Abstract
Hand aesthetics in general and aesthetic refinements of soft-tissue coverage of the hand in particular have been increasingly considered over the past few years. Advancements of microsurgery together with the traditional methods of tissue transfer have expanded the armamentarium of the reconstructive surgeon, thus shifting the reconstructive paradigm from simply ‘filling the defect’ to reconstructive refinement to provide the best functional and aesthetic results. However, drawing the boundary between what does and what does not constitute ‘aesthetic’ reconstruction of the hand is not straightforward. The selection amongst the vast amount of currently available reconstructive methods and the difficulties in objectively measuring or quantifying aesthetics has made this task complex and rather arbitrary. In this article we divide the hand into several units and subunits to simplify our understanding of the basic functional and aesthetic requirements of these regions that may ultimately bring order to complexity.
Keywords: Hand, Aesthetics, Reconstruction, Aesthetic subunits
Introduction
The primary goal of reconstructive hand surgery has traditionally been focused on restoring function. However, hand aesthetics and the key parameters that define the aesthetic goals and outcomes of soft-tissue reconstruction of the hand have not been well described in the literature.1–3 Although the greater emphasis on restoring function more than appearance is logical, hand surgeons should be mindful of a patient’s needs and desires to restore form as well as function. The hand plays an important role in social functioning, expression, productivity, and interactions with our surroundings. Hand deformities resulting from trauma or tumor resection may lead to undesired behavior such as excessive anxiety, negative self-perceptions and lowered self-esteem.4–6 Data obtained from patient-reported outcome studies have shown that improving hand appearance is one of the important factors why patients with hand deformities may seek reconstructive hand surgery.7–9 Other studies showed that improving patients’ appearance including hands improves patients’ quality of life.10–12
Given the impact of aesthetics on patients’ physical and psychological well-being, aesthetic refinements of soft-tissue coverage of the hand are essential consideration during surgical planning. In the past, the relative limited experience and concern with flap failure hampered hand surgeons from performing creative soft-tissue reconstructions that can enhance the aesthetic outcomes. In recent years, however, improvements of flap survival owing to better understanding of the vascular anatomy coupled with the advent of innovative flap designs and harvesting techniques have substantially changed the philosophy of reconstructive hand surgery.13–16 A successful soft-tissue reconstruction should no longer be measured only by the rate of flap survival or the degree of improvement of hand function. Rather soft-tissue coverage that provides optimal functional and aesthetic outcomes, minimal donor-site morbidity, and improves patients’ quality of life, is becoming the new standard of practice for reconstructive surgery.16
To date, several flaps and reconstructive techniques have been employed to provide coverage for soft-tissue defects of the hand and digits16–18, yet choosing the ideal soft-tissue cover that gives the best functional and aesthetic outcome for a particular defect can be a challenging task (Table 1). With any type of soft-tissue reconstruction, the principal objective is to restore form, function, and sensation of the hand. Patients and surgeons often lament that the soft-tissue cover is either ‘too thick’ or ‘too thin’ as a result of a bulky flap or an unsightly contour defect from skin grafting. The development of scars and skin contractures, nail deformities, and areas of color and texture mismatch may give rise to similar aesthetic concerns. Although the literature is not short of descriptions of flaps and other techniques for the soft-tissue coverage of the hand, little is known about what is deemed aesthetically pleasing in this particular part of the body. This paucity of evidence may be attributed to the majority of the published work comprising only of a small number of comparative studies, most of which have no set criteria of predefined aesthetic parameters. Additionally, the lack of an internationally accepted aesthetic guidelines and standardized outcome measures makes it difficult to further guide the aesthetic reconstruction of the hand. 19–23
Table 1.
Indications and aesthetic considerations of various reconstructive methods.
| Tissue type | Indications | Aesthetic considerations |
|---|---|---|
| Skin grafts |
|
|
| Local flaps |
|
|
| Regional pedicled flaps |
|
|
| Free flaps |
|
|
In this special article, we critically appraise the literature to identify the key parameters and determinants of aesthetics regarding soft-tissue coverage of the hand. Through various clinical applications, we will demonstrate the utility of these parameters as a conceptual framework that can further guide the decision-making process for the most appropriate type of soft-tissue reconstruction, taking into account patients’ preferences as well as the skills and preferences of the operating surgeon.
Functional aesthetic units & subunits of the hand
Anatomic and topographic considerations of the hand
An aesthetic unit is a major structural unit separated from adjacent units by junction lines that share similar skin characteristics of color, texture, thickness, contour, elasticity, pore density, hairiness and sebaceousness.24 Further breakdown of these areas into smaller units are called subunits. Gonzalez-Uloa first popularized the concept of aesthetic units for facial reconstruction,25 and later Burget and Menick26–28 further delineated these regions into smaller subunits. These pioneers advocated that by reproducing the natural contours, highlights, and landmarks of the face and hiding surgical scars within natural skin folds and creases would create the mental image of normalcy. For many years the principles of aesthetic units and subunits formed the basis of facial reconstruction and have been utilized in soft-tissue reconstruction at other regions of the body such as the breast and the gluteal region.29–31
Compared to the face and breast, the hand has special functional requirements such as mobility and sensibility. For example, when placing a skin incision or designing a skin flap, the surgeon must take into account the structure and mobility of the area it spans, the relation to deeper structures, the blood supply, and the cutaneous innervation of the integument. Tubiana32 divided the hand into several distinct regions based on functional anatomy and called these regions the ‘functional cutaneous units’ of the hand. We elaborate on the original classification made by Tubiana and refer to these regions as the ‘functional aesthetic units & subunits’ of the hand (Fig. 1, A-B), taking into consideration the principles of visual perception as well as the topographic and anatomic features of the hand, as follows:
Figure 1.
(a). Illustrating the functional aesthetic units and subunits of the volar surface of the hand. The palm can be considered as a single functional aesthetic unit that is further subdivided into 5 subunits; Thenar (T), Opposition (O) Hypothenar, Central triangular (C) and Metacarpal areas (M). The volar surface of each digit is a single functional aesthetic unit that is divided into 3 subunits (except thumb only 2 subunits). Note that the pulps (P) of fingers are highlighted to emphasize the ‘functional’ (sensory) importance of this special part of the digit. (b). Similar to the volar surface, the dorsum of the hand as highlighted in the figure (D), can be considered as a single functional aesthetic unit. The dorsum of each digit is another functional aesthetic unit that is subdivided into 3 subunits (except thumb only 2 subunits). Note that the nails (N) of the fingers are highlighted to emphasize the importance of ‘aesthetics’ (appearance) of this special part of the digit. Each region (units & subunits) of the hand has its unique functional and aesthetic requirements.
Hand size & proportions
The morphologic (shape) and morphometric (size) characteristics of the hands and digits depend on gender and race and may be influenced by the cultural background and occupation of a person3. The divine proportions of human hands are well documented in the literature and are said to be in complete harmony with the ‘golden ratio’ of the body, also found in the face and torso. Deviation from these aesthetically pleasing measurements may be perceived as less desirable.3, 33–34 Kościński recently studied the determinants of hand attractiveness by digitally manipulating hand images of healthy volunteers. He then asked subjects of opposite-sexes to indicate the images that were perceived more attractive. Both men and women preferred feminine type, slender hands of average proportions with relatively long fingers and a smooth surface. These criteria of hand attractiveness were found to match those for facial attractiveness.35
Visual stimuli
The pattern of light refection across curved surfaces, such as the hand, and the resultant highlights and shadows allows us to see an object in a three-dimensional space. The contrast between light and dark is most prominent at the intersecting planes located between the valleys and ridges that divide the form into distinct regions.36 The lightest areas are seen over convex surfaces, whereas shadows are seen over concave areas and plane-breaks.28 This predictable pattern of light reflection over curved surfaces has formed the basis of the three-dimensional artwork of many impressionist painters in the early 20th century, notably the likes of Picasso and Monet. Distortion of this elegant balance of light reflection by a scar or surface irregularities may be perceived as less visually appealing.
As we gaze at an object, the nature of saccadic eye movements causes our eyes to focus sporadically on random spots as we scan, but our neural pathways tend to ignore slight variations in our surroundings while responding to an unexpected stimuli.26 When we glance at a hand that is, or appears to be normal compared to the contralateral side, our conscious mind will likely disregard it. However, if our eyes gaze upon an abnormal hand, the brain will respond and our eyes will be drawn back to it. The field of psychology uses the term “visual search” to explain how we distinguish between target (information that should be acknowledged) and background (extraneous information that can be ignored) signals. Bootzin explains that the brain’s propensity toward “perceptual constancy” is how humans are able to focus on tasks and ignore irrelevant information.27–28,37
The visual perception of the human hand is far more complex than simply observing pattern of lights or searching the visual field. Desimone et al found neurons selective for hands/ hand-preferring regions grouped in the inferior temporal cortex of macaque monkeys that respond almost exclusively to pictures of human and monkey hands.38 By using functional MRI (fMRI) techniques, researchers have more recently discovered a region of the lateral occipitotemporal cortex in humans that is highly reactive to images of human bodies and body parts.39–40 The extrastriate body area (EBA), as this region has been termed, is specifically located in the posterior inferior temporal sulcus/middle temporal gyrus.41–42 Interestingly within this region, fMRI demonstrated that the brain was more responsive to images of the body and hands, than to images of the face and other objects.39,43 The appreciation of these concepts is essential for the reconstructive surgeon when restoring the anatomical landmarks of a complex multidimensional structure such as the hand.
Hand integument
The palmar and dorsal skin of the hand is functionally and anatomically different, each serving a specific purpose. On the dorsum of the hand, the skin is thin, supple, and mobile to allow the unrestricted movement of the underlying joints. The mobility and elasticity of dorsal skin permits the rearrangement and manipulation of tissue, such as local flaps. In an average adult hand, the skin cover of the dorsum of the hand (except fingers) measures approximately 12 cm (width) × 10 cm (length),32 but when making a full fist, the total surface area changes by approximately 21% 44. As this results in a maximal stretch of dorsal skin, it should be considered when designing and executing local flaps.
