Summary
Perioperative and palliative chemotherapy for esophageal carcinoma has undergone substantial changes in recent years. The implementation of trastuzumab in the treatment of HER2-positive advanced adenocarcinoma is a milestone as it marked the introduction of the first molecularly targeted treatment of gastric cancer. Current studies are investigating whether anti-HER2-directed treatment also proves effective in the perioperative setting. Data from the CROSS study on neoadjuvant radio-/chemotherapy with paclitaxel and carboplatin have helped to establish a new standard of care for the treatment of localized esophageal cancer. Finally, preliminary experience in potentially curative treatment approaches for oligometastatic tumor stages may offer new treatment options for patients with stage IV gastric cancer. However, some of these innovative approaches urgently require validation in larger, prospective, and controlled multicenter studies. Highly active forms of radiotherapy, radio-/chemotherapy, or chemoimmunotherapy can achieve complete tumor remissions in some patients. Despite these advances, life expectancy unfortunately continues to be very limited in the majority of patients with locally advanced or metastatic esophageal carcinoma.
Keywords: Esophageal cancer, Perioperative chemotherapy, Palliative chemotherapy, Neoadjuvant radio-/chemotherapy
Introduction
Esophageal carcinoma is a comparatively rare tumor entity and has a critical prognosis. In Germany, the Robert Koch Institute noted 6,295 new cases in 2011 [1]. The incidence of adenocarcinoma has increased markedly during the last 40 years [2]. Multimodal therapy for locally advanced carcinoma and treatment for the metastatic stage have undergone substantial changes. New agents and treatment approaches have led to marked improvements in both treatment response and overall survival. This article provides an overview of contemporary treatment approaches for each tumor stage and presents recent clinical research projects.
Perioperative Chemotherapy
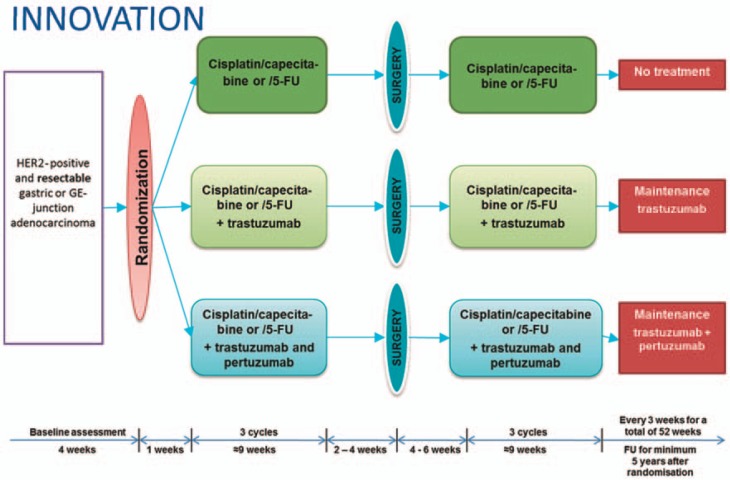
The so-called MAGIC study (on perioperative administration of platinum-based chemotherapy) and subsequently the data from the French ACCORD study established perioperative chemotherapy as a new standard of care for localized adenocarcinoma of the esophagogastric junction and for gastric carcinoma [3,4]. Both studies showed a statistically significant survival advantage for the group of patients which received perioperative chemotherapy. In the MAGIC study, the 5-year survival rate for patients receiving perioperative therapy was 36%, while in patients who underwent surgery alone it was only 23%. The findings of the HER-FLOT study were therefore eagerly awaited. This phase II study with a prospective and multicenter design tested the tolerability and activity of a combination of trastuzumab and 5-fluorouracil (5-FU)/leucovorin, oxaliplatin, and docetaxel (FLOT) chemotherapy in patients with locally advanced HER2-positive adenocarcinomas [5]. Safety and tolerability were good. With a reported histologically complete tumor remission in as many as 23% (n = 52) of the resection specimens, this therapeutic approach appears to be highly promising. The INNOVATION study of the European Organization for Research and Treatment of Cancer (EORTC) is currently being initiated in numerous countries in Europe, including several sites in Germany and in Korea, to further optimize perioperative chemotherapy in HER2-positive locally advanced carcinomas of the esophagogastric junction and stomach (fig. 1) (further information is available from the study PI in Germany, Prof. Lordick in Leipzig, and from the EORTC office at the Charité Hospital in Berlin, Ms Susen Burock, tel. +49 30 4 50 56 46 48).
Fig. 1.
INNOVATION study scheme.
