Summary
Asia is the world's largest continent comprising about 3/5 of the human population. Breast cancer is the most common type of cancer and the second leading cause of cancer-related deaths among women in Asia, accounting for 39% of all breast cancers diagnosed worldwide. The incidence of breast cancer in Asia varies widely across the continent and is still lower than in Western countries, but the proportional contribution of Asia to the global breast cancer rates is increasing rapidly in parallel to the socioeconomic development. However, the mortality-to-incidence ratios are much higher for Asia than for Western countries. Most Asian countries are low- and middle-income countries (LMICs) where breast cancer presents at a younger age and a later stage, and where patients are more likely to die from the disease than those in Western countries. Moreover, diagnostic workup, treatment and palliative services are inadequate in most Asian LMICs. In this review, we present an overview of the breast cancer risk factors and epidemiology, control measures, and cancer care among Asian countries.
Keywords: Breast cancer, Incidence, Epidemiology, Asia, Screening
Introduction
Asia as the world's largest and most populous continent comprises roughly 30% of the earth's land area and 60% of the human population. It includes 6 regions and 48 countries, most of which are classified as low- and middle-income countries (LMICs). Asia is a region with diverse ethnicities and cultures, large socioeconomic disparities, and greatly varying healthcare systems. Wide variations in breast cancer incidence and mortality exist between and within Asian countries. Overall, the incidence of breast cancer in Asia is still lower than in Western countries, but the proportional contribution of Asia to the global breast cancer rates is increasing rapidly due to rapid economic growth and urbanization and a rise in the population's socioeconomic status. Moreover, while the age-standardized incidence rate (ASR) of breast cancer in Asia is still only about 1/3 of that in the USA (29.1/100,000 in Asia versus 92.9/100,000 in the USA), the mortality-to-incidence ratios are much higher for Asia (0.35 vs. 0.16, respectively) [1].
The goal of this review is to highlight key areas of breast cancer epidemiology, care and outcomes in Asia.
Cancer Prevalence and Epidemiology in Asia
Breast Cancer Incidence
When discussing cancer incidence in Asia, it is important to note that only 20% of all Asian countries have population-based cancer registries, of which only 4 (8%), i.e. Japan, Korea, Singapore and Taiwan China, cover the entire population [2]. In the absence of representative data, informed use of the available data can still be a valuable tool for the development of cancer control policies. For example, comparable age-specific incidence rates and trends have been observed in several Asian countries [3,4,5], indicating that rough patterns showing regional or rural/urban differences as well as trends over time are likely to be valid.
In 2012, > 600,000 new breast cancer cases were reported in Asia, accounting for 39% of all breast cancers diagnosed worldwide. Based on these estimated numbers, breast cancer is certainly the most common cancer among women in Asia, accounting for 21.2% of all cancer cases in women [6]. The overall incidence of breast cancer is lower in Asia (ASR of 29.1/100,000) compared to the average world level (ASR of 43.1/100,000), and even more so compared to some developed regions such as the European Union (EU; ASR 80.3/100,000) or the USA (ASR 92.9/100,000).
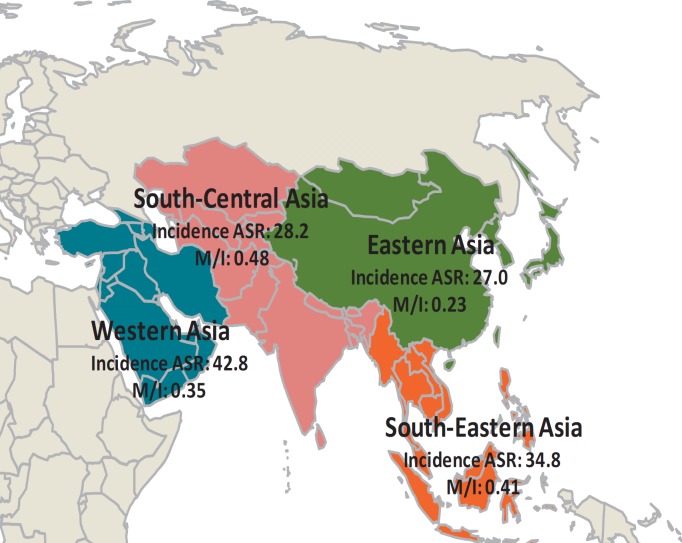
Breast cancer ASRs vary around 10-fold across Asian regions, with reported rates ranging from 27.0/100,000 in eastern Asia to 42.8/100,000 in western Asia (fig. 1, table 1). Japan and South Korea report the highest incidence rates of breast cancer for eastern Asia (51.5 and 52.1 per 100,000, respectively), whereas for south-eastern Asia the highest rate is noted in Singapore (65.7/100,000). The observed increase in breast cancer incidence rates also varies between different Asian countries, with increases of 6% per year seen in Japan compared to 3-4% per year in Shanghai China, Singapore and Thailand [7,8].
Fig. 1.
Incidence and M/I ratio of breast cancer in the Asia region. Data are adapted from [1]. ASR = Age-standardized rate (per 100,000 women), M/I = mortality-to-incidence rate ratio.
Table 1.
Incidence and mortality of breast cancer
| Incidence ASR | Mortality ASR | M/I | |
|---|---|---|---|
| World | 43.1 | 12.9 | 0.30 |
| High human development | 45.2 | 14.7 | 0.33 |
| Medium human development | 26.5 | 9.8 | 0.37 |
| Low human development | 32.6 | 17.0 | 0.52 |
| Asia | 29.1 | 10.2 | 0.35 |
| Eastern Asia | 27.0 | 6.2 | 0.23 |
| South-East Asia | 34.8 | 14.1 | 0.41 |
| South-Central Asia | 28.2 | 13.5 | 0.48 |
| Western Asia | 42.8 | 15.1 | 0.35 |
ASR = Age-standardized rates (per 100,000 women), M/I = mortality-to-incidence rate ratio.
