Abstract
Background
Men who have sex with men (MSM) are at high risk of acquiring HIV infection following diagnosis with other sexually transmitted infections (STIs). Identifying the STIs associated with the greatest risk of subsequent HIV infection could help target prevention interventions, particularly pre-exposure prophylaxis (PrEP).
Methods
Using matched HIV and STI surveillance data from Washington State from 1/1/2007–6/30/2013, we calculated the incidence of new HIV diagnoses following different STI diagnoses among MSM. Men entered observation at the time of their first STI diagnosis during the study period and exited at HIV diagnosis or 6/30/2013. Cox proportional hazards regression was used to conduct a global comparison of rates.
Results
From 1/1/2007–6/30/2013, 6577 HIV-negative MSM were diagnosed with 10,080 bacterial STIs at 8,371 unique time points and followed for 17,419 person-years. 280 (4.3%) men were subsequently diagnosed with HIV infection for an overall incidence of 1.6 per 100 person-years (95%CI=1.4–1.8). The estimated incidence of HIV diagnoses among all MSM in the state was 0.4 per 100 person-years. MSM were at the greatest risk of HIV diagnosis after being diagnosed with rectal gonorrhea (HIV incidence = 4.1 per 100 person-years), followed by early syphilis (2.8), urethral gonorrhea (1.6), rectal chlamydial infection (1.6), pharyngeal gonorrhea (1.1), late syphilis (1.0), and urethral chlamydial infection (0.6) [p<0.0001 overall].
Conclusions
MSM diagnosed with rectal gonorrhea and early syphilis were at the greatest risk of being diagnosed with HIV infection post-STI diagnosis. These men should be prioritized for more intensive prevention interventions, including PrEP.
Keywords: Men who have sex with men, sexually transmitted infections, HIV infection, pre-exposure prophylaxis, substance use
INTRODUCTION
Men who have sex with men (MSM) are disproportionately affected by HIV and other sexually transmitted infections (STIs). Being diagnosed with an STI is among the most consistent and longstanding risk factors associated with HIV acquisition1–3. The elevated risk of HIV acquisition among MSM with bacterial STIs reflects some combination of increased susceptibility2–12, sustained risk behaviors, and sexual network factors. The advent of pre-exposure prophylaxis (PrEP) has created a new impetus for identifying the populations of MSM at greatest risk for HIV infection. PrEP is efficacious13 but expensive, and cost-effectiveness analyses have consistently found that it is only cost-effective when targeted to the highest risk men14–18. Previous studies suggest that HIV-negative MSM diagnosed with early syphilis, rectal gonorrhea, and rectal chlamydial infection may be at particularly high risk of subsequent HIV infection19–22. However, these studies did not estimate HIV incidence for all bacterial STIs by anatomic site or stage of infection, were limited to STD clinics or study populations, did not estimate the proportion of all HIV infections among MSM occurring in men with a recent STI diagnosis, and measured only the incidence of HIV diagnosis rather than HIV infection. To identify MSM for prioritization of intensive HIV prevention interventions, including PrEP, we used matched HIV/STI surveillance data from Washington State to examine the incidence of new HIV diagnoses following STI diagnoses among MSM statewide and estimate the incidence of new infection among MSM diagnosed at an STD clinic and large community-based HIV/STI testing site. We used these findings to estimate the number of men who would need to receive PrEP to avert one HIV infection directly. Finally, based on a prior analysis among MSM in our STD clinic suggesting that substance use was associated with HIV risk even when controlling for STI diagnosis23, we assessed whether the risk of subsequent HIV diagnosis after STI diagnoses was modified by substance use.
MATERIALS AND METHODS
Data Sources
We used matched HIV/STI surveillance data from Washington State from January 1, 2007-June 30, 2013. STI surveillance and partner services data are routinely matched with HIV surveillance data from the Enhanced HIV/AIDS Reporting System (eHARS) through a two-step process. First, an automated probabilistic matching algorithm of all persons in the STI surveillance system and all individuals in eHARS based on legal and alias names, date of birth, and sex is run weekly. Second, Washington State Department of Health staff conduct a monthly manual review of STI cases not matched to eHARS but with an indication of HIV infection in STI surveillance or partner services data.
Population
HIV-negative or unknown status MSM diagnosed with syphilis of known stage or urethral, rectal, or pharyngeal gonorrhea or chlamydial infection were included in this analysis (98% of reported infections). Men who reported sex with men in the last year during partner services interviews, whose provider indicated male sex partners on the case report, or who were diagnosed with rectal gonorrhea or chlamydial infection were defined as MSM. (Medical providers in Washington State are legally required to complete a case report form for each person they diagnose with syphilis, gonorrhea, or chlamydial infection. This form includes gender of sex partners, which was available for 79% of all bacterial STI cases in men during the study period.) In order to include only HIV-negative or unknown status MSM, men whose HIV diagnosis date in eHARS preceded the date of their first STI diagnosis during the study period or who were diagnosed with HIV infection or reported a prior HIV diagnosis at the time of their first STI diagnosis during this period were excluded.
