can prevent ovarian cancer progression when administered at an early age, prior to ovarian cancer development.
FUTURE DIRECTIONS
The PREVENT Cancer Program will continue to optimize its resources to accelerate the cancer prevention drug pipeline. The program will actively maintain focus on developing drugs that target novel signaling pathways including the inflammatory process, repurposing approved drugs for cancer preventive indications, and developing alternative dosing strategies to reduce adverse events and drug combinations targeting different molecular pathways to augment efficacy and reduce toxicity. PREVENT will also vigorously pursue immunopreventive vaccines and will actively seek out investigators to submit proposals in this important research area, as immunoprevention appears to hold particular promise as a long-lasting prevention tool.
The PREVENT Cancer Program will also actively continue to seek out projects exploring genomic, molecular imaging, proteomic, metabolomic, glycomic, biogenomic, and epigenomic studies to identify and validate surrogate intermediate endpoints for use in Phase 2 trials. These efforts will require a multi-disciplinary approach, encompassing molecular and cellular biology, molecular epidemiology, genomics, bioinformatics, and other scientific disciplines. These studies will undoubtedly gain increasing importance as personalized medicine takes a more prominent place in treating and preventing cancer.
Acknowledgments
The authors thank the members of the External Steering Panel for advice and guidance in developing and operating the PREVENT Cancer Program, the many members of the Scientific Review Panel for their efforts in providing peer review for applications submitted to the Program, and members of the Management and Administration Committee for ongoing efforts to ensure efficient operation of the Program. We also thank the investigators for performing the studies referenced in this publication which were funded by the PREVENT Cancer Program under contracts: HHSN261201200013I; HHSN261201200015I; HSN261201200020I; HHSN261201200021I. We also thank the staff at CCS Associates for research support and editorial review of the manuscript.
Footnotes
There are no direct financial interests in the subject matter or products discussed herein.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Kanavos P. The rising burden of cancer in the developing world. Ann Oncol. 2006;17(Suppl 8):viii15–viii23. doi: 10.1093/annonc/mdl983. [DOI] [PubMed] [Google Scholar]
- 3.Malakoff D. Can treatment costs be tamed? Science. 2011;331:1545–47. doi: 10.1126/science.331.6024.1545. [DOI] [PubMed] [Google Scholar]
- 4.Mahar M. Medicare will pay $93,000 for Provenge: A big win for wall street. 2011 Apr 4; http://www.healthbeatblog.com/2011/04/medicare-will-pay-93000-for-provenge-a-big-win-for-wall-street-.html. Healthbeatblogorg 2011.
- 5.Savage N. Early detection: Spotting the first signs. Nature. 2011;471:S14–S15. doi: 10.1038/471S14a. [DOI] [PubMed] [Google Scholar]
- 6.Umar A, Dunn BK, Greenwald P. Future directions in cancer prevention. Nat Rev Cancer. 2012;12:835–48. doi: 10.1038/nrc3397. [DOI] [PubMed] [Google Scholar]
- 7.Noah-Vanhoucke J, Green LE, Dinh TA, Alperin P, Smith RA. Cost-effectiveness of chemoprevention of breast cancer using tamoxifen in a postmenopausal US population. Cancer. 2011;117:3322–31. doi: 10.1002/cncr.25926. [DOI] [PubMed] [Google Scholar]
