Abstract
Background
Global Investigation of Therapeutic Decisions in Hepatocellular Carcinoma and of Treatment with Sorafenib (GIDEON) is a worldwide, prospective, non-interventional study to evaluate the safety of sorafenib in a variety of patient subsets.
Methods
Eligible patients had unresectable hepatocellular carcinoma for whom the decision had been made to treat with sorafenib. Treatment strategies were instituted at the physician's discretion. Patient and disease characteristics, treatment practices, incidences of adverse events (AEs), and overall survival were collected.
Results
In the United States, 563 patients were evaluable for safety. Subgroup analysis was performed for patients who underwent transarterial chemoembolization (TACE) prior to the initiation of sorafenib (group A, n=158), after the initiation of sorafenib only (group B, n=29), both (group C, n=38), or did not undergo TACE (n=318). Patient demographics were similar across the groups. In group A, 29% had Child-Pugh score B or C at diagnosis, and 19% had Barcelona Clinic Liver Cancer tumor stage C or D. In group B, 48% had Child-Pugh score B or C at study entry, and 31% had BCLC stage C or D. The majority of patients in all groups initially received full-dose sorafenib. Incidences of grade ≥3 drug-related AEs were 30%, 17%, and 16% in groups A, B, and C, respectively, and 22% in patients who did not undergo TACE. No new safety signals emerged.
Conclusions
The results from GIDEON reaffirm that sorafenib can be safely used in the context of TACE.
Key Words: GIDEON, Hepatocellular carcinoma, Sorafenib, TACE, Transarterial chemoembolization
Introduction
Transarterial chemoembolization (TACE) is considered the standard of care for patients with intermediate-stage hepatocellular carcinoma (HCC) [1]. Sorafenib is the only systemic agent approved by the United States (US) Food and Drug Administration in patients with unresectable HCC [1]. The antiangiogenic activity of sorafenib suggests that it may mitigate the impact of increased serum vascular endothelial growth factor following TACE, providing a scientific rationale for using the combination to treat unresectable HCC.
Three types of approaches are used in administering sorafenib with TACE as follows: 1) it may be administered continuously throughout planned TACE procedures, 2) it may be interrupted during the time period surrounding each TACE procedure, or 3) it may be given sequentially only after TACE. All three of these methods have been tested in clinical trials [2,3,4], and additional trials are ongoing (TACE-2 [NCT01324076] and STAH [NCT01829035]; Eastern Cooperative Oncology Group (ECOG) 1208 [NCT01004978] is ongoing but closed to enrollment). In addition to the sequence in which TACE and sorafenib are used, consideration must also be given to the number and type of TACE procedures employed. It is noteworthy that in the past, most physicians would start sorafenib after TACE.
With the wide range of potential approaches to combining TACE and sorafenib, it is of interest to understand the methodologies used as well as their associated outcomes in the real-world setting. Global Investigation of Therapeutic Decisions in Hepatocellular Carcinoma and of Treatment with Sorafenib (GIDEON) is a worldwide, prospective, non-interventional study that was designed to evaluate the safety of sorafenib in a variety of patient subsets, including patients with Child-Pugh B liver classification. We have reviewed the data for patients enrolled in this registry who underwent TACE before, during, or after treatment with sorafenib. Here, we report the patient characteristics, practice patterns, safety, and efficacy for the US patient population in GIDEON.
Materials and Methods
The design and rationale for GIDEON (NCT00812175) have been previously described [5,6,7]. Data were collected for five geographic regions, with the US contributing 19% of the patients globally. Eligible patients for inclusion in GIDEON had histologically or cytologically documented or radiologically diagnosed unresectable HCC for whom the decision was made to treat with sorafenib. The patients must have had a life expectancy of more than eight weeks and have provided signed informed consent. Patient exclusion criteria were based on the approved local product information for sorafenib.
The study was conducted according to established recommendations and regulations relating to non-interventional and post-authorization safety studies [8] and according to Good Clinical Practice. Documented approval from appropriate ethics committees and institutional review boards was obtained.
All study data were collected using either electronic or paper case report forms according to study site preference. Data were collected at entry into the study (start of sorafenib) and then at intervals normally used by the prescribing physician, or if significant changes were observed in the patient's disease. Patients were followed from the start of sorafenib therapy to withdrawal, loss to follow-up, death, or final visit.
