Abstract
GORAB is a golgin that localizes predominantly at the Golgi apparatus and physically interacts with small GTPases. GORAB is ubiquitously expressed in mammalian tissues, including the skin. However, the biological function of this golgin in skin is unknown. Here, we report that disrupting the expression of the Gorab gene in mice results in hair follicle morphogenesis defects that were characterized by impaired follicular keratinocyte differentiation. This hair follicle phenotype was associated with markedly suppressed hedgehog (Hh) signaling pathway in dermal condensates in vivo. Gorab-deficient dermal mesenchymal cells also displayed significantly reduced capability to respond to Hh pathway activation in vitro. Furthermore, we found that the formation of primary cilium, a cellular organelle that is essential for the Hh pathway, was impaired in mutant dermal papilla cells, suggesting that Gorab may be required for the Hh pathway through facilitating the formation of primary cilia. Thus, data obtained from this study provided insight onto the biological functions of Gorab during embryonic morphogenesis of skin in which Hh signaling and primary cilia exert important functions.
Keywords: Gorab, Golgi, hair follicle, hedgehog signaling, primary cilia
INTRODUCTION
The Golgi apparatus is a cellular organelle essential for the post-translational processing, sorting, and transport of proteins. These diverse functions of the Golgi are mediated by a host of Golgi-associated proteins. GORAB, also called SCY1-like 1-binding protein 1 (SCYL1BP1) or N-terminal kinase-like protein binding protein 1 (NTKL-BP1) contains coiled-coil motif and localizes predominantly at the trans-Golgi network (TGN) (Liu et al., 2012). GORAB also interacts with a small GTPase, namely RAB6 (Hennies et al., 2008), a small GTPase that is extensively involved in the secretory and endocytic pathways of intracellular trafficking (Stenmark, 2009). These properties qualify GORAB as a golgin. They also suggest it functions in intracellular trafficking.
The GORAB gene is highly conserved from fly to human. Autosomal recessive mutations in the human GORAB gene cause geroderma osteodysplasticum or gerodermia osteodysplastica (GO, OMIM 231070) (Hennies et al., 2008), a congenital condition characterized by wrinkly skin and osteoporosis. Currently, the molecular mechanism underlying the pathogenesis of GO is unclear. However, the association of GORAB with the congenital phenotypes in GO strongly suggests that GORAB may play important functions in embryonic morphogenesis.
In mice, the morphogenesis of the skin initiates when cells of the surface ectoderm commit to an epidermal fate (Koster and Roop, 2007). It is then followed by a series of epidermal stratification and differentiation programs regulated by transcriptional factors, notably TRP63 (p63) (Koster and Roop, 2007). Epidermal differentiation results in the formation of the suprabasal and spinous layers of the epidermis between E14.5 and E16.5, and ultimately the cornified cell envelope and epidermal barrier. The formation of the hair follicle is initiated by hair follicle induction, the formation of the hair placodes or stage 1 hair follicles, at approximately at E14.5 (Schneider et al., 2009). It is then followed by organogenesis and cytodifferentiation, through which stage 2 hair germs mature into stage 5 hair pegs. During this process the KRT14-positive outer root sheath starts to differentiate and gives rise to the KRT75-positive companion layer (Schweizer et al., 2007). Further differentiation of embryonic hair follicles results in the formation of the inner root sheath, the hair follicle cortex, and ultimately the hair shaft (Paus et al., 1999).
Throughout the morphogenesis of hair follicles, extensive mesenchymal-epithelial interactions occur, where dermal condensate or dermal papilla cells play instructive roles (Botchkarev and Paus, 2003). Canonical Wnt and Hedgehog (Hh) signaling are among the best characterized molecular signaling pathways during hair follicle morphogenesis (Millar, 2002; Schmidt-Ullrich and Paus, 2005; Yang and Cotsarelis, 2010). Wnt signaling is believed to be the “first dermal signal” (Hardy, 1992) and essential for hair follicle induction (Andl et al., 2002; Gat et al., 1998). Hh signaling is required for cytodifferentiation of follicular keratinocytes (Chiang et al., 1999; Mill et al., 2003; St-Jacques et al., 1998; Woo et al., 2012). During skin morphogenesis, sonic hedgehog (SHH), the predominant Hh ligand in the skin, is produced by follicular keratinocytes. However, it is able to regulate Hh signaling in follicular keratinocyte and dermal papilla cells (St-Jacques et al., 1998).
The primary cilium, or nonmotile cilium, is a singular hair-like structure that protrudes from the surface of most mammalian cell types (Goetz and Anderson, 2010; Singla and Reiter, 2006), including epidermal keratinocytes and dermal papilla cells. One of the best understood functions of primary cilia during tissue morphogenesis is the processing of Hh signals. Abnormal cilia formation and function results in impaired Hh signaling and contributes to the development of ciliopathies (Badano et al., 2006; Hildebrandt et al., 2011; Tobin and Beales, 2009). Disrupting primary cilia formation during skin morphogenesis can result in severely impaired Hh signaling and hair follicle formation (Chen et al., 2015; Croyle et al., 2011; Dai et al., 2013; Dai et al., 2011; Ezratty et al., 2011; Lehman et al., 2009), suggesting that primary cilia are essential for proper Hh signaling during hair follicle morphogenesis.
To gain insight into the molecular functions of GORAB during skin morphogenesis, we engineered a mouse model in which the expression of the Gorab gene was disrupted. Striking hair follicle morphogenesis defects were observed in homozygous Gorab mutants. Further examination associated these phenotypes with disrupted Hh signaling and impaired primary cilia formation in dermal condensate cells. Data obtained from this study underscores the role of golgins in orchestrating molecular signaling during embryonic morphogenesis.
RESULTS
Gorab is expressed in embryonic skin
Gorab is highly expressed in the skin as determined by quantitative RT-PCR (Hennies et al., 2008). To further define its expression pattern in embryonic mouse skin, we performed in situ hybridization and observed that Gorab is ubiquitously expressed in basal epidermal keratinocytes, follicular keratinocytes, dermal fibroblasts, and dermal papilla cells (Figure 1a).
