Abstract
Context:
Isolated chondral and osteochondral defects of the knee are a difficult clinical challenge, particularly in younger patients for whom alternatives such as partial or total knee arthroplasty are rarely advised. Numerous surgical techniques have been developed to address focal cartilage defects. Cartilage treatment strategies are characterized as palliation (eg, chondroplasty and debridement), repair (eg, drilling and microfracture [MF]), or restoration (eg, autologous chondrocyte implantation [ACI], osteochondral autograft [OAT], and osteochondral allograft [OCA]).
Evidence Acquisition:
PubMed was searched for treatment articles using the keywords knee, articular cartilage, and osteochondral defect, with a focus on articles published in the past 5 years.
Study Design:
Clinical review.
Level of Evidence:
Level 4.
Results:
In general, smaller lesions (<2 cm2) are best treated with MF or OAT. Furthermore, OAT shows trends toward greater longevity and durability as well as improved outcomes in high-demand patients. Intermediate-size lesions (2-4 cm2) have shown fairly equivalent treatment results using either OAT or ACI options. For larger lesions (>4 cm2), ACI or OCA have shown the best results, with OCA being an option for large osteochondritis dissecans lesions and posttraumatic defects.
Conclusion:
These techniques may improve patient outcomes, though no single technique can reproduce normal hyaline cartilage.
Keywords: knee, articular cartilage, osteochondral defect, microfracture, mosaicplasty, autologous chondrocyte implantation, osteochondral autograft transfer, cartilage restoration
Focal chondral defects occur in up to two-thirds of patients undergoing knee arthroscopy.11 Symptomatic lesions may cause pain, locking, catching, swelling, and functional impairment. Their complaints may be worse than those with anterior cruciate ligament–deficient knees, and quality of life may be affected to the same extent as in patients scheduled for knee replacement.22 Isolated chondral and osteochondral defects of the knee are a difficult clinical challenge, particularly in younger patients for whom alternatives such as partial or total knee arthroplasty are rarely advised (Figure 1, a and b).
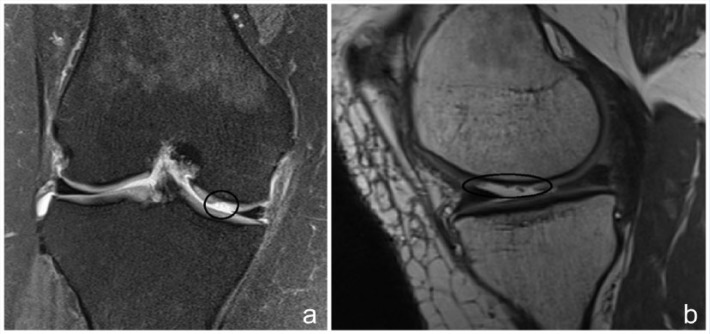
Figure 1.
(a) Coronal magnetic resonance image (MRI) demonstrating a medial femoral condyle osteochondral defect. (b) Sagittal MRI of osteochondral defect involving the weightbearing portion of the medial femoral condyle.
The infrequent healing associated with cartilage defects typically leads to the production of type I collagen and fibrocartilaginous tissue as opposed to normal hyaline cartilage. This fibrous repair tissue has diminished resiliency, less stiffness, poor wear characteristics, and a predilection for advancing arthritis.10 The “Holy Grail” for treatment of focal articular cartilage lesions is a method that restores organized hyaline cartilage through a practical, minimally invasive approach with minimal morbidity not only perioperatively but also over an extended period of time.45
Numerous surgical techniques have been developed to address focal cartilage defects. Cartilage treatment strategies can be characterized as palliation (eg, chondroplasty and debridement), repair (eg, drilling and microfracture [MF]), or restoration (eg, autologous chondrocyte implantation [ACI], osteochondral autograft transfer [OAT], and osteochondral allograft [OCA]).34 The large number of surgical options for chondral defects are evidence of the difficulty in replicating hyaline cartilage function (Table 1).
Table 1.
Surgical procedure based on size of osteochondral lesion
ACI, autologous chondrocyte implantation; OAT, osteochondral autograft transfer; OCA, osteochondral allograft.
Higher-demand patients.
Bone loss/deformity.