Treatment strategies were at the physician's discretion according to local prescribing guidelines and clinical practice. No diagnostic or monitoring interventions were mandated.
Adverse events (AEs) were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 3.0 [9]. Patients who received at least one dose of sorafenib and underwent at least one follow-up assessment were evaluable for safety. All data are summarized using descriptive statistics.
Results
Patient Disposition
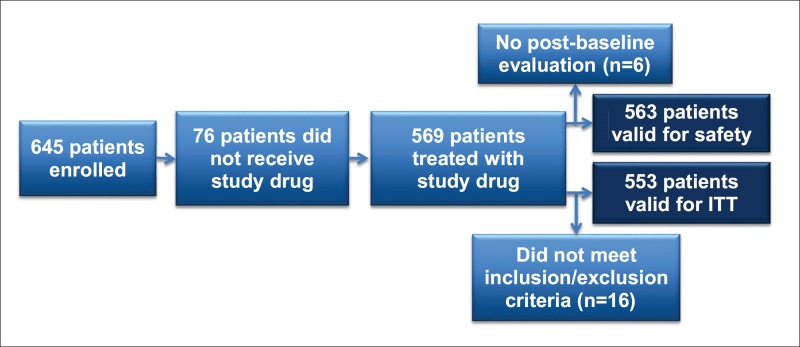
In the US, 645 patients were enrolled in GIDEON. Among these, 553 constituted the intent-to-treat (ITT) population and 563 were valid for the safety analysis (fig. 1). All results reported here refer to the safety population unless otherwise specified.
Fig. 1.
Disposition of US patients enrolled in GIDEON ITT.
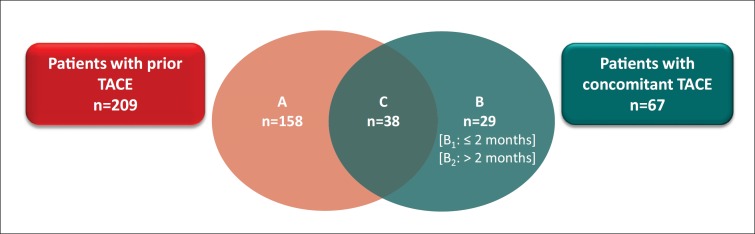
The distribution of patients by TACE approach is shown in fig. 2 Patients may have received multiple TACE treatments. In 158 patients (fig. 2, group A), TACE was administered exclusively before patients received their first dose of sorafenib (prior TACE). In 73 patients (fig. 2, group B), TACE was administered only after their first dose of sorafenib, but prior to sorafenib discontinuation (concomitant TACE). In group B, 11 patients underwent TACE ≤2 months after sorafenib initiation (group B1), and 18 underwent TACE >2 months after sorafenib initiation (group B2). Thirty-eight patients (fig. 2, group C) underwent TACE treatments both prior to and after their first dose of sorafenib (prior and concomitant TACE). A further 20 patients received TACE treatment after discontinuing sorafenib (Supplemental fig. 1); due to the very small numbers among the subgroups, data for these 20 patients are presented in the supplemental information only.
Fig. 2.
Distribution of US patients enrolled in GIDEON who underwent TACE. Diagram depicts numbers of patients who underwent TACE only prior to the initiation of sorafenib (group A), only after the initiation of sorafenib (group B), or before and after the initiation of sorafenib (group C). In group B, 11 patients underwent TACE ≤2 months after the initiation of sorafenib (group B1), and 18 underwent TACE >2 months after the initiation of sorafenib (group B2). Patients who underwent TACE after sorafenib discontinuation are not included (see Supplementary Figure 1, for all online suppl. material, see www.karger.com/doi/10.1159/000367757).
Among the 209 patients who received prior, or prior and concomitant TACE (groups A and C), 124 (59%), 55 (26%), and 30 (14%) received one, two, and three or more TACE treatments, respectively. Embolization agents included lipidiol (41%), beads (40%), microspheres (6%), and gelatin sponge (6%). The embolization agent employed was unknown or unspecified for 36 patients. The primary TACE drugs employed were doxorubicin (81%), cisplatin (30%), and mitomycin (20%). TACE drugs were not specified for 33 patients.
