Abstract
Background
Recently, sleep apnea syndrome (SAS) has been associated with hypertension, cardiovascular disease and death. Patients with chronic kidney disease (CKD) have higher rates of SAS, atherosclerotic complications and death than do patients without CKD. Although the relationship between SAS and atherosclerosis is well known, few papers have described this relationship in humans, especially in CKD patients.
Patients and Methods
This was a cross-sectional study of 110 clinically stable, non-dialysis patients with CKD who attended a CKD educational program from April 2014 to September 2015. The diagnosis of SAS and its severity were assessed using a type 3 portable monitor. Other atherosclerosis-related data were obtained from the patients' medical records in order to determine the factors associated with the severity of SAS.
Results
95 men and 15 women with a mean age of 71.4 ± 9.9 years were included in the study. The patients' mean body mass index was 24.0 ± 3.9, their mean blood pressure 134.3 ± 21.2/73.6 ± 13.4 mm Hg and their mean estimated glomerular filtration rate 19.8 ± 9.5 ml/min/1.7 m2. Adjusted plaque score was a significant predictor of severe SAS (odds ratio = 1.13, p = 0.0182). Mixed plaque was significantly associated with severe SAS (correlation ratio = 0.48, p < 0.0001).
Conclusions
Many patients with CKD also have SAS. Our findings demonstrate the relationship between plaque score and the severity of SAS.
Key Words: Plaque score, Sleep apnea syndrome, Apnea-hypopnea index, Chronic kidney disease, Atherosclerosis, Cardiovascular disease, Estimated glomerular filtration rate, Systolic function
Introduction
Recently, sleep apnea syndrome (SAS) has been shown to be associated with hypertension, cardiovascular disease (CVD) and death [1,2]. Some researchers have also suggested that SAS is related to atherosclerosis [3,4,5,6], which is a risk factor for CVD and death [7]. However, few papers describe such associations in human subjects, and which of the parameters of atherosclerosis are most strongly associated with the severity of SAS remains unknown; thus, it is clinically important to clarify how SAS induces atherosclerosis of the carotid artery. Several facts have already been established in human research: (1) Pulse wave velocity (PWV) is faster in patients with SAS than in control subjects [3]. (2) The apnea-hypopnea index (AHI) is associated with calcification of the coronary artery [4]. (3) The duration of SAS is related to intima media thickness (IMT) [5]. (4) The severity of SAS is related to IMT [6]. Although the mechanisms underlying atherosclerosis in patients with SAS remain poorly understood, some research suggests that inflammation may be important in this process. Indeed, inflammation leads to endothelial dysfunction and intermittent hypoxia and reperfusion during repetitive episodes of nocturnal apnea, which may be involved in the generation of highly reactive oxygen radicals and ischemia-reperfusion injury to the vascular wall, resulting in increased risk for atherosclerosis [8]. The prevalence of SAS is markedly higher in patients with chronic kidney disease (CKD) [9,10]. CKD has also been shown to be independently associated with the increased morbidity and mortality rates associated with CVD [11].
Although we may intuitively expect the combination of CKD and SAS to synergistically increase CVD-related morbidity and mortality rates, this relationship has yet to be determined. Herein, we examined numerous clinical parameters, including ankle-brachial index (ABI), PWV, IMT and plaque score (PS) [12] to investigate which of these was associated with the severity of SAS in patients who attended a CKD educational program.
Methods
Patients and Data Collection
This was a cross-sectional study examining 110 stable non-dialysis patients with CKD who attended a CKD educational program from April 2014 to September 2015. The program was approximately 1 week long and intended to educate patients about their kidney disease status and to provide individualized nutritional therapy, treatment options for end-stage renal disease and examination for complications. As one of the complications of CKD, we examined SAS. In this study, we excluded patients who did not undergo assessment for SAS. We collected the patients' data, including medical history, physical examination results, laboratory test results and medications from a medical record review. The term ‘cardiovascular disease event’ included ischemic heart disease, heart failure, stroke, peripheral artery disease and aortic disease.
We gathered patients' data from their physical reports, including past medical history comprising hypertension, diabetes mellitus (DM), CVD, blood tests, urinalysis, medication and use of erythropoiesis-stimulating agents (ESAs). To assess atherosclerosis, we measured maximum IMT (IMTmax) and PS with carotid ultrasonography and checked the quality of plaques, categorizing them into four groups: soft, intermediate, mixed and hard. We also evaluated ABI and PWV. In addition, we performed chest and abdominal X-rays to assess calcification of the aorta and echocardiography to assess calcification of cardiac valves.