It is still not certain which chemotherapy regimen can be described as the optimal perioperative therapy. ECF (epirubicin, cisplatin, and 5-FU) chemotherapy was used in the MAGIC study, and the CF (cisplatin and 5-FU) protocol was used in the French study. In Germany, the FLOT protocol has become established in many centers due to the treatment results of FLOT studies in palliative therapy [6,7]. The prospective and multicenter FLOT4 study is currently investigating the efficacy of the ECF/ECX (capecitabine instead of 5-FU) regimen in comparison with FLOT for the perioperative indication.
Another question that continues to be unclear is whether perioperative chemotherapy or neoadjuvant radio-/chemotherapy should be used in patients with locally advanced adenocarcinoma of the esophagus and esophagogastric junction. To date, only two small comparative studies, partly with incomplete recruitment, have been published on this issue [8,9]. Neither of them showed a significant benefit in the arms compared; as expected, the histopathological response rate was higher after radiochemotherapy. A recently published meta-analysis demonstrated that neither perioperative chemotherapy nor neoadjuvant radio-/chemotherapy leads to an increase in postoperative morbidity and mortality. This did not apply to squamous cell carcinoma of the esophagus, for which there was an increase in the postoperative and treatment-associated mortality rate. The reasons for this are multifactorial, and the comorbid conditions that patients with squamous cell carcinoma often have (chronic obstructive pulmonary disease, hepatic cirrhosis, cachexia) probably play a role here [10].
Neoadjuvant Radio-/Chemotherapy
The vast majority of randomized clinical studies in the past that have examined the value of neoadjuvant therapy for esophageal carcinoma included both patients with adenocarcinoma and those with squamous cell carcinoma. The widely varying treatment approaches and inclusion criteria used in the studies mean that it is difficult to evaluate them by using meta-analyses. A meta-analysis on 18 studies published by Gebski et al. [11] in 2007 (updated by Sjoquist et al. [12] in 2011) distinguished between the histological subtypes. The analysis showed a statistically significant survival benefit (13% after 2 years) in patients with squamous cell carcinoma of the esophagus who received neoadjuvant radio-/chemotherapy, but not in patients who received neoadjuvant chemotherapy alone. In patients with adenocarcinoma of the esophagus and esophagogastric junction, the meta-analysis showed a significant survival benefit in comparison with resection alone for neoadjuvant or perioperative chemotherapy and also for preoperative radio-/chemotherapy.
The efficacy of neoadjuvant radio-/chemotherapy in patients with operable esophageal carcinoma or carcinoma of the esophagogastric junction has been confirmed once again by the CROSS study. This randomized, multicenter phase III study included patients with locally advanced esophageal carcinomas (squamous cell carcinoma 23%, adenocarcinoma 74%) [13]. The patients received neoadjuvant radio-/chemotherapy (weekly paclitaxel 50 mg/m2, carboplatin AUC2, 41.4 Gy), followed by resection, or surgery alone. The study shows a median survival of 49 months (in the group with prior neoadjuvant therapy) in comparison with 26 months (in the group with surgery alone). The primary end point of overall survival was significantly improved both in patients with adenocarcinoma and in those with squamous cell carcinoma, although patients with squamous cell carcinoma benefited more clearly.
There is an interesting issue of whether patients benefit from surgery if they have shown complete remission after neoadjuvant radio-/chemotherapy, or whether they should receive radio-/chemotherapy alone (possibly with a higher radiation dosage) instead of surgery. Studies investigating this question have not demonstrated any survival advantage for the combination of radio-/chemotherapy and surgery [14,15]. It should be critically noted that complete remission after neoadjuvant radio-/chemotherapy is difficult to confirm in clinical practice and that the efficacy of radio-/chemotherapy cannot be assessed with certainty even weeks after the treatment has been completed. Piessen et al. [16] have now published important data on this issue. A total of 222 patients who achieved complete remission after radio-/chemotherapy were included in a ‘case-control study’. 59 of these patients who underwent definitive radio-/chemotherapy were compared with 118 patients who received neoadjuvant radio-/chemotherapy and subsequent esophageal resection. The median survival in the group of patients who received surgery was significantly longer (31 vs. 83 months), and the recurrence rate in the surgical group was lower (32.7 vs. 51%). The data show an advantage for radio-/chemotherapy with subsequent surgery, which therefore continues to be offered by a number of centers when the surgical risk is acceptable, despite the sobering data from prospective and randomized studies. However, in patients who are at an increased surgical risk due to comorbidities, definitive radio-/chemotherapy is a valid option, particularly after remission has been achieved.
The phase II/III study conducted in Great Britain (SCOPE1) shows that an addition of the anti-EGFR (epidermal growth factor receptor) antibody cetuximab to definitive radio-/chemotherapy for locally advanced esophageal carcinoma has a negative effect on survival (22.1 vs. 25.5 months without cetuximab) [17]. A recently published meta-analysis has confirmed that there is no role for cetuximab regarding this indication [18].