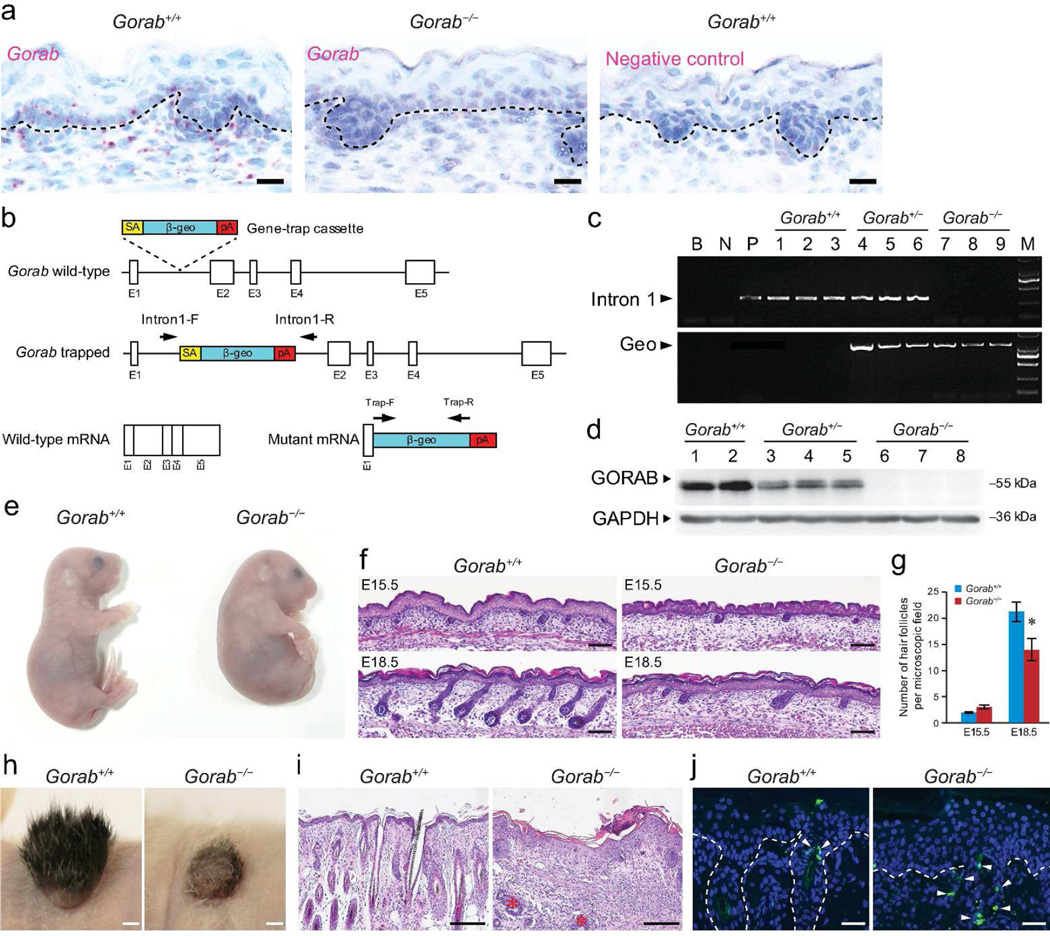
Figure 1. Gorab is involved in skin morphogenesis.
(a) Expression of Gorab in E15.5 mouse skin by in situ hybridization. Gorab+/+, wild-type; Gorab−/−, homozygous mutant. (b) Gene targeting strategy and predicted transcripts. (c) Genotyping for control and Gorab mutants. (d) Expression of GORAB by western blotting. (e) Appearance of E18.5 control and homozygous mutant. (f) Histology of dorsal skin of E15.5 and E18.5 controls and Gorab mutants. (g) Quantification of hair follicle numbers (n≥5). (h) Skin transplants at 3 weeks (n≥3). (i) Histology of skin transplants in (h). Asterisks marked hair follicle-like remnants. (j) TUNEL staining of skin transplants in (h). Arrowheads point to TUNEL-positive cells. Dotted lines illustrate epidermal-dermal junction. Scale bars: 20 µm (a); 50 µm (j); 100 µm (f); 200 µm (i); 2 mm (h).
Generation of Gorab-deficient mouse model
To gain insight into the biological function of GORAB, we generated a mutant mouse model from ES cell clone XG183 (BayGenomics consortium, CA, USA), in which a gene-trap vector (SA β-geo) was inserted into intron 1 of the Gorab locus (Figure. 1b). This trap vector is expected to interfere with splicing, resulting in a fusion transcript comprised of exon 1 of Gorab and β-geo (Figure 1b). Genotyping with Intron-F and Intron-R primers and direct genomic DNA sequencing confirmed that the β-geo cassette was inserted into intron 1 (Figure 1c and Supplemental Figure S1). In situ hybridization and western blotting with a polyclonal GORAB antibody against 1–264 amino acid of human GORAB demonstrated that Gorab transcripts and protein were absent from homozygous mutants (Figure 1a and d). Thus, this mutant model is regarded as a null allele of Gorab, hereafter referred to as Gorab−/−.
Homozygous mutants (Gorab−/−) were obtained by crossing heterozygous mutants (Gorab+/−). Wild-type (Gorab+/+), heterozygous (Gorab+/−), and homozygous (Gorab−/−) were obtained at normal Mendelian ratios at birth. The size of Gorab−/− pups appeared comparable with that of Gorab+/+ or Gorab+/− littermates (Figure 1e). However, Gorab−/− mutants displayed hunched backs and craniofacial deformities (Figure 1e). In addition, Gorab−/− mutants started to gasp for air within minutes of birth and dying. These phenotypes suggested that Gorab−/− mutants may 7 have morphogenesis defects in the musculoskeletal and respiratory systems, which will be addressed elsewhere.
Gorab is indispensable for hair follicle morphogenesis
This investigation is focused on the skin. Skins of newborn Gorab−/− pups appeared edematous but otherwise unremarkable (Figure 1e). Histological examination of the embryonic skin of Gorab−/− mutants revealed normal architecture of the epidermis (Figure 1f). In addition, immunofluorescence examination of early and late epidermal differentiation markers, keratin 1 (KRT1) and loricrin (LOR), in E18.5 skins demonstrated comparable epidermal differentiation profiles in control and Gorab−/− mutants (Supplemental Figure. S2). These observations suggested that Gorab may not play a significant role during the formation of the epidermis.
Skins of E15.5 control and Gorab−/− mutants contained comparable numbers of hair germs. In contrast, E18.5 mutant skins contained significantly reduced numbers of hair follicles (Figure 1f and g). In addition, hair follicles in the mutant skin appeared less developed, suggesting that the progression of hair follicle morphogenesis is impaired. To follow postnatal hair follicle development, skin biopsies from E18.5 embryos were transplanted onto nude mice. Three weeks later, control transplants developed abundant hair, whereas Gorab−/− transplants developed barely any hair (Figure 1h). Histology of transplants showed that hair defects in Gorab−/− transplants were associated with the lack of morphologically normal hair follicles (Figure 1i). However, hair follicle-like remnants in the mutant transplants indicated that hair follicles had nevertheless undergone significant development before degeneration (Figure 1i, asterisk). TUNEL staining revealed that apoptotic cells were restricted to the orifices of hair follicles in control skin transplant, whereas the number of apoptotic cells not only increased in Gorab−/− transplants but also dispersed into the dermal mesenchyme (Figure 1j), indicating a mechanism through which hair follicle remnants were cleared from postnatal skin. Thus, data demonstrated that Gorab is indispensable for the morphogenesis of all types of hair follicles of dorsal skin.
To further evaluate hair follicle induction, the formation of hair germs in E15.5 skins was examined by immunofluorescence labeling of KRT17. Hair germs of control and Gorab−/− mutants appeared comparable (Figure 2a). In addition, the expression of LEF1 suggested that the canonical Wnt signaling pathway, which is essential for hair follicle induction (Andl et al., 2002), was also unaffected in Gorab−/− hair follicles (Figure 2b). Furthermore, follicular keratinocyte proliferation in stage 2 hair follicles of Gorab−/− mutants, as determined by Ki67 staining, was also comparable to controls (Supplemental Figure S3). Evaluation of alkaline phosphatase (AP) activity in E18.5 skins showed that mutant dermal papilla cells are AP positive despite the mutant skin contained fewer dermal condensates or dermal papillae (Figure 2c). Thus, data obtained so far suggest that Gorab is not essential for hair follicle induction; rather, it is indispensable for later stages of hair follicle morphogenesis.