Examination of SAS
The diagnosis of SAS and its severity were evaluated using a type 3 portable monitor [13] (Apnomonitor 5; Chest M.I., Inc., Tokyo, Japan). We defined SAS severity based on the AHI (times/h) as follows: mild >5 but ≤15, moderate >15 but ≤30, and severe >30, in accordance with previous reports. Those who performed sleep study assessments were blinded to the carotid artery plaque detection.
Examination of ABI and PWV
The ABI involves the measurement of the ratio of blood pressure in the dorsalis pedis or posterior tibial artery to that in the brachial artery [14]. PWV was measured using a volume-plethysmographic apparatus (BP-203RPE III; OMRON Colin Co., Ltd., Tokyo, Japan). Briefly, this apparatus records the phonocardiogram, electrocardiogram and volume pulse form and blood pressure at both left and right brachia and ankles. PWV was calculated by time phase analysis between brachial and volume waveforms at ankles [3].
Examination of Carotid Artery Ultrasonography
Carotid artery ultrasonography was performed by a real-time B-mode ultrasound imager (ProSound α-10; Hitachi Aloka Medical, Ltd., Tokyo, Japan). The IMT measurements were performed within 1 cm above the bifurcation of the common artery. IMT was defined as the distance between two parallel echogenic lines of the vessel in the sonography. The PS was calculated by previously reported methods [15,16]. It was computed by adding the maximal thickness of plaques (in mm) in each segment of both sides. Segment 1 was the region of the internal carotid artery that was 15 mm distal to its bifurcation from the common carotid artery. Segment 2 was the region of the internal carotid artery and the common carotid artery that was 15 mm proximal to the bifurcation. Segment 3 was the region of the common carotid artery that was 15 and 30 mm proximal to the bifurcation. Segment 4 was the region of the common carotid artery that was 30 mm proximal to the bifurcation and below the flow divider. Consequently, a high PS means existence of plaques in many segments. Plaque quality is classified into hypo-, iso-, hyperechoic and mixed groups. Hypoechoic group indicates soft plaque, isoechoic intermediate plaque and hyperechoic hard plaque.
Statistical Analysis
Baseline characteristics were presented descriptively and tested using Student's t test or χ2 test. Univariate correlations were examined using Spearman's rank correlation coefficient. Multiple linear regression analysis of several markers for the AHI was also performed. Logistic regression analysis was undertaken to examine the relation of atherosclerotic parameters to severe SAS without and with adjustment for potential confounding variables. Receiver operating characteristic (ROC) curve was used to determine the ability of PS to discriminate between those with and without severe AHI. p values <0.05 were considered statistically significant.
Results
Baseline Characteristics
Table 1 shows the patients' baseline characteristics. 95 men and 15 women with a mean age of 71.4 ± 9.9 years were included. The patients' mean body mass index (BMI) was 24.0 ± 3.9, and there was no tendency of obesity. Among these patients, the mean AHI was markedly high at 25.2 ± 13.9. Only 7 patients (6.4%) were not diagnosed with SAS. 23 patients (20.9%) had mild SAS, 39 (35.5%) moderate SAS and 41 (37.3%) severe SAS. The prevalence of SAS is higher among patients with CKD than among those with other diseases [17,18,19,20].
Table 1.
Baseline characteristics of all patients with CKD (n = 110)
| Parameter | |
| Male, % | 86.4 |
| Age, years | 71.4 ± 9.9 |
| Primary disease | |
| Nephrosclerosis, % | 45.5 |
| Diabetic nephropathy, % | 24.5 |
| Chronic glomerulonephritis, % | 14.5 |
| Unknown, % | 9.1 |
| Others, % | 6.4 |
| DM, % | 56.4 |
| Hypertension, % | 97.3 |
| History of CVD, % | 45.5 |
| BMI | 24.0 ± 3.9 |
| Systolic blood pressure, mm Hg | 134.3 ± 21.2 |
| Diastolic blood pressure, mm Hg | 73.6 ± 13.4 |
| Creatinine, mg/dl | 3.1 ± 1.4 |
| Estimated glomerular filtration rate, ml/min/1.7 m2 | 19.8 ± 9.5 |
| C-reactive protein, mg/dl | 0.38 ± 0.59 |
| AHI | 25.2 ± 13.9 |
Clinical Characteristics of Patients with and without Severe SAS
Table 2 shows the clinical characteristics of patients with and without severe SAS. Although patients in the severe SAS group tended to be older, this tendency was not statistically significant. There was no significant difference in sex between those with and without severe SAS, nor were there differences in blood pressure, physique, past medical history, medication or renal function. Brain natriuretic peptide and cardiothoracic ratio were significantly higher among patients in the severe SAS group. The percentage of ESA users was significantly higher in the severe SAS group. Low-density lipoprotein cholesterol (LDL-C) tended to be higher in the severe group, but not significantly so. On the contrary, high-density lipoprotein cholesterol (HDL-C) and triglyceride were not higher among patients in the severe group. There were no significant differences in parameters of atherosclerosis. Only PS was significantly higher in the severe SAS group.