- 8.Cuzick J, Forbes JF, Sestak I, Cawthorn S, Hamed H, Holli K, Howell A. Long-term results of tamoxifen prophylaxis for breast cancer--96-month follow-up of the randomized IBIS-I trial. J Natl Cancer Inst. 2007;99:272–82. doi: 10.1093/jnci/djk049. [DOI] [PubMed] [Google Scholar]
- 9.Meyskens FL, Jr, Curt GA, Brenner DE, Gordon G, Herberman RB, Finn O, Kelloff GJ, Khleif SN, Sigman CC, Szabo E. Regulatory approval of cancer risk-reducing (chemopreventive) drugs: Moving what we have learned into the clinic. Cancer Prev Res. 2011;4:311–23. doi: 10.1158/1940-6207.CAPR-09-0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Steward WP, Brown K. Cancer chemoprevention: A rapidly evolving field. Br J Cancer. 2013;109:1–7. doi: 10.1038/bjc.2013.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jacoby RF, Seibert K, Cole CE, Kelloff G, Lubet RA. The cyclooxygenase-2 inhibitor celecoxib is a potent preventive and therapeutic agent in the Min mouse model of adenomatous polyposis. Cancer Res. 2000;60:5040–44. [PubMed] [Google Scholar]
- 12.Reddy BS, Kawamori T, Lubet RA, Steele VE, Kelloff GJ, Rao CV. Chemopreventive efficacy of sulindac sulfone against colon cancer depends on time of administration during carcinogenic process. Cancer Res. 1999;59:3387–91. [PubMed] [Google Scholar]
- 13.Bertagnolli MM, Eagle CJ, Zauber AG, Redston M, Solomon SD, Kim K, Tang J, Rosenstein RB, Wittes J, Corle D, Hess TM, Woloj GM, Boisserie F, Anderson WF, Viner JL, Bagheri D, Burn J, Chung DC, Dewar T, Foley R, Hoffman N, Macrae F, Pruitt R, Saltzman J, Salzberg B, Sylwestrowicz T, Gordon G, Hawk E. Celecoxib for the prevention of sporadic colorectal adenomas. N Engl J Med. 2006;355:873–84. doi: 10.1056/NEJMoa061355. [DOI] [PubMed] [Google Scholar]
- 14.Solomon SD, Wittes J, Finn PV, Fowler R, Viner J, Bertagnolli MM, Arber N, Levin B, Meinert CL, Martin B, Pater JL, Goss PE, Lance P, Obara S, Chew EY, Kim J, Arndt G, Hawk E. Cardiovascular risk of celecoxib in 6 randomized placebo-controlled trials: The cross trial safety analysis. Circulation. 2008;117:2104–13. doi: 10.1161/CIRCULATIONAHA.108.764530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meyskens FL, Jr, McLaren CE, Pelot D, Fujikawa-Brooks S, Carpenter PM, Hawk E, Kelloff G, Lawson MJ, Kidao J, McCracken J, Albers CG, Ahnen DJ, Turgeon DK, Goldschmid S, Lance P, Hagedorn CH, Gillen DL, Gerner EW. Difluoromethylornithine plus sulindac for the prevention of sporadic colorectal adenomas: A randomized placebo-controlled, double-blind trial. Cancer Prev Res. 2008;1:32–38. doi: 10.1158/1940-6207.CAPR-08-0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goss PE, Ingle JN, Ales-Martinez JE, Cheung AM, Chlebowski RT, Wactawski-Wende J, McTiernan A, Robbins J, Johnson KC, Martin LW, Winquist E, Sarto GE, Garber JE, Fabian CJ, Pujol P, Maunsell E, Farmer P, Gelmon K, Tu D. Exemestane for breast-cancer prevention in postmenopausal women. N Engl J Med. 2011;364:2381–91. doi: 10.1056/NEJMoa1103507. [DOI] [PubMed] [Google Scholar]
- 17.Cuzick J, Sestak I, Forbes JF, Dowsett M, Knox J, Cawthorn S, Saunders C, Roche N, Mansel RE, von Minckwitz G, Bonanni B, Palva T, Howell A. Anastrozole for prevention of breast cancer in high-risk postmenopausal women (IBIS-II): An international, double-blind, randomised placebo-controlled trial. Lancet. 2014;383:1041–48. doi: 10.1016/S0140-6736(13)62292-8. [DOI] [PubMed] [Google Scholar]