In contrast to dorsal skin, the palmar skin is thick, durable, glabrous (hairless), and relatively inelastic skin, designed to withstand the shearing forces encountered during daily activities of the hand. The epidermis of palmar skin, similar to plantar skin, consists of well-defined layers of stratum lucidum and stratum corneum45 and a thick layer of dermis rich in exocrine sweat glands, blood vessels, and sensory nerve endings. Furthermore, the mobility of the skin along with the pattern of blood supply and sensation across the palm and digits are not evenly distributed. On the radial side, the skin over the thenar eminence is mobile and relatively well vascularized. However, on the ulnar side, the skin over the hypothenar eminence and the metacarpal arch is less mobile. The central triangular area of the palm (located between the thenar and hypothenar eminences and the projection of the metacarpal arch) is the least mobile and least vascular.32
There are numerous sensory nerve endings and rapidly adapting mechanoreceptors exclusively found in the glabrous skin, namely the Meissner and Pacinian corpusles, that are responsible for tactile gnosis and two-point discrimination.46 Using immuno-histochemistry techniques on biopsy samples obtained from palmar skin of volunteers with no neuro-sensory deficits, Kelly and colleagues quantified a significantly higher density of Meissner corpuscles at the fingertips (pulp) compared to the palm47. This possibly accounts for the higher critical sensibility and representation of the fingertips at the cerebral cortex as opposed to the palm and dorsum of the hand where sensory nerve endings responsible for protective sensibility (pain and temperature) are more abundant.48
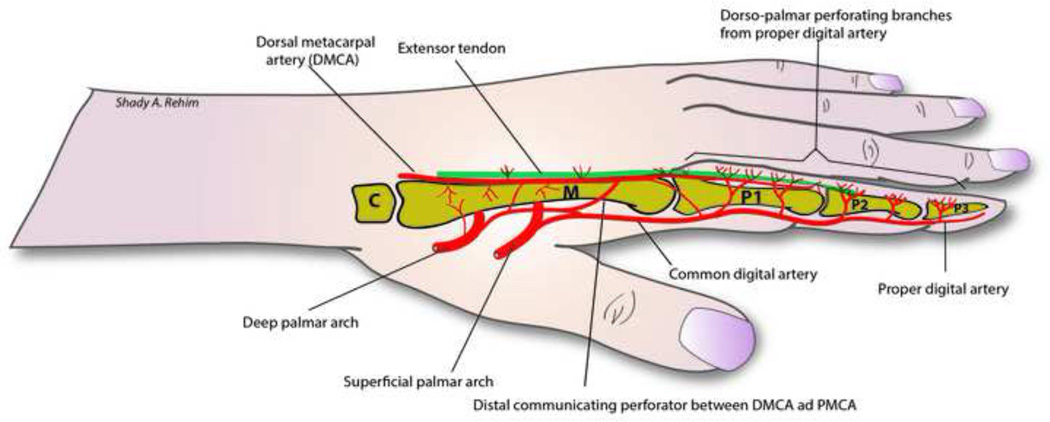
The blood supply of the hand and integument comes from two main arteries, the radial (RA) and ulnar arteries (UA) and contributions from anterior and posterior interosseus arteries. The anastomosis between these four arteries forms 4 arches; the superficial palmar arch (SPA), and deep palmar arch (DPA) as well as the dorsal and palmar carpal arches. The palmar aspect of the hand is predominantly supplied by the SPA and its major contributor the UA, whereas the dorsum of the hand is supplied by the RA through the dorsal metacarpal arteries. Communications exists between the palmar and dorsal systems. On the palmar side, the SPA gives off common digital arteries that subsequently divide into two proper digital arteries at the level of the webspace and a branch that runs dorsally to communicate with the dorsal circulation. The DPA gives I-IV palmar metacarpal arteries (PMCA) that run along the volar intermetacarpal spaces. On the dorsal surface, the dorsal carpal arch gives off II-V dorsal metacarpal arteries (DMCA) that run on the facial pockets of dorsal interosseus muscles. Just distal to the junctura tendinae, the DMCA gives its first and only major cutaneous perforator (0.5–1cm proximal to MCPJ) and receives a perforator branch from the palmar metacarpal artery. At the level of MCP Joint, the DMCA bifurcate into two dorsal arteries to supply the dorsum of the fingers (up to the PIP joint) and adjoin a confluence between common digital artery and proper digital arteries, thus forming a junction between these four vessels. Distal to this point, anastomosis between four dorso-palmar arteries and their venae commitantes exist along the three phalanges of the fingers (Fig. 2). 15, 32,49–52 This complex pattern of blood supply of the hand forms the basis of several local flaps on the dorsum of the hand such as the DMCA perforator flap and its modifications (see below).
Figure 2.
Illustrating vascular communications between palmar and dorsal circulation of the hand and digits.
Clinical applications and discussion
To provide the most suitable soft-tissue cover for a given defect, it is imperative for the surgeon to have a thorough understanding of tissue requirements and appreciation of the three-dimensional construct of the different regions of the hand. The concept of the functional aesthetic units and subunits of the hand incorporates what the mind perceives with what is actually present (or in most cases with what is missing), thus provides the surgeon with a roadmap for selecting the most appropriate type of reconstruction that is both functional and desirable.
Soft-tissue coverage of the hand can be optimized by matching the color, texture, soft-tissue volume, symmetry and donor-recipient tissue interface, as well as by hiding and camouflaging resultant scars within the borders of the functional aesthetic units and subunits of the hand (Table 3). The surgeon should also address individual patient variations, by providing a soft-tissue cover that resembles patient’s contralateral ‘normal’ hand. Examples of innovative approaches and refinements of reconstructive methods, with a special emphasis on enhancing aesthetics, can be distilled from the literature in respect to the various functional aesthetic units and subunits of the hand.
Table 3.
Summary of important aesthetic parameters in soft-tissue reconstruction of the hand
|
Fingers
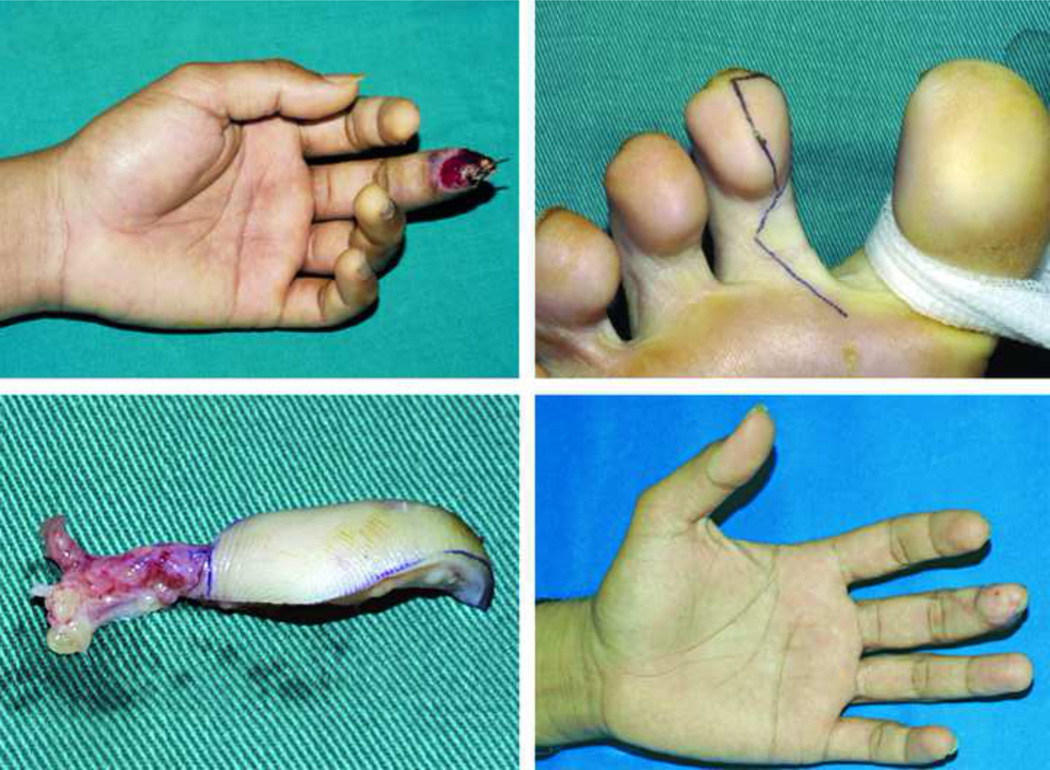
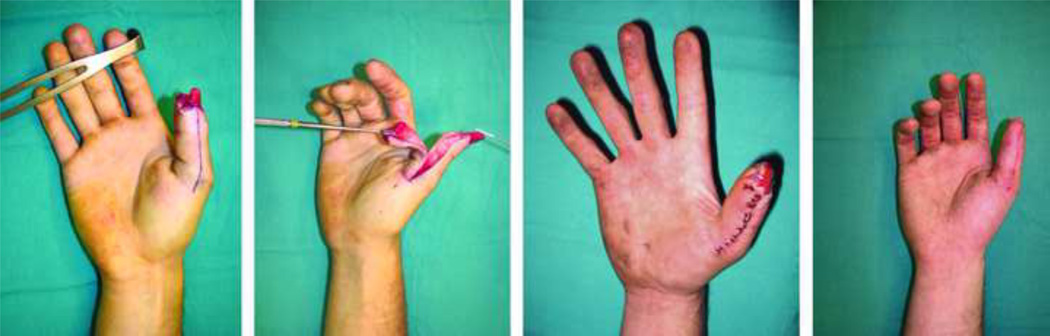
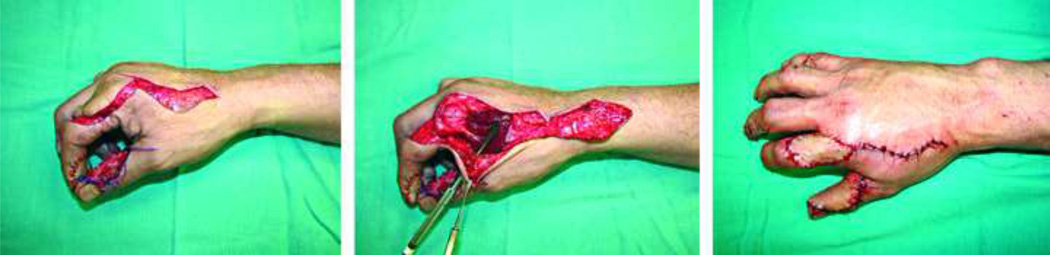
The finger pulp is a highly specialized subunit of the finger essential for prehension and tactile gnosis. Fingertip injuries are common and often result in a loss of this precious part of the digit. Soft-tissue coverage to restore finger pulp volume and sensation becomes essential when distal bone is exposed. Lee et al. published a large series of 929 flaps to reconstruct fingertip injuries using a short-pedicle partial medial second toe pulp free flap (Level of Evidence: Therapeutic, IV).53 An excellent flap survival rate of 99.7% and an average static two-point discrimination (S2PD) in reconstructed digits of 8mm (normal range: 3.0–5.0)13 was reported. The toe-pulp is round and has sufficient tissue to restore the volume of finger pulp, thus provide excellent aesthetic and functional results (Fig. 3). Despite the good results achieved by this technique, it requires highly specialized microsurgical skills, which is not always feasible or available.
Figure 3.