Table 2.
Clinical characteristics of patients with and without severe SAS
| Parameter | AHI >30 | AHI ≤30 | p value |
|---|---|---|---|
| Age, years | 73.4 ± 10.4 | 70.2 ± 9.5 | 0.10175 |
| Male, % | 87.8 | 85.5 | 0.73421 |
| BMI | 23.8 ± 3.9 | 24.2 ± 4.0 | 0.56466 |
| Systolic blood pressure, mm Hg | 131.9 ± 22.4 | 135.7 ± 20.9 | 0.36088 |
| Diastolic blood pressure, mm Hg | 73.2 ± 15.6 | 73.9 ± 12.0 | 0.78003 |
| History of CVD, % | 41.5 | 47.8 | 0.51697 |
| History of DM, % | 58.5 | 55.1 | 0.72316 |
| History of hypertension, % | 97.6 | 97.1 | 0.88623 |
| Angiotensin II receptor blocker, % | 73.1 | 71.0 | 0.80796 |
| Angiotensin converting enzyme inhibitor, % | 4.9 | 11.6 | 0.23611 |
| Calcium channel blocker, % | 78.0 | 69.1 | 0.33404 |
| β-Blocker, % | 31.7 | 29.0 | 0.76333 |
| Loop diuretics, % | 36.6 | 29.0 | 0.40797 |
| ESA, % | 36.6 | 17.4 | 0.02371 |
| Vitamin D receptor activator, % | 4.9 | 10.1 | 0.32981 |
| Statin, % | 51.2 | 55.0 | 0.69519 |
| NaHCO3, % | 19.5 | 18.8 | 0.95965 |
| White blood cells, /μl | 6,251.2 ± 1,636.2 | 6,220.3 ± 1,689.6 | 0.92535 |
| Hemoglobin, g/dl | 11.1 ± 1.9 | 11.4 ± 1.5 | 0.37400 |
| Albumin, g/dl | 3.8 ± 0.5 | 3.9 ± 0.5 | 0.37216 |
| Alanine transaminase, U/l | 16.6 ± 15.1 | 18.2 ± 12.0 | 0.52631 |
| Alkaline phosphatase, U/l | 246.1 ± 77.0 | 236.2 ± 77.0 | 0.51387 |
| Uric acid, mg/dl | 7.1 ± 1.7 | 7.2 ± 1.9 | 0.86089 |
| Blood urea nitrogen, mg/dl | 44.3 ± 17.0 | 42.8 ± 15.8 | 0.63909 |
| Creatinine, mg/dl | 3.2 ± 1.3 | 3.1 ± 1.4 | 0.72728 |
| eGFR, ml/min/m2 | 19.2 ± 9.5 | 20.2 ± 9.8 | 0.58407 |
| Na, mEq/l | 138.9 ± 3.0 | 138.8 ± 2.5 | 0.78026 |
| K, mEq/l | 4.8 ± 0.6 | 4.7 ± 0.6 | 0.57355 |
| Adjusted Ca, mg/dl | 9.2 ± 0.5 | 9.3 ± 0.6 | 0.67205 |
| P, mg/dl | 3.9 ± 1.0 | 3.9 ± 0.8 | 0.92155 |
| C-reactive protein, mg/dl | 0.32 ± 0.35 | 0.42 ± 0.70 | 0.39543 |
| LDL-C, mg/dl | 101.6 ± 40.6 | 88.38 ± 30.0 | 0.05310 |
| HDL-C, mg/dl | 42.39 ± 9.9 | 44.4 ± 11.5 | 0.33099 |
| Triglyceride, mg/dl | 146.2 ± 81.7 | 182.3 ± 198.4 | 0.27131 |
| Intact parathyroid hormone, pg/ml | 167.3 ± 107.9 | 150.7 ± 107.4 | 0.43 |
| Brain natriuretic peptide, pg/ml | 216.6 ± 326.0 | 100.3 ± 135.7 | 0.01071 |
| pH | 7.360 ± 0.040 | 7.360 ± 0.040 | 0.79913 |
| HCO3−, mEq/l | 20.4 ± 2.7 | 20.5 ± 3.0 | 0.81865 |
| Cardiothoracic ratio, % | 52.8 ± 6.5 | 49.1 ± 5.7 | 0.00223 |
| Urinary protein, g/day | 1.6 ± 1.9 | 1.5 ± 1.7 | 0.68431 |
| Thoracic aortic calcification, % | 46.3 | 31.9 | 0.10477 |
| Abdominal aortic calcification, % | 55.6 | 67.2 | 0.24750 |
| Valve calcification, % | 48.8 | 37.7 | 0.25381 |
| Lower ABI | 1.08 ± 0.16 | 1.12 ± 0.13 | 0.23058 |
| Faster PWV, cm/s | 1,920.5 ± 522.5 | 1,902.9 ± 380.6 | 0.84400 |
| IMTmax, mm | 2.82 ± 1.07 | 2.68 ± 1.49 | 0.58825 |
| PS | 12.8 ± 5.96 | 9.38 ± 5.47 | 0.00265 |
eGFR = Estimated glomerular filtration rate.