Patient Demographics and Disease Characteristics
Baseline characteristics by TACE subgroup are shown in table 1. Patient characteristics were similar among the subgroups except that patients who underwent both prior and concomitant TACE (group C) tended to have better ECOG performance status. In addition, hepatitis C virus infection was a more prevalent etiology of HCC in patients who underwent concomitant TACE with or without prior TACE (groups B and C), and alcohol use was more prevalent in those who underwent concomitant TACE only (group B). The median age (range) at the start of sorafenib therapy was 60 (20-86) years for patients who did not undergo TACE, 62 (38-87) years for group A, 59 (48-72) years for group B1, 61 (47-79) years for group B2, and 57 (31-83) years for group C.
Table 1.
Demographic and baseline characteristics of US patients enrolled in GIDEON by TACE subgroup
| n (%) | No TACE n=318 | Group A Prior TACE n=158 | Group B Concomitant TACE n=29 | Group C Prior and Concomitant TACE n=38 |
|---|---|---|---|---|
| Sex | ||||
| Male | 247 (78) | 131 (83) | 22 (76) | 28 (74) |
| Age, years | ||||
| <65 | 201 (63) | 98 (62) | 20 (69) | 30 (79) |
| 65–<75 | 81 (26) | 36 (23) | 8 (28) | 5 (13) |
| ≥75 | 36 (11) | 24 (15) | 1 (3) | 3 (8) |
| ECOG PSa | ||||
| 0/1 | 206 (65) | 102 (65) | 20 (69) | 32 (85) |
| ≥2 | 63 (21) | 35 (22) | 5 (17) | 5 (13) |
| Etiologyb | ||||
| Hepatitis B virus infection | 51 (16) | 18 (11) | 2 (7) | 4 (11) |
| Hepatitis C virus infection | 166 (52) | 88 (56) | 21 (72) | 24 (63) |
| Alcohol use | 124 (39) | 59 (37) | 15 (52) | 16 (42) |
| Other | 41 (13) | 25 (16) | 2 (7) | 5 (13) |
| Unknown | 36 (11) | 14 (9) | 1 (3) | 3 (8) |
Data refer to the safety population (n=563).
ECOG missing for 78 (14%) patients.
Patients may have >1.
Table 2 summarizes the tumor and disease characteristics for patients in each of the subgroups at diagnosis and at the start of sorafenib therapy. Both are included since values at diagnosis may be more relevant in considering practice patterns prior to treatment with sorafenib, whereas values at the start of therapy may be more relevant to those after the initiation of sorafenib. Child-Pugh score and Barcelona Clinic Liver Cancer (BCLC) stage were unknown at diagnosis or considered not evaluable at entry to the study for a substantial number of patients. Nonetheless, it is interesting to note that at least 29% of patients in group A were classified as having Child-Pugh B or C liver function at diagnosis, and 19% of patients were staged as BCLC C or D, respectively. In group B, 48% of patients were classified as Child-Pugh B or C at study entry, and 31% had BCLC stage C or D, repsectively. At the start of sorafenib therapy, the median tumor size (range) was 6 (0-23) cm for patients who did not undergo TACE, 4 (0-20) cm for group A, 5 (3-9) cm for group B1, 4 (2-13) cm for group B2, and 4 (1-16) cm for group C. Data were missing for 58 patients.
Table 2.