Microfracture
Microfracture is a marrow stimulation technique considered the first-line treatment given its minimally invasive nature, technical ease, limited surgical morbidity, and relatively low cost (Figures 1 and 2).52
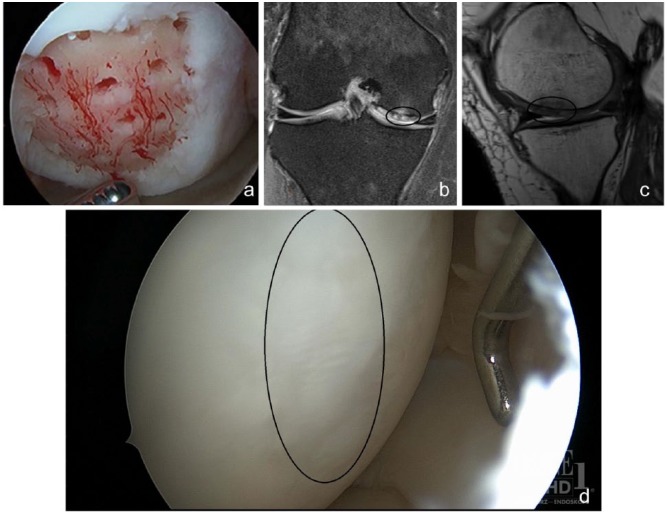
Figure 2.
(a) Arthroscopic view after microfracture treatment of the medial femoral condyle in patient from Figure 1. (b, c) Follow-up coronal and sagittal magnetic resonance images 1 year after microfracture showing filling of osteochondral defect. (d) Second-look arthroscopy 1 year after microfracture demonstrating filling of previous defect with reparative tissue.
At a mean 7-year follow-up, 80% of patients rated themselves as improved after MF, with patients younger than 35 years showing the most improvement.50 The mean size of chondral defect was 2.8 cm2. Of 25 National Football League players who underwent MF for treatment of full-thickness chondral lesions, three-fourths were able to return to football the following season for an average of almost 5 additional seasons.51 Biopsies after MF have noted that approximately 10% had hyaline cartilage, with the majority having predominantly fibrocartilage.25 Lesions less than 4 cm2 were likely to respond better to MF in the first 2 years. Systematic reviews have similarly demonstrated clear improvement in knee function at 24 months after MF but inconclusive durability and treatment failure beyond 5 years.16,37
Autologous Matrix-Induced Chondrogenesis
Autologous matrix-induced chondrogenesis (AMIC) combines MF surgery with the application of a bilayer collagen membrane that physically stabilizes the clot and may guide and enhance marrow-derived repair. A multicenter, randomized controlled trial compared BST-CarGel (Piramal Life Sciences, Bio-Orthopaedic Division) treatment with MF alone in the repair of cartilage lesions in the knee.49 At 12 months, BST-CarGel treatment resulted in greater lesion filling and superior repair tissue quality compared with MF treatment alone; however, clinical symptoms were equivalent between groups.
Osteochondral Autograft Transfer
With the OAT technique, defects can be filled immediately with mature, hyaline articular cartilage.20,33 The area to be treated should not exceed 4 cm2, and donor site morbidity can be a concern.20 Perpendicular access to the cartilage surface, either arthroscopically or via a mini-open technique, is critical to allow the donor plug to be flush to re-create the normal articular contour and contact pressures (Figure 3).12,26,40
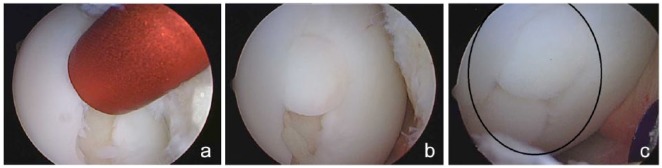
Figure 3.
(a) Arthroscopic view of cylindrical sizer used to characterize defect and pattern for osteochondral autograft plug transfer. (b) First of 2 plugs sunk flush with the surrounding articular cartilage. (c) Two plugs have been transferred to fill the osteochondral defect.
At up to 10 years postoperatively, good or excellent results were obtained in approximately 90% of patients undergoing femoral condyle or tibial plateau mosaicplasty.20 Outcomes vary greatly depending on age, sex, and size of the lesion, with increased failure rates in patients older than 40 years, women, and defect size greater than 3 cm2.47
Autologous Chondrocyte Implantation
Autologous chondrocyte implantation is most useful for younger patients who have single defects larger than 2 cm2.4,43 Disadvantages include the need for 2 stages and an open arthrotomy, expense, and a significant rate of reoperation for graft hypertrophy, specifically with first-generation ACI treatments. Second- (porcine membrane) and third-generation (matrix-associated) ACI treatments have not been approved in the United States at this time.