Correlations between AHI and Evaluated Parameters
Table 3 shows the non-adjusted odds ratios (ORs) for severe SAS with the evaluated parameters. Only PS was significantly different. There were no significant changes in any of the other parameters measured, including markers of atherosclerosis.
Table 3.
Non-adjusted ORs for severe SAS with the evaluated parameters
| Parameter | OR | 95% CI | p value |
|---|---|---|---|
| Thoracic aortic calcification | 1.8450 | 0.8326–4.0886 | 0.1314 |
| Abdominal aortic calcification | 0.6512 | 0.2787–1.5216 | 0.3219 |
| Valve calcification | 1.5751 | 0.7203–3.4441 | 0.2551 |
| Lower ABI | 0.1905 | 0.0126–2.8772 | 0.2313 |
| Faster PWV | 1.0001 | 0.9992–1.0010 | 0.8422 |
| IMTmax | 1.0823 | 0.8134–1.4401 | 0.5873 |
| PS | 1.1108 | 1.0339–1.1934 | 0.0041 |
Table 4 shows the ORs for severe SAS after adjustment for numerous parameters, including age, sex, BMI, systolic blood pressure, comorbid conditions and parameters of kidney function and plasma lipids [21]. Of the seven parameters of atherosclerosis evaluated, only PS was a significant predictor of severe SAS (OR = 1.13, 95% confidence interval [CI] 1.0214-1.2559, p = 0.0182).
Table 4.
Adjusted ORs for severe SAS with the evaluated parameters
| Parameter | OR | 95% CI | p value |
|---|---|---|---|
| Thoracic aortic calcification | 1.6902 | 0.5598–5.1028 | 0.3519 |
| Abdominal aortic calcification | 0.3310 | 0.1007–1.0882 | 0.0686 |
| Valve calcification | 1.2703 | 0.4703–3.4309 | 0.6370 |
| Lower ABI | 0.7966 | 0.0242–26.1959 | 0.8984 |
| Faster PWV | 0.9996 | 0.9982–1.0009 | 0.5279 |
| IMTmax | 1.0583 | 0.7367–1.5203 | 0.7590 |
| PS | 1.1326 | 1.0214–1.2559 | 0.0182 |
Adjusted for age, sex, BMI, systolic blood pressure, DM, CVD, ESA, estimated glomerular filtration rate, LDL-C, brain natriuretic peptide and cardiothoracic ratio.