Data are adapted from [1].
Even within countries, there are notable differences in the incidence of breast cancer: In China, the highest rate occurs at the socioeconomically developed east coast region (e.g., an ASR of 46.6/100,000 in Guangzhou) [9]. By contrast, the ASR is estimated to be as low as 7.9/100,000 in the less developed south-western regions. In addition, the breast cancer incidence rates also vary by ethnicity: In Malaysia, a multi-ethnical country, the incidences rates are 59.7/100,000 in the Chinese population, 55.8/100,000 in Indians, and 33.9/100,000 in native Malaysians [10].
Age at Diagnosis
In Asian countries, the age at diagnosis of breast cancer is substantially lower than in high-income countries [11], with a median of 53.9 years in Japan, 51 years in Korea [12], 48-50 years in China [8], and 48.3 years in Thailand [13]. For Asian-Arab countries, the median age at breast cancer diagnosis is even below 50 years, with 48 years in Oman [14], 45 years in Saudi Arabia [15], and 44 years in Jordan [16]. In Saudi Arabia, 26.4% of the breast cancers are diagnosed before the age of 40 years, compared to 6.5% in the USA [15].
The age at presentation has increased over time in Asian countries, which may be due to the fact that women of successive generations are increasingly exposed to risk factors, as well as to the introduction of mammography and ultrasound in women older than 50 years and an increased life expectancy. In Japan, the median age at diagnosis increased from 48 years in 1946-1959 to 53.9 years in 2000-2001. Similarly, in China, the median age at diagnosis increased from 47.5 years in 1990 to 50 years in 2007 [8], and the median age at diagnosis in Saudi Arabia increased from 40 years in 1981-1990 [17] to 45 years in 2002 [15].
Breast Cancer Mortality
In 2012, an estimated 231,013 women in Asia died from breast cancer, accounting for 7% of all deaths, and 40.8% of the cancer deaths, ranking second behind lung cancer in women. The age-standardized mortality rate of breast cancer in Asia is 10.2/100,000, which is lower than the global level of 12.9/100,000 and matching countries with medium human development. Similar to the differences in the incidence rates there are also large differences in the breast cancer mortality rates across Asia, ranging from 6.2/100,000 in eastern Asia to 15.1/100,000 in western Asia (fig. 1, table 1) [6]. In Malaysia (25%), the Philippines (23%), Indonesia (22%), Singapore (20%), and Japan (19%), breast cancer is the leading cause of cancer-related deaths. In these countries, the breast cancer mortality rates have been stable in recent years. In contrast, the mortality rates have increased in several countries, with the highest recorded increases noted in Thailand (7% per year from 2000 to 2006) and Malaysia (6% per year from 1997 to 2008) [18].
The most suitable measure to compare outcomes for patients across countries is the age-standardized mortality-to-incidence rate (M/I) ratio. For breast cancer in Asia, it is 0.35, which is higher than the world average of 0.30, and also higher than expected from Asia's human development index (0.33; see fig. 1, table 1) [6].
Breast Cancer Survival
Similar to incidence data, cancer survival data are not widely available for many Asian countries, and concerns regarding data accuracy apply as discussed above for incidence.
A recent worldwide cancer survival study compared the 5-year survival of cancer from 67 countries, including 16 Asian countries [19]. For women diagnosed from 2005 to 2009, the 5-year survival from breast cancer was 80% or higher in 5 countries within Asia, including China (80.9%), Israel (86.7%), Japan (84.7%), Korea (82.7%), and Qatar (85.3%). Survival was lower than 70% in Malaysia (67.8%) and India (60.4%), and very low in Mongolia (56.5%). The greatest increase in survival was observed in China, rising from 53.8% in 1995-1999 to 80.9% in 2005-2009. Survival increased little in Malaysia from 64.8% in 1995-1999 to 67.8% in 2005-2009, and similarly slowly, but starting at a much higher level, in Japan (81.8% in 1995-1999, 84.2% in 2000-2004, and 84.7% in 2005-2009) [19]. The breast cancer survival rates across Asia were consistent with the development of cancer health services and the gross national income (GNI) per head in the countries or regions.
Apart from between-country differences, disparities in survival can also be observed between different socioeconomic regions within Asian countries. For example, in China, the 5-year survival of breast cancer in Qidong was 58% in 1992, compared to 78% in its rural neighboring area of Shanghai [20]. In India, large differences were likewise noted in the survival of most cancers between rural, semi-urban, or small urban areas, whereas no differences were noted between different metropolitan areas, where healthcare services are more generally available [21]. These large variations in survival within populations reflect large geographic, rural/urban, and socioeconomic disparities with varying levels of development of cancer health services. In addition, ethnic differences in breast cancer survival exist: In South-East Asia, the 5-year breast cancer overall survival is highest in Chinese women (75.8%), followed by Indians (68.0%) and Malaysians (58.5%) [22].
Risk Factors
In Asian populations, breast cancer is associated with some of the risk factors known from Western populations [23], including early menarche, late menopause, older age at first full-term pregnancy, and no breast-feeding, regardless of the region [24,25,26,27,28,29]. However, not all of these factors are similarly relevant in Asian populations. For example, a recent meta-analysis suggests that, although hormone replacement therapy is an important risk factor in Caucasians (odds ratio (OR) 1.13, 95% confidence interval (CI) 1.05-1.22), it is not associated with an elevated breast cancer risk in Asian women (OR 0.96, 95% CI 0.44-2.09) [30].
As for lifestyle factors, 2 meta-analyses have implicated soy and high isoflavone to be associated with a decreased risk of breast cancer [31,32], with the greatest protective effects for hormone receptor-positive tumors [33]. Diabetes mellitus (OR 1.52, 95% CI 1.30-1.78) [30] and smoking were reported to increase the breast cancer risk [34], but not to a similar extent for all regions [35]. In contrast to reports from developed countries [36,37], a higher body mass index is not associated with premenopausal breast cancer, but is linked to postmenopausal breast cancer in Asian populations [38].