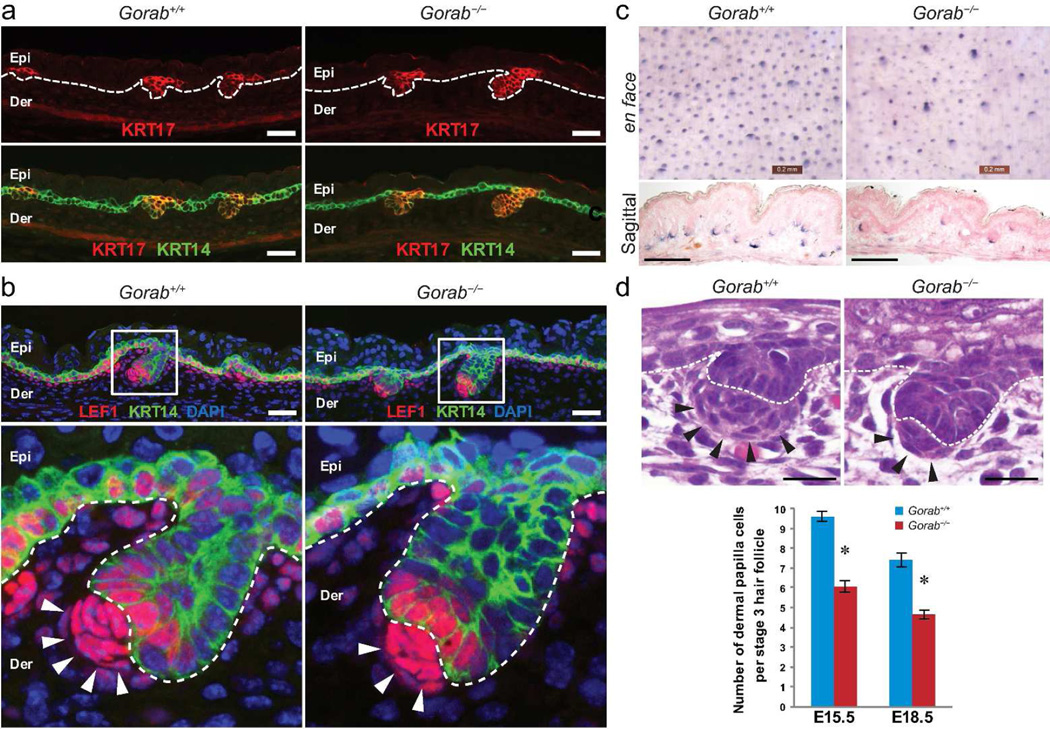
Figure 2. Hair follicle induction in Gorab mutants.
(a) Detection of hair germs in E15.5 dorsal skin of control (Gorab+/+) and homozygous Gorab mutants (Gorab−/−) by keratin 17 (KRT17, red). Keratin 14 (KRT14) was labeled green. Dotted lines outline the basement membrane. (b) Examination of LEF1 (red) in E15.5 skin. KRT14 was labeled green; nuclei were stained with DAPI (blue). (c) Flat-mount (upper panels) and sagittal sections (lower panels) of alkaline phosphatase (AP) stained E18.5 dorsal skins. (d) H&E staining of stage 2 hair follicles in E15.5 embryos and quantification. Note that the number of dermal papilla cells was reduced in homozygous mutants. Arrows point to dermal condensate cells. Scale bars: 50 µm (a, b, and d); 200 µm (c).
Throughout this study, we noticed that hair follicles in E15.5 and E18.5 Gorab−/− mutants harbored consistently fewer dermal condensate cells (Figure. 2b–d). Quantification (based on H&E staining) of cells in mesenchymal condensates of stage 2 and stage 3 hair follicles confirmed that mutant hair follicles contained significantly reduced number of dermal condensate cells in the mutant in comparison to those in controls (Figure. 2d). More interestingly, we found that dermal condensate cells of Gorab−/− mutants were not readily detectable by dermal condensate cell markers, such as nerve growth factor receptor (NGFR, also known as p75 neurotrophin receptor) (Figure 3a and Supplemental Figure S4), suggesting that the dermal condensates in mutant skin were quantitatively and qualitatively different than those in controls.
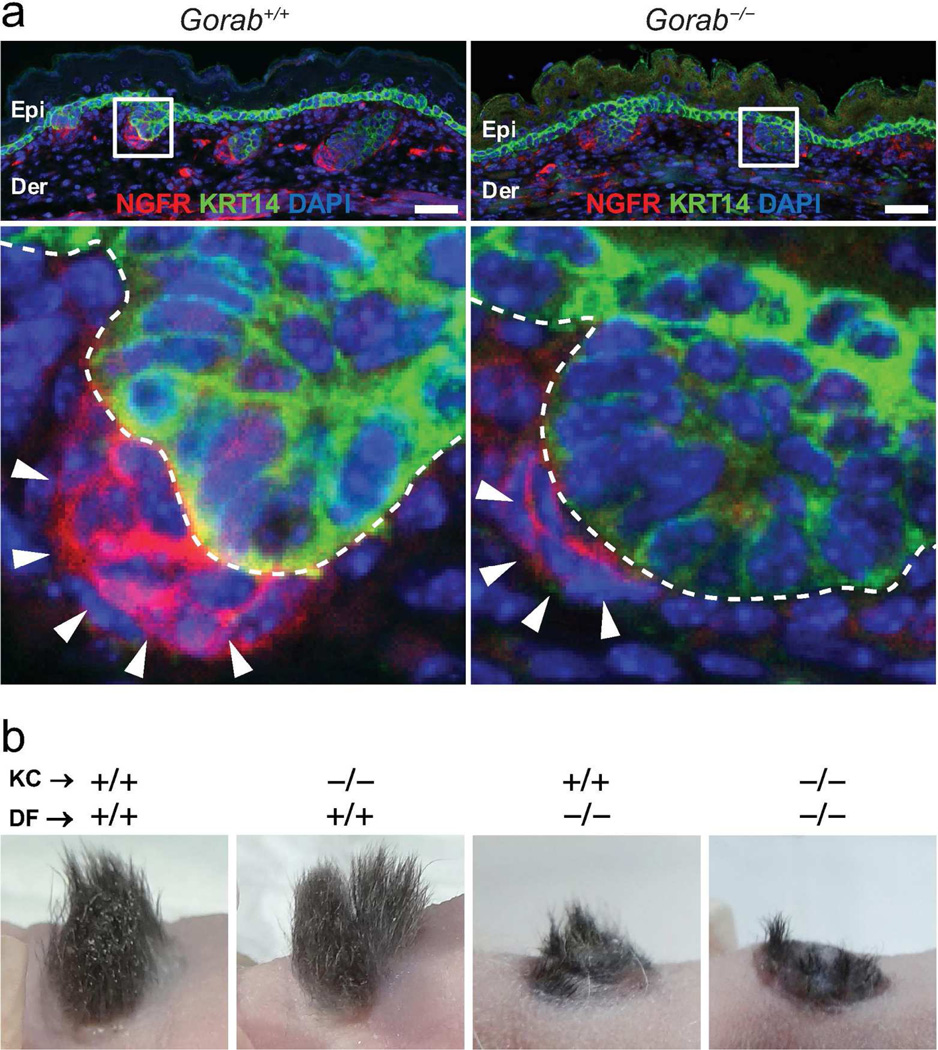
Figure 3. Abnormality in dermal mesenchymal cells during hair follicle formation.