Relationship between AHI and PS
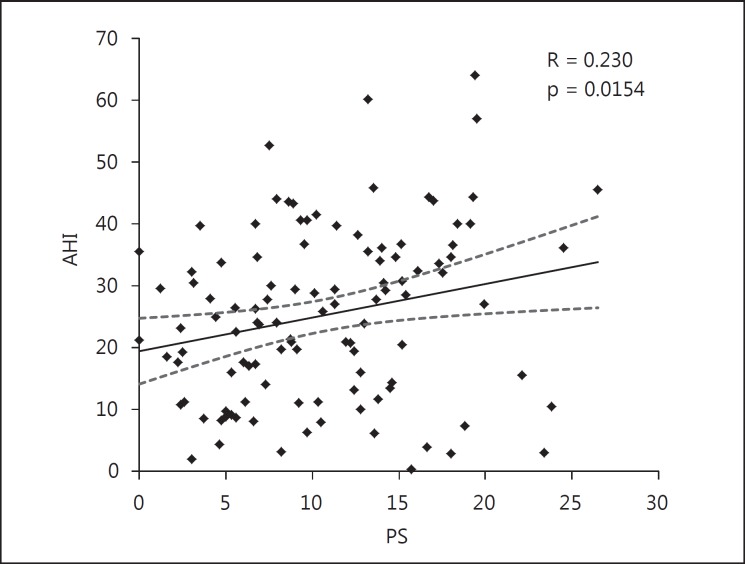
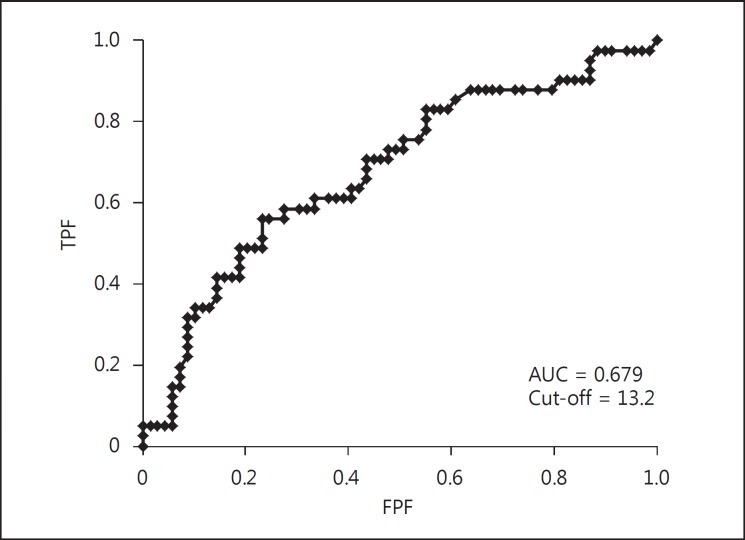
Figure 1 graphically represents the positive correlation between AHI and PS. This association suggests that PS is associated with the severity of SAS. Figure 2 shows the ROC curve for prediction of severe SAS using the PS. The area under the curve (AUC) was 0.679 and the cut-off value of PS was 13.2. Therefore, it seems that PS by itself is not a strong indicator for severe SAS.
Fig. 1.
Correlation of AHI and PS (n = 110). A positive correlation was observed between PS and AHI. The mean regression line is indicated by the solid line and the 95% CIs are indicated by the dotted lines.
Fig. 2.
ROC curve for severe SAS using the PS. The AUC was 0.679 and the cut-off value of PS was 13.2.
Association between Plaque Quality and Severity of SAS
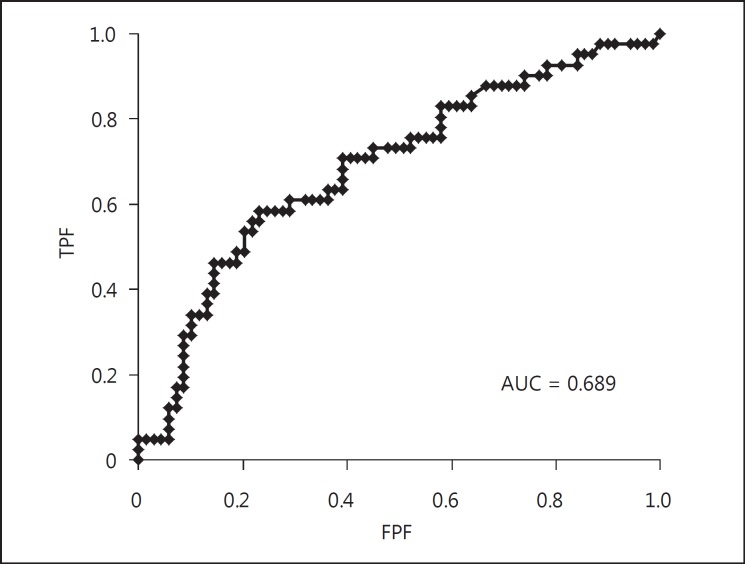
Table 5 shows the relationship between the severity of SAS and plaque quality. More severe SAS was associated with higher likelihood of mixed and hard plaque quality. Using these two categories (mixed and hard plaque quality) we conducted a logistic regression analysis using AHI >30 (severe SAS) as the criterion variable. Table 6 shows the non-adjusted ORs for severe SAS for mixed and hard plaque quality, and table 7 shows the ORs for severe SAS for mixed and hard plaque quality adjusted for age, sex, BMI, systolic blood pressure, DM, CVD, ESAs, estimated glomerular filtration rate, LDL-C, brain natriuretic peptide and cardiothoracic ratio. Only mixed plaque was significantly associated with severe SAS before and after adjustment. We used a prediction formula for AHI using PS and mixed plaque quality: AHI = 0.1072 × PS + 0.2567 × mixed (with mixed plaque = 1, without mixed plaque = 0). However, the ROC curve with the formula (fig. 3) did not significantly increase the prediction accuracy (AUC = 0.689). Therefore, we evaluated the relationship between PS and quality of plaque. We found that PS was significantly correlated with the presence of mixed plaque (table 8, correlation ratio = 0.48, p < 0.0001). This most likely indicates that there is an overlap between these two factors that results in no increase in the prediction accuracy of severe SAS.