Analyses
Incidence of HIV diagnosis by STI type
We calculated incidences of HIV diagnosis following STI diagnosis defined by pathogen and anatomic site or stage of infection (STI type) by dividing the total number of new HIV diagnoses within a given STI stratum by the total time at risk within that stratum (see below for a description of how we defined these strata). We conducted a global comparison of incidences using Cox proportional hazards regression including all STI types as a time-varying covariate. The time scale for the Cox model was calendar time. Men entered observation on the date of their first STI diagnosis during the study period and exited either on the date of HIV diagnosis from eHARS or June 30, 2013 (administrative censoring). Prior to the analyses, we created a hierarchy of STI types by calculating the incidence of HIV diagnosis following STI diagnosis separately for each type and ranking them in descending order as follows: rectal gonorrhea, early syphilis (primary, secondary, and early latent), rectal chlamydial infection, urethral gonorrhea, late syphilis, pharyngeal gonorrhea, urethral chlamydial infection, and pharyngeal chlamydial infection. Because interventions would be targeted based on an individual’s highest-risk STI, MSM diagnosed with concurrent infections or infections at ≥1 site were assigned the higher-risk STI for that time point, and men with multiple infections over time could move up, but not down, the STI hierarchy. In a parallel analysis, we examined the incidence of HIV diagnosis following co-infections with the three highest risk STI types by incorporating them into the hierarchy as follows: rectal gonorrhea and early syphilis, rectal chlamydial infection and early syphilis, and rectal gonorrhea and rectal chlamydial infection.
Incidence of HIV diagnosis by substance use
Partner services staff elicit substance use (methamphetamine, inhaled nitrites, and erectile dysfunction [ED] drugs) in the year prior to STI diagnosis during interviews. We calculated the incidence of HIV diagnosis following STI diagnosis by substance use by dividing the total number of new HIV diagnoses within a given substance use stratum, by the total time at risk within that stratum. We compared incidences using Cox proportional hazards regression with substance use and STI types as time-varying covariates. The time scale for the Cox model was calendar time. Men entered observation on the date of their first STI diagnosis during the study period at which substance use was ascertained and exited on their HIV diagnosis date or June 30, 2013. Men diagnosed with multiple STIs over time could be recategorized from non-users to users of substances, but not from users to non-users.
Comparison with general population of MSM
For comparison, we estimated the incidence of HIV diagnosis among sexually-active HIV-negative MSM in Washington State during the study period. We used the number of new HIV diagnoses among MSM in eHARS as the numerator and 3.9% of men age 15 and older from Washington State census estimates (based on U.S. Centers for Disease Control and Prevention estimate of proportion of men who have had sex with men in the past 5 years24) minus the number of MSM known to be living with HIV from eHARS as the denominator. Because HIV risk in Washington is concentrated among MSM under 65 years of age, we also compared the incidence of HIV diagnosis post-STI diagnosis among MSM aged 15–64 (99.5% of STI cases; 99% of HIV diagnoses among MSM with STIs) to the incidence among sexually-active HIV-negative MSM in Washington State aged 15–64 (99.6% of HIV cases among MSM).
Number needed to treat (NNT) and population-level impact
We calculated the NNT with PrEP for one year to prevent one HIV infection among PrEP recipients as follows: 1 ÷ (HIV incidence × PrEP effectiveness)25. To address differences in PrEP effectiveness due to adherence, we used three efficacy estimates from iPrEx: the overall estimate of 44% reduction in HIV acquisition, the estimate among MSM reporting ≥90% adherence of 73%, and the estimate among MSM with detectable blood levels of emtricitabine, tenofovir, or their metabolites of 92%13.
To provide an upper bound for the potential direct population-level impact of a prevention intervention targeted towards MSM diagnosed with bacterial STIs, we calculated the proportion of HIV cases reported among MSM in the last two years of the study period who had been diagnosed with an STI in the two years prior to HIV diagnosis.
Sensitivity analyses
The primary analysis has the potential to underestimate the true HIV incidence and overestimate NNTs because men who were not tested for HIV infection at the time of STI diagnosis may have been misclassified as uninfected, passive follow-up may fail to identify new HIV infections, and incidence of HIV diagnosis is sensitive to the lag between HIV acquisition and diagnosis. To address these limitations, we conducted an analysis among STI cases diagnosed at two publicly-funded HIV/STI testing programs (Public Health–Seattle & King County STD Clinic and Gay City) unless partner services data indicated that cases were not HIV tested at STI diagnosis. At these sites, near-universal HIV testing at STI diagnosis reduced misclassification of persons at study entry, and frequent HIV testing should diminish the influence of passive follow-up and the lag between HIV acquisition and diagnosis. In addition, we calculated an incidence of HIV infection (rather than diagnosis) by estimating the infection date as the midpoint between the last negative and first positive HIV test from surveillance or partner services data. Men entered observation on the date of their first STI diagnosis at these sites during the study period and exited on the date of HIV infection or June 30, 2013.
Analyses were conducted using SAS 9.3 (SAS Institutes, Cary, NC) and Stata 11.0 (Stata Corp, College Station, TX). These analyses were conducted as part of public health program activities and not considered human subjects research.
RESULTS
From January 2006–June 2013, 10,080 bacterial STIs were reported among 6,577 HIV-negative or unknown status MSM in Washington State at 8,371 unique time points. These men were followed for a total of 17,419 person-years (median=2.4 years, interquartile range=1.1 to 4.0), and 280 (4.3%) were diagnosed with HIV infection during follow-up for an overall incidence of 1.61 diagnoses per 100 person-years (95% confidence interval [CI]=1.43–1.81), four times greater than the estimated incidence of diagnoses among all HIV-negative MSM statewide (0.41 per 100 person-years). Restricted to MSM ages 15–64, incidence of HIV diagnosis was 1.60 per 100 person-years among men following STI diagnoses, more than three-fold higher than the 0.48 per 100 person-years among all HIV-negative MSM. Sociodemographic characteristics at each individual’s initial reported STI during the study period and HIV testing history are presented in Table 1.
Table 1.