A pulp defect of the long finger (A) reconstructed with an innervated partial medial second toe pulp free flap (B–C), achieving good aesthetic results (D). (With permissions from PRS:- Lee DC, Kim JS, Ki SH, Roh SY, Yang JW, Chung KC. Partial second toe pulp free flap for fingertip reconstruction. Plast Reconstr Surg. 2008 Mar;121(3):899–907)
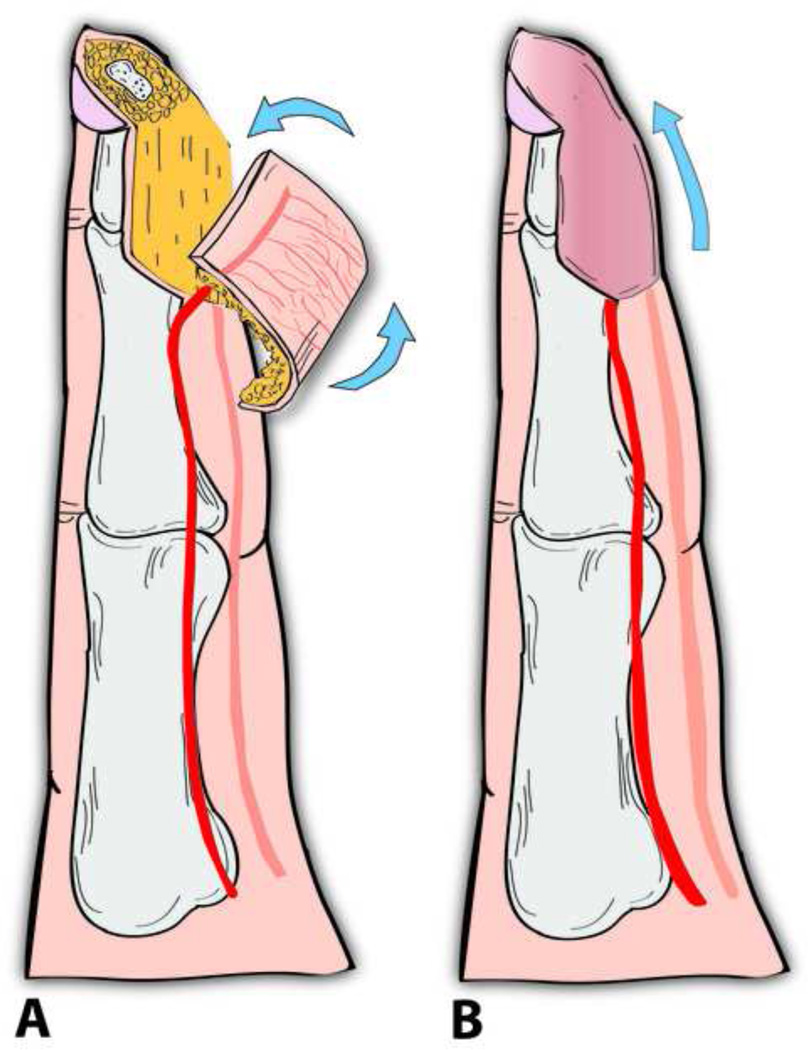
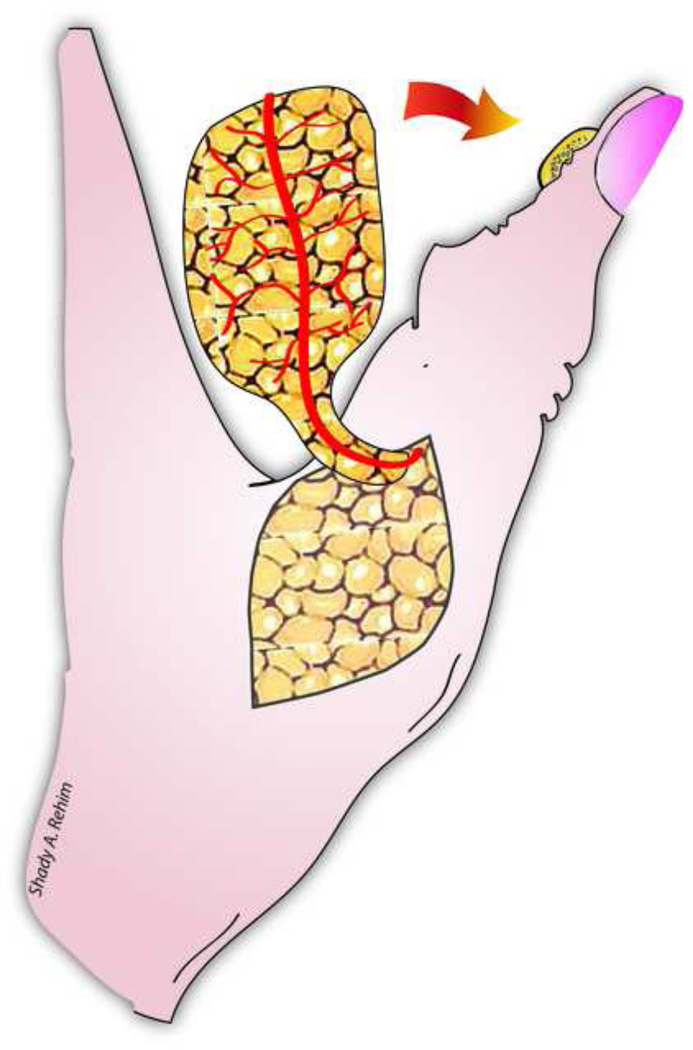
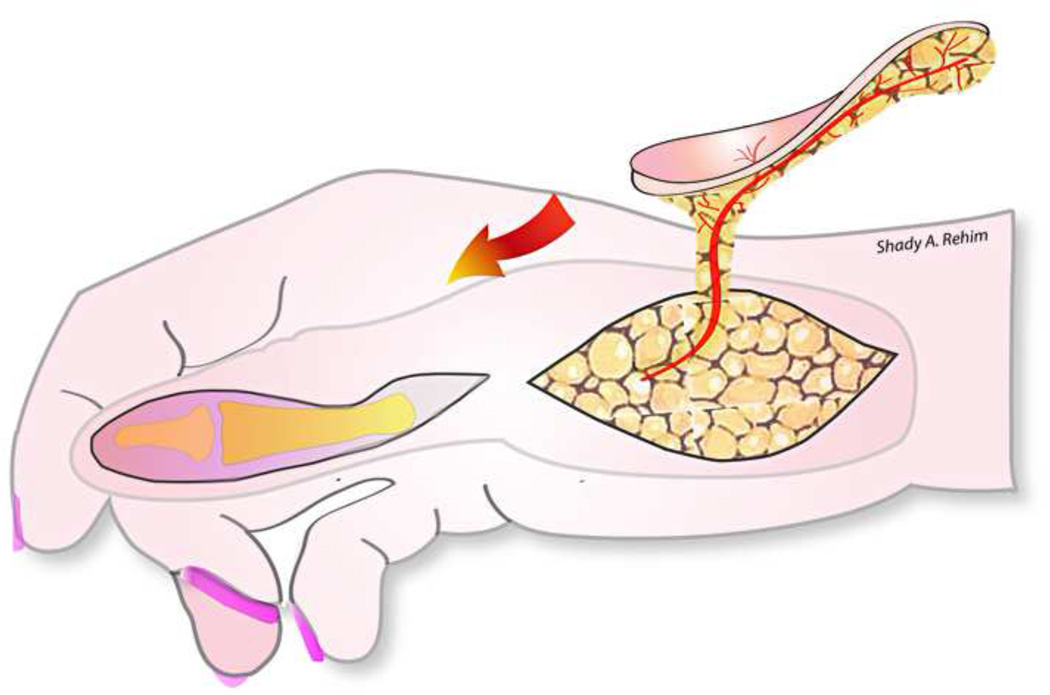
Apart from free flaps, Yam et al and later Feng and colleagues have demonstrated the utility of a local ‘palmar pivot’ flap for reconstructing fingers’ palmar defects and fingertip injuries (Level of Evidence: Therapeutic, IV).54–55 Originally described by Yam et al, the pivot flap is an axial pattern flap based on the transverse subcutaneous branches of the digital artery on the same side as the defect. The flap incorporates a neurovascular bundle on one side and is designed just proximal and adjacent to the defect (Fig. 4ab). The flap is raised over the tendon sheath, then is pivoted on the digital neurovascular bundle and rotated approximately 90 degrees to cover the defect. Any resultant marginal donor-site defects can be grafted by a small full thickness skin graft. In their series, Feng and colleagues reported a mean S2PD of 4.7 mm and good aesthetic results 11 month postoperatively.55
Figure 4.
Illustrating a fingertip amputation injury reconstructed by a pivot flap (A). The flap is pivoted on a single neurovascular pedicle and then rotated 90 degrees to cover the defect.
Another group of versatile flaps that have been widely used to restore soft-tissue finger defects are the distally based DMCA perforator flaps. These pedicled skin flaps, first described by Quaba and Davison in 1990, are designed on the dorsum of the hand and then transposed to cover dorsal finger defects. In their original description, Quaba and Davison based their flap on the main palmar-dorsal perforator which arises from the deep palmar arch and can be easily identified at the level of the metacarpal neck in the dorsal intermetacarpal space.56 Several modifications have since been designed based on the vascular anatomy of the DMCA and the more distal dorso-palmar digital cutaneous perforators in order to increase the span of the flap to reach more distal defects.57–59 Additionally, as any perforator flap, the DMCA flaps have been harvested as a fascicutaneous, fascial, adipofascial and a compound flaps (including bone and tendon).52,60–61
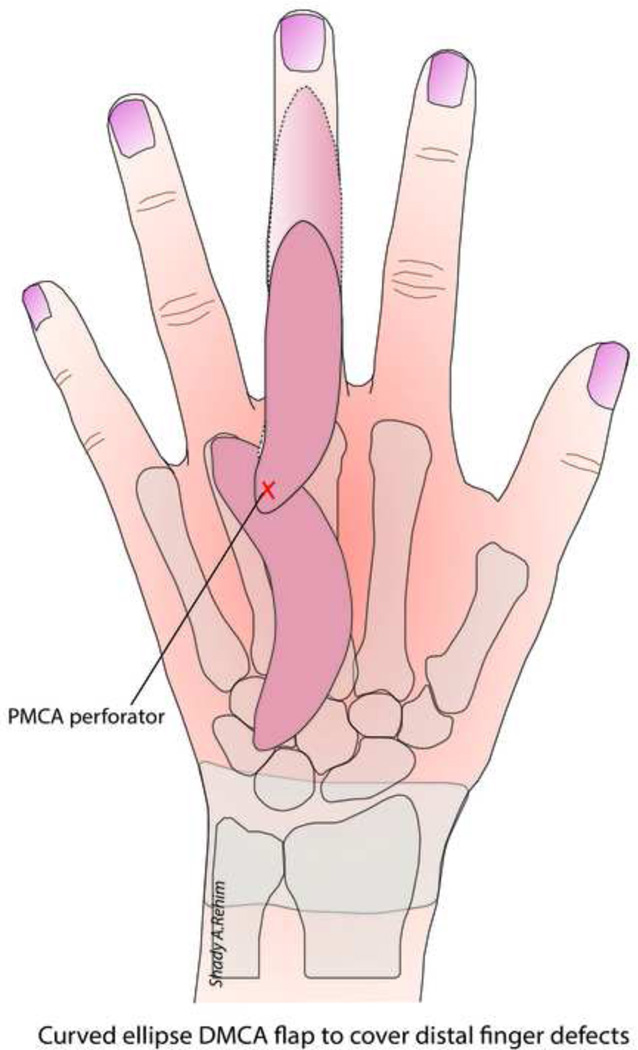
Sebastin et al demonstrated the utility of the DMCA perforator flaps for reconstructing a variety of dorsal, webspace and palmar finger defects.62 The authors preferred using a flap based on the main palmar-dorsal perforator (as described by Quaba & Davison, Fig. 5) because of perforator constant anatomy as compared to the DMCA and larger caliber as opposed to the more distal dorso-palmar finger perforators. To cover more distal defects (i.e. below the nail fold), the authors designed the flap as a curved ellipse rather than a straight ellipse, which adds 8–10 mm of extra length after the flap is straightened owing to the elasticity of dorsal skin (Fig. 6). Another maneuver the authors described involved dividing the DMCA proximal to the main perforator, thus providing an additional 5–8mm in length. DMCA flaps are a good choice for reconstructing dorsal finger defects because they provide excellent color, texture and soft-tissue volume match in a single-staged procedure. However, despite the versatility of these flaps, from the aesthetic standpoint there are two major drawbacks; 1) The resultant linear scar spans several plane-breaks across the dorsal subunits of the hand and fingers, distorting the natural pattern of light reflection which result in a conspicuous scar, 2) When the DMCA flaps are used to reconstruct palmar finger defects, the transposition of thin, hairy, pigmented dorsal skin is not an ideal match to the glabrous, lighter-colored skin of the palmar surface of the fingers.
Figure 5.
A 20-year-old male who sustained a complex laceration injury over the dorsoulnar aspect of the right little finger. A dorsal metacarpal perforator artery flap measuring 3 cm × 2cm was designed to cover dorsal defect. The location of the constant perforator from the volar artery is marked with a ‘cross’ at the intermetacarpal space (top left). The DMCA can be ligated to increase the reach of the flap (top right). Despite an adequate soft-tissue coverage, an obvious resultant linear scar usually traverses the dorsal unit and subunits of the fingers (bottom).
Figure 6.
The skin paddle of the DMCA flap can be designed as a curved ellipse to increase the reach of the flap to cover more distal defects.