- 18.Hanahan D, Weinberg RA. Hallmarks of cancer: The next generation. Cell. 2011;144:646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 19.Schneider T, Hoffmann H, Dienemann H, Schnabel PA, Enk AH, Ring S, Mahnke K. Non-small cell lung cancer induces an immunosuppressive phenotype of dendritic cells in tumor microenvironment by upregulating B7-H3. J Thorac Oncol. 2011;6:1162–68. doi: 10.1097/JTO.0b013e31821c421d. [DOI] [PubMed] [Google Scholar]
- 20.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 21.Siveen KS, Sikka S, Surana R, Dai X, Zhang J, Kumar AP, Tan BK, Sethi G, Bishayee A. Targeting the STAT3 signaling pathway in cancer: Role of synthetic and natural inhibitors. Biochim Biophys Acta. 2014;1845:136–54. doi: 10.1016/j.bbcan.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 22.Siddiquee K, Zhang S, Guida WC, Blaskovich MA, Greedy B, Lawrence HR, Yip ML, Jove R, McLaughlin MM, Lawrence NJ, Sebti SM, Turkson J. Selective chemical probe inhibitor of Stat3, identified through structure-based virtual screening, induces antitumor activity. Proc Natl Acad Sci USA. 2007;104:7391–96. doi: 10.1073/pnas.0609757104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu J, Liang Q, Frost-Pineda K, Muhammad-Kah R, Rimmer L, Roethig H, Mendes P, Sarkar M. Relationship between biomarkers of cigarette smoke exposure and biomarkers of inflammation, oxidative stress, and platelet activation in adult cigarette smokers. Cancer Epidemiol Biomarkers Prev. 2011;20:1760–69. doi: 10.1158/1055-9965.EPI-10-0987. [DOI] [PubMed] [Google Scholar]
- 24.Parimon T, Chien JW, Bryson CL, McDonell MB, Udris EM, Au DH. Inhaled corticosteroids and risk of lung cancer among patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2007;175:712–19. doi: 10.1164/rccm.200608-1125OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kiri VA, Fabbri LM, Davis KJ, Soriano JB. Inhaled corticosteroids and risk of lung cancer among COPD patients who quit smoking. Respir Med. 2009;103:85–90. doi: 10.1016/j.rmed.2008.07.024. [DOI] [PubMed] [Google Scholar]
- 26.Wattenberg LW, Wiedmann TS, Estensen RD, Zimmerman CL, Galbraith AR, Steele VE, Kelloff GJ. Chemoprevention of pulmonary carcinogenesis by brief exposures to aerosolized budesonide or beclomethasone dipropionate and by the combination of aerosolized budesonide and dietary myo-inositol. Carcinogenesis. 2000;21:179–82. doi: 10.1093/carcin/21.2.179. [DOI] [PubMed] [Google Scholar]
- 27.Pereira MA, Li Y, Gunning WT, Kramer PM, Al-Yaqoub F, Lubet RA, Steele VE, Szabo E, Tao L. Prevention of mouse lung tumors by budesonide and its modulation of biomarkers. Carcinogenesis. 2002;23:1185–92. doi: 10.1093/carcin/23.7.1185. [DOI] [PubMed] [Google Scholar]
- 28.Estensen RD, Jordan MM, Wiedmann TS, Galbraith AR, Steele VE, Wattenberg LW. Effect of chemopreventive agents on separate stages of progression of benzo[α]pyrene induced lung tumors in A/J mice. Carcinogenesis. 2004;25:197–201. doi: 10.1093/carcin/bgg196. [DOI] [PubMed] [Google Scholar]