In a retrospective analysis, Chiu et al. [19] investigated the influence of the interval length between neoadjuvant radio-/chemotherapy and surgery. A total of 138 patients were included in each arm of the study. It was found that patients who underwent surgery within 8 weeks had a significantly better 5-year survival in comparison with patients who had delayed operations (50 vs. 35%). A research group in Lille (France) investigated whether prolonging the interval between radiotherapy and surgery improves the efficacy of the prior treatment [20]. A prospective database including 257 patients who received trimodal therapy between 1997 and 2011 was evaluated retrospectively (161 squamous cell carcinomas, 96 adenocarcinomas). The patients were divided into two groups: time point of surgery < 7 weeks or > 7 weeks after neoadjuvant radio-/chemotherapy. The two groups were comparable with regard to demographic data. The ypT0 and R0 resection rates were similar, as were the postoperative course, median long-term survival, and the incidence and distribution pattern of recurrences. The multivariate analysis also showed no evidence of improved efficacy for neoadjuvant therapy resulting from a prolongation of the interval to surgery.
Examined as a whole, these data signify that the optimal interval between the completion of radio-/chemotherapy and surgery cannot be clearly determined yet. A period of 4-8 weeks has proved its value in everyday clinical practice in most centers. Complete recovery from the acute toxicities associated with radio-/chemotherapy is an absolute necessity at the time of surgery.
Encouraging data for innovative approaches have been provided by the multicenter FLOT3 study. With a three-armed design (in locally advanced tumors, tumors with limited metastases, metastatic tumors of the esophagogastric junction, and gastric carcinomas), the study suggests that with good patient selection, patients may also benefit from surgery even at the stage of limited metastases [21]. However, the true value of cytoreductive surgery in patients with oligometastases needs to be investigated in prospective and randomized studies before it is uncritically transferred to clinical routine.
Palliative Therapy
Adenocarcinoma
The meta-analysis by Wagner et al. [22] showed that in regards to survival and quality of life, chemotherapy is superior to purely symptom-oriented therapy. Older patients also benefit from treatment [23]. If the patient's state of health is good enough, treatment should consist of a combination therapy with 5-FU [22].
The established standard for first-line therapy of irresectable or metastatic adenocarcinoma of the esophagogastric junction is a platinum analogue (cisplatin or oxaliplatin) in combination with a fluoropyrimidine (5-FU or capecitabine or S-1); oral fluoropyrimidines are not inferior to 5-FU therapy [22,24]. A further increase in efficacy can be achieved through additional administration of docetaxel. However, this is at the cost of greater toxicity [6,25,26].
Anti-HER2-Directed Therapy
HER2 assessment must be carried out before chemotherapy is started. Trastuzumab in combination with cisplatin and 5-FU or capecitabine has been approved since 2010 for the first-line treatment of advanced HER2-positive gastric carcinoma and adenocarcinoma of the esophagogastric junction (HER2 immunohistochemistry (IHC) score 3+ or IHC 2+ and positive on in situ hybridization (ISH) + ratio of HER2 gene/chromosome 7 centromere ≥ 2). The Trastuzumab for GAstric cancer (ToGA) study published in Lancet compared chemotherapy alone with a combination of chemotherapy and trastuzumab in patients with HER2-positive gastric carcinoma (defined as IHC score 3+ or ISH+) [27]. A significantly longer survival was achieved in the antibody treatment group (median 13.8 vs. 11.1 months). The post-hoc analysis also showed that there was an even longer survival (16 months) in the subgroup of patients whose tumors showed HER2 receptor overexpression according to the current approval criteria for trastuzumab (see above), in comparison with 11.8 months in patients without meeting these criteria.
Anti-Angiogenic Therapy
Following the REGARD study, the RAINBOW study is now the second phase III study that has demonstrated the efficacy of ramucirumab in second-line therapy for metastatic gastric carcinoma [28,29]. Ramucirumab is a monoclonal human immunoglobulin G antibody directed against vascular endothelial growth factor receptor (VEGFR) 2. In RAINBOW, a combination of ramucirumab with weekly paclitaxel was compared with paclitaxel alone. The patients had received platinum- and fluoropyrimidine-containing chemotherapy as their first-line treatment. Only patients with an Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1 were included. The vast majority of the patients (>79%) had gastric carcinoma, while less than 21% had adenocarcinoma of the esophagogastric junction. The primary end point was overall survival. The overall survival in the combination arm was more than 2 months longer (9.6 vs. 7.4 months; hazard ratio 0.81; p = 0.017). The improved median progression-free survival and a significantly higher response rate also confirm the superior efficacy of the drug combination. Adverse events (grade 3 and 4 in the Common Terminology Criteria) included neutropenia (40.7 vs. 18.8%), leukopenia, high blood pressure, anemia, and fatigue.