Characteristics of 6577 HIV-negative men who have sex with men diagnosed with bacterial sexually transmitted infections (STI) from January 2007 to June 2013 in Washington State
| Characteristics at initial STI diagnosis during study period and HIV testing history | Median or Number | IQR or % |
|---|---|---|
| Age (years) | 28 | 23 to 37 |
| Race/Ethnicity | ||
| White | 3966 | 60% |
| Hispanic/Latino (any race) | 969 | 15% |
| Black | 575 | 9% |
| Asian/Pacific Islander | 394 | 6% |
| Native American/Alaska Native | 65 | 1% |
| Multiple race | 143 | 2% |
| Unknown | 420 | 6% |
| King County residence | 4202 | 64% |
| HIV testing status* | ||
| Tested HIV-negative at the time of or following initial STI diagnosis | 3371 | 51% |
| Tested HIV-negative prior to initial STI diagnosis or at unknown time | 865 | 13% |
| Reported no prior HIV test | 237 | 4% |
| No HIV testing history available | 2105 | 32% |
| Years of follow-up | 2.4 | 1.1 to 4.0 |
| HIV diagnosis during follow-up | 280 | 4.3% |
| Tested HIV-negative at time of or following initial STI diagnosis and prior to HIV diagnosis^ | 221 | 79% |
IQR = Interquartile range.
Based on self-reported HIV testing history from STI partner services interviews.
Based on self-reported HIV testing history or medical record review from HIV surveillance, HIV partner services, or STI partner services.
The incidence and cumulative hazard of HIV diagnosis following STI diagnosis by STI type are presented in Table 2A and Figure 1, respectively. MSM were at the greatest risk of acquiring HIV after diagnosis with rectal gonorrhea (incidence=4.1 per 100 person-years), followed by early syphilis (2.8), urethral gonorrhea (1.6), rectal chlamydial infection (1.6), pharyngeal gonorrhea (1.1), late syphilis (1.0), and urethral chlamydial infection (0.6) [p<0.0001 overall]. Consequently, the NNT with PrEP was lowest for rectal gonorrhea and early syphilis (Table 2A; Figure 2A). If PrEP reduces the risk of acquiring HIV by 44%, 55 and 80 MSM diagnosed with rectal gonorrhea and early syphilis, respectively, would need to be treated with PrEP for one year to prevent one new infection among PrEP recipients. Using the highest estimate of PrEP efficacy (92% risk reduction), 26 and 38 MSM with rectal gonorrhea and early syphilis would need to be treated.
Table 2.
HIV incidence following sexually transmitted infections by infection type and associated number needed to treat with pre-exposure prophylaxis to prevent one HIV infection
| Panel A: Primary analysis – Incidence of HIV diagnosis among MSM with STIs in Washington State | |||||||
|---|---|---|---|---|---|---|---|
| Total number of STIs | No. of STIs based on order* | Subsequent HIV diagnoses | Incidence of HIV diagnosis per 100 person-yrs (95% CI) | Number needed to treat with PrEP by estimate of efficacy^ | |||
| 44% | 73% | 92% | |||||
| Overall | 10080 | 7064 | 280 | 1.61 (1.43–1.81) | 141 | 85 | 68 |
| Rectal GC | 985 | 871 | 79 | 4.13 (3.31–5.15) | 55 | 33 | 26 |
| Early syphilis | 771 | 675 | 51 | 2.83 (2.15–3.72) | 80 | 48 | 38 |
| Rectal CT | 1480 | 939 | 29 | 1.59 (1.10–2.29) | 143 | 86 | 68 |
| Urethral GC | 2087 | 1537 | 66 | 1.63 (1.28–2.07) | 140 | 84 | 67 |
| Late syphilis | 188 | 155 | 4 | 1.04 (0.39–2.78) | 218 | 131 | 104 |
| Pharyngeal GC | 1205 | 528 | 11 | 1.08 (0.60–1.95) | 210 | 127 | 100 |
| Urethral CT | 3124 | 2283 | 39 | 0.62 (0.45–0.84) | 369 | 222 | 176 |
| Pharyngeal CT | 240 | 76 | 1 | 1.08 (0.15–7.65) | 211 | 127 | 101 |
| Panel B: Sensitivity analysis – Incidence of HIV infection among MSM diagnosed with STIs at publicly funded HIV/STI testing programs | ||||||
|---|---|---|---|---|---|---|
| No. of STIs based on order* | Subsequent HIV diagnoses | Incidence of HIV infection† per 100 person-yrs (95% CI) | Number needed to treat with PrEP by estimate of efficacy^ | |||
| 44% | 73% | 92% | ||||
| Overall | 2223 | 135 | 2.48 (2.10–2.94) | 92 | 55 | 44 |
| Rectal GC | 541 | 54 | 4.36 (3.34–5.70) | 52 | 31 | 25 |
| Early syphilis | 204 | 19 | 3.74 (2.38–5.86) | 61 | 37 | 29 |
| Rectal CT | 515 | 27 | 2.31 (1.59–3.37) | 98 | 59 | 47 |
| Urethral GC | 303 | 17 | 1.99 (1.24–3.21) | 114 | 69 | 55 |
| Late syphilis | 39 | 3 | 3.06 (0.99–9.49) | 74 | 45 | 36 |
| Pharyngeal GC | 290 | 8 | 1.26 (0.63–2.51) | 181 | 109 | 86 |
| Urethral CT | 298 | 7 | 0.77 (3.69–1.62) | 293 | 177 | 140 |
| Pharyngeal CT | 33 | 0 | 0 | N/A | N/A | N/A |
STI = sexually transmitted infection. MSM = men who have sex with men. GC = gonorrhea. CT = chlamydial infection. CI = confidence interval. PrEP = HIV pre-exposure prophylaxis.
STI types are presented in hierarchical order based on prior analyses identifying descending incidence of HIV diagnosis. Men diagnosed with concurrent infections or infections at >1 site were assigned the higher order STI for that time point. Repeat infections of the same type were not included in this count.