Table 5.
Distribution regarding severity of SAS in each plaque quality
| Severity | Soft | Intermediate | Mixed | Hard |
|---|---|---|---|---|
| AHI ≤5 (n = 7) | 0 (0.0%) | 6 (85.7%) | 4 (57.1%) | 0 (0.0%) |
| 5 < AHI ≤ 15 (n = 23) | 1 (4.3%) | 23 (100.0%) | 8 (34.8%) | 1 (4.3%) |
| 15 < AHI ≤ 30 (n = 39) | 0 (0.0%) | 38 (97.4%) | 20 (51.3%) | 2 (5.1%) |
| AHI >30 (n = 41) | 0 (0.0%) | 40 (97.6%) | 30 (73.2%) | 6 (14.6%) |
Table 6.
Non-adjusted ORs for AHI >30 in each plaque quality
| Parameter | OR | 95% CI | p value |
|---|---|---|---|
| Mixed | 3.1534 | 1.3650–7.2851 | 0.0072 |
| Hard | 3.7714 | 0.8889–16.0015 | 0.0718 |
Table 7.
Adjusted ORs for AHI >30 in each plaque quality
| Parameter | OR | 95% CI | p value |
|---|---|---|---|
| Mixed | 7.0881 | 1.7684–28.4114 | 0.0057 |
| Hard | 2.3393 | 0.4435–12.3396 | 0.3165 |
Adjusted for age, sex, BMI, systolic blood pressure, DM, CVD, ESA, estimated glomerular filtration rate, LDL-C, brain natriuretic peptide and cardiothoracic ratio.
Fig. 3.
ROC curve for severe SAS using PS and plaque quality. Prediction formula for the AHI with the information of PS and with/without mixed plaque quality: AHI = 0.1072 × PS + 0.2567 × mixed (with mixed plaque = 1, without mixed plaque = 0). The AUC was 0.689.
Table 8.
Correlation of AHI >30 in each plaque quality
| Parameter | Correlation ratio | p value |
|---|---|---|
| Soft | 0.0629 | 0.5140 |
| Intermediate | 0.2613 | 0.0058 |
| Mixed | 0.4800 | <0.0001 |
| Hard | 0.1240 | 0.1970 |
Discussion
We examined numerous parameters of atherosclerosis, including ABI, PWV, IMT and PS, in order to investigate which of them was associated with the severity of SAS among patients who attended a CKD educational program. We found a relationship only between PS in the carotid artery and the severity of SAS among patients with CKD. PWV has been shown to be faster among SAS patients than among control subjects [3]. In addition, AHI is known to be related to calcification of the coronary artery [4], the duration of SAS is related to IMT [5], and the severity of SAS is associated with IMT [6]. In the present study, our findings did not show a relationship between PWV, IMT or ABI and the severity of SAS. However, our findings show a significant association between PS and the severity of SAS. All patients in this research had CKD since they attended a CKD educational program. Therefore, the baseline condition of the carotid artery was already worse than that in the general population. This fact must have affected the result that we could not find a correlation between some atherosclerotic parameters such as PWV, IMT or ABI and SAS. We strongly believe that PS has a high sensitivity in detecting atherosclerosis as compared to other atherosclerotic factors. All of the CKD patients already had worse carotid arteries than the general population, and our study compared CKD patients with SAS and CKD patients without SAS, so this fact might have masked atherosclerosis-detecting abilities that some parameters have in a way. To our knowledge, ours is the first study to have investigated the association between comprehensive parameters of atherosclerosis and the severity of SAS in patients with CKD. Although previous studies have focused on a single parameter, a strength of our investigation is that we examined multiple ones and found PS to be the marker most strongly correlated with the severity of SAS. Although the mechanisms underlying atherosclerosis in patients with SAS remain poorly understood, some evidence suggests that inflammation may be an important mechanism of atherosclerosis in SAS. Chronic intermittent hypoxia caused by SAS is a major stimulus for oxidative stress with the production of systemic inflammation. This inflammatory process with highly reactive oxygen radicals and ischemia-reperfusion injury to the vascular wall during repetitive episodes of nocturnal apnea may result in an increased risk for atherosclerosis [22]. The percentage of ESA users was higher in the group with severe SAS (AHI >30) despite the fact that there was no significant difference in the amount of hemoglobin between the groups. This finding may indicate that intermittent hypoxia caused inflammation, resulting in resistance to ESAs [23,24], which may be the cause of the increased number of ESA users among the severe group. As other reports show, patients with mixed plaque tend to have severe carotid artery stenosis as compared to those with hypo- and hyperechoic plaque [25]. This we believe is the reason why we detected a significant difference among these three qualities.