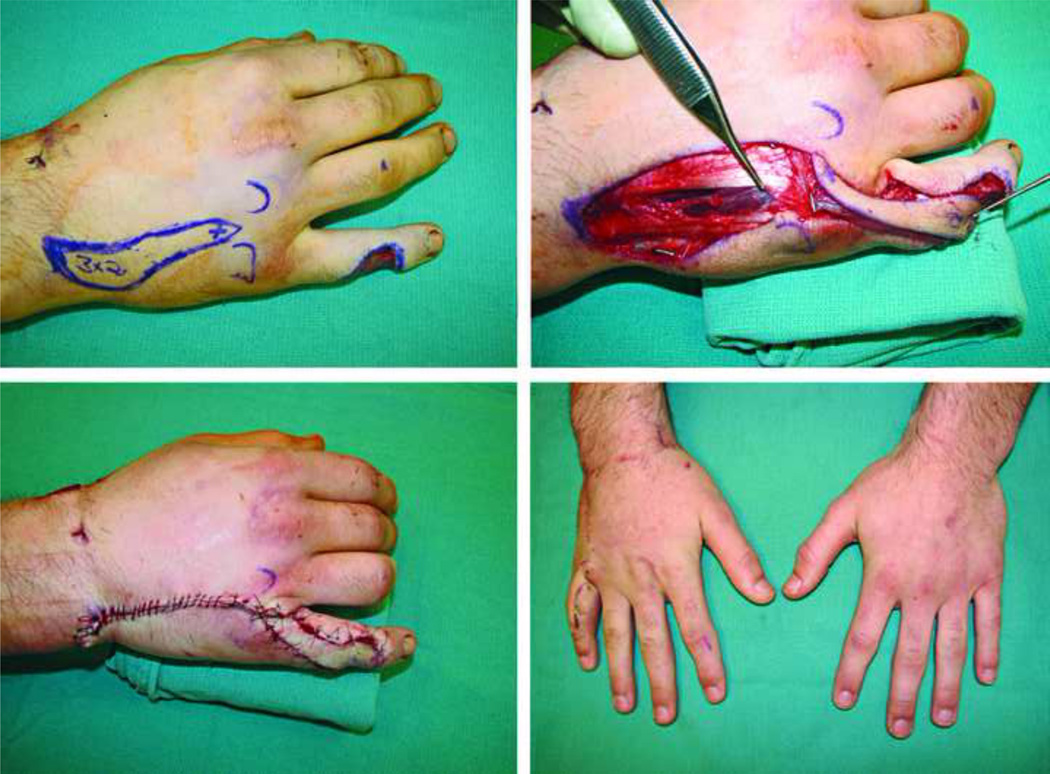
Dorsum of the hand
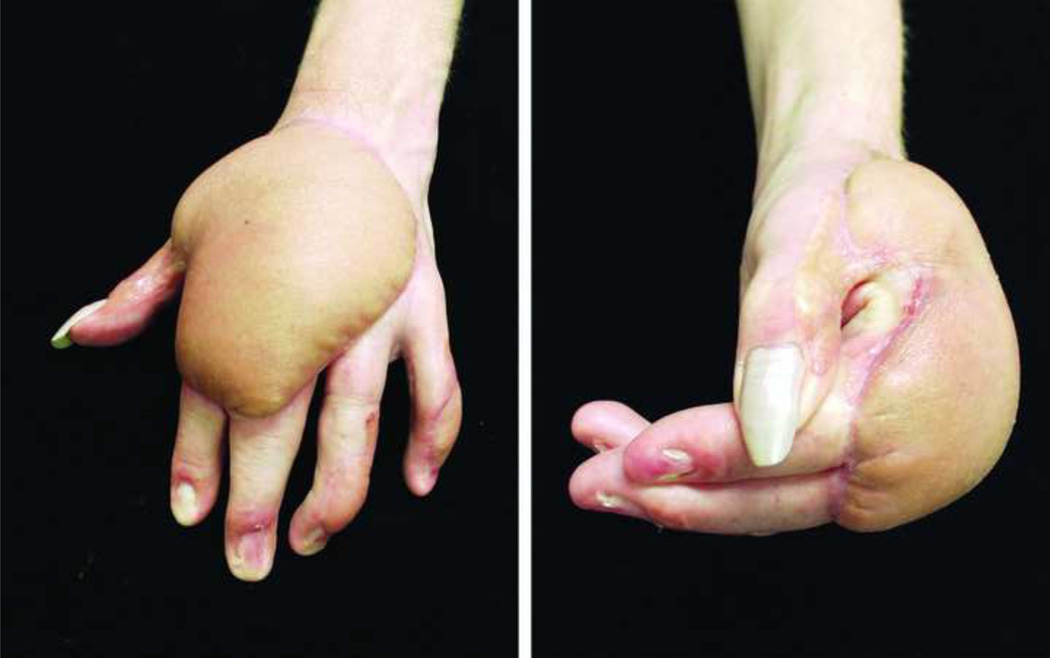
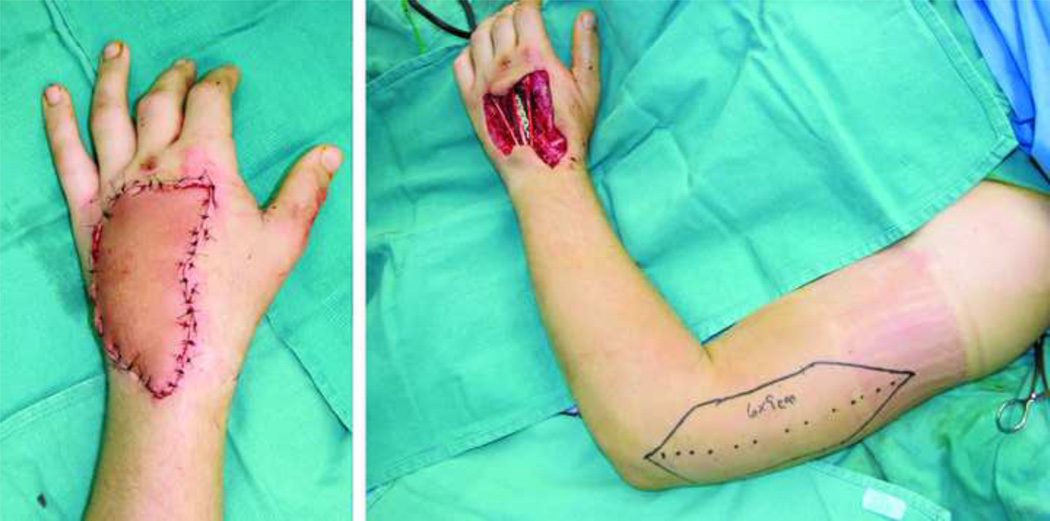
On the dorsal unit of the hand, two problems commonly arise following flap surgery; 1) A bulky flap, that is unsightly and can be functionally limiting. 2) A demarcated donor-recipient tissue interface that may result in a patchwork appearance (Fig. 7). Parrett et al., demonstrated their experience and assessed the aesthetic outcomes in a large series of muscle, fasciocutaneous, fascial and forearm venous free flaps, and their subtypes (e.g. rectus, latissimus, gracilis, anterolateral thigh, radial forearm, lateral arm flaps etc.) that were used to cover dorsal hand defects (Level of Evidence: Therapeutic, III).63 Evaluated measures included; flap survival, donor-site morbidity and the need for debulking procedures. In addition, a panel of plastic surgeons and non-physician aestheticians subjectively analyzed the final appearance of the flaps. In particular, the panel assessed the color, texture and contour match as well as the appearance of meshed versus sheet split thickness skin grafts that were used to cover muscle and fascial flaps. All the defects were matched for size and location. The results of this interesting study showed that venous flaps had the best aesthetic results in terms of color, contour and texture match with no donor-site morbidity and no need for debulking procedures. Fasciocutaneous flaps had the worst results in all of aforementioned mentioned parameters as well as a higher demand for debulking procedures (67% of fasciocutaneous flaps required debulking despite initial thinning before flap inset) and greater donor-site morbidity in terms of skin graft coverage and wound breakdown. Muscle and fascial flaps were considered second best to venous flaps with no significant differences among them in all categories, with the exception of an increased need for debulking of muscle flaps. In their series, partial muscle flaps were found to require significantly less need for debulking compared to traditional muscle free flaps (7% versus 37%, respectively). Despite the need for debulking procedures in some of the muscle flaps, the contour match was comparable to venous and fascial flaps, owing to muscle atrophy that results in a good contour match. Finally, sheet split thickness skin grafts (STSG) used for fascial and muscle flaps were found to have much better aesthetic results than meshed STSG. In spite of this, it should be emphasized that flap aesthetics was not the sole determinant of flap choice and the authors correctly pointed out the indications and selection of different types of flaps were based on size, type, and location of the defect, among other parameters. Figure 8–Figure 11, demonstrates several examples of soft-tissue coverage of dorsal skin defects. (Video 1) (INSERT VIDEO GRAPHIC 1. See supplemental digital content 1, which displays the lateral arm flap for reconstruction of a dorsal soft-tissue defect of the hand. This video is available in the “Related Videos” section of the Full-Text article on PRSJournal.com or available at INSERT LINK HERE.)
Figure 7.
Volume discrepancy between donor and recipient tissue (usually obvious on dorsum of the hand) results in a well demarcated bulky soft-tissue reconstruction that is undesirable and can be functionally limiting.
Figure 8.
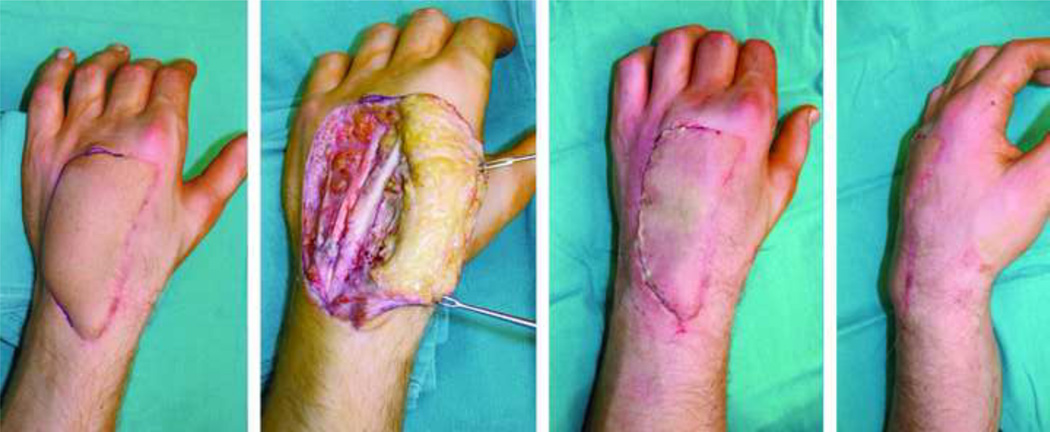
A 22-year-old male who suffered an accidental gunshot wound on dorsum of the hand that was covered with a lateral arm flap.
Figure 11.