This represents an estimate of the number of HIV-negative MSM with each infection that would need to be treated with PrEP for one year in order to prevent one HIV infection among PrEP recipients based on three estimates of PrEP effectiveness from iPrex17: overall (44%), with at least 90% adherence (73%), and with detectable blood levels of PrEP drug (92%).
The date of HIV infection was estimated as the midpoint between the last negative and first positive HIV test from HIV surveillance, HIV partner services, or STI partner services data.
Figure 1. Cumulative hazard of HIV diagnosis following bacterial sexually transmitted infections (STI).
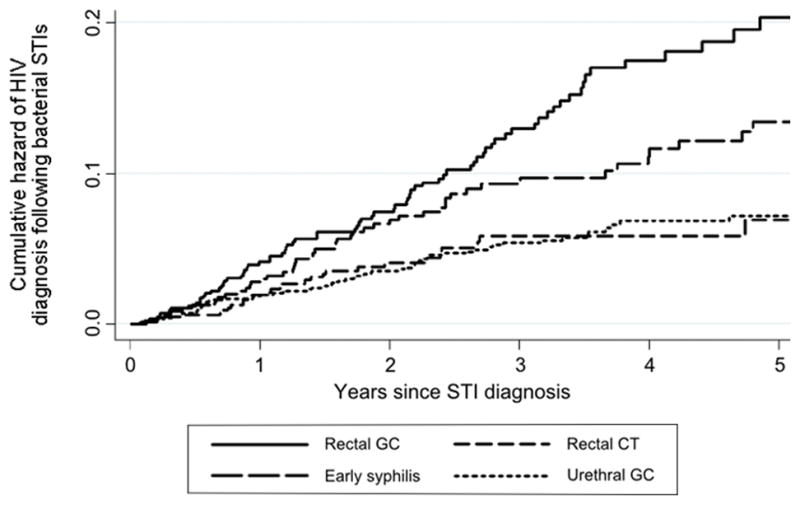
GC = gonorrhea. CT = chlamydial infection.
Figure 2. Number needed to treat (NNT) with HIV pre-exposure prophylaxis to directly prevent one new HIV infection by sexually transmitted infection type.
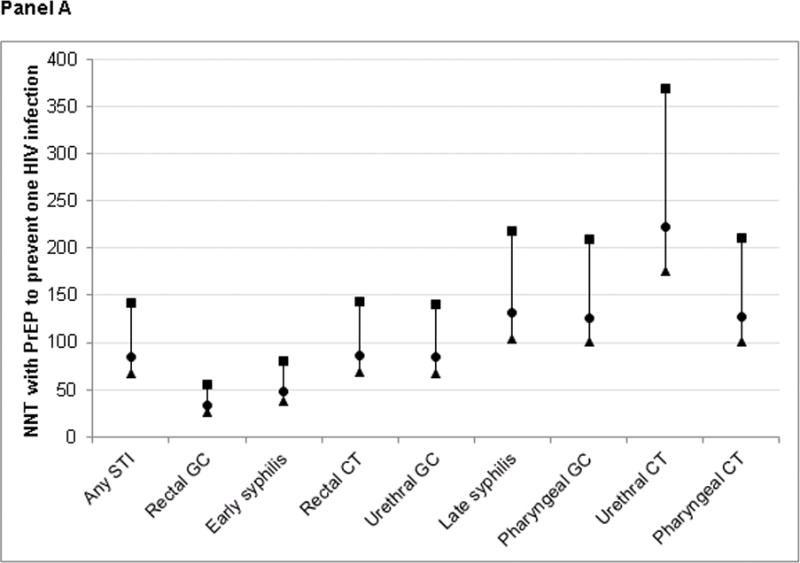
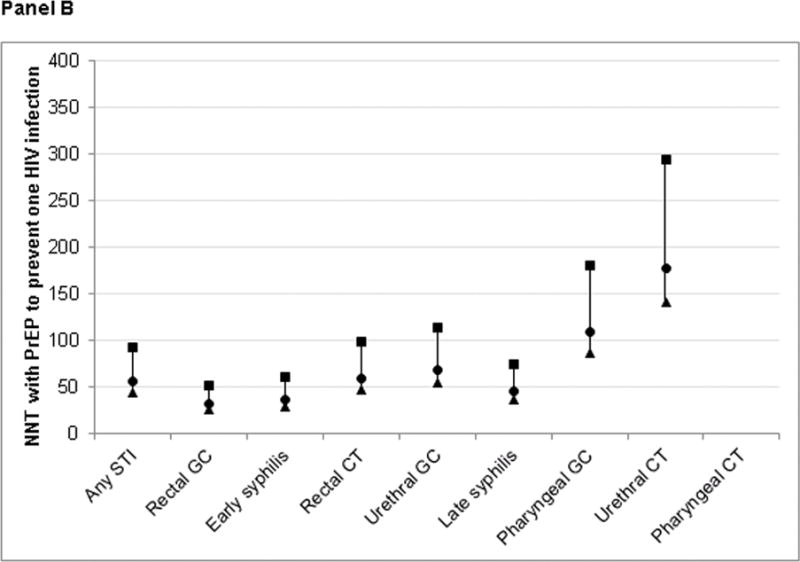
Panel A: Primary analysis – Estimated from incidence of HIV diagnosis in Washington State
Panel B: Sensitivity analysis – Estimated from incidence of HIV infection among MSM diagnosed at publicly funded HIV/STI testing programs
MSM = men who have sex with men. GC = gonorrhea. CT = chlamydial infection. PrEP = HIV pre-exposure prophylaxis.