Following sociocultural changes towards Westernized lifestyles, Asian women are now characterized by delayed childbearing and fewer children [8], less breast-feeding, a more sedentary workforce, and other Westernized dietary and lifestyle patterns [39]. These changes increase the relevance of ‘Western’ breast cancer risk factors. Modifiable health behaviors in Asian populations thus include the maintenance of a traditional dietary pattern (high in rice, fresh vegetables, and soy) thought to be protective, in addition to enhanced physical activity and maintaining body weight [40].
Screening and Early Detection
As discussed, Asian populations display a peak in breast cancer incidence between 40 and 49 years of age. Therefore, screening programs that have been shown to be successful in Western countries probably cannot be implemented in a similar way in Asia because the efficacy of mammographic screening might be questionable in populations with a different epidemiology of breast cancer regarding age, risk factors, and ethnicity. In addition, cost also needs to be addressed in less developed populations with different cost-effectiveness thresholds. Finally, even if theoretically (cost-)effective, inadequate resources for a population-based screening equipment and the lack of human resources and quality control might still hamper the implementation of effective screening [23]. Finally, compliance with a population-based mammographic screening program is needed before showing a reduction in breast cancer mortality (as in Canada), and it is therefore unlikely for age, biologic and compliance reasons to achieve a reduction in mortality in Asia rivaling that shown in Western countries from these programs.
In most Asian countries, there are no national population-based breast cancer screening programs [23]. However, some Asian countries, including Korea [41], Japan [42], Singapore [43], China [44], and Taiwan [44,45], have initiated population breast cancer screening programs: In Korea, biennial mammographic screening starting at age 50 years is being recommended [46]. Chinese recommendations include annual mammography for women aged 40-49 years, and every 1-2 years for those aged 50-69 years [47].
Chinese patients are often reluctant to visit doctors for regular health screening, frequently presenting only when advanced symptoms arise [48]. In several regions of Asia, including, for example, China and Malaysia, delayed presentation of breast cancer is often due to cancer fatalism, which leads to difficulties in the uptake of healthcare interventions, particularly those targeted at primary prevention [21,49]. In addition, the belief in traditional and alternative therapies is widespread and sometimes hampers effective therapies [21,49]. Reluctance to adopt the available healthcare is prevalent not only in eastern Asia: For example, in Saudi Arabia, 92% of the women aged above 50 years have never had a screening mammogram although health services are free of charge [50].
Strategies to improve early diagnosis of breast cancer in Asian populations are needed. In a study from India, clinical breast examination (CBE) done annually for women aged 40-60 years was estimated to reduce the mortality by 23.3%, similar to reductions achieved by biannual mammographic screening (25.8% for the same age group), but potentially at half the cost [51]. On the other hand, a randomized trial from Shanghai reported no reduction in mortality from breast self-examination (BSE) after 10-11 years of follow-up, but a costly increase in benign breast biopsies [52]. Overall, most researchers agree that teaching BSE is useful for promoting the benefits of early diagnosis via enhancing public awareness [53].
If screening is found to be a cost-effective way of early diagnosis for specific Asian populations, different screening techniques from those used in Western populations might be indicated. In particular, it has been reported that a higher breast density in Asian populations might limit the sensitivity of mammography screening so that ultrasound screening, which is superior in dense breast tissue, might be more sensitive in Asian populations. However, evidence is scarce to support its use within a national screening strategy. A nationwide randomized trial in China has been undertaken comparing efficacy and cost in high-risk women aged 30-65 years screened by mammography, ultrasound, or both. Although ultrasound was shown to be superior in terms of accuracy and sensitivity [54], there is presently no sound evidence to justify the routine use of ultrasonography as an adjunct screening tool in Asian women at an average population risk for breast cancer [55]. Population-based studies of clinical breast examination combined with diagnostic ultrasound are ongoing in China [56].
Taken together, these factors indicate that mammography screening should be adopted prudently in Asian countries, considering regional risk factors, patterns of healthcare uptake, epidemiologic characteristics, and human and financial resources. In the current transition period of Asian economies, birth-cohort effects should be incorporated to quantify risk and identify high-risk groups. Furthermore, known regional, socioeconomic and particularly urban-rural disparities need to be addressed when screening programs are designed and implemented throughout Asia [21].
Stage at Presentation and Delayed Detection
Presenting with more advanced stages of breast cancer is related to poor prognosis and higher treatment cost [57]. The stage at presentation of breast cancer varies widely throughout Asian countries and within these countries. In less developed regions and countries, late stage at presentation is very common: For example, in India, more than half of all breast cancer patients present with stage III-IV disease [58]; a nationwide study in China showed that 15.7% of patients were diagnosed at stage I, 44.9% at stage II, 18.7% at stage III, and 2.4% at stage IV [59]. The proportion of stage IV patients in this study was likely greatly underestimated, as the presenting stage of disease is generally kept within surgical databases, which do not keep records of stage IV presentations. As another example of late-stage presentations, in Oman, 34.9% and 15.8% of patients were reported to have stage III or IV disease, respectively [14]. In contrast, in more developed countries or regions of Asia, women present with earlier stages of breast cancer: In Korea, 56% of breast cancers are diagnosed at a localized stage [60], and in Japan 90% of patients present with stages 0-II [61].
Across Asia, data on the stage at presentation from within countries also reflect large regional, ethnic, and socioeconomic disparities: In China, women living in Beijing are more likely to present with stage I breast cancer (27%) and are less likely to have stage IV disease (0.3% - which again is likely underestimated) compared to less developed regions (3% stage I and 5% stage IV) [62].