Our study has some limitations. First, this was a single-center study, so there may be some bias, such as features of the facility and the patient population. Second, this was a cross-sectional study; thus, we can determine relationships but not causality. Third, this study included only Japanese patients. Consequently, it is not clear whether our findings are applicable to all ethnicities.
Conclusions
Many patients with CKD also have severe SAS. We found that high PS is correlated with severe SAS. Our findings suggest that patients with CKD should be monitored for SAS. In addition, patients with CKD and severe SAS may have a higher PS and may thus be at higher risk for cardiovascular events.
Statement of Ethics
This study was approved by the Ethical Committee of the Institutional Review Board of the Japanese Red Cross Nagoya Daini Hospital and conducted under the Declaration of Helsinki.
Disclosure Statement
The authors declare no conflicts of interest.
References
- 1.Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342:1378–1384. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- 2.Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365:1046–1053. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]
- 3.Nagahama H, Soejima M, Uenomachi H, Higashi Y, Yotsumoto K, Samukawa T, et al. Pulse wave velocity as an indicator of atherosclerosis in obstructive sleep apnea syndrome patients. Intern Med. 2004;43:184–188. doi: 10.2169/internalmedicine.43.184. [DOI] [PubMed] [Google Scholar]
- 4.Sorajja D, Gami AS, Somers VK, Behrenbeck TR, Garcia-Touchard A, Lopez-Jimenez F. Independent association between obstructive sleep apnea and subclinical coronary artery disease. Chest. 2008;133:927–933. doi: 10.1378/chest.07-2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ciccone MM, Scicchitano P, Mitacchione G, Zito A, Gesualdo M, Caputo P, et al. Is there a correlation between OSAS duration/severity and carotid intima-media thickness? Respir Med. 2012;106:740–746. doi: 10.1016/j.rmed.2011.12.016. [DOI] [PubMed] [Google Scholar]
- 6.Suzuki T, Nakano H, Maekawa J, Okamoto Y, Ohnishi Y, Yamauchi M, et al. Obstructive sleep apnea and carotid-artery intima-media thickness. Sleep. 2004;27:129–133. doi: 10.1093/sleep/27.1.129. [DOI] [PubMed] [Google Scholar]
- 7.Chambless LE, Heiss G, Folsom AR, Rosamond W, Szklo M, Sharrett AR, et al. Association of coronary heart disease incidence with carotid arterial wall thickness and major risk factors: the Atherosclerosis Risk in Communities (ARIC) Study, 1987-1993. Am J Epidemiol. 1997;146:483–494. doi: 10.1093/oxfordjournals.aje.a009302. [DOI] [PubMed] [Google Scholar]
- 8.Arik B, Inci MF, Gumus C, Varol K, Ege MR, Dogan OT, et al. Advanced age and apnea-hypopnea index predict subclinical atherosclerosis in patients with obstructive sleep apnea syndrome. Multidiscip Respir Med. 2013;8:9. doi: 10.1186/2049-6958-8-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hanly PJ, Ahmed SB. Sleep apnea and the kidney: is sleep apnea a risk factor for chronic kidney disease? Chest. 2014;146:1114–1122. doi: 10.1378/chest.14-0596. [DOI] [PubMed] [Google Scholar]
- 10.Roumelioti ME, Buysse DJ, Sanders MH, Strollo P, Newman AB, Unruh ML. Sleep-disordered breathing and excessive daytime sleepiness in chronic kidney disease and hemodialysis. Clin J Am Soc Nephrol. 2011;6:986–994. doi: 10.2215/CJN.05720710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sarnak MJ, Levey AS, Schoolwerth AC, Coresh J, Culleton B, Hamm LL, et al. Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Circulation. 2003;108:2154–2169. doi: 10.1161/01.CIR.0000095676.90936.80. [DOI] [PubMed] [Google Scholar]