This figure presents estimates of the number of HIV-negative MSM with each infection that would need to be treated with PrEP for one year in order to prevent one HIV infection among PrEP recipients (NNT) based on three estimates of PrEP effectiveness from iPrex17: overall (44%; ■), with at least 90% adherence (73%; ●), and with detectable blood levels of PrEP drug (92%; ▲). Panel A shows NNTs estimated from the incidence of HIV diagnosis following STI diagnoses among all MSM in Washington State (primary analysis). Panel B presents estimates from the incidence of HIV infection among MSM diagnosed at publicly funded HIV/STI testing programs (sensitivity analysis). The date of HIV infection was estimated as the midpoint between the last negative and first positive HIV test from HIV surveillance, HIV partner services, or STI partner services data.
Men were concurrently diagnosed with more than one bacterial pathogen at 810 time points (9.7% of 8,371), including 24 early syphilis-rectal gonorrhea, 29 early syphilis-rectal chlamydia, and 251 rectal gonorrhea-rectal chlamydia co-infections. The incidences of HIV diagnosis following these co-infections were the greatest we observed: 11.8, 10.8, and 5.9 per 100 person-years, respectively.
Table 2B and Figure 2B describe the HIV incidence and NNTs from the sensitivity analysis conducted among STI cases from publicly-funded testing programs and estimating the incidence of HIV infection. Overall, HIV incidence following STI diagnosis was 2.48 per 100 person-years, a 54% increase from 1.61 in the primary analysis. The absolute difference between estimated incidence of diagnosis and infection was small for gonococcal infections regardless of anatomic site and urethral chlamydial infection, but higher for early and latent syphilis and rectal chlamydial infection. Despite these differences, MSM continued to be at greatest risk of acquiring HIV after diagnosis with rectal gonorrhea (incidence=4.4 per 100 person-years) and early syphilis (3.7).
From July 2011–June 2013, 736 MSM were diagnosed with HIV infection in Washington State, of whom 104 (14%) had a history of bacterial STI in the two years prior to HIV diagnosis, including 47 (6.4%) with a history of early syphilis or rectal gonorrhea (Figure 3).
Figure 3. Proportion of 736 MSM newly diagnosed with HIV infection July 2011-June 2013 with a reported sexually transmitted infection diagnosis in the 2 years prior to HIV diagnosis.
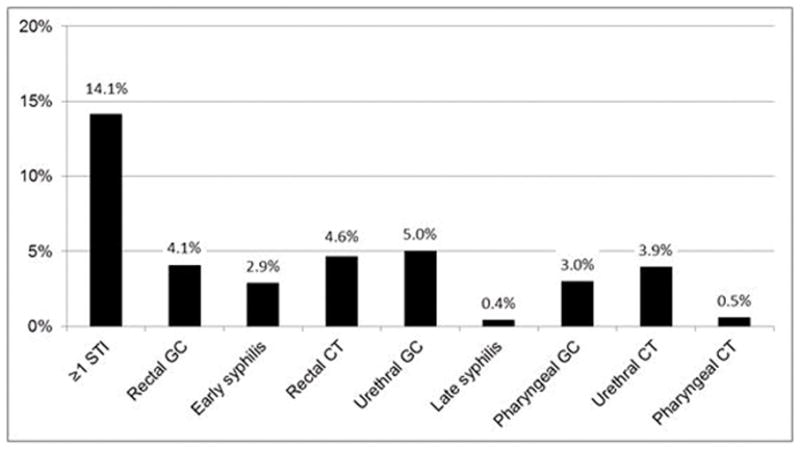
GC = gonorrhea. CT = chlamydial infection.
Among 3715 men (56% of total) for whom information regarding substance use was available from ≥1 STI diagnosis, 306 (8.2%) reported using methamphetamine, 632 (17%) inhaled nitrites, and 401 (11%) ED medications in the year prior to STI diagnosis. In bivariable analyses, men who reported using each of these substances experienced greater incidences of HIV diagnosis following STI diagnosis than men who denied using these substances (methamphetamine=5.09 vs. 1.65 per 100 person-years, inhaled nitrites=4.40 vs. 1.53, ED drugs=3.91 vs. 1.71; p<0.001 for all). In multivariable analyses including all three substances and STI type, methamphetamine and inhaled nitrite use remained significantly associated with incidence of HIV diagnosis [adjusted hazard ratios=1.92 (95%CI=1.29–2.84) and 2.2 (1.6–3.1), respectively; p<0.001 for both].
DISCUSSION
In Washington State, MSM diagnosed with any bacterial STI experienced three- to four-fold greater incidence of HIV diagnosis following their STI than MSM overall, with the greatest risks observed among MSM diagnosed with rectal gonorrhea and early syphilis or reporting methamphetamine or inhaled nitrite use. However, only one in every seven MSM newly diagnosed with HIV infection had been diagnosed with a bacterial STI in the two years prior to HIV diagnosis, and only 6.4% had a recent history of rectal gonorrhea or syphilis. These results suggest that targeting HIV prevention interventions to MSM diagnosed with STIs, specifically rectal gonorrhea and early syphilis and methamphetamine or inhaled nitrite users, has the potential to reach very high risk men but will only directly affect a relatively small subset of MSM who will ultimately acquire HIV infection. These findings highlight the potential value of using specific STIs to target resource-intensive interventions such as PrEP but suggest how such a highly-targeted approach might have limited direct population-level impact.