Second-Line Chemotherapy
The effectiveness of second-line chemotherapy has been confirmed by three randomized studies [30,31,32]. An increase in survival by 1.5 months can be expected. Two of the studies also assessed symptom control and showed that active antineoplastic treatment improved symptom control. A phase III study in Japan compared whether irinotecan or paclitaxel in second-line therapy after failure of 5-FU/platinum treatment is more effective in metastatic gastric carcinoma. This prospective and randomized study did not show any significant survival difference between the two regimens; thus, taxanes (docetaxel or paclitaxel) and irinotecan must be regarded as effective forms of chemotherapy for the second-line treatment of gastric carcinoma [33].
Other Biological Agents
Other biological agents cannot currently be recommended - with negative results for bevacizumab in the AVAGAST study [34], for cetuximab in the EXPAND study [35], for panitumumab in the REAL-3 study [36], and for temsirolimus in the GRANITE-1 study [37].
Lapatinib, a tyrosine kinase inhibitor directed against anti-HER2/EGFR, did not show sufficient efficacy in second-line therapy, although there were positive trends in patients with strong HER2 immunoreactivity (IHC 3+) [38].
Treatment with the anti-hepatocyte growth factor (anti-HGF)/MET inhibitor rilotumumab appeared to be very promising; there was a positive efficacy signal in a randomized phase II study [39]. However, according to a recent press release by the manufacturers, Amgen, the intermediate results of the current phase III study, RILOMET-1, were negative and forced the study to be stopped [40].
Squamous Cell Carcinoma
The chemotherapy protocol by Herskovic et al. [41], dating back to 1992 (cisplatin, 5-FU), was applied to the metastatic situation by the EORTC in 1997 and still continues to be the standard form of treatment for metastatic squamous cell carcinoma. There are also only limited data on the effect of treatment with paclitaxel monotherapy or vinorelbine monotherapy [42,43].
A phase II study by the Arbeitsgemeinschaft Internistische Onkologie (AIO) of the German Cancer Society compared a regimen containing cisplatin/5-FU alone or in combination with cetuximab in patients with metastatic esophageal carcinoma [44]. The primary end point of this study was the response rate. 62 patients were included. The overall response rate was 19% in the cetuximab group compared with 13% in the standard treatment group, and there was a signal for improved efficacy regarding survival end points. This provided the basis for a subsequent phase III study (POWER) that is testing the efficacy of the EGFR antibody panitumumab.
Conclusions
There is no doubt that new agents and new chemotherapy approaches are needed in order to further improve the treatment successes in esophagogastric cancer. The highest medical need exists in the realm of squamous cell cancer of the esophagus, for which the treatment options are often limited and the evidence for the existing options is often scarce.
For future prospective studies on neoadjuvant and perioperative therapy it is mandatory that the quality of all of the modalities used must be controlled to allow for outcome improvements as well as for clear conclusions. A recently published meta-analysis showed enormous differences in the quality of care, including differences in local recurrence rates and hospital mortality [45]. It appears to be clear that the best results in the treatment of locally advanced esophageal and gastric carcinoma are achieved with a multimodal therapeutic approach. Several exciting results for these indications can be expected from ongoing studies during the next few years.
Disclosure Statement
A. Behrens and C. Ell: No disclosures.
F. Lordick: Advisor for BioGanymed, Lilly, and Roche. Lectures on behalf of Amgen, Lilly, and Roche. Scientific projects supported by GSK and Fresenius Biotech. Travel expenses by Bayer, Lilly, Merck, and Taiho.