- 29.Lazzeroni M, Guerrieri-Gonzaga A, Serrano D, Varricchio MC, Veronesi G, Radice D, Feroce I, Nardi-Pantoli A, Lippman SM, Szabo E, Bonanni B. Budesonide versus placebo in high-risk population with screen-detected lung nodules: Rationale, design and methodology. Contemp Clin Trials. 2010;31:612–19. doi: 10.1016/j.cct.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Veronesi G, Szabo E, Decensi A, Guerrieri-Gonzaga A, Bellomi M, Radice D, Ferretti S, Pelosi G, Lazzeroni M, Serrano D, Lippman SM, Spaggiari L, Nardi-Pantoli A, Harari S, Varricchio C, Bonanni B. Randomized phase II trial of inhaled budesonide versus placebo in high-risk individuals with CT screen-detected lung nodules. Cancer Prev Res. 2011;4:34–42. doi: 10.1158/1940-6207.CAPR-10-0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van den Berg RM, Teertstra HJ, van Zandwijk N, van Tinteren H, Visser C, Pasic A, Sutedja TG, Baas P, Golding RP, Postmus PE, Smit EF. CT detected indeterminate pulmonary nodules in a chemoprevention trial of fluticasone. Lung Cancer. 2008;60:57–61. doi: 10.1016/j.lungcan.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 32.Newman S, Salmon A, Nave R, Drollmann A. High lung deposition of 99mTc-labeled ciclesonide administered via HFA-MDI to patients with asthma. Respir Med. 2006;100:375–84. doi: 10.1016/j.rmed.2005.09.027. [DOI] [PubMed] [Google Scholar]
- 33.Leach CL, Bethke TD, Boudreau RJ, Hasselquist BE, Drollmann A, Davidson P, Wurst W. Two-dimensional and three-dimensional imaging show ciclesonide has high lung deposition and peripheral distribution: A nonrandomized study in healthy volunteers. J Aerosol Med. 2006;19:117–26. doi: 10.1089/jam.2006.19.117. [DOI] [PubMed] [Google Scholar]
- 34.Chopra D, Bhandari B, Wardhan N. Ciclesonide--a novel corticosteroid for the management of asthma. Curr Clin Pharmacol. 2012;7:73–77. doi: 10.2174/157488412800228901. [DOI] [PubMed] [Google Scholar]
- 35.Singas E, Karpel JP. Profile of ciclesonide for the maintenance treatment of asthma. Ther Clin Risk Manag. 2011;7:351–58. doi: 10.2147/TCRM.S5433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fernandez-Garcia I, Ortiz-de-Solorzano C, Montuenga LM. Telomeres and telomerase in lung cancer. J Thorac Oncol. 2008;3:1085–88. doi: 10.1097/JTO.0b013e3181886713. [DOI] [PubMed] [Google Scholar]
- 37.Lantuejoul S, Salon C, Soria J-C, Brambilla E. Telomerase expression in lung preneoplasia and neoplasia. Int J Cancer. 2007;120:1835–41. doi: 10.1002/ijc.22473. [DOI] [PubMed] [Google Scholar]
- 38.Brunsvig PF, Kyte JA, Kersten C, Sundstrom S, Moller M, Nyakas M, Hansen GL, Gaudernack G, Aamdal S. Telomerase peptide vaccination in NSCLC: A phase II trial in stage III patients vaccinated after chemoradiotherapy and an 8-year update on a phase I/II trial. Clin Cancer Res. 2011;17:6847–57. doi: 10.1158/1078-0432.CCR-11-1385. [DOI] [PubMed] [Google Scholar]
- 39.Middleton G, Silcocks P, Cox T, Valle J, Wadsley J, Propper D, Coxon F, Ross P, Madhusudan S, Roques T, Cunningham D, Falk S, Wadd N, Harrison M, Corrie P, Iveson T, Robinson A, McAdam K, Eatock M, Evans J, Archer C, Hickish T, Garcia-Alonso A, Nicolson M, Steward W, Anthoney A, Greenhalf W, Shaw V, Costello E, Naisbitt D, Rawcliffe C, Nanson G, Neoptolemos J. Gemcitabine and capecitabine with or without telomerase peptide vaccine GV1001 in patients with locally advanced or metastatic pancreatic cancer (TeloVac): An open-label, randomised, phase 3 trial. Lancet Oncol. 2014;15:829–40. doi: 10.1016/S1470-2045(14)70236-0. [DOI] [PubMed] [Google Scholar]