This population-based study supports conclusions from previous studies in clinical and research settings that MSM with rectal infections and early syphilis are at extremely high risk of subsequently acquiring HIV, though our absolute risk estimates are lower than those previously reported19–22. Similar to a study in New York City STD clinics19, we observed higher HIV incidence following rectal gonorrhea than rectal chlamydia diagnosis, but HIV incidence in both groups was greater in New York City than in Washington State (7.1 vs. 4.1 per 100 person-years for rectal gonorrhea; 5.9 vs. 1.6 for rectal chlamydial infection). In iPrEx21, which was conducted primarily in South America, MSM experienced an HIV incidence of 8.0 per 100 person-years following syphilis diagnosis, more than twice the 2.8 per 100 person-years observed among MSM in Washington State. Variations in absolute risk across studies may reflect differences in HIV incidence among MSM in these geographic areas, differences in risk in the study populations (i.e. population-based vs. STD clinic clients or study participants), or in the case of iPrEx, greater ascertainment of infection through active follow-up.
The relationship between HIV and STI acquisition is complex and multidirectional. Some evidence suggests that genital tract inflammation caused by bacterial STIs increases the risk of HIV transmission by increasing HIV shedding by HIV-infected partners and causing breaches in the genital tract epithelium and recruitment of targets cells to this area in HIV-susceptible partners.2–12 However, these biological mechanisms because they require the pathogen to be present at the time of HIV exposure. Rather, our findings are likely a result of the association between different STI and subsequent behaviors and sexual network factors that result in exposure to HIV. Rectal infections are direct markers of condomless receptive anal sex, which is associated with a greater risk of HIV acquisition than oral or insertive anal sex26–28, and because the syphilis epidemic in the U.S. and other developed countries is concentrated among MSM living with HIV29, early syphilis may be a marker of condomless sex within sexual networks including high-risk MSM living with HIV. It is also possible that men at higher risk of acquiring HIV are more likely to seek STI screening in general, in clinical settings where extragenital testing is available, or are more likely to recognize or seek care in response to symptoms. Exploring the reasons that STI diagnoses predict future HIV acquisition may help identify additional targets for prevention interventions.
PrEP implementation is resource-intensive, and several cost-effectiveness analyses have found that, despite its potential impact on HIV incidence, providing PrEP to general populations of MSM in the U.S. and Australia is probably not cost-effective at current medication costs14–17,30. Prioritizing sub-populations of MSM with higher HIV incidence, however, may be cost-effective in some situations14–18. With that in mind, we designed our analysis to identify populations at high risk for HIV infection for PrEP prioritization and assess the potential population-level effects of such an approach. NNTs can be used to compare the effectiveness of targeting interventions to subpopulations with different levels of risk, intervention adherence, and other factors. Similar to an analysis from iPrEx31, relatively few MSM diagnosed with syphilis would need to be treated with PrEP to prevent one new HIV infection among PrEP recipients, but the proportion of HIV infections occurring in men with a prior syphilis diagnosis was small. In iPrEx, condomless receptive anal sex with a partner of any HIV status was associated with a similar NNT but a much larger population attributable fraction, suggesting that offering PrEP to all men reporting condomless receptive anal sex would have a larger population-level impact than offering it to MSM with rectal gonorrhea or early syphilis alone at a similar cost per case directly averted. Our study does not directly address the relative benefits of targeting MSM with syphilis or rectal gonorrhea versus all MSM who engage in condomless receptive anal sex. However, in a prior analysis among MSM attending an STD clinic, we found that condomless receptive anal sex was not associated with HIV incidence when adjusting for diagnosis with a bacterial STI and methamphetamine or inhaled nitrite use in the prior year23, suggesting that NNTs may be substantially higher in a group defined solely by condomless receptive anal sex and not stronger risk factors. Neither the iPrEx study population (primarily South American MSM) nor MSM in Washington State is likely to provide accurate estimates of HIV risk for prioritizing PrEP among MSM in other settings. PrEP targeting criteria should be context-specific and may not be appropriate in settings where HIV risk in the general population of MSM is extremely high. Regardless, offering PrEP to MSM diagnosed with higher-risk STIs may help increase the cost-effectiveness of PrEP programs.
This study has several limitations. First, without being able to measure the true date of HIV infection, the primary analysis relied on the date of the first positive test. This approach likely led us to underestimate the true incidence of HIV infection post-STI diagnosis for several reasons: the lag between HIV acquisition and diagnosis; failure to identify infections in men who did not test during follow-up; and underascertainment of infections in men who migrated out of the area. Our approach may also have misclassified HIV-infected MSM who tested HIV-negative during acute infection or did not test at the time of their initial STI diagnosis, potentially leading to an overestimate of the true HIV incidence in the population. To partially address these limitations, we undertook an analysis restricted to MSM diagnosed with STIs in large publicly-funded testing programs and estimated time of HIV infection based on cases’ HIV testing history. NNTs were somewhat lower in this analysis, suggesting that relying on HIV diagnosis as a surrogate for HIV infection led us to overestimate NNTs in our main analysis. Some of this variation, however, may reflect differences between persons seen in an STD clinic or community-based program and the general population. In addition, many bacterial STIs in MSM are asymptomatic, and it is possible that the elevated rate of HIV diagnoses we observed overall and following specific STI types reflects a pattern of more frequent testing in men with these STIs rather than a true elevation of HIV risk. While we cannot completely exclude this possibility, the fact that some asymptomatic STIs (e.g. urethral chlamydial infection and pharyngeal gonorrhea) were associated with a relatively low risk of subsequent HIV acquisition argues against the idea that our findings are simply a result of ascertainment bias. Moreover, from a public health perspective, our findings clearly identify groups at high risk for HIV acquisition that could benefit from PrEP. Missing data regarding substance use may affect estimates of the association with subsequent HIV diagnosis and limited our ability to calculate the potential population-level effects of such prioritization. Furthermore, our NNTs were limited to the direct effects of PrEP on individuals taking the medications and did not take into account potential infections averted among partners and sexual networks. Lastly, the utility of PrEP efficacy estimates from iPrEx for calculating NNTs may be affected by differences in behavior and adherence between MSM in Washington State versus the study population due to differential knowledge regarding PrEP effectiveness, access to intensive risk-reduction or adherence counseling, or frequency and intensity of HIV exposures.