References
- 1.Robert Koch Institute. www.krebsdaten.de
- 2.Simard EP, Ward EM, Siegel R, Jemal A. Cancers with increasing incidence trends in the United States: 1999 through 2008. Cancer J Clin. 2012;62:118–128. doi: 10.3322/caac.20141. [DOI] [PubMed] [Google Scholar]
- 3.Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJ, Nicolson M, Scarffe JH, Lofts FJ, Falk SJ, Iveson TJ, Smith DB, Langley RE, Verma M, Weeden S, Chua YJ, MAGIC Trial Participants Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;6(355):11–20. doi: 10.1056/NEJMoa055531. [DOI] [PubMed] [Google Scholar]
- 4.Boige V, Pignon J, Saint-Aubert B. Final results of a randomized trial comparing preoperative 5- floururacil(F)/cisplatin (P) to surgery alone in adenocarcinoma of stomach and lower esophagus: FNLCC ACCORD07-FFCD 9703 trial. J Clin Oncol. 2007;25(suppl):4510. [Google Scholar]
- 5.Hofheinz R, Hegewisch-Becker S, Thuss-Patience PC, et al. HER-FLOT: Trastuzumab in combination with FLOT as perioperative treatment for patients with HER2-positive locally advanced esophagogastric adenocarcinoma: a phase II trial of the AIO Gastric Cancer Study Group. J Clin Oncol. 2014;32(suppl):4073. [Google Scholar]
- 6.Al-Batran SE, Hartmann JT, Hofheinz R, Homann N, Rethwisch V, Probst S, Stoehlmacher J, Clemens MR, Mahlberg R, Fritz M, Seipelt G, Sievert M, Pauligk C, Atmaca A, Jäger E. Biweekly fluorouracil, leucovorin, oxaliplatin, and docetaxel (FLOT) for patients with metastatic adenocarcinoma of the stomach or esophagogastric junction: a phase II trial of the Arbeitsgemeinschaft Internistische Onkologie. Ann Oncol. 2008;19:1882–1887. doi: 10.1093/annonc/mdn403. [DOI] [PubMed] [Google Scholar]
- 7.Homann N, Pauligk C, Luley K, Werner Kraus T, Bruch HP, Atmaca A, Noack F, Altmannsberger HM, Jäger E, Al-Batran SE. Pathological complete remission in patients with oesophagogastric cancer receiving preoperative 5-fluorouracil, oxaliplatin and docetaxel. Int J Cancer. 2012;130:1706–1713. doi: 10.1002/ijc.26180. [DOI] [PubMed] [Google Scholar]
- 8.Luc G, Vendrely V, Terrebonne E, Chiche L, Collet D. Neoadjuvant chemoradiotherapy improves histological results compared with perioperative chemotherapy in locally advanced esophageal adenocarcinoma. Ann Surg Oncol. 2015;22:604–609. doi: 10.1245/s10434-014-4005-y. [DOI] [PubMed] [Google Scholar]
- 9.Stahl M, Walz MK, Stuschke M, Lehmann N, Meyer HJ, Riera-Knorrenschild J, Langer P, Engenhart-Cabillic R, Bitzer M, Königsrainer A, Budach W, Wilke H. Phase III comparison of preoperative chemotherapy compared with chemoradiotherapy in patients with locally advanced adenocarcinoma of the esophagogastric junction. J Clin Oncol. 2009;27:851–856. doi: 10.1200/JCO.2008.17.0506. [DOI] [PubMed] [Google Scholar]
- 10.Kumagai K, Rouvelas I, Tsai JA, Mariosa D, Klevebro F, Lindblad M, Ye W, Lundell L, Nilsson M. Meta-analysis of postoperative morbidity and perioperative mortality in patients receiving neoadjuvant chemotherapy or chemoradiotherapy for resectable oesophageal and gastro-oesophageal junctional cancers. Br J Surg. 2014;101:321–338. doi: 10.1002/bjs.9418. [DOI] [PubMed] [Google Scholar]
- 11.Gebski V, Burmeister B, Smithers BM, Foo K, Zalcberg J, Simes J, Australasian Gastro-Intestinal Trials Group Survival benefits from neoadjuvant chemoradiatiotherapy in oesophageal carcinoma: a meta-analysis. Lancet Oncol. 2007;8:226–234. doi: 10.1016/S1470-2045(07)70039-6. [DOI] [PubMed] [Google Scholar]
- 12.Sjoquist KM, Burmeister BH, Smithers BM, Zalcberg JR, Simes RJ, Barbour A, Gebski V, Australasian Gastro-Intestinal Trials Group Survival after neoadjuvant chemotherapy or chemoradiotherapy for resectable oesophageal carcinoma: an updated meta-analysis. Lancet Oncol. 2011;12:681–692. doi: 10.1016/S1470-2045(11)70142-5. [DOI] [PubMed] [Google Scholar]
- 13.Van Hagen P, Hulshof MC, van Lanschot JJ, et al. CROSS Group Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012;31(366):2074–2084. doi: 10.1056/NEJMoa1112088. [DOI] [PubMed] [Google Scholar]