As Asian countries develop socioeconomically, offering better education and access to healthcare, a shift in stage at breast cancer diagnosis can be noted: For example, in Shanghai, cases detected early are increasing, with the proportion of stages 0-II rising from 78.6% in 1990 to 93.3% in 2007, whereas the share of stage III breast cancer gradually decreases. Correspondingly, the proportion of asymptomatic breast cancer gradually increased from 0.94% in 1990 to 6.8% in 2007, and non-invasive breast cancers increased markedly, representing 14.7% of all cases in 2007 [8].
Besides delays in diagnosis in less developed regions, long waiting times before initiation of treatment for newly diagnosed breast cancer are common in Asia. In Thailand, 17% of patients report a delay in medical consultation of longer than 3 months, and 42% of longer than 3 months, before seeing a doctor. Family income, education, previous breast symptoms, self-treatment, and travel time to the hospital were associated with a delay of treatment initiation [63]. In China, the median time delay for treatment is 1 month in wealthy areas, compared to 94 days in less developed western and central regions. In some undeveloped regions of China, delays of more than 1 year occur in up to 12-17% of patients [64,65,66]. These numbers are causing concern, as any effort to increase awareness and early diagnosis of breast cancer becomes irrelevant if timely treatment cannot be offered to patients.
Breast Cancer Treatment
Because of its cost and the need for specialized multimodality infrastructure and human resources, the variability in treatment standards offered to breast cancer patients in Asia is even greater than that of screening options. Of a total of 51 Asian countries, only 20 have some form of national cancer center, with research capacities in only a minority of these [2]. Treatment standards are strongly associated with socioeconomic development, whereby Asian countries can be classified into 3 categories according to per capita GNI income level: low-resource, middle-resource, and high-resource countries.
In low-resource countries such as Nepal, Pakistan, Mongolia, and Iraq, cancer diagnosis and treatment facilities are extremely scarce [2] and prognosis is correspondingly poor [67]. The biggest challenges in these countries are little community awareness of breast cancer, inadequate pathology facilities, and fragmented care systems with respect to radiotherapy and systemic treatment [68]. In Nepal, for example, there is only 1 national cancer hospital in addition to 5 specialized cancer hospitals, for a population of ∼30 million people [69]. Half of all breast cancer patients present at late stages, with only 1% in situ cancers, 7% stage I, 41% stage II, 40% stage III, and 10% stage IV [70]. Only 4 hospitals in the country have radiation equipment, which is available at very high cost to patients, and novel diagnostic procedures and targeted therapies are unavailable [69].
Most Asian countries such as China, India, Malaysia, Turkey, and Iran are categorized as middle-resource countries [2]. For these countries, the main challenges include the low quality of data registries, inadequate multidisciplinary coordination, and a lack of resource-appropriate prioritization of breast cancer control programs [68]. In parallel to the growing socioeconomic status, promising improvements in breast cancer treatment have been recorded in these countries over the past few decades. For example, in China, less invasive surgical modalities have been adopted and doxorubicin-based regimens are increasingly being used so that 81.4% of all patients with invasive breast cancer receive adjuvant chemotherapy [64], and the rate of breast-conserving surgery has climbed from 12.1% in 2005 to 24.3% by 2008 [64,71]. However, treatment often does not concur with current international guidelines: Of those patients who receive breast-conserving surgery, 16.3% do not receive radiation therapy as per standard guidelines [72], and in China, 12.1% of the patients who receive adjuvant chemotherapy are given less than 4 cycles of therapy [64]. A Beijing study also reported that only 80.1% of hormone receptor-positive patients receive adjuvant endocrine therapy, while 9.2% of patients with hormone receptor-negative tumors are improperly prescribed endocrine therapy [59,64].
Many drugs are not covered by insurance, leading to prohibitively high out-of-pocket expenses [73,74] and limited treatment options, particularly for metastatic disease [75]. For example, ∼80% of Chinese patients with human epidermal growth factor receptor 2 (HER2)-positive disease are not offered trastuzumab and newer targeted agents are not covered by insurance [64]. Consequently, patients with metastatic breast cancer have limited options, with only 40% receiving second-line treatment, and only 15% receiving third-line therapy for metastatic breast cancer. In comparison, 80% of breast cancer patients receive second-line treatment in Japan and 65% third-line therapy in the USA [76].
With these limitations and a lack of implementation of standardized management, the survival rates of breast cancer in Asia's middle-resource countries are considerably lower than those in high-resource countries. In addition, wide disparities between subpopulations within middle-resource countries remain, so that numbers on a national level often neither reflect the poor outcomes of a large share of disenfranchised populations nor the good outcomes of urban elites.
Japan, Korea, Singapore, and Taiwan belong to the group of high-resource Asian countries, with high breast cancer incidences and relatively favorable breast cancer survival rates [2]. These countries have well-established cancer control and cancer care systems with sufficient financial resources at the national level. The clinical characteristics and outcomes of breast cancer in these countries are quite similar to those in Western countries and treatment is standardized to a large extent [77].
Palliative Care
Although palliative care has become an integral component of healthcare systems in many developed countries of the world, it has yet to be established in most low- and middle-resource countries. The most recent global classification of palliative care provision categorizes countries into 6 groups. Within Asia, only Japan, Singapore, Taiwan, and Hong Kong have achieved advanced palliative care integration, while most Asian countries offer either limited or no palliative care services. China and India, which comprise about 2/5 of the world population, are categorized under Group 4a (‘preliminary integration') and Group 3b (‘generalized palliative care provision’), respectively. As yet, no Asian country offers ambulatory palliation, which has been shown in the Western literature to provide better quality of life for patients and their families, to be more cost effective and to prolong survival when compared to established in-patient palliation. Afghanistan, Bhutan, North Korea, and Laos have no known palliative care activities [78]. Even within countries offering some extent of palliative care on a national level, palliative services may only be available to urban populations [23].