(a) Examination of dermal condensate cells in E15.5 embryos by NGFR (red). KRT14 was labeled green; nuclei were stained with DAPI (blue). Lower panels are enlargements of boxed areas in upper panels. Arrows point to dermal condensate cells. (b) Skin and hair follicle reconstitution assay. Genotypes of keratinocytes (KC) and dermal fibroblasts (DF) were indicated for each graft. +/+, wild-type; −/−, homozygous. n ≥ 3. Epi, epidermis; Der, dermis. Scale bars: 50 µm.
To functionally determine which cell type, i.e, keratinocyte or dermal condensate cells, is responsible for the hair follicle morphogenesis defects in Gorab−/− mutants, mismatched hair follicle re-constitution assays were performed. Specifically, Gorab−/− keratinocytes or dermal fibroblasts were mixed with wild-type dermal fibroblasts or keratinocytes, respectively, and grafted on nude mice (Foxn1nu) to regenerate the skin and hair follicles. Skin grafts regenerated with wild-type dermal fibroblasts, irrespective of the genotype of keratinocytes, formed abundant hair (Figure 3b). In contrast, skin grafts regenerated with Gorab−/− dermal fibroblasts displayed severely compromised hair regeneration (Figure 3b). This experiment demonstrated that it is mutant dermal condensate cells, but not keratinocytes, that were associated with the hair follicle morphogenesis defects in Gorab−/− mutants.
Gorab is required for proper Hh signaling in dermal papilla cells
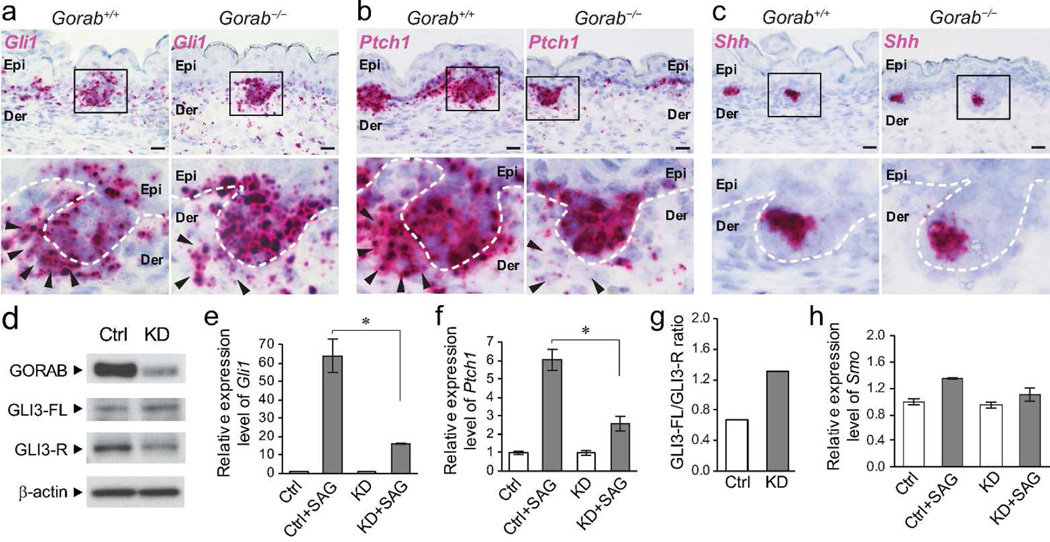
By E18.5 fewer hair follicles in the dorsal skin of Gorab−/− mutants developed beyond stage 4 (Figure. 1f) and expressed hair follicle differentiation markers, such as KRT75 (a companion layer marker) and AE13 (an acidic hair cortex keratins marker (Lynch et al., 1986)) (Supplemental Figure S5). These observations suggested that the cytodifferentiation of mutant hair follicles was disrupted in Gorab−/− mutants. Because the Hh pathway is one of the most important molecular signaling required for hair follicles differentiation (Botchkarev and Paus, 2003), and the hair follicle phenotypes of the Gorab−/− mutants resemble those observed in Hh mutant mice (Chiang et al., 1999; Gat et al., 1998; Mill et al., 2003; St-Jacques et al., 1998; Woo et al., 2012), we investigated Hh signaling in Gorab−/− mutants. In situ hybridization revealed that dermal condensates of mutant skin express remarkably reduced levels of Hh responsive genes, such as Gli1 and Ptch1 (Figure 4a and b). In contrast, the expression of these genes did not seem to be affected in mutant follicular keratinocytes (Figure 4a and b). Furthermore, the expression of Shh, which encodes the ligand of the Hh pathway, was unaffected in follicular keratinocytes (Figure 4c). These results associated defective Hh signaling pathway with hair follicles defects in Gorab−/− mutant mice. They also suggested that Gorab may be specifically required for the activation of Hh signaling in dermal condensate cells.
Figure 4. Hh signaling pathways is impaired in mutant mesenchymal cells.
(a–c) Expression of Gli1, Ptch1, and Shh in E15.5 skins of control (Gorab+/+) and homozygous Gorab mutants (Gorab−/−) by in situ hybridization. Dotted lines illustrate the basement membrane. Arrowheads point to dermal papilla cells. n=4. (d) Expression of GORAB, full-length GLI3 (GLI3-FL, ≈190 kDa), and repressor form of GLI3 (GLI3-R, ≈85 kDa) in Gorab knockdown (KD) mouse embryonic fibroblasts (MEFs) by western blotting. (e and f) Quantification of Gli1 and Ptch1 mRNA by quantitative RT-PCR after SAG treatment in control (Ctrl) and KD MEFs. Asterisk (*) indicates p < 0.01. (g) Quantification GLI3-FL/GLI3-R ratio in control and KD MEFs as shown in a. (h) Quantification of Smo mRNA by quantitative RT-PCR in SAG-treated control and KD MEFs. All experiments were performed three times. Scale bars: 20 µm.
To further dissect the Hh signaling pathway, Gorab was knocked down in mouse embryonic fibroblasts (MEFs), a cell type frequently used for the examination of Hh responsiveness (Figure 4d–h). The Hh pathway was subsequently activated with SAG, a Smo agonist (Chen et al., 2002). Gorab-knockdown was confirmed by western blotting (Figure 4d). Quantitative RT-PCR of Hh responsive gene Gli1 and Ptch1 demonstrated a significantly reduced response to Hh pathway activation in knockdown cells (Figure 4e and f). Furthermore, GLI3 processing, as determined by the full-length GLI3 (GLI3-FL) and GLI3 repressor (GLI3-R) ratio in western blotting, was also disrupted in knockdown cells (Figure 4d and g). In contrast, the expression levels of the Smo receptor in control and Gorab-knockdown cells were comparable (Figure 4h). Taken together, these in vivo and in vitro data suggested that the hair follicle phenotype in Gorab−/− mutant are likely associated with disrupted Hh pathway activation in dermal mesenchymal cells.
GORAB is enriched at the Golgi apparatus but not essential for Golgi structure in fibroblasts
GORAB is enriched at the Golgi. To determine whether Gorab expression is required for Golgi architecture or function, we examined the expression of Golgi resident proteins in Gorab-knockdown MEFs. Immunofluorescence labeling demonstrated that GORAB is almost completely absent in knockdown cells (Supplemental Figure S6). Examination of trans-Golgi marker Golgin-97 (GOLGA1) and cis-medial Golgi marker lectin Helix pomatia agglutinin (HPA) showed that the expression pattern these Golgi-associated proteins and the morphology of the Golgi apparatus appeared comparable in control and Gorab-knockdown cells (Supplemental Figure S6). These findings were consistent with previous observations (Hennies et al., 2008), and suggested GORAB is not essential for maintaining Golgi structure or the localization of Golgi resident proteins in dermal fibroblasts. Rather, it may function in post-Golgi trafficking of proteins.