- 12.Moriwaki H, Matsumoto M, Handa N, Isaka Y, Hashikawa K, Oku N, et al. Functional and anatomic evaluation of carotid atherothrombosis. A combined study of indium 111 platelet scintigraphy and B-mode ultrasonography. Arterioscler Thromb Vasc Biol. 1995;15:2234–2240. doi: 10.1161/01.atv.15.12.2234. [DOI] [PubMed] [Google Scholar]
- 13.Santos-Silva R, Sartori DE, Truksinas V, Truksinas E, Alonso FF, Tufik S, et al. Validation of a portable monitoring system for the diagnosis of obstructive sleep apnea syndrome. Sleep. 2009;32:629–636. doi: 10.1093/sleep/32.5.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shanmugasundaram M, Ram VK, Luft UC, Szerlip M, Alpert JS. Peripheral arterial disease - what do we need to know? Clin Cardiol. 2011;34:478–482. doi: 10.1002/clc.20925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Handa N, Matsumoto M, Maeda H, Hougaku H, Ogawa S, Fukunaga R, et al. Ultrasonic evaluation of early carotid atherosclerosis. Stroke. 1990;21:1567–1572. doi: 10.1161/01.str.21.11.1567. [DOI] [PubMed] [Google Scholar]
- 16.Holdsworth RJ, McCollum PT. Ultrasonographic carotid plaque morphology in predicting stroke risk. Br J Surg. 1996;83:1479–1480. doi: 10.1002/bjs.1800831051. [DOI] [PubMed] [Google Scholar]
- 17.Kales A, Bixler EO, Cadieux RJ, Schneck DW, Shaw LC, 3rd, Locke TW, et al. Sleep apnoea in a hypertensive population. Lancet. 1984;2:1005–1008. doi: 10.1016/s0140-6736(84)91107-3. [DOI] [PubMed] [Google Scholar]
- 18.Schafer H, Koehler U, Ewig S, Hasper E, Tasci S, Luderitz B. Obstructive sleep apnea as a risk marker in coronary artery disease. Cardiology. 1999;92:79–84. doi: 10.1159/000006952. [DOI] [PubMed] [Google Scholar]
- 19.Gami AS, Howard DE, Olson EJ, Somers VK. Day-night pattern of sudden death in obstructive sleep apnea. N Engl J Med. 2005;352:1206–1214. doi: 10.1056/NEJMoa041832. [DOI] [PubMed] [Google Scholar]
- 20.Sampol G, Romero O, Salas A, Tovar JL, Lloberes P, Sagales T, et al. Obstructive sleep apnea and thoracic aorta dissection. Am J Respir Crit Care Med. 2003;168:1528–1531. doi: 10.1164/rccm.200304-566OC. [DOI] [PubMed] [Google Scholar]
- 21.Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta K, et al. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009;53:982–992. doi: 10.1053/j.ajkd.2008.12.034. [DOI] [PubMed] [Google Scholar]
- 22.Toraldo DM, De Nuccio F, De Benedetto M, Scoditti E. Obstructive sleep apnoea syndrome: a new paradigm by chronic nocturnal intermittent hypoxia and sleep disruption. Acta Otorhinolaryngol Ital. 2015;35:69–74. [PMC free article] [PubMed] [Google Scholar]
- 23.Tanaka Y, Joki N, Hase H, Iwasaki M, Ikeda M, Ando R, et al. Effect of erythropoietin-stimulating agent on uremic inflammation. J Inflamm (Lond) 2012;9:17. doi: 10.1186/1476-9255-9-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kanbay M, Perazella MA, Kasapoglu B, Koroglu M, Covic A. Erythropoiesis stimulatory agent-resistant anemia in dialysis patients: review of causes and management. Blood Purif. 2010;29:1–12. doi: 10.1159/000245041. [DOI] [PubMed] [Google Scholar]
- 25.AbuRahma AF, Wulu JT, Jr, Crotty B. Carotid plaque ultrasonic heterogeneity and severity of stenosis. Stroke. 2002;33:1772–1775. doi: 10.1161/01.str.0000019127.11189.b5. [DOI] [PubMed] [Google Scholar]