One measure for the quality of palliative care across the world is opioid consumption, which is used by the World Health Organization (WHO) as a surrogate for pain management, particularly in cancer patients with severe pain. The morphine equivalence (ME) metric by WHO region (adjusted for population) allows for equianalgesic comparisons between countries of the aggregate consumption of major opioids. These data show that opioid consumption in any Asian country has not nearly reached the extent of use in Europe or North America and it is particularly low in southern Asia (fig. 2).
Fig. 2.
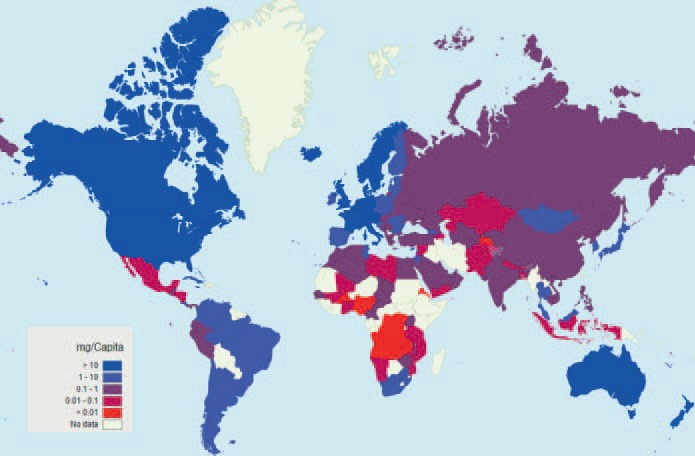
Opioid consumption maps - morphine, mg/capita, 2012 [79].
Conclusion
In parallel to the socioeconomic development in Asia and the subsequent shift from communicable to non-communicable diseases, cancer is moving to the forefront of health threats to Asian middle-resource populations. With respect to breast cancer, the incidence rates are increasing, while healthcare systems are often ill prepared to deal with diseases such as this, requiring multidisciplinary, comprehensive care. While cancer care in some developed countries is comparable to US or European standards, late stage at presentation, cancer fatalism, poor diagnostic workup, and inadequate treatment and palliative services hamper most low- and middle-resource Asian countries.
Women with breast cancer in developing Asian countries present at a younger age, at later stages, and are more likely to die from the disease than those in Western countries. Furthermore, discrepancies in incidence, mortality, age at diagnosis, and stage at presentation exist within and between Asian countries. Breast cancer control efforts therefore need to be tailored to the local breast cancer epidemiology and healthcare resources and should include not only healthcare delivery but also promote awareness and early detection. In addition, breast cancer control strategies in developing Asian countries need to address the existing vast geographic and urban/rural disparities and specifically focus on the needs of underserved populations.
Disclosure Statement
The authors declare no conflicts of interest.
References
- 1.Bridges JF, Anderson BO, Buzaid AC, et al. Identifying important breast cancer control strategies in Asia, Latin America and the Middle East/North Africa. BMC Health Serv Res. 2011;11:227. doi: 10.1186/1472-6963-11-227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yoo KY. Cancer prevention in the Asia Pacific region. Asian Pac J Cancer Prev. 2010;11:839–844. [PubMed] [Google Scholar]
- 3.Shen YC, Chang CJ, Hsu C, Cheng CC, Chiu CF, Cheng AL. Significant difference in the trends of female breast cancer incidence between Taiwanese and Caucasian Americans: implications from age-period-cohort analysis. Cancer Epidemiol Biomarkers Prev. 2005;14:1986–1990. doi: 10.1158/1055-9965.EPI-04-0932. [DOI] [PubMed] [Google Scholar]
- 4.Wong IO, Cowling BJ, Schooling CM, Leung GM. Age-period-cohort projections of breast cancer incidence in a rapidly transitioning Chinese population. Int J Cancer. 2007;121:1556–1563. doi: 10.1002/ijc.22731. [DOI] [PubMed] [Google Scholar]
- 5.Leung GM, Thach TQ, Lam TH, et al. Trends in breast cancer incidence in Hong Kong between 1973 and 1999: an age-period-cohort analysis. Br J Cancer. 2002;87:982–988. doi: 10.1038/sj.bjc.6600583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.GLOBOCAN 2012 http://globocan.iarc.fr/Pages/fact_sheets_population.aspx [last accessed July 11, 2015].