Gorab is involved in primary cilia formation in dermal condensate cells
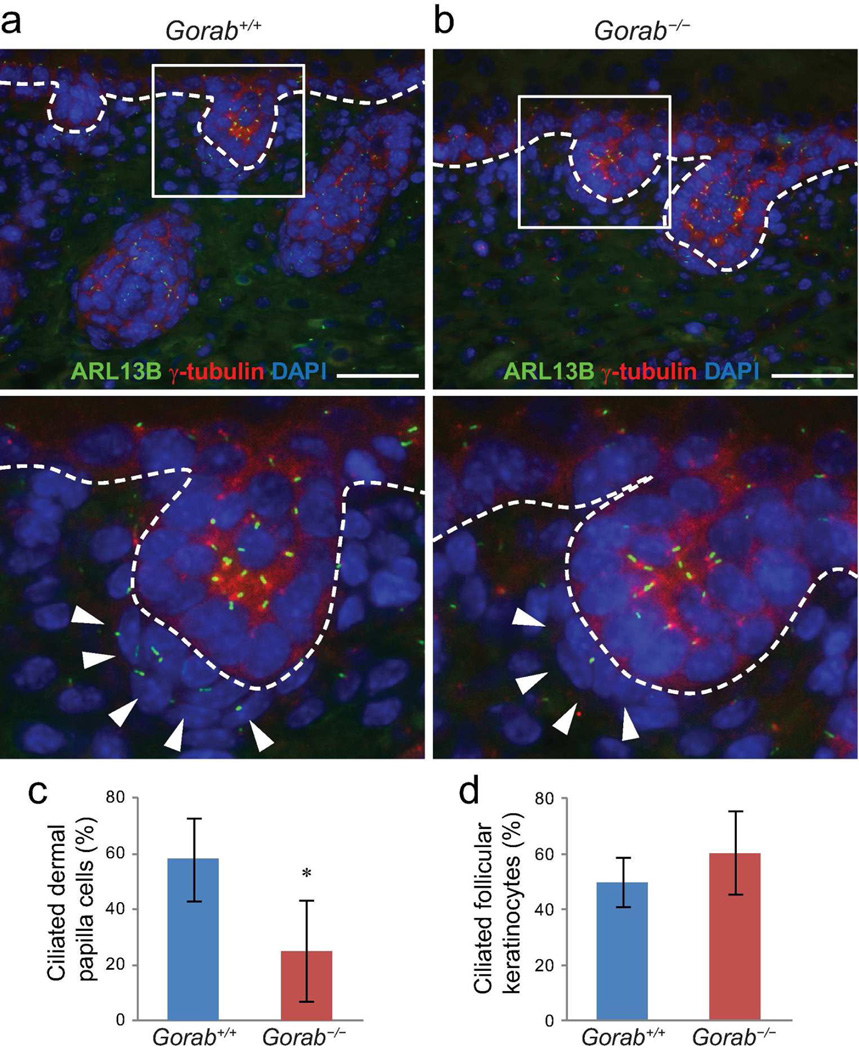
The primary cilium is essential for the Hh signaling pathway and is indispensable for dermal papilla cells during hair follicle morphogenesis (Dai et al., 2011; Lehman et al., 2009; Woo et al., 2012). In fact, hair follicle phenotypes observed in Gorab−/− mutants herein phenocopied those in a number of cilia mutants (Chen et al., 2015; Croyle et al., 2011; Dai et al., 2013; Dai et al., 2011; Ezratty et al., 2011; Lehman et al., 2009). To determine whether Hh signaling defects in Gorab−/− mutants was associated with primary cilia abnormalities, we examined primary cilia formation in Gorab−/− mutants and found that the formation of primary cilia was severely impaired in dermal condensate cells in E15.5 and E18.5 Gorab−/− skins (Figure 5a and b; Supplemental Figure S7), such that 57.88 ± 24.72% dermal condensate cells of stage 2 and stage 3 hair follicles were ciliated in control skin, and only 18.02 ± 14.87% were ciliated in Gorab−/− mutants (Figure 5c). Interestingly, primary cilia in keratinocytes of either the epidermis or the hair follicles appeared comparable between controls and Gorab−/− mutants (Figure 5; Supplemental Figure S7). These observations suggested that disrupted primary cilia formation is likely responsible for the disrupted Hh signaling pathway in mutant dermal papilla cells, thereby contributing to the hair follicle morphogenesis defects in Gorab−/− mutant mice.
Figure 5. Formation of primary cilia is impaired in dermal condensate cells of Gorab mutants.
(a) Immunofluorescence labeling of primary cilia with ARL13B (green) in dorsal skin of E18.5 control (Gorab+/+) and homozygous Gorab mutants (Gorab−/−). Basal bodies labeled with γ-tubulin (red); nuclei were stained with DAPI (blue). Lower panels are magnified boxed hair germs in the upper panels. Dotted lines illustrate the basement membrane. Arrowheads point to dermal papilla cells. (c and d) Quantification of ciliated dermal condensate cells (c) and follicular keratinocytes (d). A minimum of three animals were evaluated for each genotype. Asterisk (*) indicates p<0.05. Scale bars: 50 µm.
DISCUSSION
The Golgi apparatus is a vital organelle of the cell. The biological functions of Golgi during skin morphogenesis are not well understood. In this study we demonstrated that GORAB, a Golgi-associated protein, is required for proper Hh signaling during embryonic morphogenesis of hair follicles, and suggested that GORAB may participate in Hh signaling through facilitating primary cilia formation. Thus, findings obtained from this study provided an example of how Golgi-associated proteins regulate skin morphogenesis.
The Hh pathway is a key molecular signaling mechanism of skin morphogenesis (Driskell et al., 2011; Lee and Tumbar, 2012; Millar, 2002; Oro and Higgins, 2003; Schmidt-Ullrich and Paus, 2005; Yang and Cotsarelis, 2010). In vertebrates, the activation of the Hh signaling pathway is typically mediated in a paracrine fashion, in which the epithelial cells produce the ligand to signal the adjacent mesenchymal cells (Ingham and McMahon, 2001). In this study, we found that the expression of Shh, the Hh ligand, was unaffected in follicular keratinocytes in Gorab mutants. The expression of Hh responsive genes was also undisrupted in follicular keratinocytes. In contrast, the Hh pathway was significantly impaired in Gorab-deficient dermal mesenchymal cells in vitro and in vivo. These observations suggest that Gorab is specifically required for the activation of Hh signaling pathway in recipient cells in the mesenchyme, at least in the context of hair follicle morphogenesis. Thus, data obtained from this study provided an example of how molecular signaling is spatially regulated during morphogenesis. How Gorab achieves tissue- or cell type-specific regulation of Hh signaling remains to be determined.
Disrupting Gorab affected the number of dermal papilla cells and their capability of expressing NGFR, responding to Hh pathway activation, and forming primary cilia. However, these abnormalities were relatively subtle. It remains to be further determined whether other mechanisms, such as disrupted dermal papilla cell proliferation and differentiation through impaired p75 NGFR signaling (Botchkareva et al., 1999) or a more general effect associated with this golgin, have collectively contributed to hair follicle morphogenesis defects.