- 14.Chiu PW, Chan AC, Leung SF, Leong HT, Kwong KH, Li MK, Au-Yeung AC, Chung SC, Ng EK. Multicenter prospective randomized trial comparing standard esophagectomy with chemoradiotherapy or treatment of squamous esophageal cancer: early results from the Chinese University Research Group for Esophageal Cancer (CURE) J Gastrointest Surg. 2005;9:794–802. doi: 10.1016/j.gassur.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 15.Bedenne L, Michel P, Bouché O, Milan C, Mariette C, Conroy T, Pezet D, Roullet B, Seitz JF, Herr JP, Paillot B, Arveux P, Bonnetain F, Binquet C. Chemoradiation followed by surgery compared with chemoradiation alone in squamous cell cancer of the esophagus. J Clin Oncol. 2007;25:1160–1168. doi: 10.1200/JCO.2005.04.7118. [DOI] [PubMed] [Google Scholar]
- 16.Piessen G, Messager M, Mirabel X, Briez N, Robb WB, Adenis A, Mariette C. Is there a role for surgery for patients with a complete clinical response after chemoradiation for esophageal cancer? An intention-to-treat case-control study. Ann Surg. 2013;258:793–799. doi: 10.1097/SLA.0000000000000228. [DOI] [PubMed] [Google Scholar]
- 17.Crosby T, Hurt CN, Falk S, Gollins S, Mukherjee S, Staffurth J, Ray R, Bashir N, Bridgewater JA, Geh JI, Cunningham D, Blazeby J, Roy R, Maughan T, Griffiths G. Chemoradiotherapy with or without cetuximab in patients with oesophageal cancer (SCOPE1): a multicentre, phase 2/3 randomised trial. Lancet Oncol. 2013;14:627–637. doi: 10.1016/S1470-2045(13)70136-0. [DOI] [PubMed] [Google Scholar]
- 18.Tian X, Zhou JG, Zeng Z, Shuai T, Yi LJ, Ma L, Wang Y, Cao H, Song GM. Cetuximab in patients with e-sophageal cancer: a systematic review and meta-analysis of randomized controlled trials. Med Oncol. 2015;32:127. doi: 10.1007/s12032-015-0521-2. [DOI] [PubMed] [Google Scholar]
- 19.Chiu CH, Chao YK, Chang HK, Tseng CK, Chan SC, Liu YH, Chen WH. Interval between neoadjuvant chemoradiotherapy and surgery for esophageal squamous cell carcinoma: does delayed surgery impact outcome? Ann Surg Oncol. 2013;20:4245–4251. doi: 10.1245/s10434-013-3139-7. [DOI] [PubMed] [Google Scholar]
- 20.Tessier W, Gronnier C, Messager M, Hec F, Mirabel X, Robb WB, Piessen G, Mariette C. Does timing of surgical procedure after neoadjuvant chemoradiation affect outcomes in esophageal cancer? Ann Thorac Surg. 2014;97:1181–1189. doi: 10.1016/j.athoracsur.2013.12.026. [DOI] [PubMed] [Google Scholar]
- 21.Sunan K, Hofheinz R, Homann N, Illerhaus G, Mönig SP, Pauligk C, Jäger E, Kraus TW, Al Batran SE, Arbeitsgemeinschaft Internistische Onkologie (AIO) Gibt es Kandidaten für eine bimodale Behandlungsstrategie beim metastasierten Magenkarzinom? - Endergebnisse der prospektiven multizentrischen FLOT3-Studie der AIO. 130. Kongress der Deutschen Gesellschaft für Chirurgie. München. 2013 Meeting Abstract;DOI: 10.3205/13dgch152. [Google Scholar]
- 22.Wagner AD, Unverzagt S, Grothe W, Kleber G, Grothey A, Haerting J, Fleig WE. Chemotherapy for advanced gastric cancer. Cochrane Database Syst Rev. 2010;3:CD004064. doi: 10.1002/14651858.CD004064.pub3. [DOI] [PubMed] [Google Scholar]
- 23.Trumper M, Ross PJ, Cunningham D, Norman AR, Hawkins R, Seymour M, Harper P, Iveson T, Nicolson M, Hickish T. Efficacy and tolerability of chemotherapy in elderly patients with advanced oesophago-gastric cancer: a pooled analysis of three clinical trials. Eur J Cancer. 2006;42:827–834. doi: 10.1016/j.ejca.2005.08.044. [DOI] [PubMed] [Google Scholar]
- 24.Ajani JA, Rodriguez W, Bodoky G, Moiseyenko V, Lichinitser M, Gorbunova V, Vynnychenko I, Garin A, Lang I, Falcon S. Multicenter phase III comparison of cisplatin/S-1 with cisplatin/infusional fluorouracil in advanced gastric or gastroesophageal adenocarcinoma study: the FLAGS trial. J Clin Oncol. 2010;28:1547–1553. doi: 10.1200/JCO.2009.25.4706. [DOI] [PubMed] [Google Scholar]