- 40.Lubet RA, Szabo E, Iwata KK, Gill SC, Tucker C, Bode A, Steele VE, Juliana MM, Nicastro HL, Grubbs CJ. Effect of intermittent dosing regimens of erlotinib on methylnitrosourea-induced mammary carcinogenesis. Cancer Prev Res. 2013;6:448–54. doi: 10.1158/1940-6207.CAPR-12-0322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Milton DT, Azzoli CG, Heelan RT, Venkatraman E, Gomez JE, Kris MG, Krug LM, Pao W, Rizvi NA, Dunne M, Miller VA. A phase I/II study of weekly high-dose erlotinib in previously treated patients with nonsmall cell lung cancer. Cancer. 2006;107:1034–41. doi: 10.1002/cncr.22088. [DOI] [PubMed] [Google Scholar]
- 42.Grommes C, Oxnard GR, Kris MG, Miller VA, Pao W, Holodny AI, Clarke JL, Lassman AB. “Pulsatile” high-dose weekly erlotinib for CNS metastases from EGFR mutant non-small cell lung cancer. Neuro Oncol. 2011;13:1364–69. doi: 10.1093/neuonc/nor121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moser AR, Dove WF, Roth KA, Gordon JI. The Min (multiple intestinal neoplasia) mutation: Its effect on gut epithelial cell differentiation and interaction with a modifier system. J Cell Biol. 1992;116:1517–26. doi: 10.1083/jcb.116.6.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rothwell PM, Wilson M, Elwin C-E, Norrving B, Algra A, Warlow CP, Meade TW. Long-term effect of aspirin on colorectal cancer incidence and mortality: 20-year follow-up of five randomised trials. Lancet. 2010;376:1741–50. doi: 10.1016/S0140-6736(10)61543-7. [DOI] [PubMed] [Google Scholar]
- 45.Penman ID, El-Omar E, McGregor JR, Hillan KJ, O’Dwyer PJ, McColl KEL. Omeprazole inhibits colorectal carcinogenesis induced by azoxymethane in rats. Gut. 1993;34:1559–65. doi: 10.1136/gut.34.11.1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim YJ, Lee JS, Hong KS, Chung JW, Kim JH, Hahm KB. Novel application of proton pump inhibitor for the prevention of colitis-induced colorectal carcinogenesis beyond acid suppression. Cancer Prev Res. 2010;3:963–74. doi: 10.1158/1940-6207.CAPR-10-0033. [DOI] [PubMed] [Google Scholar]
- 47.Patlolla JM, Zhang Y, Li Q, Steele VE, Rao CV. Anti-carcinogenic properties of omeprazole against human colon cancer cells and azoxymethane-induced colonic aberrant crypt foci formation in rats. Int J Oncol. 2012;40:170–75. doi: 10.3892/ijo.2011.1214. [DOI] [PubMed] [Google Scholar]
- 48.Matsumoto T, Endo I. Eldecalcitol for the treatment of osteoporosis. Drugs Today. 2012;48:189–96. doi: 10.1358/dot.2012.48.3.1745223. [DOI] [PubMed] [Google Scholar]
- 49.American Cancer Society. Cancer Facts & Figures 2014. Atlanta: American Cancer Society; 2014. Available at: http://www.cancer.org/acs/groups/content/@research/documents/webcontent/acspc-042151.pdf. [Google Scholar]
- 50.Chang K, Pastan I. Molecular cloning of mesothelin, a differentiation antigen present on mesothelium, mesotheliomas, and ovarian cancers. Proc Natl Acad Sci USA. 1996;93:136–40. doi: 10.1073/pnas.93.1.