In conclusion, we found that MSM with rectal gonorrhea and syphilis and MSM with STIs who use methamphetamine or inhaled nitrites were at very high risk for future HIV infection. Gonorrhea and syphilis are reportable throughout the U.S. and, insofar as gonococcal infections are reportable with data on anatomic site of infection, the population with these infections is readily defined and could be targeted by public health agencies for specific prevention outreach, particularly promoting frequent HIV/STI testing and PrEP. At a minimum, clinicians should discuss PrEP as an HIV prevention option with MSM with rectal gonorrhea, early syphilis, or who report methamphetamine or inhaled nitrite use concurrent with any STI diagnosis.
SUMMARY.
A population-based study in Washington State found that men who have sex with men were at very high risk of acquiring HIV following diagnosis with rectal gonorrhea or early syphilis.
Acknowledgments
Sources of Funding: This evaluation reported in this publication was supported by the U.S. Centers for Disease Control and Prevention (CDC PS12-1201) and by NIAID, NCI, NIMH, NIDA, NICHD, NHLBI, NIA, NIGMS, NIDDK, of the National Institutes of Health under award number P30AI027757.
We would like to thank the disease intervention specialists of Washington State for their work conducting partner services and promoting HIV testing in persons with STIs; Julieann Simon, Claire LaSee, and Jason Carr of the Washington State Department of Health for providing STD surveillance and partner services data; Amy Bennett and Christina Thibault of Public Health – Seattle & King County for providing HIV testing history data from HIV surveillance; and Dr. James Hughes of the University of Washington for biostatistics support.
Footnotes
Requests for reprints should be addressed to corresponding author.
Note: This work was presented in part at the 2014 National STD Prevention Conference in Atlanta, GA.
Conflicts of Interest
Dr. Golden has received research support from Cempra Pharmaceuticals and Melina Pharmaceuticals. The remaining authors have no potential conflicts of interest to declare.
References
- 1.Piot P, Laga M. Genital ulcers, other sexually transmitted diseases, and the sexual transmission of HIV. BMJ. 1989;298(6674):623–4. doi: 10.1136/bmj.298.6674.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fleming DT, Wasserheit JN. From epidemiological synergy to public health policy and practice: the contribution of other sexually transmitted diseases to sexual transmission of HIV infection. Sex Transm Infect. 1999;75(1):3–17. doi: 10.1136/sti.75.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ward H, Ronn M. Contribution of sexually transmitted infections to the sexual transmission of HIV. Curr Opin HIV AIDS. 2010;5(4):305–10. doi: 10.1097/COH.0b013e32833a8844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cohen CR, Plummer FA, Mugo N, et al. Increased interleukin-10 in the the endocervical secretions of women with non-ulcerative sexually transmitted diseases: a mechanism for enhanced HIV-1 transmission? AIDS. 1999;13(3):327–32. doi: 10.1097/00002030-199902250-00004. [DOI] [PubMed] [Google Scholar]
- 5.de Jong MA, de Witte L, Oudhoff MJ, Gringhuis SI, Gallay P, Geijtenbeek TB. TNF-alpha and TLR agonists increase susceptibility to HIV-1 transmission by human Langerhans cells ex vivo. The Journal of clinical investigation. 2008;118(10):3440–52. doi: 10.1172/JCI34721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Henning T, Butler K, Mitchell J, et al. Development of a rectal sexually transmitted infection--HIV coinfection model utilizing Chlamydia trachomatis and SHIVSF162p3. Journal of medical primatology. 2014;43(3):135–43. doi: 10.1111/jmp.12103. [DOI] [PubMed] [Google Scholar]
- 7.Henning TR, Butler K, Hanson D, et al. Increased susceptibility to vaginal simian/human immunodeficiency virus transmission in pig-tailed macaques coinfected with Chlamydia trachomatis and Trichomonas vaginalis. J Infect Dis. 2014;210(8):1239–47. doi: 10.1093/infdis/jiu240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaul R, Pettengell C, Sheth PM, et al. The genital tract immune milieu: an important determinant of HIV susceptibility and secondary transmission. Journal of reproductive immunology. 2008;77(1):32–40. doi: 10.1016/j.jri.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 9.Masson L, Mlisana K, Little F, et al. Defining genital tract cytokine signatures of sexually transmitted infections and bacterial vaginosis in women at high risk of HIV infection: a cross-sectional study. Sex Transm Infect. 2014;90(8):580–7. doi: 10.1136/sextrans-2014-051601. [DOI] [PubMed] [Google Scholar]
- 10.Ramsey KH, Schneider H, Cross AS, et al. Inflammatory cytokines produced in response to experimental human gonorrhea. J Infect Dis. 1995;172(1):186–91. doi: 10.1093/infdis/172.1.186. [DOI] [PubMed] [Google Scholar]
- 11.Schust DJ, Ibana JA, Buckner LR, et al. Potential mechanisms for increased HIV-1 transmission across the endocervical epithelium during C. trachomatis infection. Current HIV research. 2012;10(3):218–27. doi: 10.2174/157016212800618093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sellati TJ, Wilkinson DA, Sheffield JS, Koup RA, Radolf JD, Norgard MV. Virulent Treponema pallidum, lipoprotein, and synthetic lipopeptides induce CCR5 on human monocytes and enhance their susceptibility to infection by human immunodeficiency virus type 1. J Infect Dis. 2000;181(1):283–93. doi: 10.1086/315209. [DOI] [PubMed] [Google Scholar]