- 25.Van Cutsem E, Moiseyenko VM, Tjulandin S, Majlis A, Constenla M, Boni C, Rodrigues A, Fodor M, Chao Y, Voznyi E, Risse ML, Ajani JA, V325 Study Group Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: a report of the V325 Study Group. J Clin Oncol. 2006;24:4991–4997. doi: 10.1200/JCO.2006.06.8429. [DOI] [PubMed] [Google Scholar]
- 26.Lorenzen S, Hentrich M, Haberl C, Heinemann V, Schuster T, Seroneit T, Roethling N, Peschel C, Lordick F. Split-dose docetaxel, cisplatin and leucovorin/fluorouracil as first-line therapy in advanced gastric cancer and adenocarcinoma of the gastroesophageal junction: results of a phase II trial. Ann Oncol. 2007;18:1673–1679. doi: 10.1093/annonc/mdm269. [DOI] [PubMed] [Google Scholar]
- 27.Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T, Aprile G, Kulikov E, Hill J, Lehle M, Rüschoff J, Kang YK, ToGA Trial Investigators Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 2010;376:687-697. Erratum in: Lancet. 2010;376:1302. doi: 10.1016/S0140-6736(10)61121-X. [DOI] [PubMed] [Google Scholar]
- 28.Wilke H, Muro K, Van Cutsem E, RAINBOW Study Group Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol. 2014;15:1224–1235. doi: 10.1016/S1470-2045(14)70420-6. [DOI] [PubMed] [Google Scholar]
- 29.Fuchs CS, Tomasek J, Yong CJ, et al. Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): an international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet. 2014;383:31–39. doi: 10.1016/S0140-6736(13)61719-5. [DOI] [PubMed] [Google Scholar]
- 30.Thuss-Patience PC, Kretzschmar A, Bichev D, Deist T, Hinke A, Breithaupt K, Dogan Y, Gebauer B, Schumacher G, Reichardt P. Survival advantage for irinotecan versus best supportive care as second-line chemotherapy in gastric cancer - a randomised phase III study of the Arbeitsgemeinschaft Internistische Onkologie (AIO) Eur J Cancer. 2011;47:2306–2314. doi: 10.1016/j.ejca.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 31.Kang JH, Lee SI, Lim do H, Park KW, Oh SY, Kwon HC, Hwang IG, Lee SC, Nam E, Shin DB, Lee J, Park JO, Park YS, Lim HY, Kang WK, Park SH. Salvage chemotherapy for pretreated gastric cancer: a randomized phase III trial comparing chemotherapy plus best supportive care with best supportive care alone. J Clin Oncol. 2012;30:1513–1518. doi: 10.1200/JCO.2011.39.4585. [DOI] [PubMed] [Google Scholar]
- 32.Ford HE, Marshall A, Bridgewater JA, Janowitz T, Coxon FY, Wadsley J, Mansoor W, Fyfe D, Madhusudan S, Middleton GW, Swinson D, Falk S, Chau I, Cunningham D, Kareclas P, Cook N, Blazeby JM, Dunn JA, COUGAR-02 Investigators Docetaxel versus active symptom control for refractory oesophagogastric adenocarcinoma (COUGAR-02): an open-label, phase 3 randomised controlled trial. Lancet Oncol. 2014;15:78–86. doi: 10.1016/S1470-2045(13)70549-7. [DOI] [PubMed] [Google Scholar]
- 33.Hironaka S, Ueda S, Yasui H, Nishina T, Tsuda M, Tsumura T, Sugimoto N, Shimodaira H, Tokunaga S, Moriwaki T, Esaki T, Nagase M, Fujitani K, Yamaguchi K, Ura T, Hamamoto Y, Morita S, Okamoto I, Boku N, Hyodo I. Randomized, open-label, phase III study comparing irinotecan with paclitaxel in patients with advanced gastric cancer without severe peritoneal metastasis after failure of prior combination chemotherapy using fluoropyrimidine plus platinum: WJOG 4007 Trial. J Clin Oncol. 2013;31:4438–4444. doi: 10.1200/JCO.2012.48.5805. [DOI] [PubMed] [Google Scholar]
- 34.Ohtsu A, Shah MA, Van Cutsem E, Rha SY, Sawaki A, Park SR, Lim HY, Yamada Y, Wu J, Langer B, Starnawski M, Kang YK, Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer a randomized, double-blind, placebo-controlled phase III study. J Clin Oncol. 2011;29:3968–3976. doi: 10.1200/JCO.2011.36.2236. [DOI] [PubMed] [Google Scholar]
- 35.Lordick F, Kang YK, Chung HC, Salman P, Oh SC, Bodoky G, Kurteva G, Volovat C, Moiseyenko VM, Gorbunova V, Park JO, Sawaki A, Celik I, Götte H, Melezínková H, Moehler M, Arbeitsgemeinschaft Internistische Onkologie and EXPAND Investigators Capecitabine and cisplatin with or without cetuximab for patients with previously untreated advanced gastric cancer (EXPAND): a randomised, open-label phase 3 trial. Lancet Oncol. 2013;14:490–499. doi: 10.1016/S1470-2045(13)70102-5. [DOI] [PubMed] [Google Scholar]