Tumor and disease characteristics of US patients enrolled in GIDEON at diagnosis and at the start of sorafenib by TACE subgroup
| n (%) | At Diagnosis |
At Start of Sorafenib Therapy |
||||||
|---|---|---|---|---|---|---|---|---|
| No TACE n=318 | Group A Prior TACE n=158 | Group B Concomitant TACE n=29 | Group C Prior and Concomitant TACE n=38 | No TACE n=318 | Group A Prior TACE n =158 | Group B Concomitant TACE n=29 | Group C Prior and Concomitant TACE n=38 | |
| Child-Pugh scorea | ||||||||
| A | 113 (36) | 56 (35) | 6 (21) | 17 (45) | 105 (33) | 56 (35) | 10 (34) | 14 (37) |
| B | 99 (31) | 31 (22) | 9 (31) | 9 (24) | 101 (32) | 45 (29) | 10 (34) | 14 (37) |
| C | 31 (10) | 8 (5) | 2 (7) | 1 (3) | 27 (9) | 9 (6) | 4 (14) | 1 (3) |
| UK/NE | 75 (24) | 60 (38) | 12 (41) | 11 (29) | 85 (27) | 48 (30) | 5 (17) | 8 (21) |
| BCLC stagea,b | ||||||||
| A | 30 (9) | 43 (27) | 8 (28) | 11 (29) | 14 (4) | 27 (17) | 7 (24) | 7 (18) |
| B | 33 (10) | 19 (12) | 3 (10) | 7 (18) | 34 (11) | 19 (12) | 4 (14) | 8 (21) |
| C | 112 (35) | 23 (15) | 1 (3) | 7 (18) | 139 (44) | 45 (29) | 5 (17) | 10 (26) |
| D | 22 (7) | 7 (4) | 2 (7) | 1 (3) | 40 (13) | 18 (11) | 4 (14) | 2 (5) |
| UK/NE | 104 (33) | 55 (35) | 11 (38) | 10 (26) | 91 (29) | 49 (31) | 9 (31) | 10 (26) |
| Portal vein thrombosisc | 93 (29) | 17 (11) | 6 (21) | 4 (11) | 92 (29) | 33 (21) | 7 (24) | 7 (18) |
| Number of lesionsd | ||||||||
| 1-3 | 202 (64) | 122 (77) | 22 (76) | 27 (71) | 171 (54) | 92 (58) | 22 (76) | 27 (71) |
| >3 | 101 (32) | 29 (18) | 6 (21) | 10 (26) | 115 (36) | 52 (33) | 7 (24) | 10 (26) |
| AFPe, ng/mL | ||||||||
| <400 | 151 (48) | 81 (51) | 13 (45) | 21 (55) | 152 (48) | 78 (49) | 15 (52) | 24 (63) |
| ≥400 | 112 (35) | 39 (25) | 11 (38) | 8 (21) | 109 (34) | 54 (34) | 11 (38) | 6 (16) |
| Extrahepatic spread | NR | NR | NR | NR | 112 (35) | 45 (29) | 4 (14) | 5 (13) |
Data refer to the safety population (n=563).
Missing for 1 patient at the start of therapy.
Missing for 35 patients at diagnosis.
Unknown 121 patients at diagnosis and 94 patients at the start of therapy (data missing for 1).
Missing for 24 patients at diagnosis and 49 patients at the start of therapy.
Unknown for 114 patients at diagnosis and 97 patients at the start of therapy. AFP=alpha-fetoprotein; NR=not recorded; UK/NE=unknown or not evaluable.
Administration of Sorafenib
The median (range) time in months from the initial diagnosis to the start of treatment with sorafenib was two (0-72) for patients who did not undergo TACE, nine (1-126) for group A, one (<1-6) for group B, and four (1-44) for group C. In group A, the median (range) time from the most recent TACE to the start of treatment with sorafenib was three (<1-77) months, and the corresponding value for group C was one (0-3) month.
In this observational study, the initial dose of sorafenib that was selected as well as the methods for dose interruptions and modifications were determined according to the judgment of the treating physician at each institution. Initial dose selections for patients in each of the TACE subgroups are shown in table 3. The majority of patients initially received full-dose (400 mg twice daily) sorafenib, and this proportion was similar in patients who underwent TACE and those who did not. The median average daily dose (range) was 586 (112-800) mg for patients who did not undergo TACE, 489 (156-800) mg for group A, 675 (229-800) mg and 492 (200-800) mg for groups B1 and B2, respectively, and 487 (175-800) mg for group C. The average daily dose was determined within patient-based actual days on the study drug excluding interruptions. Data were missing for 33 patients. The median duration of treatment (range) was 10 (<1-131) weeks for patients who did not undergo TACE, 13 (<1-112) weeks for group A, 25 (3-67) weeks for group B1, 36 (9-82) weeks for group B2, and 38 (7-93) weeks for group C. Data were missing for 12 patients.
Table 3.
Initial daily sorafenib dose administered to US patients enrolled in GIDEON by TACE subgroup
| n (%) | No TACE n=318 | Group A Prior TACE n=158 | Group B Concomitant TACE n=29 | Group C Prior and Concomitant TACE n=38 |
|---|---|---|---|---|
| Initial dosea | ||||
| 800 mg/day | 182 (57) | 86 (54) | 14 (48) | 17 (45) |
| 400 mg/day | 109 (34) | 52 (33) | 12 (41) | 14 (37) |
| Otherb | 26 (8) | 20 (13) | 3 (10) | 7 (18) |
Data refer to the safety population (n=563).