Gorab is also expressed in epidermal keratinocytes. Our investigation did not reveal overt epidermal abnormalities, suggesting that Gorab may not play a significant role during epidermal morphogenesis. Interestingly, GORAB has been linked to the regulation of TRP53 (p53) expression during neurite outgrowth (Liu et al., 2012) and tumor cell growth in vitro (Hu et al., 2012; Yang et al., 2014), in which GORAB acts in MDM2-mediated polyubiquitination and proteasome degradation of p53 (Yan et al., 2010; Zhang et al., 2005). While regulating p53 stability by GORAB unlikely plays a significant role during epidermal morphogenesis, it remains to be determined whether GORAB is involved in post-natal skin homeostasis by regulating the level of p53 at the post-translational level.
Recent progress in understanding the biological functions of primary cilium substantially expanded our understanding on how Hh signal pathway is regulated during morphogenesis (Berbari et al., 2009; Goetz and Anderson, 2010; Oro, 2007; Veland et al., 2009; Wong and Reiter, 2008). Data obtained from this study suggests that participating in primary cilia formation is a candidate mechanism through which Gorab is involved in Hh signaling. The precise molecular mechanism through which GORAB participates in primary cilia formation remains to be determined in future studies. However, based on the knowledge that GORAB is enriched at the Golgi and interacts with RAB6, a small GTPase that is extensively involved in intracellular trafficking (Stenmark, 2009), it is conceivable that GORAB participates in ciliogenesis through regulating the Golgi localization or intracellular trafficking of ciliogenic proteins.
Mutations in the human GORAB gene are associated with GO. Disease-causing mutations in the GORAB gene are predominantly missense mutations located across the coding sequence (Al-Dosari and Alkuraya, 2009; Hennies et al., 2008), often resulting in complete absence of the GORAB protein (Hennies et al., 2008). The mutant Gorab mouse model engineered in this study does not express detectable levels of Gorab mRNA or protein. Therefore, it is likely to be able to mimic GO at the genetic level. Although the characteristic pre-mature aging phenotypes of GO, specifically wrinkly skin and osteoporosis, remains to be characterized in this mouse model, data obtained from this study suggest that impaired Hh signaling may contribute to the development of GO-related skin and bone phenotypes. In comparison to strong hair follicle phenotypes and pathway suppression in Hh mutants (Chiang et al., 1999; Mill et al., 2003; St-Jacques et al., 1998; Woo et al., 2012), Gorab mutants displayed relatively mild Hh phenotypes. Thus, it is probable that Gorab may perform functions beyond controlling Hh signaling or primary cilia formation. In light of recent reports which demonstrated that the GORAB protein physically interacts with RAB6 and ARF5, small GTPases involved in anterograde and retrograde intracellular trafficking (Egerer et al., 2015; Hennies et al., 2008), it is conceivable that GORAB performs important functions along the secretory and endocytic pathways.
In summary, this study linked the function of GORAB, a golgin, to Hh signaling and primary cilia formation during hair follicle morphogenesis. It not only provided important insight into the biological function of GORAB during embryonic morphogenesis but also provided an example of how skin morphogenesis is regulated at the Golgi level. Further understanding of the molecular functions of GORAB in the context of Hh signaling and primary cilia formation may provide important insight into how golgins coordinate intracellular trafficking of proteins.
MATERIALS AND METHODS
Generation of Gorab mutant mouse model
ES cell clone XG183, containing the trap vector pGT1Lxf, was obtained from BayGenomics consortium (CA, USA). ES cells were cultured as recommended by the supplier and injected into blastocysts (C57BL6) before implantation in surrogate moms to generate chimeric mice. Chimeric mice were backcrossed to the C57BL6 mice. F1 mice were identified by PCR-based genotyping of the Gorab locus with primers Intron1-F and Intron1-R (Figure. 1 and Supplemental Figure S1).
Mice used in this study were maintained on a mixed genetic background of 129 and C57BL6. Homozygous Gorab mutants (Gorab−/−) were obtained by crossing heterozygous (Gorab+/−) mice. Wild-type (Gorab+/+) littermates were used as controls. E15.5 and E18.5 fetuses were obtained by timed-mating. All procedures related to mice were approved by the Institutional Animal Care and Use Committee of ILAS and Stony Brook University.
Tissue processing and histology analyses
Freshly isolated tissues were fixed immediately in formalin and embedded in paraffin and sectioned and processed for routine hematoxylin and eosin (H&E) staining or other examinations. Specimens were examined on an Olympus BX40 (Olympus, Tokyo, Japan) or Nikon 80i (Melville, NY) microscope.
Alkaline phosphatase staining
Alkaline phosphatase (AP) staining was performed on fresh skin with the NBT/BCIP method as previously described (Tsai et al., 2010). After staining, skins were either immediately imaged on a LEICA M125 dissecting microscope fitted with a LEICA DFC450 camera or embedded in OCT, sectioned at 10 µm, and counter stained with 0.1% nuclear fast red before being mounted on glycerol and photographed.
In situ hybridization
In situ hybridization was carried out on formalin-fixed paraffin embedded tissue sections using the RNAScope system (Advanced Cell Diagnostic, Hayward, CA) per manufacturer’s instructions and as previously described (Chen et al., 2015; Wang et al., 2012).
Cell culture and in vitro assays
The isolation of primary keratinocytes and dermal fibroblasts from E18.5 embryos was conducted as described elsewhere (Dai et al., 2011). Primary mouse embryonic fibroblasts (MEF) were generated by disrupting E12.5 embryos in trypsin. Fibroblasts were cultured in Dulbecco’s modified Eagle’s medium (4.5 g/l glucose) supplemented with 10% fetal bovine serum and antibiotics. Knockdown was performed by 15 nM siRNA (IDT) and RNAi/MAX transfection per manufacturer’s recommendation. To examine Hh signaling, MEFs were serum-starved for 24 hours, treated with 100 nM SAG (Calbiochem) for another 24 hours, before being lysed in RLT buffer (Qiagen) for RNA extraction or RIPA buffer for western blotting.
RNA isolation and quantitative RT-PCR
RNA was isolated with the RNeasy kit (Qiagen) and quantitative RT-PCR analyses were performed as described previously (Dai et al., 2013). Complementary DNA was synthesized from 2 µg of total mRNA using a High Capacity cDNA Reverse Transcription kit (Applied Biosystems) and random hexameric primers. Real-time qRT-PCR was performed on ABI Prism 7500 with the following TaqMan probes: Gli1, Mm00494645_m1; Gli2, Mm01293111_m1; Ptch1, Mm00436026_m1; Smo, Mm01162710_m1; and β-actin, Mm00607939_m1 (Life Technologies). Results were analyzed using the ΔΔCt method. Relative expression levels of target genes were determined by comparing with wild type or treatment controls after normalizing with β-actin.
Protein Analysis
Protein was extracted in cold RIPA lysis buffer (50 mM Tris-HCl pH 7.4, 150 mM NaCl, 1% Triton X-100, 1% sodium deoxycholate, and 0.1% SDS) supplemented with proteinase inhibitors. Tissue or cell lysates were cleared and separated by 10% SDS-PAGE and transferred to polyvinylidene fluoride (PVDF, Millipore) or Hybond nitrocellulose (GE Healthcare) membranes, following standard procedures. Blots were probed with the primary antibodies which were then detected with HRP-conjugated secondary antibodies (BD biosciences) and SuperSignal substrates (Thermo Scientific). Enhanced chemiluminescent (ECL) substrate (Pierce, Rockford, IL USA) and CL-XPosure film (Thermo Scientific) were used for detection. The following primary antibodies were used: GORAB, 1:1,000 (Proteintech); β-actin, 1:1,000 (Santa Cruz); GLI1, 1:250 (clone V812, Cell Signaling); GLI3 (1 µg/ml, Cell Signaling). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) or β-actin was used as a loading control. Quantification was performed with densitometry and ImageJ software (1.43u, NIH).