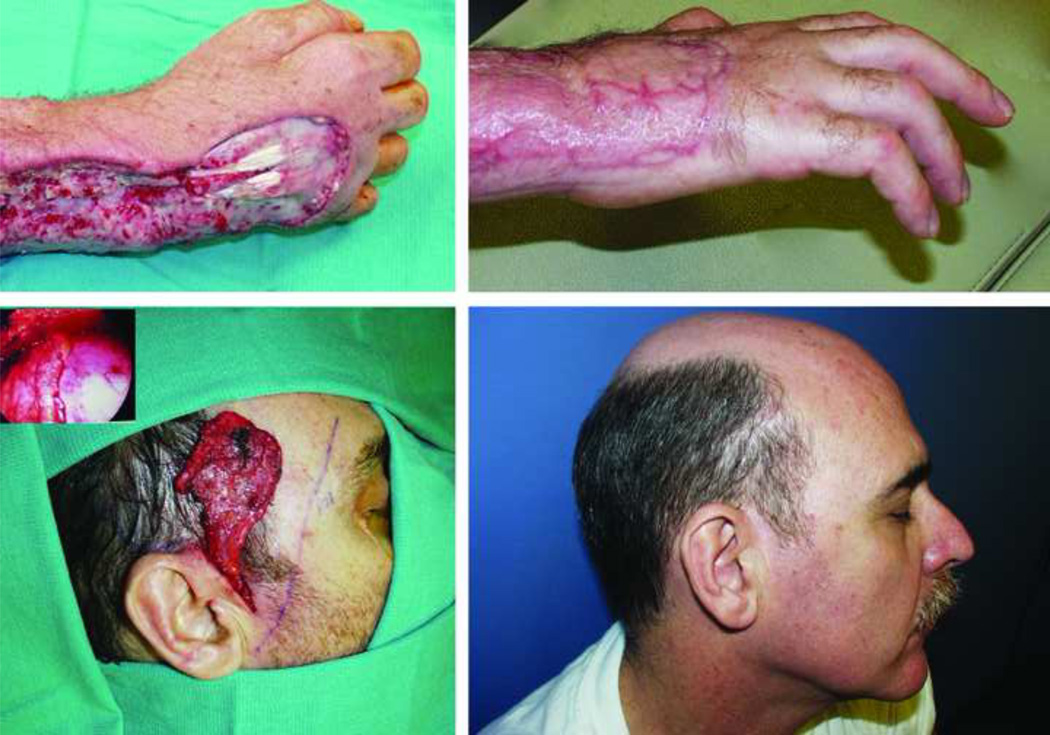
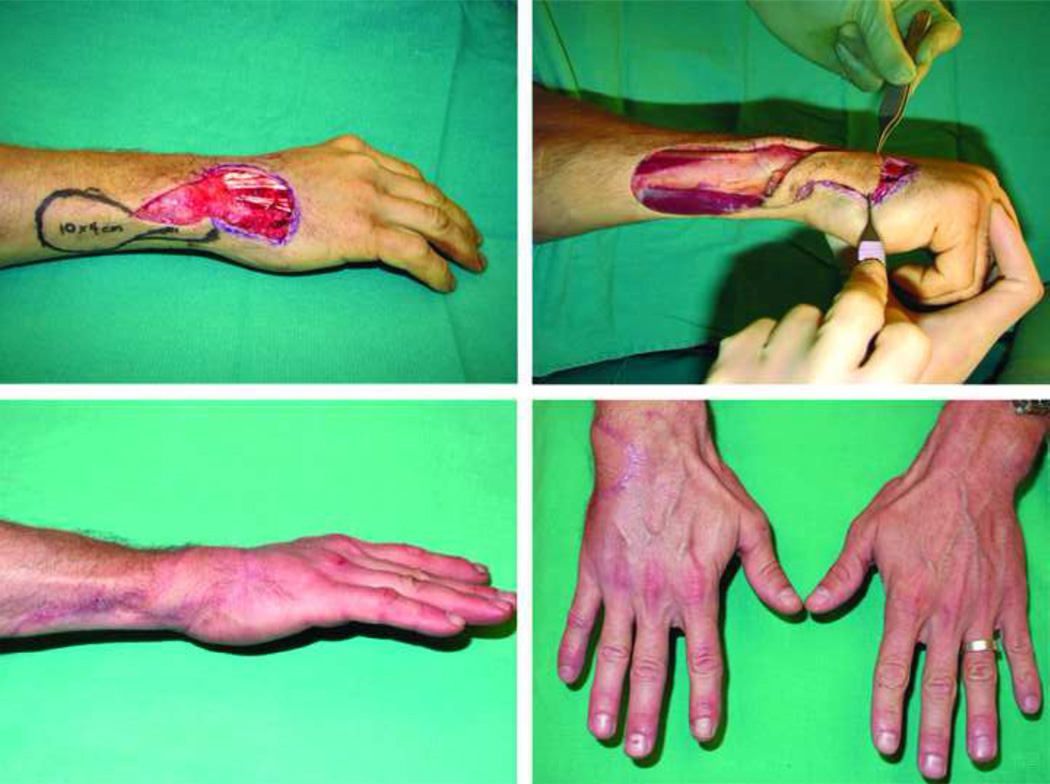
A 52-year-old male who sustained an extensive degloving injury of the dorsum of his right hand leaving exposed tendons (top left). A right endoscopic assisted temporoparietal fascia free flap (bottom left) was harvested to cover the defect. Note the endoscope picture delineating the distal extent of the flap. A smooth contour (top right) between the fascial flap and dorsal skin was achieved as well as an inconspicuous donor-site, hidden within patient’s hairline (bottom right).
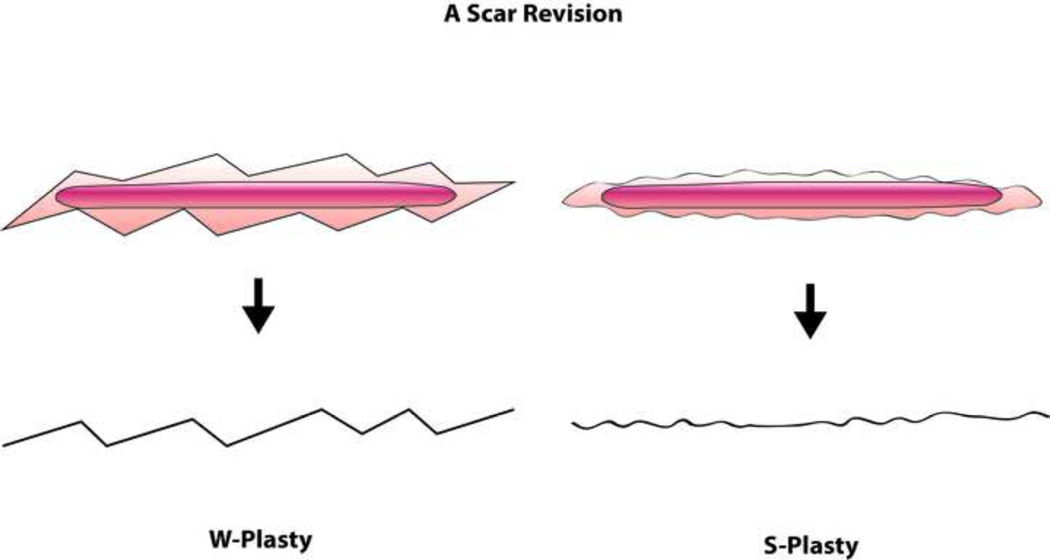
Occasionally a depressed scar develops after a flap inset at the junction between the flap and surrounding skin. Volume discrepancy and cluttering lights across the flap-skin interface may result in a more conspicuous scar line. Akin to W-plasty, S-plasty is one way of revising scars by making multiple curvilinear small S-shaped incisions that break tension lines (Fig. 12), creating a less depressed tension-free scar at the junction lines.64–65
Figure 12.
Both W-plasty and S-plasty result in a minimal gain of length and a decreased tension on the scar-line. However the final scar appearance following scar revision by S-plasty results in a less noticeable scar than the right-angled zig-zag lines resulting from W-plasty.
Palm of the hand
The special tissue requirements of the palmar skin present the surgeon with several challenges. The availability is limited of a suitable donor-tissue that is a glabrous, sensate (for protective sensibility), durable (to resist ulceration) and relatively immobile skin (for mechanical stability) yet does not restrict grip and mobility.66 The relative inelasticity of palmar skin, in addition to its vascular supply via short vertical cutaneous perforators, almost precludes the use of local flaps.67 Additionally, restoration of palmar defects with skin grafts may result in color mismatch and development of skin contractures.68 Although muscle and fascial flaps covered with skin grafts may be suitable alternatives, muscle flaps are generally insensate and facial flaps may ulcerate due to shear stress. In an attempt of finding the ideal match for restoring palmar defects, Engelhardt and colleagues69 devised an algorithm based on careful ‘defect-analysis’ of the functional units and subunits of the palm as described earlier. For areas where sensation is regarded essential such as the thenar and hypothenar subunits, the authors recommended the use of free and pedicled sensate faciocutaneous flaps such as radial forearm flap, dorsal pedis artery flap, lateral arm flap or medial plantar artery flap (instep flap).70 However aesthetic disadvantages of fasciocutaneous flaps in the palm are well-recognized and include bulkiness and hair growth. For the central subunit of the hand the authors suggested thin fascial (e.g. temprofascial) or muscle flap (e.g. serratus or gracilis) combined with intermediate thickness plantar skin graft, noting that plantar skin grafts (similar to instep flap) provide good aesthetic results in terms of scarring, color and texture match.
Thumb
The thumb represents 40–50% of hand function71–72,, thus restoring thumb (especially pulp) defects is essential for pulp-to-pulp and lateral pinch grip. A wide variety of flaps are available for thumb reconstruction, but four flaps are particularly interesting from the functional and aesthetic viewpoints; 1.) Advancement neurovascular flap (Moberg 1964)73 2.) First dorsal metacarpal artery flap (Foucher 1979)74–75, 3.) Dorsoulnar collateral artery flap (Brunelli 1993)76–78 4.) Dorsoradial collateral artery flap (Moschella 1996) (Table 2). 79–80 Aesthetically, the Moberg flap is an excellent choice for small volar and pulp defects because it follows the principle of replacing ‘like-with-like’ and provide excellent sensibility, leaving only inconspicuous scars along the non-tension midlateral axis of the thumb. Foucher’s kite flap is probably the most commonly utilized flap for palmar and dorsal thumb defects. However aesthetic disadvantages include conspicuous skin grafted donor-site and hair growth on volar surface of the thumb. The Brunelli and Moschella flaps can therefore be considered as good alternatives to the Foucher flap, as hair growth on the dorsoradial and dorsoulnar sides of the thumb is rare and the skin closely resembles the palmar glabrous skin of the thumb. Moreover the donor-site of these flaps can be closed primarily leaving inconspicuous scar-lines.83
Table 2.
Comparison between four types of skin island flaps for coverage of thumb soft-tissue defects.
| Type of Flap | Indication* | Donor Area | Vascular Supply |
Cutaneous Innervation |
Reported Static two- point discrimination (mm) |
Cortical Re-orintation‡ |
|---|---|---|---|---|---|---|
|
Moberg flap Fig. 13 |
Soft-tissue coverage of thumb volar/pulp defects (medium-size defects approximately 1.5cm) |
Volar surface of the thumb. No donor-site defect created unless the flap is designed as an island flap, the resultant donor site over base of thumb can then be covered with full thickness skin graft |
Palmar collateral arteries |
Palmar digital nerves |
5–4 mm | 100% immediate post-operative81 |
|
Foucher flap (Kite flap). Fig. 14 |
Soft-tissue coverage of variable sized defects such as distal amputation, loss of palmar surface (including pulp), or loss of dorsal substance and at a more proximal level to reconstruct loss of dorsal surface. |
Skin island from the dorsal surface of the adjacent index finger. The donor site is usually covered by a full thickness skin graft |
First dorsal metacarpal artery |
A branch of superficial radial nerve |
10.8 mm75,82 | 100% 1 year post-operative 82 |
|
Brunelli flap (Dorsoulnar thumb flap). Fig. 15 |
Medium-size defects such as distal amputation, loss of palmar surface (including pulp), or loss of dorsal substance and at a more proximal level to reconstruct loss of dorsal surface. |
Skin island over the dorsoulnar aspect of the thumb. The donor defect can be closed primarily. |
Ulnar dorsocollateral artery of the thumb |
Dorsocollateral branch of the radial nerve |
10 mm 77 | _ |
|
Moschella flap (Dorsoradial thumb flap). Fig. 16 |
Medium-size defects (up to 5cm × 4cm) such as distal amputation, loss of palmar surface, or loss of dorsal substance and at a more proximal level to reconstruct loss of dorsal surface. |
Skin island over the dorsoradial aspect of the thumb. The donor site can be closed primarily or covered by a full thickness skin graft. |
Radial dorsocollateral artery of the thumb. |
Dorsocollateral branch of the radial nerve |
9.7 mm 80 | _ |
All flaps are indicated for soft-tissue coverage of the thumb when there is exposed bone, tendon or nerves.
Cortical re-orientation: is the ability of a patients’ brain to recognize a sensory stimulus from the flap area to be originating from the recipient-site (e.g. thumb) and not from the donor finger.
(-) Not reported.
Conclusion
The current substantial gaps in quantifying, collecting and reporting aesthetic outcomes hinder meaningful comparisons and the ability of surgeons to provide a more standardized approach for some of commonly performed reconstructive procedures of the hand. Hence, we feel that there is a need to implement guiding principles on the practice of aesthetic surgery. The development of validated outcome measures that are based on rigorous study methodologies may achieve this goal and facilitate an evidence-based approach that ensures high quality of care and minimize disparity amongst plastic surgeons in the future. However till the development of a universally accepted aesthetic assessment tool in hand surgery, adherence to basic principles of function and aesthetics that are catered to individual patient’s needs and preferences is the most likely to improve patient’s outcomes and satisfaction with surgery.
Supplementary Material
Figure 9.
The same patient in figure 8, note volume discrepancy between the flap and dorsum of the hand (flap bulkiness), can be substantially reduced by flap debulking procedure.
Figure 10.
A 39-year-old male sustained a crush injury resulting in an extensive soft-tissue loss and exposed tendons. A dorsal forearm ulnar artery perforator flap was raised to cover the defect. Optimal aesthetic and functional results were achieved due to similarity between the skin on dorsal unit of the hand and the dorsal skin of the forearm.