Missing for 1 patient.
Other doses included 100, 200, and 600 mg/day.
Safety
The primary reasons for the discontinuation of treatment with sorafenib are shown in table 4. In patients who underwent only prior TACE (group A), 25% discontinued the drug due to AEs; the proportion was fewer than 15% in the other subgroups. Other reasons for discontinuation of the drug in >10% of patients in any subgroup were due to progression of the tumor and death.
Table 4.
Primary reason for sorafenib discontinuation among US patients enrolled in GIDEON
| n (%) | No TACE n=322 | Group A Prior TACE n=160 | Group B Concomitant TACE n=29 | Group C Prior and Concomitant TACE n=38 |
|---|---|---|---|---|
| OLT/other treatment | 4 (1) | 5 (3) | 3 (10) | 2 (5) |
| Progression of liver diseasea | 25 (8) | 12 (8) | 2 (7) | 0 |
| Death | 58 (18) | 18 (11) | 4 (14) | 3 (8) |
| Progression/recurrence/relapse | 72 (22) | 30 (19) | 2 (7) | 8 (21) |
| Adverse event | 42 (13) | 40 (25) | 4 (14) | 2 (5) |
| Otherb | 121 (38) | 55 (34) | 16 (5) | 23 (61) |
Data refer to the enrolled population (n=645).
Not tumor progression.
Including, but not limited to, deterioration of general condition, lost to follow-up, patient decision, liver transplant. OLT=orthotopic liver transplantation.
Safety data are presented in table 5. The incidences of overall and grade 3/4 AEs were similar among the groups. The most common grade 3 drug-related AEs were hand-foot skin reaction (HFSR), fatigue, diarrhea, and hyperbilirubinemia, all of which occurred in ≤5% of patients except HFSR, which occurred in 11% of patients in group A and in 10% of patients in group C. One patient in group A and one patient who did not undergo TACE experienced grade 4 hyperbilirubinemia that was considered drug-related. No other grade 4 drug-related AEs or deaths occurred. In patients who did not undergo treatment with TACE, 78% of all AEs occurred in the first four weeks of sorafenib treatment; corresponding values for groups A, B, and C were 76%, 79%, and 71%, respectively.
Table 5.
Treatment-emergent AEs in US patients enrolled in GIDEON by TACE group
| n (%) | No TACE n=318 | Group A Prior TACE n=158 | Group B Concomitant TACE n=29 | Group C Prior and Concomitant TACE n=38 |
|---|---|---|---|---|
| AEs (all grades) | 311 (98) | 156 (99) | 29 (100) | 38 (100) |
| Grade 3 | 85 (27) | 60 (38) | 11 (38) | 12 (32) |
| Grade 4 | 21 (7) | 11 (7) | 1 (3) | 2 (5) |
| Serious AEs (all grades) | 198 (62) | 72 (46) | 18 (62) | 17 (45) |
| Drug-related AEs (all grades) | 215 (68) | 121 (77) | 22 (76) | 30 (79) |
| Grade 3 | 61 (19) | 44 (28) | 5 (17) | 6 (16) |
| Grade 4 | 9 (3) | 3 (2) | 0 | 0 |
| AEs resulting in permanent discontinuation of sorafenib | 116 (37) | 64 (41) | 7 (24) | 8 (21) |
| Deatha | 131 (41) | 41 (26) | 9 (31) | 6 (16) |
Data refer to the safety population (n=563).
Death while on treatment and up to 30 days of last dose collected from all available sources.
Efficacy
In the ITT population, the median (95% confidence interval) overall survival (OS) from the start of treatment with sorafenib in group A was 8.4 (7.6-10.3) months. The corresponding OS for patients who did not undergo treatment with TACE was 5.9 (4.6-7.2) months. The median (range) time from initial diagnosis of HCC until death was 19.0 (15.6-25.5) months for patients in group A and 9.8 (8.4-11.7) months for patients who did not undergo treatment with TACE. The sample sizes in groups B and C were insufficient to address OS.