- 7.Seow A, Duffy SW, McGee MA, Lee J, Lee HP. Breast cancer in Singapore: trends in incidence 1968-1992. Int J Epidemiol. 1996;25:40–45. doi: 10.1093/ije/25.1.40. [DOI] [PubMed] [Google Scholar]
- 8.Fan L, Zheng Y, Yu KD, et al. Breast cancer in a transitional society over 18 years: trends and present status in Shanghai, China. Breast Cancer Res Treat. 2009;117:409–416. doi: 10.1007/s10549-008-0303-z. [DOI] [PubMed] [Google Scholar]
- 9.National Cancer Center and Disease Prevention and Control Bureau, Ministry of Health . Chinese cancer registry annual report. Beijing: Military Medical Sciences Press; 2012. [Google Scholar]
- 10.Verma M. Cancer Epidemiology. Vol. 471. Totowa, NJ: Humana Press; 2009. Methods in Molecular Biology. [DOI] [PubMed] [Google Scholar]
- 11.U.S. Preventive Services Task Force Screening for breast cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2009;151:716–726. doi: 10.7326/0003-4819-151-10-200911170-00008. W-236. [DOI] [PubMed] [Google Scholar]
- 12.Kim Z, Min SY, Yoon CS, et al. The basic facts of Korean breast cancer in 2012: results from a nationwide survey and breast cancer registry database. J Breast Cancer. 2015;18:103–111. doi: 10.4048/jbc.2015.18.2.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aphinives P, Punchai S, Vajirodom D, Bhudhisawasdi V. Breast cancer: five-year survival in Srinagarind Hospital, Thailand. J Med Assoc Thai. 2010;93(suppl 3):S25–S29. [PubMed] [Google Scholar]
- 14.Al-Moundhri M, Al-Bahrani B, Pervez I, et al. The outcome of treatment of breast cancer in a developing country - Oman. Breast. 2004;13:139–145. doi: 10.1016/j.breast.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 15.Elkum N, Dermime S, Ajarim D, et al. Being 40 or younger is an independent risk factor for relapse in operable breast cancer patients: the Saudi Arabia experience. BMC Cancer. 2007;7:222. doi: 10.1186/1471-2407-7-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abu-Salem OT. Fine needle aspiration biopsy (FNAB) of breast lumps: comparison study between pre- and post-operative histological diagnosis. Arch Inst Pasteur Tunis. 2002;79:59–63. [PubMed] [Google Scholar]
- 17.al-Idrissi HY, Ibrahim EM, Kurashi NY, Sowayan SA. Breast cancer in a low-risk population. The influence of age and menstrual status on disease pattern and survival in Saudi Arabia. Int J Cancer. 1992;52:48–51. doi: 10.1002/ijc.2910520111. [DOI] [PubMed] [Google Scholar]
- 18.Shin HR, Carlos MC, Varghese C. Cancer control in the Asia Pacific region: current status and concerns. Jpn J Clin Oncol. 2012;42:867–881. doi: 10.1093/jjco/hys077. [DOI] [PubMed] [Google Scholar]
- 19.Allemani C, Weir HK, Carreira H, et al. Global surveillance of cancer survival 1995-2009: analysis of individual data for 25,676,887 patients from 279 population-based registries in 67 countries (CONCORD-2) Lancet. 2015;385:977–1010. doi: 10.1016/S0140-6736(14)62038-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.World Health Organization Cancer survival in Qidong, China, 1992-2000. http://survcan.iarc.fr/survivalchap6.php?Id=6 [last accessed November 18, 2015].
- 21.Goss PE, Strasser-Weippl K, Lee-Bychkovsky BL, et al. Challenges to effective cancer control in China, India, and Russia. Lancet Oncol. 2014;15:489–538. doi: 10.1016/S1470-2045(14)70029-4. [DOI] [PubMed] [Google Scholar]
- 22.Bhoo-Pathy N, Hartman M, Yip CH, et al. Ethnic differences in survival after breast cancer in South East Asia. PLoS One. 2012;7:e30995. doi: 10.1371/journal.pone.0030995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lertkhachonsuk AA, Yip CH, Khuhaprema T, et al. Cancer prevention in Asia: resource-stratified guidelines from the Asian Oncology Summit 2013. Lancet Oncol. 2013;14:e497–e507. doi: 10.1016/S1470-2045(13)70350-4. [DOI] [PubMed] [Google Scholar]
- 24.Long N, Moore MA, Chen W, et al. Cancer epidemiology and control in North-East Asia - past, present and future. Asian Pac J Cancer Prev. 2010;11(suppl 2):107–148. [PubMed] [Google Scholar]
- 25.Moore MA, Attasara P, Khuhaprema T, et al. Cancer epidemiology in mainland South-East Asia - past, present and future. Asian Pac J Cancer Prev. 2010;11(suppl 2):67–80. [PubMed] [Google Scholar]
- 26.Moore MA, Manan AA, Chow KY, et al. Cancer epidemiology and control in peninsular and island South-East Asia - past, present and future. Asian Pac J Cancer Prev. 2010;11(suppl 2):81–98. [PubMed] [Google Scholar]
- 27.Salim EI, Moore MA, Bener A, Habib OS, Seif-Eldin IA, Sobue T. Cancer epidemiology in South-West Asia - past, present and future. Asian Pac J Cancer Prev. 2010;11(suppl 2):33–48. [PubMed] [Google Scholar]
- 28.Moore MA, Ariyaratne Y, Badar F, et al. Cancer epidemiology in South Asia - past, present and future. Asian Pac J Cancer Prev. 2010;11(suppl 2):49–66. [PubMed] [Google Scholar]
- 29.Moore MA, Eser S, Igisinov N, et al. Cancer epidemiology and control in North-Western and Central Asia - past, present and future. Asian Pac J Cancer Prev. 2010;11(suppl 2):17–32. [PubMed] [Google Scholar]
- 30.Anothaisintawee T, Wiratkapun C, Lerdsitthichai P, et al. Risk factors of breast cancer: a systematic review and meta-analysis. Asia Pac J Public Health. 2013;25:368–387. doi: 10.1177/1010539513488795. [DOI] [PubMed] [Google Scholar]
- 31.Wu AH, Yu MC, Tseng CC, Pike MC. Epidemiology of soy exposures and breast cancer risk. Br J Cancer. 2008;98:9–14. doi: 10.1038/sj.bjc.6604145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Qin LQ, Xu JY, Wang PY, Hoshi K. Soyfood intake in the prevention of breast cancer risk in women: a meta-analysis of observational epidemiological studies. J Nutr Sci Vitaminol (Tokyo) 2006;52:428–436. doi: 10.3177/jnsv.52.428. [DOI] [PubMed] [Google Scholar]
- 33.Suzuki T, Matsuo K, Tsunoda N, et al. Effect of soybean on breast cancer according to receptor status: a case-control study in Japan. Int J Cancer. 2008;123:1674–1680. doi: 10.1002/ijc.23644. [DOI] [PubMed] [Google Scholar]
- 34.Ceber E, Sogukpinar N, Mermer G, Aydemir G. Nutrition, lifestyle, and breast cancer risk among Turkish women. Nutr Cancer. 2005;53:152–159. doi: 10.1207/s15327914nc5302_4. [DOI] [PubMed] [Google Scholar]