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Scholler N, Fu N, Yang Y, Ye Z, Goodman GE, Hellstrom KE, Hellstrom I. Soluble member(s) of the mesothelin/megakaryocyte potentiating factor family are detectable in sera from patients with ovarian carcinoma. Proc Natl Acad Sci USA. 1999;96:11531–36. doi: 10.1073/pnas.96.20.11531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Politi K, Zakowski MF, Fan P-D, Schonfeld EA, Pao W, Varmus HE. Lung adenocarcinomas induced in mice by mutant EGF receptors found in human lung cancers respond to a tyrosine kinase inhibitor or to down-regulation of the receptors. Genes Dev. 2006;20:1496–510. doi: 10.1101/gad.1417406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xu X, Wagner KU, Larson D, Weaver Z, Li C, Ried T, Hennighausen L, Wynshaw-Boris A, Deng CX. Conditional mutation of Brca1 in mammary epithelial cells results in blunted ductal morphogenesis and tumour formation. Nat Genet. 1999;22:37–43. doi: 10.1038/8743. [DOI] [PubMed] [Google Scholar]
- 54.Perets R, Wyant GA, Muto KW, Bijron JG, Poole BB, Chin KT, Chen JY, Ohman AW, Stepule CD, Kwak S, Karst AM, Hirsch MS, Setlur SR, Crum CP, Dinulescu DM, Drapkin R. Transformation of the fallopian tube secretory epithelium leads to high-grade serous ovarian cancer in Brca;Tp53;Pten models. Cancer Cell. 2013;24:751–65. doi: 10.1016/j.ccr.2013.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rowse GJ, Tempero RM, VanLith ML, Hollingsworth MA, Gendler SJ. Tolerance and immunity to MUC1 in a human MUC1 transgenic murine model. Cancer Res. 1998;58:315–21. [PubMed] [Google Scholar]
- 56.Hingorani SR, Petricoin EF, Maitra A, Rajapakse V, King C, Jacobetz MA, Ross S, Conrads TP, Veenstra TD, Hitt BA, Kawaguchi Y, Johann D, Liotta LA, Crawford HC, Putt ME, Jacks T, Wright CV, Hruban RH, Lowy AM, Tuveson DA. Preinvasive and invasive ductal pancreatic cancer and its early detection in the mouse. Cancer Cell. 2003;4:437–50. doi: 10.1016/s1535-6108(03)00309-x. [DOI] [PubMed] [Google Scholar]
- 57.Yonezawa S, Higashi M, Yamada N, Goto M. Precursor lesions of pancreatic cancer. Gut Liver. 2008;2:137–54. doi: 10.5009/gnl.2008.2.3.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Crum CP, Drapkin R, Kindelberger D, Medeiros F, Miron A, Lee Y. Lessons from BRCA: The tubal fimbria emerges as an origin for pelvic serous cancer. Clin Med Res. 2007;5:35–44. doi: 10.3121/cmr.2007.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Karst AM, Levanon K, Drapkin R. Modeling high-grade serous ovarian carcinogenesis from the fallopian tube. Proc Natl Acad Sci USA. 2011;108:7547–52. doi: 10.1073/pnas.1017300108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lee Y, Miron A, Drapkin R, Nucci MR, Medeiros F, Saleemuddin A, Garber J, Birch C, Mou H, Gordon RW, Cramer DW, McKeon FD, Crum CP. A candidate precursor to serous carcinoma that originates in the distal fallopian tube. J Pathol. 2007;211:26–35. doi: 10.1002/path.2091. [DOI] [PubMed] [Google Scholar]
- 61.Medeiros F, Muto MG, Lee Y, Elvin JA, Callahan MJ, Feltmate C, Garber JE, Cramer DW, Crum CP. The tubal fimbria is a preferred site for early adenocarcinoma in women with familial ovarian cancer syndrome. Am J Surg Pathol. 2006;30:230–36. doi: 10.1097/01.pas.0000180854.28831.77. [DOI] [PubMed] [Google Scholar]
- 62.Kindelberger DW, Lee Y, Miron A, Hirsch MS, Feltmate C, Medeiros F, Callahan MJ, Garner EO, Gordon RW, Birch C, Berkowitz RS, Muto MG, Crum CP. Intraepithelial carcinoma of the fimbria and pelvic serous carcinoma: Evidence for a causal relationship. Am J Surg Pathol. 2007;31:161–69. doi: 10.1097/01.pas.0000213335.40358.47. [DOI] [PubMed] [Google Scholar]