- 36.Waddell T, Chau I, Cunningham D, et al. Epirubicin, oxaliplatin, and capecitabine with or without panitumumab for patients with previously untreated advanced oesophagogastric cancer (REAL3): a randomised, open-label phase 3 trial. Lancet Oncol. 2013;14:481–489. doi: 10.1016/S1470-2045(13)70096-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ohtsu A, Ajani JA, Bai YX, Bang YJ, Chung HC, Pan HM, Sahmoud T, Shen L, Yeh KH, Chin K, Muro K, Kim YH, Ferry D, Tebbutt NC, Al-Batran SE, Smith H, Costantini C, Rizvi S, Lebwohl D, Van Cutsem E. Everolimus for previously treated advanced gastric cancer: results of the randomized, double-blind, phase III GRANITE-1 study. J Clin Oncol. 2013;31:3935–3943. doi: 10.1200/JCO.2012.48.3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hecht JR, Bang Y-J, Qin S, et al. Lapatinib in combination with capecitabine plus oxaliplatin (CapeOx) in HER2-positive advanced or metastatic gastric, esophageal, or gastroesophageal adenocarcinoma (AC): the TRIO-013/LOGiC Trial. J Clin Oncol. 2013;31(suppl) doi: 10.1200/JCO.2015.62.6598. abstr LBA4001. [DOI] [PubMed] [Google Scholar]
- 39.Iveson T, Donehower RC, Davidenko I, Tjulandin S, Deptala A, Harrison M, Nirni S, Lakshmaiah K, Thomas A, Jiang Y, Zhu M, Tang R, Anderson A, Dubey S, Oliner KS, Loh E. Rilotumumab in combination with epirubicin, cisplatin, and capecitabine as first-line treatment for gastric or oesophagogastric junction adenocarcinoma: an open-label, dose de-escalation phase 1b study and a double-blind, randomised phase 2 study. Lancet Oncol. 2014;15:1007–1018. doi: 10.1016/S1470-2045(14)70023-3. [DOI] [PubMed] [Google Scholar]
- 40.Doshi S, Gisleskog PO, Zhang Y, Zhu M, Oliner KS, Loh E, Ruixo JJ. Rilotumumab exposure-response relationship in patients with advanced or metastatic gastric cancer. Clin Cancer Res. 2015;21:2453–2461. doi: 10.1158/1078-0432.CCR-14-1661. [DOI] [PubMed] [Google Scholar]
- 41.Herskovic A, Martz K, al-Sarraf M, Leichman L, Brindle J, Vaitkevicius V, Cooper J, Byhardt R, Davis L, Emami B. Combined chemotherapy and radiotherapy compared with radiotherapy alone in patients with cancer of the esophagus. N Engl J Med. 1992;326:1593–1598. doi: 10.1056/NEJM199206113262403. [DOI] [PubMed] [Google Scholar]
- 42.Ajani JA, Ilson DH, Daugherty K, Kelsen DP. Paclitaxel in the treatment of carcinoma of the esophagus. Semin Oncol. 1995;22(suppl 6):35–40. [PubMed] [Google Scholar]
- 43.Conroy T, Etienne PL, Adenis A, Ducreux M, Paillot B, Oliveira J, Seitz JF, Francois E, Van Cutsem E, Wagener DJ, Kohser F, Daamen S, Praet M, Gorlia T, Baron B, Wils J, European Organisation for Research and Treatment of Cancer Gastrointestinal Tract Cancer Cooperative Group Vinorelbine and cisplatin in metastatic squamous cell carcinoma of the esophagus: response, toxicity, quality of life and survival. Ann Oncol. 2002;13:721–729. doi: 10.1093/annonc/mdf063. [DOI] [PubMed] [Google Scholar]
- 44.Lorenzen S, Schuster T, Porschen R, Al-Batran SE, Hofheinz R, Thuss-Patience P, Moehler M, Grabowski P, Arnold D, Greten T, Müller L, Röthling N, Peschel C, Langer R, Lordick F. Cetuximab plus cisplatin-5-fluorouracil versus cisplatin-5-fluorouracil alone in first-line metastatic squamous cell carcinoma of the esophagus: a randomized phase II study of the Arbeitsgemeinschaft Internistische Onkologie. Ann Oncol. 2009;20:1667–1673. doi: 10.1093/annonc/mdp069. [DOI] [PubMed] [Google Scholar]
- 45.Markar SR, Wiggins T, Ni M, Steyerberg EW, Van Lanschot JJ, Sasako M, Hanna GB. Assessment of the quality of surgery within randomised controlled trials for the treatment of gastro-oesophageal cancer: a systematic review. Lancet Oncol. 2015;16:e23–e31. doi: 10.1016/S1470-2045(14)70419-X. [DOI] [PubMed] [Google Scholar]