Figure 13. Moberg flap.
Soft-tissue coverage of thumb volar/pulp defects (medium-size defects approximately 1.5cm)
Figure 14. Kite flap.
Soft-tissue coverage of variable sized defects such as distal amputation, loss of palmar surface (including pulp), or loss of dorsal substance and at a more proximal level to reconstruct loss of dorsal surface
Figure 15. Dorsoulnar thumb flap.
Medium-size defects such as distal amputation, loss of palmar surface (including pulp), or loss of dorsal substance and at a more proximal level to reconstruct loss of dorsal surface.
Figure 16. Dorsoradial thumb flap.
Medium-size defects (up to 5cm × 4cm) such as distal amputation, loss of palmar surface, or loss of dorsal substance and at a more proximal level to reconstruct loss of dorsal surface.
Learning objectives.
After reading this article and viewing the video, the participant should be able to:
Have an in-depth understanding of the functional and aesthetic requirements of different regions of the hand
Describe the anatomic blood supply and vascular territories of the hand
Describe the advantages and disadvantages of different types of soft-tissue reconstructions for different anatomic regions of the hand e.g. digits, thumb, palm and dorsum of the hand.
Summary.
Soft-tissue coverage of the hand has evolved dramatically over the past few decades. To achieve the best aesthetic and functional results, the aesthetic hand surgeon should be aware of various flap designs and recognize limitations of each type of flap reconstruction. Finally develop a systematic evaluation of the defect size, site and composition with respect to aesthetic units and subunits of the hand to guide flap choice.
Acknowledgments
Research reported in this publication was supported in part by the National Institute of Arthritis and Musculoskeletal and Skin Diseases and National Institute on Aging (R01 AR062066), the National Institute of Arthritis and Musculoskeletal and Skin Diseases (2R01 AR047328-06), and a Midcareer Investigator Award in Patient-Oriented Research (K24 AR053120) The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Multiple Choice Questions
1. Compared to the palmar skin, the pulp of the fingers contains a higher density of which of the following sensory receptor?
Merkel discs
Ruffini corpusles
Meissner corpuscles
Golgi-Mazzoni corpusles
Correct answer: (C) Meissner corpuscles
Discussion: Meissner corpuscles are encapsulated ovoid receptors that are exclusively present within the dermal papillae of glabrous skin such as palmar surface of the hand and sole of the foot. Meissner corpuscles are rapidly adapting mechanoreceptors that respond to vibration and pressure, therefore are responsible for fingers’ tactile gnosis and two-point discrimination. Moberg indicated that a minimum of 6 mm two-discrimination is required for an optimal finger prehension. “Fu Chan Wei showed statistically significant correlation between moving two-point discrimination and the number of regenerated Meissner corpuscles in the transplanted toe wrap-around flaps in children undergoing a debulking procedure of the flap at secondary surgery”. Although the functional role of Meissner is well established, more recently studies have assessed the quantity of Meissner corpuscles across the palm and fingertips and found a significantly higher number of Meissner corpuscles at the pulp of fingers. These findings emphasize the special function of the finger pulp that should be carefully considered during any reconstructive procedure.
References:
1. Kelly EJ, Terenghi G, Hazari A, Wiberg M. Nerve fibre and sensory end organ density in the epidermis and papillary dermis of the human hand. Br J Plast Surg. 2005 Sep;58(6):774-9.
2. Wei FC, Carver N, Lee YH, Chuang DC, Cheng SL. Sensory recovery and Meissner corpuscle number after toe-to-hand transplantation. Plast Reconstr Surg 2000;105:2405–11.
2. Which of the following coverage options provides an optimal aesthetic and functional results when used to resurface a soft-tissue defect of the hand?
Skin graft
Local flap
Regional pedicled flap
Distant free flap
Correct answer: (B)
Discussion: When the surrounding tissue is healthy and the defect can be closed without distortion of the natural anatomical landmarks of the hand, local flaps usually offer the best aesthetic and functional results given the histological similarity between the flap and surrounding skin. The use of skin grafts and pedicled regional flaps have generally fallen out of favor as a primary method of soft-tissue reconstruction, however, the usefulness of these methods for reconstruction cannot be underestimated and remain viable options in many circumstances especially when microsurgery is not available or contraindicated. Free flaps can virtually be harvested from any part of the body and tailored to match the function and appearance of the recipient site. The surgeon should weight the ‘pros’ and ‘cons’ of each type of reconstruction depending on several variables including; wound characteristics (e.g. site, size, location and type of tissue loss) and patient characteristics (e.g. preferences, body habitus, compliance, medical and surgical co-morbidities and contraindications etc.) in order to provide the most suitable soft-tissue cover. Careful planning is the key for a successful soft-tissue reconstruction of the hand.
References:
1. The reconstruction of the mutilated hand. Neumeister M, Hegge T, Amalfi A, Sauerbier M. Semin Plast Surg. 2010 Feb;24(1):77-102.
2. Saint-Cyr M, Gupta A. Indications and selection of free flaps for soft tissue coverage of the upper extremity. Hand Clin. 2007 Feb;23(1):37–48.
3. Giessler GA, Erdmann D, Germann G. Soft tissue coverage in devastating hand injuries. Hand Clin. 2003 Feb;19(1):63–71, vi.
3. Which of the following best describes the blood supply of the skin paddle of the dorsal metacarpal artery flap also known as the Quaba flap?
A dorsal perforator artery arising from the proper digital artery.
A dorsal perforator artery arising from the common digital artery.
A dorsal perforator artery originating from the superficial palmar arch.
A dorsal perforator artery arising from the deep palmar arch.
Correct answer: (D)
Discussion: The DMCA flaps can be generally classified according to their feeding blood vessel into proximal and distally based DMCA flaps. The proximally based DMCA flap was first described by Early and Milner (1987) and is based on the first and second dorsal metacarpal artery as it dives deep to the extensor tendon. This flap was utilized to cover soft-tissue defects on the dorsum of the proximal phalanx of the fingers. However, in 1990 the distally based metacarpal artery flaps were described by Quaba and Davidson as well as by Maryuama to can cover more distal finger and webspace defects. Quaba and Davidson based their flap on a constant perforator arising from the deep palmar arch of the hand that forms an anastomosis between the dorsal metacarpal artery and the palmar arterial system, whereas the DMCA flap designed by Maryuama included the less constant dorsal metacarpal artery itself and not the perforator arising from the palmar vessels. The dorsal metacarpal arteries do not have constant anatomy; the second DMCA is present in 97% of the cases, the third in 93% of the cases and the fifth DMCA is present in 70-83%. Because of the variable anatomy of the dorsal metacarpal arteries, refinements and modifications of DMCA flap described thereafter were principally based on the direct cutaneous perforators from dorsal metacarpal artery and palmar circulation along the hand and digits (Fig. 2) such as the reverse dorsal digital and metacarpal flaps described by Pelissier in 1999.
References:
1. Quaba AA, Davison PM. The distally-based dorsal hand flap. Br J Plast Surg. 1990 Jan;43(1):28–39.
2. Maruyama Y. The reverse dorsal metacarpal flap. Br J Plast Surg 1990;43:24—7.
3. Pelissier P, Casoli V, Bakhach J, Martin D, Baudet J. Reverse dorsal digital and metacarpal flaps: a review of 27 cases. Plast Reconstr Surg. 1999 Jan;103(1):159-65.
4. A 40-year-old man who is a professional pianist sustained a laceration resulting in a soft-tissue loss of the tip of the long finger of his dominant right hand. The wound was left to heal by secondary intention. One year later, the patient reports that the loss of soft tissue on the fingertip prevents her from working. Physical examination shows thin adherence to the underlying bone. The full length of bone is preserved, and active and passive motion is within normal limits. Sensation is decreased in the area of scarring. Which of the following types of reconstruction is most likely to provide the best improvement in soft-tissue bulk and sensation?
Groin flap
Cross finger flap.
V-Y advancement flap
Toe pulp flap
Thenar flap
Correct answer: (D)
Discussion: Many techniques have been described for reconstruction of fingertip injuries, including simple revision amputation, skin grafting, local flaps, island flaps, distant flaps, and free flaps. When bone is not exposed healing by secondary intention may yield surprisingly good results. However when there is significant soft tissue loss there might be insufficient padding for the fingertips. The toe pulp free flap replaces the glabrous tissue over the fingertip with a similar tissue and restores both soft tissue bulk and sensibility. Partial second toe pulp free flap, for example, is a single staged procedure that has shown to achieve these goals while resulting in minimal donor-site morbidity. The partial second toe flap is nourished by the medial digital artery and one or two subcutaneous veins. The flap is innervated by the palmar digital nerves of the toe. An average of static two-point discrimination of 8mm (range 4–15mm) can be obtained by using this technique. On the other hand, thenar and groin flaps are two staged procedures, although they provide soft tissue cover they do not provide sensation. Other disadvantages may include PIP joint stiffness that is associated with thenar flap especially in middle-aged men. A sensory cross finger flap may provide soft tissue bulk and sensation however the results are not superior to toe pulp flap, moreover transfer of hair follicles from dorsal skin to the pulp of the finger is not desirable. The V-Y advancement flap can be a good option for small volar defects, however with significant loss of pulp, the choice of V-Y advancement flap has limited mobility to cover the whole defect and is usually considered suboptimal compared to other techniques.
References:
1. Lee DC, Kim JS, Ki SH, Roh SY, Yang JW, Chung KC. Partial second toe pulp free flap for fingertip reconstruction. Plast Reconstr Surg. 2008 Mar;121(3):899–907.
2. Lin CH, Lin YT, Sassu P, Lin CH, Wei FC. Functional assessment of the reconstructed fingertips after free toe pulp transfer. Plast Reconstr Surg. 2007 Oct;120(5):1315-21.
5. A 36-year-old woman sustained a circular saw injury to the volar surface of the tip of her left thumb. On physical examination there was a soft tissue defect measuring approximately 1.5 cm with exposed bone. Thumb vascularity as well as active and passive range of motion is within normal range. Which of the following is the most appropriate type of soft tissue cover for this defect?
Moberg flap.
Healing by secondary intention.
Cross finger flap.
Skin graft.
Correct answer: (A)
Discussion: The Moberg flap is an excellent choice for coverage of small soft tissue defects measuring approximately 1.5 cm. Advancement of volar glabrous skin of the thumb provides good soft tissue match and sensibility as opposed to other above-mentioned donor-sites options. Healing by secondary intention is slow and inappropriate in this case especially when there is an exposed bone. Skin graft is also inappropriate because skin grafts require a graftable bed (i.e. intact peritenon and/or intact periosteoum) for the skin graft to take. A cross finger flap can be used in this case but both outcomes at the recipient and donor-sites are inferior to Moberg flap.
References:
1. Baumeister S, Menke H, Wittemann M, Germann G. Functional outcome after the Moberg advancement flap in the thumb. J Hand Surg Am. 2002 Jan;27(1):105-14.
6. Which of the following blood vessels supply the lateral arm flap?
Axillary artery.
Posterior interosseous artery.
Posterior branch of the radial collateral artery.
Profunda brachii.
Correct answer: (C)
Discussion: The anterior and posterior radial collateral intermuscular perforators arise from the profunda brachii artery in the middle of the upper arm midway between the acromion and the lateral epicondyle. The posterior radial collateral artery is the nourishing artery for the lateral arm flap. The posterior radial collateral artery courses through the radial groove of the humerus and gives rise to four to five septocutaneous perforators. The most constant branch can be found about 9 cm above the lateral epicondyle. The skin paddle of the lateral arm flap can be extended to proximal forearm because of the rich vascular communications between vessels of the arm and upper forearm.
References:
1. Sauerbier M, Giessler GA. The free lateral arm flap for hand and wrist coverage. In: Cooney WP, Moran SL, editors. Master techniques in orthopedic surgery: soft tissue. Philadelphia: Lippincott Williams & Wilkins; 2008. p. 179–89.