Immunofluorescence labeling and microscopy
Immunofluorescence labeling of tissue specimens was performed as described previously (Dai et al., 2013). Cells were fixed in 4% PFA and blocked in 1% BSA prior to incubating with primary antibodies. The following primary antibodies were used: GORAB, 1:500 (Proteintech); KRT14, 1:1,000 (Covance); KRT1, 1:2000 (Roop et al., 1987); KRT17, 1:200 (Abcam); LOR, 1:500 (Covance); NGFR, 1:200 (Promega); LEF1, 1:100 (Cell Signaling, Danvers, MA); acetylated α-tubulin, 1:600 (Sigma); γ-tubulin, 1:500 (Abcam); ARL13B, 1:100 (#73–287, NeuroMab); lectin Helix pomatia agglutinin (HPA), 1:1,000 (Invitrogen); Golgin97, 1:1,000 (Molecular Probes), and Ki67, 1:1000 (BD Pharmingen). AlexaFluor-conjugated secondary antibodies (1:250) were from Life Technologies. Sections were sealed in mounting medium with DAPI (Vector Laboratories). TUNEL staining were performed with DeadEnd Fluorometric TUNEL System (Promega). Images were acquired by Nikon 80i fitted with Nikon DS-Qi1Mc camera and processed with Photoshop 5.5 CS.
Skin transplantation and hair follicle reconstitution assay
Dorsal skin obtained from E18.5 embryos were transplanted onto the back of 8 – 12 week old nude mice (Foxn1−/−) as described previously (Dai et al., 2011). Keratinocytes and dermal fibroblasts isolated from control and homozygous mutants were used immediately in skin and hair follicle reconstitution assays as described in (Dai et al., 2011). Briefly, 2 × 106 keratinocytes were mixed with 2 × 106 fibroblasts of the same or mis-matched genotypes before the cell slurry was seeded in silicon grafting chambers placed on the back of nude mice. For skin transplantation and reconstitution assays, grafting chambers were removed 10 days after placement and skin grafts were harvested two weeks thereafter. Experiments were performed at least three times.
Statistical analyses
All quantifications are presented as mean ± S.D. Student t-test was used unless otherwise stated. One-way ANOVA and two-way ANOVA were conducted using the GraphPad software. P < 0.05 was considered statistically significant.
Supplementary Material
ACKNOWLEDGEMENT
We would like to thank Yanfeng Xu, Yuanwu Ma, Lan Huang, David Naimzadeh, and Mallory Korman for technical assistance. This study was supported by the National Natural Science Foundation of China (Grant No. 31301928 and 81472899), the Fundamental Research Funds for the Central Universities (Grant No. DWS201201), start-up funds provided by the Department of Pathology and the Cancer Center of Stony Brook University, and a research grant from NIH/NIAMS (AR061485 to JC).
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
REFERENCES
- Al-Dosari M, Alkuraya FS. A novel missense mutation in SCYL1BP1 produces geroderma osteodysplastica phenotype indistinguishable from that caused by nullimorphic mutations. Am J Med Genet A. 2009;149A:2093–2098. doi: 10.1002/ajmg.a.32996. [DOI] [PubMed] [Google Scholar]
- Andl T, Reddy ST, Gaddapara T, et al. WNT signals are required for the initiation of hair follicle development. Dev Cell. 2002;2:643–653. doi: 10.1016/s1534-5807(02)00167-3. [DOI] [PubMed] [Google Scholar]
- Badano JL, Mitsuma N, Beales PL, et al. The ciliopathies: an emerging class of human genetic disorders. Annu Rev Genomics Hum Genet. 2006;7:125–148. doi: 10.1146/annurev.genom.7.080505.115610. [DOI] [PubMed] [Google Scholar]
- Berbari NF, O'Connor AK, Haycraft CJ, et al. The primary cilium as a complex signaling center. Curr Biol. 2009;19:R526–R535. doi: 10.1016/j.cub.2009.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botchkarev VA, Paus R. Molecular biology of hair morphogenesis: development and cycling. J Exp Zool B Mol Dev Evol. 2003;298:164–180. doi: 10.1002/jez.b.33. [DOI] [PubMed] [Google Scholar]
- Botchkareva NV, Botchkarev VA, Chen LH, et al. A role for p75 neurotrophin receptor in the control of hair follicle morphogenesis. Dev Biol. 1999;216:135–153. doi: 10.1006/dbio.1999.9464. [DOI] [PubMed] [Google Scholar]
- Chen J, Laclef C, Moncayo A, et al. The ciliopathy gene Rpgrip1l is essential for hair follicle development. J Invest Dermatol. 2015;135:701–709. doi: 10.1038/jid.2014.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JK, Taipale J, Young KE, et al. Small molecule modulation of Smoothened activity. Proc Natl Acad Sci U S A. 2002;99:14071–14076. doi: 10.1073/pnas.182542899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang C, Swan RZ, Grachtchouk M, et al. Essential role for Sonic hedgehog during hair follicle morphogenesis. Dev Biol. 1999;205:1–9. doi: 10.1006/dbio.1998.9103. [DOI] [PubMed] [Google Scholar]
- Croyle MJ, Lehman JM, O'Connor AK, et al. Role of epidermal primary cilia in the homeostasis of skin and hair follicles. Development. 2011;138:1675–1685. doi: 10.1242/dev.060210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai D, Li L, Huebner A, et al. Planar cell polarity effector gene Intu regulates cell fate-specific differentiation of keratinocytes through the primary cilia. Cell Death Differ. 2013;20:130–138. doi: 10.1038/cdd.2012.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai D, Zhu H, Wlodarczyk B, et al. Fuz controls the morphogenesis and differentiation of hair follicles through the formation of primary cilia. J Invest Dermatol. 2011;131:302–310. doi: 10.1038/jid.2010.306. [DOI] [PubMed] [Google Scholar]
- Driskell RR, Clavel C, Rendl M, et al. Hair follicle dermal papilla cells at a glance. J Cell Sci. 2011;124:1179–1182. doi: 10.1242/jcs.082446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egerer J, Emmerich D, Fischer-Zirnsak B, et al. GORAB Missense Mutations Disrupt RAB6 and ARF5 Binding and Golgi Targeting. J Invest Dermatol. 2015;135:2368–2376. doi: 10.1038/jid.2015.192. [DOI] [PubMed] [Google Scholar]
- Ezratty EJ, Stokes N, Chai S, et al. A role for the primary cilium in Notch signaling and epidermal differentiation during skin development. Cell. 2011;145:1129–1141. doi: 10.1016/j.cell.2011.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gat U, DasGupta R, Degenstein L, et al. De Novo hair follicle morphogenesis and hair tumors in mice expressing a truncated beta-catenin in skin. Cell. 1998;95:605–614. doi: 10.1016/s0092-8674(00)81631-1. [DOI] [PubMed] [Google Scholar]