- 35.Lin Y, Kikuchi S, Tamakoshi K, et al. Active smoking, passive smoking, and breast cancer risk: findings from the Japan Collaborative Cohort Study for Evaluation of Cancer Risk. J Epidemiol. 2008;18:77–83. doi: 10.2188/jea.18.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eliassen AH, Colditz GA, Rosner B, Willett WC, Hankinson SE. Adult weight change and risk of postmenopausal breast cancer. JAMA. 2006;296:193–201. doi: 10.1001/jama.296.2.193. [DOI] [PubMed] [Google Scholar]
- 37.Colditz GA, Rosner B. Cumulative risk of breast cancer to age 70 years according to risk factor status: data from the Nurses' Health Study. Am J Epidemiol. 2000;152:950–964. doi: 10.1093/aje/152.10.950. [DOI] [PubMed] [Google Scholar]
- 38.Turati F, La Vecchia C. Risk factors for breast cancer in China: similarities and differences with Western populations. Arch Med Sci. 2012;8:179–182. doi: 10.5114/aoms.2012.28542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Porter P. ‘Westernizing’ women's risks? Breast cancer in lower-income countries. N Engl J Med. 2008;358:213–216. doi: 10.1056/NEJMp0708307. [DOI] [PubMed] [Google Scholar]
- 40.Shu XO, Zheng Y, Cai H, et al. Soy food intake and breast cancer survival. JAMA. 2009;302:2437–2443. doi: 10.1001/jama.2009.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim Y, Jun JK, Choi KS, Lee HY, Park EC. Overview of the national cancer screening programme and the cancer screening status in Korea. Asian Pac J Cancer Prev. 2011;12:725–730. [PubMed] [Google Scholar]
- 42.Kikuchi M, Tsunoda H, Koyama T, et al. Opportunistic breast cancer screening by mammography in Japan for women in their 40s at our preventive medical center: harm or benefit? Breast Cancer. 2014;21:135–139. doi: 10.1007/s12282-012-0367-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang SC. The Singapore National Breast Screening Programme: principles and implementation. Ann Acad Med Singapore. 2003;32:466–476. [PubMed] [Google Scholar]
- 44.Huang CS, Chang KJ, Shen CY. Breast cancer screening in Taiwan and China. Breast Dis. 2001;13:41–48. doi: 10.3233/bd-2001-13106. [DOI] [PubMed] [Google Scholar]
- 45.Leong SP, Shen ZZ, Liu TJ, et al. Is breast cancer the same disease in Asian and Western countries? World J Surg. 2010;34:2308–2324. doi: 10.1007/s00268-010-0683-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Woo PP, Kim JJ, Leung GM. What is the most cost-effective population-based cancer screening program for Chinese women? J Clin Oncol. 2007;25:617–624. doi: 10.1200/JCO.2006.06.0210. [DOI] [PubMed] [Google Scholar]
- 47.Committee of Breast Cancer Society CA-CA China Anti-Cancer Association guidelines for breast cancer diagnosis and treatment. China Oncol. 2013;23:637–684. [Google Scholar]
- 48.Kwok C, Sullivan G. The concepts of health and preventive health practices of Chinese Australian women in relation to cancer screening. J Transcult Nurs. 2007;18:118–126. doi: 10.1177/1043659606298503. [DOI] [PubMed] [Google Scholar]
- 49.Taib NA, Yip CH, Low WY. Recognising symptoms of breast cancer as a reason for delayed presentation in Asian women - the psycho-socio-cultural model for breast symptom appraisal: opportunities for intervention. Asian Pac J Cancer Prev. 2011;12:1601–1608. [PubMed] [Google Scholar]
- 50.El Bcheraoui C, Basulaiman M, Wilson S, et al. Breast cancer screening in Saudi Arabia: free but almost no takers. PLoS One. 2015;10:e0119051. doi: 10.1371/journal.pone.0119051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Okonkwo QL, Draisma G, der Kinderen A, Brown ML, de Koning HJ. Breast cancer screening policies in developing countries: a cost-effectiveness analysis for India. J Natl Cancer Inst. 2008;100:1290–1300. doi: 10.1093/jnci/djn292. [DOI] [PubMed] [Google Scholar]
- 52.Thomas DB, Gao DL, Ray RM, et al. Randomized trial of breast self-examination in Shanghai: final results. J Natl Cancer Inst. 2002;94:1445–1457. doi: 10.1093/jnci/94.19.1445. [DOI] [PubMed] [Google Scholar]
- 53.Plesnicar A, Golicnik M, Fazarinc IK, Kralj B, Kovac V, Plesnicar BK. Attitudes of midwifery students towards teaching breast-self examination. Radiol Oncol. 2010;44:52–56. doi: 10.2478/v10019-010-0009-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shen S, Zhou Y, Xu Y, et al. A multi-centre randomised trial comparing ultrasound vs mammography for screening breast cancer in high-risk Chinese women. Br J Cancer. 2015;112:998–1004. doi: 10.1038/bjc.2015.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gartlehner G, Thaler K, Chapman A, et al. Mammography in combination with breast ultrasonography versus mammography for breast cancer screening in women at average risk. Cochrane Database Syst Rev. 2013;(4):CD009632. doi: 10.1002/14651858.CD009632.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hussein MA, Saleh M, Ravandi F, Mason J, Rifkin RM, Ellison R. Phase 2 study of arsenic trioxide in patients with relapsed or refractory multiple myeloma. Br J Haematol. 2004;125:470–476. doi: 10.1111/j.1365-2141.2004.04941.x. [DOI] [PubMed] [Google Scholar]
- 57.Anderson BO, Braun S, Carlson RW, et al. Overview of breast health care guidelines for countries with limited resources. Breast J. 2003;9(suppl 2):S42–S50. doi: 10.1046/j.1524-4741.9.s2.3.x. [DOI] [PubMed] [Google Scholar]
- 58.Hebert JR, Ghumare SS, Gupta PC. Stage at diagnosis and relative differences in breast and prostate cancer incidence in India: comparison with the United States. Asian Pac J Cancer Prev. 2006;7:547–555. [PubMed] [Google Scholar]
- 59.Li J, Zhang BN, Fan JH, et al. A nation-wide multicenter 10-year (1999-2008) retrospective clinical epidemiological study of female breast cancer in China. BMC Cancer. 2011;11:364. doi: 10.1186/1471-2407-11-364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Korea Central Cancer Registry Annual report of cancer statistics in Korea in 2010 Seoul, Korea Central Cancer Registry National Cancer Center and Ministry of Health & Welfare; 2012.