2. Sauerbier M, Unglaub F. Perforator flaps in the upper extremity. Clin Plast Surg. 2010 Oct;37(4):667-76, vii.
7. A 31-year-old man presented to the emergency room after sustaining an injury to the dorsal surface of his non-dominant thumb. Physical examination shows 1.5 cm×1 cm soft tissue loss over the dorsum of the proximal phalanx with an exposed but intact EPL tendon. Which of the following is the most appropriate management?
Healing by secondary intention.
First dorsal metacarpal artery flap (kite flap).
Split thickness skin graft.
Full thickness skin graft.
Correct answer: (B)
Discussion: The kite flap is one of the most commonly utilized local flaps in the hand. Kite flap is suitable for all dorsal thumb defects and can also be used to restore sensation over the pulp by incorporating the sensory branch of the radial nerve. The flap is based on the first dorsal metacarpal artery (FDMA), which arises from the radial artery in the first intermetacarpal space, just distal to the tendon of the extensor pollicis longus. The artery divides into the radial branch to the thumb, the intermediate branch to the first web space, and the ulnar branch to the index finger. The flap is harvested from the dorsal aspect of the index finger incorporating the skin and fascia over the first interosseus muscle which contains the vascular pedicle. It is important to preserve the paratenon over underlying tendon in order to close donor-site defect with a skin graft. The flap is then rotated and secured over the defect on the dorsum of the thumb. In this case the significant loss of skin and subcutaneous tissue precludes healing by secondary intention. Additionally, the presence of an exposed tendon without paratenon makes coverage with a skin graft a non-viable option for this type of wound reconstruction.
References:
1. Foucher G, Braun JB. A new island flap transfer from the dorsum of the index to the thumb. Plast Reconstr Surg. 1979;63:344–349.
2. Germann G, Biedermann N, Levin SL. Intrinsic flaps in the hand. Clin Plast Surg. 2011 Oct;38(4):729-38.
3. Muyldermans T, Hierner R. First dorsal metacarpal artery flap for thumb reconstruction: a retrospective clinical study. Strategies Trauma Limb Reconstr. 2009 Apr;4(1):27–33.
Footnotes
Financial disclosure:
None of the authors has a financial interest in any of the products, devices, or drugs mentioned in this manuscript.
References
- 1.McCash RC. Cosmetic aspects of hand surgery. The Hand. 1969 Mar;1(1):67–71. [Google Scholar]
- 2.Manske PR. Aesthetic hand surgery. J Hand Surg Am. 2002;27:383–384. doi: 10.1053/jhsu.2002.33938. [DOI] [PubMed] [Google Scholar]
- 3.Jakubietz RG, Jakubietz MG, Kloss D, Gruenert JG. Defining the Basic Aesthetics of the Hand. Aesthetic Plast Surg. 2005 Nov-Dec;29(6):546–551. doi: 10.1007/s00266-005-0100-6. [DOI] [PubMed] [Google Scholar]
- 4.Rumsey N, Harcourt D. Body image and disfigurement: issues and interventions. Body Image. 2004 Jan;1(1):83–97. doi: 10.1016/S1740-1445(03)00005-6. [DOI] [PubMed] [Google Scholar]
- 5.Magee L. Traumatic Injuries and Body Image. Encyclopedia of Body Image and Human Appearance. 2012:790–797. [Google Scholar]
- 6.Bradbury E. The psychological and social impact of disfigurement to the hand in children and adolescents. Dev Neurorehabil. 2007 Apr-Jun;10(2):143–148. doi: 10.1080/17518420701281122. [DOI] [PubMed] [Google Scholar]
- 7.Chung KC, Kotsis SV, Kim HM, Burke FD, Wilgis EF. Reasons why rheumatoid arthritis patients seek surgical treatment for hand deformities. J Hand Surg Am. 2006 Feb;31(2):289–294. doi: 10.1016/j.jhsa.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 8.Bogoch ER, Escott BG, Ronald K. Hand appearance as a patient motivation for surgery and a determinant of satisfaction with metacarpophalangeal joint arthroplasty for rheumatoid arthritis. J Hand Surg Am. 2011 Jun;36(6):1007–1014. e1–e4. doi: 10.1016/j.jhsa.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 9.Bogoch ER, Judd MG. The hand: a second face? J Rheumatol. 2002 Dec;29(12):2477–2483. [PubMed] [Google Scholar]
- 10.Papadopulos NA, Kovacs L, Krammer S, Herschbach P, Henrich G, Biemer E. Quality of life following aesthetic plastic surgery: a prospective study. J Plast Reconstr Aesthet Surg. 2007;60(8):915–921. doi: 10.1016/j.bjps.2007.01.071. [DOI] [PubMed] [Google Scholar]
- 11.Von Soest T, Kvalem IL, Roald HE, Skolleborg KC. The effects of cosmetic surgery on body image, self-esteem, and psychological problems. J Plast Reconstr Aesthet Surg. 2009 Oct;62(10):1238–1244. doi: 10.1016/j.bjps.2007.12.093. [DOI] [PubMed] [Google Scholar]
- 12.Kovacs L, Grob M, Zimmermann A, Eder M, Herschbach P, Henrich G, Zimmer R, Biemer E, Papadopulos NA. Quality of life after severe hand injury. J Plast Reconstr Aesthet Surg. 2011 Nov;64(11):1495–1502. doi: 10.1016/j.bjps.2011.05.022. [DOI] [PubMed] [Google Scholar]
- 13.Upton J, Havlik RJ, Khouri RK. Refinements in hand coverage with microvascular free flaps. Clin Plast Surg. 1992 Oct;19(4):841–857. [PubMed] [Google Scholar]
- 14.Fisher M, Dorafshar A, Bojovic B, Manson PN. RodriguezThe evolution of critical concepts in aesthetic craniofacial microsurgical reconstruction. Plast Reconstr Surg. 2012 Aug;130(2):389–398. doi: 10.1097/PRS.0b013e3182589e12. [DOI] [PubMed] [Google Scholar]
- 15.Germann G, Biedermann N, Levin SL. Intrinsic flaps in the hand. Clin Plast Surg. 2011 Oct;38(4):729–738. doi: 10.1016/j.cps.2011.07.007. [DOI] [PubMed] [Google Scholar]
- 16.Hamdi M, Van Landuyt K, Blondeel P, Monstrey S. Aesthetic and functional considerations in the reconstruction of large skin defects with free flaps. Clin Plast Surg. 2004 Jan;31(1):39–48. doi: 10.1016/s0094-1298(03)00116-0. [DOI] [PubMed] [Google Scholar]
- 17.Saint-Cyr M, Gupta A. Indications and selection of free flaps for soft tissue coverage of the upper extremity. Hand Clin. 2007 Feb;23(1):37–48. doi: 10.1016/j.hcl.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 18.Friedrich JB, Katolik LI, Vedder NB. Soft tissue reconstruction of the hand. J Hand Surg Am. 2009 Jul-Aug;34(6):1148–1155. doi: 10.1016/j.jhsa.2009.04.035. [DOI] [PubMed] [Google Scholar]
- 19.Davis Sears E, Burns PB, Chung KC. The outcomes of outcome studies in plastic surgery: a systematic review of 17 years of plastic surgery research. Plast Reconstr Surg. 2007 Dec;120(7):2059–2065. doi: 10.1097/01.prs.0000287385.91868.33. [DOI] [PubMed] [Google Scholar]
- 20.Alderman A, Chung KC. Measuring outcomes in aesthetic surgery. Clin Plast Surg. 2013 Apr;40(2):297–304. doi: 10.1016/j.cps.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 21.Ching S, Thoma A, McCabe RE, Antony MM. Measuring outcomes in aesthetic surgery: a comprehensive review of the literature. Plast Reconstr Surg. 2003 Jan;111(1):469–480. doi: 10.1097/01.PRS.0000036041.67101.48. discussion 481–2. [DOI] [PubMed] [Google Scholar]
- 22.Ching S, Rockwell G, Thoma A, Antony MM. Clinical research in aesthetic surgery. Clin Plast Surg. 2008 Apr;35(2):269–273. doi: 10.1016/j.cps.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 23.Goh C. The need for evidence-based aesthetic dermatology practice. J Cutan Aesthet Surg. 2009 Jul;2(2):65–71. doi: 10.4103/0974-2077.58518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Summers BK, Siegle RJ. Facial cutaneous reconstructive surgery: general aesthetic principles. J Am Acad Dermatol. 1993 Nov;29(5 Pt 1):669–681. doi: 10.1016/0190-9622(93)70230-q. quiz 682–3. [DOI] [PubMed] [Google Scholar]
- 25.Gonzalez-Uloa M. Restoration of the face covering by means of selected skin in regional aesthetic units. Br J Plast Surg. 1956 Oct;9(3):212–221. doi: 10.1016/s0007-1226(56)80036-2. [DOI] [PubMed] [Google Scholar]
- 26.Burget GC, Menick FJ. The subunit principle in nasal reconstruction. Plast Reconstr Surg. 1985 Aug;76(2):239–247. doi: 10.1097/00006534-198508000-00010. [DOI] [PubMed] [Google Scholar]
- 27.Menick FJ. Artistry in aesthetic surgery. Aesthetic perception and the subunit principle. Clin Plast Surg. 1987 Oct;14(4):723–735. [PubMed] [Google Scholar]
- 28.Thompson S, Menick FJ. Aesthetic facial reconstruction: blending human perception and the facial subunit theory. Plast Surg Nurs. 1994 Winter;14(4):211–216. doi: 10.1097/00006527-199401440-00004. 224. [DOI] [PubMed] [Google Scholar]
- 29.Spear SL, Davison SP. Aesthetic subunits of the breast. Plast Reconstr Surg. 2003 Aug;112(2):440–447. doi: 10.1097/01.PRS.0000070486.35968.38. [DOI] [PubMed] [Google Scholar]
- 30.Bailey SH, Saint-Cyr M, Oni G, Maia M, Andry D, Shirvani A, Nguyen V, Wong C, Zhang S, Leitch AM, Euhus D, Rao R, Rohrich R. Aesthetic subunit of the breast: an analysis of women’s preference and clinical implications. Ann Plast Surg. 2012 Mar;68(3):240–245. doi: 10.1097/SAP.0b013e318216b563. [DOI] [PubMed] [Google Scholar]
- 31.Centeno RF. Gluteal aesthetic unit classification: a tool to improve outcomes in body contouring. Aesthet Surg J. 2006 Mar-Apr;26(2):200–208. doi: 10.1016/j.asj.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 32.Tubiana R. Functional anatomy: Functional cutaneous units. In: Tubiana R, Thomine JM, Mackin E, editors. Examination of the Hand and Wrist. 2nd. London: Martin Dunitz; 1996. pp. 145–146. [Google Scholar]
- 33.Garrett JW. The adult human hand: some anthropometric and biomechanical considerations. Hum Factors. 1971 Apr;13(2):117–131. doi: 10.1177/001872087101300204. [DOI] [PubMed] [Google Scholar]
- 34.Kanchan T, Krishan K. Anthropometry of hand in sex determination of dismembered remains - A review of literature. J Forensic Leg Med. 2011 Jan;18(1):14–7. doi: 10.1016/j.jflm.2010.11.013. [DOI] [PubMed] [Google Scholar]
- 35.Kościński K. Determinants of hand attractiveness--a study involving digitally manipulated stimuli. Perception. 2011;40(6):682–694. doi: 10.1068/p6960. [DOI] [PubMed] [Google Scholar]
- 36.Clark DP. Aesthetics and reconstruction seeing before cutting. Semin Cutan Med Surg. 2003 Dec;22(4):236–243. doi: 10.1016/S1085-5629(03)00073-7. [DOI] [PubMed] [Google Scholar]