- Goetz SC, Anderson KV. The primary cilium: a signalling centre during vertebrate development. Nat Rev Genet. 2010;11:331–344. doi: 10.1038/nrg2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy MH. The secret life of the hair follicle. Trends Genet. 1992;8:55–61. doi: 10.1016/0168-9525(92)90350-d. [DOI] [PubMed] [Google Scholar]
- Hennies HC, Kornak U, Zhang H, et al. Gerodermia osteodysplastica is caused by mutations in SCYL1BP1, a Rab-6 interacting golgin. Nat Genet. 2008;40:1410–1412. doi: 10.1038/ng.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrandt F, Benzing T, Katsanis N. Ciliopathies. N Engl J Med. 2011;364:1533–1543. doi: 10.1056/NEJMra1010172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu L, Liu M, Chen L, et al. SCYL1 binding protein 1 promotes the ubiquitin-dependent degradation of Pirh2 and has tumor-suppressive function in the development of hepatocellular carcinoma. Carcinogenesis. 2012;33:1581–1588. doi: 10.1093/carcin/bgs162. [DOI] [PubMed] [Google Scholar]
- Ingham PW, McMahon AP. Hedgehog signaling in animal development: paradigms and principles. Genes Dev. 2001;15:3059–3087. doi: 10.1101/gad.938601. [DOI] [PubMed] [Google Scholar]
- Koster MI, Roop DR. Mechanisms regulating epithelial stratification. Annu Rev Cell Dev Biol. 2007;23:93–113. doi: 10.1146/annurev.cellbio.23.090506.123357. [DOI] [PubMed] [Google Scholar]
- Lee J, Tumbar T. Hairy tale of signaling in hair follicle development and cycling. Semin Cell Dev Biol. 2012;23:906–916. doi: 10.1016/j.semcdb.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehman JM, Laag E, Michaud EJ, et al. An essential role for dermal primary cilia in hair follicle morphogenesis. J Invest Dermatol. 2009;129:438–448. doi: 10.1038/jid.2008.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Chen Y, Lu X, et al. SCYL1BP1 modulates neurite outgrowth and regeneration by regulating the Mdm2/p53 pathway. Mol Biol Cell. 2012;23:4506–4514. doi: 10.1091/mbc.E12-05-0362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch MH, O'Guin WM, Hardy C, et al. Acidic and basic hair/nail ("hard") keratins: their colocalization in upper cortical and cuticle cells of the human hair follicle and their relationship to "soft" keratins. J Cell Biol. 1986;103:2593–2606. doi: 10.1083/jcb.103.6.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mill P, Mo R, Fu H, et al. Sonic hedgehog-dependent activation of Gli2 is essential for embryonic hair follicle development. Genes Dev. 2003;17:282–294. doi: 10.1101/gad.1038103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar SE. Molecular mechanisms regulating hair follicle development. J Invest Dermatol. 2002;118:216–225. doi: 10.1046/j.0022-202x.2001.01670.x. [DOI] [PubMed] [Google Scholar]
- Oro AE. The primary cilia, a 'Rab-id' transit system for hedgehog signaling. Curr Opin Cell Biol. 2007;19:691–696. doi: 10.1016/j.ceb.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oro AE, Higgins K. Hair cycle regulation of Hedgehog signal reception. Dev Biol. 2003;255:238–248. doi: 10.1016/s0012-1606(02)00042-8. [DOI] [PubMed] [Google Scholar]
- Paus R, Muller-Rover S, Van Der Veen C, et al. A comprehensive guide for the recognition and classification of distinct stages of hair follicle morphogenesis. J Invest Dermatol. 1999;113:523–532. doi: 10.1046/j.1523-1747.1999.00740.x. [DOI] [PubMed] [Google Scholar]
- Schmidt-Ullrich R, Paus R. Molecular principles of hair follicle induction and morphogenesis. Bioessays. 2005;27:247–261. doi: 10.1002/bies.20184. [DOI] [PubMed] [Google Scholar]
- Schneider MR, Schmidt-Ullrich R, Paus R. The hair follicle as a dynamic miniorgan. Curr Biol. 2009;19:R132–R142. doi: 10.1016/j.cub.2008.12.005. [DOI] [PubMed] [Google Scholar]
- Schweizer J, Langbein L, Rogers MA, et al. Hair follicle-specific keratins and their diseases. Exp Cell Res. 2007;313:2010–2020. doi: 10.1016/j.yexcr.2007.02.032. [DOI] [PubMed] [Google Scholar]
- Singla V, Reiter JF. The primary cilium as the cell's antenna: signaling at a sensory organelle. Science. 2006;313:629–633. doi: 10.1126/science.1124534. [DOI] [PubMed] [Google Scholar]
- St-Jacques B, Dassule HR, Karavanova I, et al. Sonic hedgehog signaling is essential for hair development. Curr Biol. 1998;8:1058–1068. doi: 10.1016/s0960-9822(98)70443-9. [DOI] [PubMed] [Google Scholar]
- Stenmark H. Rab GTPases as coordinators of vesicle traffic. Nat Rev Mol Cell Biol. 2009;10:513–525. doi: 10.1038/nrm2728. [DOI] [PubMed] [Google Scholar]
- Tobin JL, Beales PL. The nonmotile ciliopathies. Genet Med. 2009;11:386–402. doi: 10.1097/GIM.0b013e3181a02882. [DOI] [PubMed] [Google Scholar]
- Tsai SY, Clavel C, Kim S, et al. Oct4 and klf4 reprogram dermal papilla cells into induced pluripotent stem cells. Stem Cells. 2010;28:221–228. doi: 10.1002/stem.281. [DOI] [PubMed] [Google Scholar]
- Veland IR, Awan A, Pedersen LB, et al. Primary cilia and signaling pathways in mammalian development, health and disease. Nephron Physiol. 2009;111:p39–p53. doi: 10.1159/000208212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Flanagan J, Su N, et al. RNAscope: a novel in situ RNA analysis platform for formalin-fixed, paraffin-embedded tissues. J Mol Diagn. 2012;14:22–29. doi: 10.1016/j.jmoldx.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong SY, Reiter JF. The primary cilium at the crossroads of mammalian hedgehog signaling. Curr Top Dev Biol. 2008;85:225–260. doi: 10.1016/S0070-2153(08)00809-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo WM, Zhen HH, Oro AE. Shh maintains dermal papilla identity and hair morphogenesis via a Noggin-Shh regulatory loop. Genes Dev. 2012;26:1235–1246. doi: 10.1101/gad.187401.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan J, Zhang D, Di Y, et al. A newly identified Pirh2 substrate SCYL1-BP1 can bind to MDM2 and accelerate MDM2 self-ubiquitination. FEBS Lett. 2010;584:3275–3278. doi: 10.1016/j.febslet.2010.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang CC, Cotsarelis G. Review of hair follicle dermal cells. J Dermatol Sci. 2010;57:2–11. doi: 10.1016/j.jdermsci.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang ZP, Xie YH, Ling DY, et al. SCYL1BP1 has tumor-suppressive functions in human lung squamous carcinoma cells by regulating degradation of MDM2. Asian Pac J Cancer Prev. 2014;15:7467–7471. doi: 10.7314/apjcp.2014.15.17.7467. [DOI] [PubMed] [Google Scholar]
- Zhang L, Li J, Wang C, et al. A new human gene hNTKL-BP1 interacts with hPirh2. Biochem Biophys Res Commun. 2005;330:293–297. doi: 10.1016/j.bbrc.2005.02.156. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.