Discussion
Current treatment guidelines recommend the use of locoregional therapies, including TACE, for patients with Child-Pugh A or B classification and unresectable HCC who are not candidates for liver transplantation. Guidelines also indicate that sorafenib may be appropriate following arterially directed therapies in patients with adequate liver function once bilirubin returns to baseline if there is evidence of residual/recurrent tumor that is not amenable to additional local therapies [1].
In the US population enrolled in GIDEON, nearly half underwent TACE during the course of treatment for HCC. The majority of these patients (35%) had treatment with TACE prior to sorafenib (groups A and C), while a smaller group (12%) underwent TACE after their first dose of sorafenib (groups B and C). A sizeable proportion of patients who received treatment with TACE, particularly after the initiation of sorafenib therapy, had BCLC stage C or D tumors. Similarly, more than a fifth of patients in each TACE subgroup had Child-Pugh B liver stage, and patients with Child-Pugh C stage were also treated with TACE, although the number was small. These observations may reflect a surprisingly high level of comfort with the use of TACE and sorafenib in certain patients with compromised liver function and more advanced tumors.
Perhaps in keeping with this observation, the initial dose of sorafenib chosen by physicians to treat patients with prior or concomitant TACE was very similar to that used in patients who did not undergo TACE. In patients who had undergone prior TACE, this finding might be interpreted to mean that practitioners were not greatly concerned with incremental toxicity in the setting of the co-administration of sorafenib and TACE when selecting an initial dose of sorafenib and/or that clinical or laboratory factors, that may not have been captured in this analysis, influenced the choice of the initial dose. Median average daily doses were similar in all subgroups with the exception of those who underwent TACE ≤2 months after initiation of sorafenib; in this small group of 11 patients, the average daily dose appeared higher.
No new safety signals were observed in patients who underwent TACE prior to and/or concomitantly with sorafenib treatment. Although drug-related AEs were more frequent in patients who received any treatment with TACE, these apparently did not lead to an increase in permanent discontinuations, and most reported AEs were grade 1 or 2 in severity. Interestingly, serious AEs and death were less frequent in patients who underwent prior TACE. These results likely reflect selection bias among subgroups, but may also indicate that physicians exhibited competency in adjusting the doses of sorafenib and managing AEs on an individual patient basis.
Due to the small size of many subgroups, OS results are reported only for patients who underwent prior TACE (group A) and those who had no treatment with TACE. Patients who underwent TACE exhibited a longer time from diagnosis until death, and longer OS from the initiation of therapy with sorafenib than patients who had no treatment with TACE. However, it is extremely important to interpret these results with caution since GIDEON was not a comparative study and these observations likely reflect, in large part, selection bias toward using TACE in patients most likely to benefit from the procedure, and who had a perceived ability to successfully undergo the procedure. One hypothesis that may result from this observation is that, in appropriately chosen patients, TACE may be an effective debulking approach. In a large number of instances, TACE is used in the US for downstaging cancers. Many US centers and regions accept adequately downstaged tumors for transplantation even if their original tumor burden was beyond the San Francisco criteria. This observation could explain a substantial number of TACE procedures that were done in patients whose liver function was classified as Child-Pugh B or C. An additional global observational study, OPTIMIS (NCT01933945), is ongoing to evaluate OS in patients receiving sorafenib following TACE [10].
GIDEON is a non-interventional registry designed to evaluate the safety of sorafenib in a variety of patient subsets. As an observational study, patient populations were heterogeneous and treatment protocols were not mandated. In addition, several of the subgroups in this analysis had small sample sizes. Thus, the analyses presented here are exploratory in nature and should not be used to draw firm conclusions, particularly with respect to differences among subgroups. Nonetheless, GIDEON provides important information on physician practice patterns and the safety and efficacy of sorafenib in the real world. This analysis indicates that the co-administration of TACE and sorafenib is not uncommon in the real world, and it reinforces the notion that the combination is safe in appropriately selected patients.
Disclosures
JFG and PMG are consultants for, and have received research funding from, Bayer HealthCare; PM and RCGM are consultants for Bayer HealthCare; EZ is an employee of Onyx Pharmaceuticals, an Amgen subsidiary; JI is a former employee of Onyx Pharmaceuticals, an Amgen subsidiary; SB is an employee of Bayer HealthCare; PKF received funding from Onyx Pharmaceuticals, an Amgen subsidiary, for data analysis and manuscript preparation.