- 61.Minami Y, Tsubono Y, Nishino Y, Ohuchi N, Shibuya D, Hisamichi S. The increase of female breast cancer incidence in Japan: emergence of birth cohort effect. Int J Cancer. 2004;108:901–906. doi: 10.1002/ijc.11661. [DOI] [PubMed] [Google Scholar]
- 62.Wang Q, Li J, Zheng S, et al. Breast cancer stage at diagnosis and area-based socioeconomic status: a multicenter 10-year retrospective clinical epidemiological study in China. BMC Cancer. 2012;12:122. doi: 10.1186/1471-2407-12-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Poum A, Promthet S, Duffy SW, Parkin DM. Factors associated with delayed diagnosis of breast cancer in Northeast Thailand. J Epidemiol. 2014;24:102–108. doi: 10.2188/jea.JE20130090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yuan XM, Wang N, Ouyang T, et al. Current status of diagnosis and treatment of primary breast cancer in Beijing, 2008. Chin J Cancer Res. 2011;23:38–42. doi: 10.1007/s11670-011-0038-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang G, Jiang X. Help-seeking delay by breast cancer patients in Sichuan province. Chin J Evid-based Med. 2007;7:702–705. [Google Scholar]
- 66.Jiang DX, Luo XP, Liang ML, Hou XC. The status and intervention of medical treatment delay of breast cancer patients in Nanhai District. Chin Gen Pract Nurs. 2012;10:1233–1234. [Google Scholar]
- 67.Pradhan GB, Shrestha R, Shrestha S, Khadka P, Bhattachan CL. Outcome analysis of breast cancer patients treated at Nepal Medical College. Nepal Med Coll J. 2012;14:93–95. [PubMed] [Google Scholar]
- 68.Anderson BO, Cazap E, El Saghir NS, et al. Optimisation of breast cancer management in low-resource and middle-resource countries: executive summary of the Breast Health Global Initiative consensus, 2010. Lancet Oncol. 2011;12:387–398. doi: 10.1016/S1470-2045(11)70031-6. [DOI] [PubMed] [Google Scholar]
- 69.Piya MK, Acharya SC. Oncology in Nepal. South Asian J Cancer. 2012;1:5–8. doi: 10.4103/2278-330X.96490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Singh YP, Sayami P. Management of breast cancer in Nepal. JNMA J Nepal Med Assoc. 2009;48:252–257. [PubMed] [Google Scholar]
- 71.Yu KD, Di GH, Wu J, et al. Development and trends of surgical modalities for breast cancer in China: a review of 16-year data. Ann Surg Oncol. 2007;14:2502–2509. doi: 10.1245/s10434-007-9436-2. [DOI] [PubMed] [Google Scholar]
- 72.Wang SL, Li YX, Zhang BN, et al. Epidemiologic study of radiotherapy use in China in patients with breast cancer between 1999 and 2008. Clin Breast Cancer. 2013;13:47–52. doi: 10.1016/j.clbc.2012.09.012. [DOI] [PubMed] [Google Scholar]
- 73.Lambertenghi-Deliliers G, Annaloro C, Oriani A, Soligo D. Myelodysplastic syndrome associated with bone marrow fibrosis. Leuk Lymphoma. 1992;8:51–55. doi: 10.3109/10428199209049817. [DOI] [PubMed] [Google Scholar]
- 74.Johnston SR, Hickish T, Houston S, Ellis PA, Howes AJ, Thibault A. Efficacy and tolerability of two dosing regimens of R115777 (Zarnestra), a farnesyl protein transferase inhibitor, in patients with advanced breast cancer. Proc Am Soc Clin Oncol. 2002;21 doi: 10.1200/JCO.2003.10.064. abstr 138. [DOI] [PubMed] [Google Scholar]
- 75.Steensma DP, Mesa RA, Li CY, Gray L, Tefferi A. Etanercept, a soluble tumor necrosis factor receptor, palliates constitutional symptoms in patients with myelofibrosis with myeloid metaplasia: results of a pilot study. Blood. 2002;99:2252–2254. doi: 10.1182/blood.v99.6.2252. [DOI] [PubMed] [Google Scholar]
- 76.Fan L, Strasser-Weippl K, Li JJ, et al. Breast cancer in China. Lancet Oncol. 2014;15:e279–e289. doi: 10.1016/S1470-2045(13)70567-9. [DOI] [PubMed] [Google Scholar]
- 77.Son BH, Kwak BS, Kim JK, et al. Changing patterns in the clinical characteristics of Korean patients with breast cancer during the last 15 years. Arch Surg. 2006;141:155–160. doi: 10.1001/archsurg.141.2.155. [DOI] [PubMed] [Google Scholar]
- 78.Lynch T, Connor S, Clark D. Mapping levels of palliative care development: a global update. J Pain Symptom Manage. 2013;45:1094–1106. doi: 10.1016/j.jpainsymman.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 79.Opioid Consumption Maps https://ppsg.medicine.wisc.edu/ [last accessed July 11, 2015].