- 37.Green RT, Courtis MC. Information theory and figure perception: the metaphor that failed. Acta Psychol (Amst) 1966;25:12–35. doi: 10.1016/0001-6918(66)90003-5. [DOI] [PubMed] [Google Scholar]
- 38.Desimone R, Albright TD, Gross CG, Bruce C. Stimulus-selective properties of inferior temporal neurons in the macaque. J Neurosci. 1984;4:2051–2062. doi: 10.1523/JNEUROSCI.04-08-02051.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Peelen MV, Downing PE. The neural basis of visual body perception. Nat Rev Neurosci. 2007;8:636–648. doi: 10.1038/nrn2195. [DOI] [PubMed] [Google Scholar]
- 40.Downing PE, Jiang Y, Shuman M, Kanwisher N. A cortical area selective for visual processing of the human body. Science. 2001;293:2470–2473. doi: 10.1126/science.1063414. [DOI] [PubMed] [Google Scholar]
- 41.Peelen MV, Downing PE. Within-subject reproducibility of category-specific visual activation with functional MRI. Hum Brain Mapp. 2005;25:402–408. doi: 10.1002/hbm.20116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Spiridon M, Fischl B, Kanwisher N. Location and spatial profile of category-specific regions in human extrastriate cortex. Hum Brain Mapp. 2006;27:77–89. doi: 10.1002/hbm.20169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Peelen MV, Atkinson AP, Andersson F, Vuilleumier P. Emotional modulation of body-selective visual areas. Soc Cogn Affect Neurosci. 2007;2:274–283. doi: 10.1093/scan/nsm023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cowan RJ, Conley MM. Surface area of the hand and digits. Can J Surg. 1973 May;16(3):187–194. [PubMed] [Google Scholar]
- 45.Eryilmaz T, Tellioglu AT, Ozakpinar HR, Oktem HF, Sen T, Albayrak A, Alper M. Correction of hyperpigmented palmar grafts with full-thickness skin grafts from the lateral aspect of the foot. J Plast Surg Hand Surg. 2013 Apr 3; doi: 10.3109/2000656X.2013.773517. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 46.Halbert CF, Wei FC. Neurosensory free flaps. Digits and hand. Hand Clin. 1997 May;13(2):251–262. [PubMed] [Google Scholar]
- 47.Kelly EJ, Terenghi G, Hazari A, Wiberg M. Nerve fibre and sensory end organ density in the epidermis and papillary dermis of the human hand. Br J Plast Surg. 2005 Sep;58(6):774–779. doi: 10.1016/j.bjps.2004.12.017. [DOI] [PubMed] [Google Scholar]
- 48.Swartz WM. Restoration of sensibility in mutilating hand injuries. Clin Plast Surg. 1989 Jul;16(3):515–29. [PubMed] [Google Scholar]
- 49.Morris SF, Tang M, Almutari K, Geddes C, Yang D. The anatomic basis of perforator flaps. Clin Plast Surg. 2010 Oct;37(4):553–570. doi: 10.1016/j.cps.2010.06.006. xi. [DOI] [PubMed] [Google Scholar]
- 50.Sauerbier M, Unglaub F. Perforator flaps in the upper extremity. Clin Plast Surg. 2010 Oct;37(4):667–676. doi: 10.1016/j.cps.2010.06.010. vii. [DOI] [PubMed] [Google Scholar]
- 51.Taylor GI, Palmer JH. The vascular territories (angiosomes) of the body: experimental study and clinical applications. Br J Plast Surg. 1987 Mar;40(2):113–141. doi: 10.1016/0007-1226(87)90185-8. [DOI] [PubMed] [Google Scholar]
- 52.Vuppalapati G, Oberlin C, Balakrishnan G. “Distally based dorsal hand flaps”: clinical experience, cadaveric studies and an update. Br J Plast Surg. 2004 Oct;57(7):653–667. doi: 10.1016/j.bjps.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 53.Lee DC, Kim JS, Ki SH, Roh SY, Yang JW, Chung KC. Partial second toe pulp free flap for fingertip reconstruction. Plast Reconstr Surg. 2008 Mar;121(3):899–907. doi: 10.1097/01.prs.0000299945.03655.0d. [DOI] [PubMed] [Google Scholar]
- 54.Yam A, Peng YP, Pho RW. “Palmar pivot flap” for resurfacing palmar lateral defects of the fingers. J Hand Surg Am. 2008 Dec;33(10):1889–1893. doi: 10.1016/j.jhsa.2008.08.015. [DOI] [PubMed] [Google Scholar]
- 55.Ni F, Appleton SE, Chen B, Wang B. Aesthetic and functional reconstruction of fingertip and pulp defects with pivot flaps. J Hand Surg Am. 2012 Sep;37(9):1806–11. doi: 10.1016/j.jhsa.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 56.Quaba AA, Davison PM. The distally-based dorsal hand flap. Br J Plast Surg. 1990 Jan;43(1):28–39. doi: 10.1016/0007-1226(90)90042-x. [DOI] [PubMed] [Google Scholar]
- 57.Maruyama Y. The reverse dorsal metacarpal flap. Br J Plast Surg. 1990;43:24–7. doi: 10.1016/0007-1226(90)90041-w. [DOI] [PubMed] [Google Scholar]
- 58.Pelissier P, Casoli V, Bakhach J, Martin D, Baudet J. Reverse dorsal digital and metacarpal flaps: a review of 27 cases. Plast Reconstr Surg. 1999 Jan;103(1):159–165. doi: 10.1097/00006534-199901000-00025. [DOI] [PubMed] [Google Scholar]
- 59.Massimo DD, et al. Reverse dorsal digital island flap. Plast Reconstr Surg. 1994;93:552. [PubMed] [Google Scholar]
- 60.Santa Comba A, et al. Reverse dorsal metacarpal osteocutaneous flaps. Br J Plast Surg. 1997;50(7):555–558. doi: 10.1016/s0007-1226(97)91305-9. [DOI] [PubMed] [Google Scholar]
- 61.Gregory H, Heitmann C, Germann G. The evolution and refinements of the distally based dorsal metacarpal artery (DMCA) flaps. J Plast Reconstr Aesthet Surg. 2007;60(7):731–739. doi: 10.1016/j.bjps.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 62.Sebastin SJ, Mendoza RT, Chong AK, Peng YP, Ono S, Chung KC, Lim AY. Application of the dorsal metacarpal artery perforator flap for resurfacing soft-tissue defects proximal to the fingertip. Plast Reconstr Surg. 2011 Sep;128(3):166e–178e. doi: 10.1097/PRS.0b013e318221ddfa. [DOI] [PubMed] [Google Scholar]
- 63.Parrett BM, Bou-Merhi JS, Buntic RF, Safa B, Buncke GM, Brooks D. Refining outcomes in dorsal hand coverage: consideration of aesthetics and donor-site morbidity. Plast Reconstr Surg. 2010 Nov;126(5):1630–8. doi: 10.1097/PRS.0b013e3181ef8ea3. [DOI] [PubMed] [Google Scholar]
- 64.Ono S, Sebastin SJ, Ogawa R, Hyakusoku H. Letter to the editor S-Plasty Method for Secondary Scar Revision After Flap Surgery. Eplasty. 2011;11:e31. Epub 2011 Jun 27. [PMC free article] [PubMed] [Google Scholar]
- 65.Hyakusoku H, Ogawa R. The small-wave incision for long keloids. Plast Reconstr Surg. 2003 Feb;111(2):964–965. doi: 10.1097/00006534-200302000-00101. [DOI] [PubMed] [Google Scholar]
- 66.Ninkovíc MM, Schwabegger AH, Wechselberger G, Anderl H. Reconstruction of large palmar defects of the hand using free flaps. J Hand Surg Br. 1997 Oct;22(5):623–630. doi: 10.1016/s0266-7681(97)80361-0. [DOI] [PubMed] [Google Scholar]
- 67.Beasley RW. Surgical anatomy of the hand: Skin of the hand. In: Beasley RW, editor. Beasley’s surgery of the hand. New York: Thieme; 2003. pp. 8–9. [Google Scholar]
- 68.Bunyan AR, Mathur BS. Medium thickness plantar skin graft for the management of digital and palmar flexion contractures. Burns. 2000 Sep;26(6):575–580. doi: 10.1016/s0305-4179(00)00014-0. [DOI] [PubMed] [Google Scholar]
- 69.Engelhardt TO, Rieger UM, Schwabegger AH, Pierer G. Functional resurfacing of the palm: flap selection based on defect analysis. Microsurgery. 2012 Feb;32(2):158–166. doi: 10.1002/micr.20951. Epub 2011 Nov 28. [DOI] [PubMed] [Google Scholar]
- 70.Ninković M, Wechselberger G, Schwabegger A, Anderl H. The instep free flap to resurface palmar defects of the hand. Plast Reconstr Surg. 1996 Jun;97(7):1489–1493. doi: 10.1097/00006534-199606000-00030. [DOI] [PubMed] [Google Scholar]
- 71.Dong Huang, Hong-Gang Wang, Wei-Zhi Wu, Hui-Ru Zhang, Hao Lin. Functional and aesthetic results of immediate reconstruction of traumatic thumb defects by toe-to-thumb transplantation. nt Orthop. 2011 Apr;35(4):543–547. doi: 10.1007/s00264-010-1044-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bunnell S. Reconstruction of the thumb. Am J Surg. 1958 Feb;95(2):168–172. doi: 10.1016/0002-9610(58)90496-3. [DOI] [PubMed] [Google Scholar]
- 73.Moberg E. Aspects of sensation in reconstructive surgery of the upper extremity. J Bone Joint Surg Am. 1964 Jun;46:817–825. [PubMed] [Google Scholar]
- 74.Foucher G, Braun JB. A new island flap transfer from the dorsum of the index to the thumb. Plast Reconstr Surg. 1979;63:344–349. doi: 10.1097/00006534-197903000-00008. [DOI] [PubMed] [Google Scholar]
- 75.Tränkle M, Sauerbier M, Heitmann C, Germann G. Restoration of thumb sensibility with the innervated first dorsal metacarpal artery island flap. J Hand Surg Am. 2003 Sep;28(5):758–66. doi: 10.1016/s0363-5023(03)00369-1. [DOI] [PubMed] [Google Scholar]
- 76.Brunelli F. Le lambeau dorso-cubital du pouce. Ann Chir Main. 1993;12:105–114. doi: 10.1016/s0753-9053(05)80084-5. [DOI] [PubMed] [Google Scholar]
- 77.Brunelli F, Vigasio A, Valenti P, Brunelli GR. Arterial anatomy and clinical application of the dorsoulnar flap of the thumb. J Hand Surg Am. 1999 Jul;24(4):803–811. doi: 10.1053/jhsu.1999.0803. [DOI] [PubMed] [Google Scholar]
- 78.Terán P, Carnero S, Miranda R, Trillo E, Estefanía M. Refinements in dorsoulnar flap of the thumb: 15 cases. J Hand Surg Am. 2010 Aug;35(8):1356–1359. doi: 10.1016/j.jhsa.2010.05.016. [DOI] [PubMed] [Google Scholar]
- 79.Moschella F, Cordova A, Pirrello R, Brunelli F. Anatomic basis for the dorsal radial flap of the thumb: Clinical applications. Surg. Radiol. Anat. 1996;18:179. doi: 10.1007/BF02346124. [DOI] [PubMed] [Google Scholar]
- 80.Moschella F, Cordova A. Reverse homodigital dorsal radial flap of the thumb. Plast Reconstr Surg. 2006 Mar;117(3):920–926. doi: 10.1097/01.prs.0000200665.73624.6d. [DOI] [PubMed] [Google Scholar]
- 81.Foucher G, Delaere O, Citron N, Molderez A. Long-term outcome of neurovascular palmar advancement flaps for distal thumb injuries. Br J Plast Surg. 1999 Jan;52(1):64–68. doi: 10.1054/bjps.1997.3005. [DOI] [PubMed] [Google Scholar]
- 82.Delikonstantinou IP, Gravvanis AI, Dimitriou V, Zogogiannis I, Douma A, Tsoutsos DA. Foucher first dorsal metacarpal artery flap versus littler heterodigital neurovascular flap in resurfacing thumb pulp loss defects. Ann Plast Surg. 2011 Aug;67(2):119–122. doi: 10.1097/SAP.0b013e3181ef6f6d. [DOI] [PubMed] [Google Scholar]
- 83.Bruner JM. Incisions for plastic and reconstructive (non-septic) surgery of the hand. Br J Plast Surg. 1951 Apr;4(1):48–55. doi: 10.1016/s0007-1226(51)80006-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.