Supplementary Material
Supplementary Figure
Supplementary data
Acknowledgements
We thank the patients, their caregivers, and the investigators who participated in GIDEON. This study was funded, designed, and executed by Bayer HealthCare Pharmaceuticals and Onyx Pharmaceuticals, an Amgen subsidiary. Funding for medical writing was provided by Onyx Pharmaceuticals, an Amgen subsidiary, and both institutions provided final approval.
References
- 1.National Comprehensive Cancer Network NCCN Clinical Practice Guidelines in Oncology: hepatobiliary cancersVersion 1.2015. http://www.nccn.org/professionals/physician_gls/pdf/hepatobiliary.pdf [DOI] [PMC free article] [PubMed]
- 2.Lencioni R, Llovet J, Han G, Tak W-Y, Yang J, Leberre M-A, Niu W, Nicholson K, Meinhardt G, Bruix J. Sorafenib or placebo in combination with transarterial chemoembolization (TACE) with doxorubicin-eluting beads (DEBDOX) for intermediate-stage hepatocellular carcinoma (HCC): Phase II, randomized, double-blind SPACE trial [abstract] J Clin Oncol. 2012;30 abstract LBA154. [Google Scholar]
- 3.Kudo M, Imanaka K, Chida N, Nakachi K, Tak WY, Takayama T, Yoon JH, Hori T, Kumada H, Hayashi N, Kaneko S, Tsubouchi H, Suh DJ, Furuse J, Okusaka T, Tanaka K, Matsui O, Wada M, Yamaguchi I, Ohya T, Meinhardt G, Okita K. Phase III study of sorafenib after transarterial chemoembolisation in Japanese and Korean patients with unresectable hepatocellular carcinoma. Eur J Cancer. 2011;47:2117–2127. doi: 10.1016/j.ejca.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 4.Sansonno D, Lauletta G, Russi S, Conteduca V, Sansonno L, Dammacco F. Transarterial chemoembolization plus sorafenib: a sequential therapeutic scheme for HCV-related intermediate-stage hepatocellular carcinoma: a randomized clinical trial. Oncologist. 2012;17:359–366. doi: 10.1634/theoncologist.2011-0313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lencioni R, Kudo M, Ye SL, Bronowicki JP, Chen XP, Dagher L, Furuse J, Geschwind JF, Ladrón de Guevara L, Papandreou C, Sanyal AJ, Takayama T, Yoon SK, Nakajima K, Cihon F, Heldner S, Marrero JA. First interim analysis of the GIDEON (Global Investigation of therapeutic decisions in hepatocellular carcinoma and of its treatment with sorafeNib) non-interventional study. Int J Clin Pract. 2012;66:675–683. doi: 10.1111/j.1742-1241.2012.02940.x. [DOI] [PubMed] [Google Scholar]
- 6.Lencioni R, Marrero J, Venook A, Ye SL, Kudo M. Design and rationale for the non-interventional Global Investigation of Therapeutic DEcisions in Hepatocellular Carcinoma and Of its Treatment with Sorafenib (GIDEON) study. Int J Clin Pract. 2010;64:1034–1041. doi: 10.1111/j.1742-1241.2010.02414.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lencioni R, Venook A, Marrero J, Kudo M, Ye SL, Nakajima K, Cihon F. Second interim results of the GIDEON (Global Investigation of therapeutic DEcisions in HCC and Of its Treatment with sorafeNib) study: Barcelona-Clinic Liver Cancer (BCLC) stage subgroup analysis Presented at: European Multidisciplinary Cancer Congress (ECCO-ESMO); September 23-27, 2011; Stockholm, Sweden.
- 8.European Commission EUDRALEX: The rules governing medicinal products in the European Union. http://ec.europa.eu/enterprise/newsroom/cf/itemdetail.cfm?&item_id=897.2013
- 9.Cancer Therapy Evaluation Program Common Terminology Criteria for Adverse Events v3.0 (CTCAE). Bethesda, MD: National Cancer Institute, US National Institutes of Health, 2006. http://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcaev3.pdf
- 10.Peck-Radosavljevic M, Raoul JL, Lee HC, Kudo M, Nakajima K, Cheng AL. OPTIMIS: An international observational study to assess the use of sorafenib after transarterial chemoembolization (TACE) in patients with hepatocellular carcinoma (HCC) J Clin Oncol. 2014;32:TPS4155. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure
Supplementary data