- 13.Grant RM, Lama JR, Anderson PL, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010;363(27):2587–99. doi: 10.1056/NEJMoa1011205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Juusola JL, Brandeau ML, Owens DK, Bendavid E. The cost-effectiveness of preexposure prophylaxis for HIV prevention in the United States in men who have sex with men. Ann Intern Med. 2012;156(8):541–50. doi: 10.1059/0003-4819-156-8-201204170-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kessler J, Myers JE, Nucifora KA, et al. Evaluating the impact of prioritization of antiretroviral pre-exposure prophylaxis in New York City. AIDS. 2014 doi: 10.1097/QAD.0000000000000460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paltiel AD, Freedberg KA, Scott CA, et al. HIV preexposure prophylaxis in the United States: impact on lifetime infection risk, clinical outcomes, and cost-effectiveness. Clin Infect Dis. 2009;48(6):806–15. doi: 10.1086/597095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schneider K, Gray RT, Wilson DP. A cost-effectiveness analysis of HIV preexposure prophylaxis for men who have sex with men in Australia. Clin Infect Dis. 2014;58(7):1027–34. doi: 10.1093/cid/cit946. [DOI] [PubMed] [Google Scholar]
- 18.Desai K, Sansom SL, Ackers ML, et al. Modeling the impact of HIV chemoprophylaxis strategies among men who have sex with men in the United States: HIV infections prevented and cost-effectiveness. AIDS. 2008;22(14):1829–39. doi: 10.1097/QAD.0b013e32830e00f5. [DOI] [PubMed] [Google Scholar]
- 19.Pathela P, Braunstein SL, Blank S, Schillinger JA. HIV incidence among men with and those without sexually transmitted rectal infections: estimates from matching against an HIV case registry. Clin Infect Dis. 2013;57(8):1203–9. doi: 10.1093/cid/cit437. [DOI] [PubMed] [Google Scholar]
- 20.Bernstein KT, Marcus JL, Nieri G, Philip SS, Klausner JD. Rectal gonorrhea and chlamydia reinfection is associated with increased risk of HIV seroconversion. J Acquir Immune Defic Syndr. 2010;53(4):537–43. doi: 10.1097/QAI.0b013e3181c3ef29. [DOI] [PubMed] [Google Scholar]
- 21.Solomon MM, Mayer KH, Glidden DV, et al. Syphilis predicts HIV incidence among men and transgender women who have sex with men in a pre-exposure prophylaxis trial. Clin Infect Dis. 2014;59(7):1020–6. doi: 10.1093/cid/ciu450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kelley CF, Vaughan AS, Luisi N, et al. The Effect of High Rates of Bacterial Sexually Transmitted Infections on HIV Incidence in a Cohort of Black and White Men Who Have Sex with Men in Atlanta, Georgia. AIDS Res Hum Retroviruses. 2015;31(6):587–92. doi: 10.1089/aid.2015.0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Menza TW, Hughes JP, Celum CL, Golden MR. Prediction of HIV acquisition among men who have sex with men. Sex Transm Dis. 2009;36(9):547–55. doi: 10.1097/OLQ.0b013e3181a9cc41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Purcell DW, Johnson CH, Lansky A, et al. Estimating the population size of men who have sex with men in the United States to obtain HIV and syphilis rates. Open AIDS J. 2012;6:98–107. doi: 10.2174/1874613601206010098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zulman DM, Vijan S, Omenn GS, Hayward RA. The Relative Merits of Population-Based and Targeted Prevention Strategies. Milbank Q. 2008;86(4):557–80. doi: 10.1111/j.1468-0009.2008.00534.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Varghese B, Maher JE, Peterman TA, Branson BM, Steketee RW. Reducing the risk of sexual HIV transmission: quantifying the per-act risk for HIV on the basis of choice of partner, sex act, and condom use. Sex Transm Dis. 2002;29(1):38–43. doi: 10.1097/00007435-200201000-00007. [DOI] [PubMed] [Google Scholar]
- 27.Baggaley RF, White RG, Boily MC. HIV transmission risk through anal intercourse: systematic review, meta-analysis and implications for HIV prevention. Int J Epidemiol. 2010;39(4):1048–63. doi: 10.1093/ije/dyq057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vittinghoff E, Douglas J, Judson F, McKirnan D, MacQueen K, Buchbinder SP. Per-contact risk of human immunodeficiency virus transmission between male sexual partners. Am J Epidemiol. 1999;150(3):306–11. doi: 10.1093/oxfordjournals.aje.a010003. [DOI] [PubMed] [Google Scholar]
- 29.Garcia-Garcia L, Gonzalez-Escalada JR, Ariza-Megia MC, Gil De Miguel A, Gil-Prieto R. Syphilis: An Epidemiological Review. Curr Womens Health Rev. 2012;8(3):231–41. [Google Scholar]
- 30.Koppenhaver RT, Sorensen SW, Farnham PG, Sansom SL. The cost-effectiveness of pre-exposure prophylaxis in men who have sex with men in the United States: an epidemic model. J Acquir Immune Defic Syndr. 2011;58(2):e51–2. doi: 10.1097/QAI.0b013e31822b74fe. [DOI] [PubMed] [Google Scholar]
- 31.Buchbinder SP, Glidden DV, Liu AY, et al. HIV pre-exposure prophylaxis in men who have sex with men and transgender women: a secondary analysis of a phase 3 randomised controlled efficacy trial. Lancet Infect Dis. 2014;14(6):468–75. doi: 10.1016/S1473-3099(14)70025-8. [DOI] [PMC free article] [PubMed] [Google Scholar]