Results of 23 patients at 39-month follow-up showed good or excellent clinical results in 70% of cases (femoral condylar defects had greater rates of healing, nearly 90%) (Figure 4, a and b).4 Almost 3 of 4 biopsies had hyaline-like appearance, but the repair tissue is not identical to hyaline cartilage, and fibrocartilage was found in some.44 Durable clinical results have been shown up to 11 years after ACI treatment.42 First-generation ACI for large (mean, 5.33 cm2), full-thickness, symptomatic chondral defects of the knee showed significant improvements in pain relief and functional outcome measures at 8-year follow-up.3
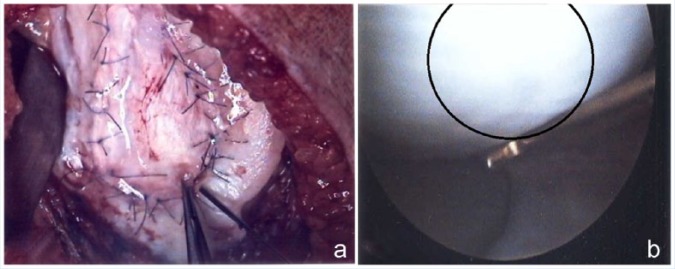
Figure 4.
(a) Arthoscopic view of autologous chondrocyte implantation (ACI) with sutured patch. (b) Second-look arthoscopy 1 year after ACI demonstrating filling of defect with reparative tissue.
In patients with at least 1 previously failed non-ACI treatment for an osteochondritis dissecans knee lesion, significant reduction in pain and improved function was noted for up to 4 years after ACI, despite the complexity and severity of these lesions.8 However, treatment failure occurred in 1 of 5 patients in this series. ACI after failed microfracture is associated with a significantly greater failure rate and inferior clinical outcome when compared with ACI as a first-line treatment.41
In a series of more than 200 patients treated with ACI for larger lesions (mean defect size, 8.4 ± 5.5 cm2), ACI provided durable outcomes with a survivorship of 71% at 10 years and improved function in 75% of patients.36 A history of prior marrow stimulation as well as the treatment of very large defects was associated with an increased risk of failure. Magnetic resonance imaging (MRI) is used to evaluate the cartilage preoperatively and at final follow-up using the Magnetic Resonance Observation of Cartilage Repair Tissue (MOCART) score.32 In 23 patients (mean age, 30.5 ± 8.2 years) with full-thickness chondral lesions of the distal femur, ACI resulted in a substantial improvement in all clinical outcome parameters at 1 year and a mean 9.9 years postoperatively.38 Younger patients with a shorter duration of preoperative symptoms and smaller defect sizes benefitted the most. MRI findings confirmed complete defect filling in one-half of patients at final follow-up.
Comparison of Techniques
A comparison of the cartilage repair and restoration techniques is outlined in Table 2.
Table 2.
Comparison of cartilage repair and restoration techniques
| Site of Lesion | No. of Knees | Mean Age, y | Median Follow-up (Range), y | Mean Lesion Size (Range), cm2 | Outcomes | |
|---|---|---|---|---|---|---|
| MF vs OAT | ||||||
| Krych et al28 | Femoral condyle, trochlea | MF = 48 OAT = 48 |
MF = 32.5 OAT = 29.7 |
4.4 (2.0-10.0) | MF = 2.6 (1.0-6.3) OAT = 2.7 (1.0-6.3) |
OAT: superior activity level No difference in PROM |
| Gudas et al18 | Femoral condyle | MF = 29 OAT = 28 |
MF = 24.3 OAT = 24.6 |
3.1 (3.0-3.2) | MF = 2.8 (1.0-4.0) OAT = 2.8 (1.0-4.0) |
OAT: higher PROM and ICRS scores postop, greater % hyaline cartilage Achieved preinjury level of activity: OAT (93%), MF (52%) |
| Ulstein et al54 | Femoral condyle, trochlea | MF = 11 OAT = 14 |
MF = 31.7 OAT = 32.7 |
9.8 (4.9-11.4) | MF = 2.6 (2.0-5.2) OAT = 3.0 (2.0-6.0) |
No difference in PROM, muscle strength, or radiological outcome |
| MF vs ACI | ||||||
| Knutsen et al25 | Femoral condyle | MF = 40 ACI = 40 |
MF = 31.1 ACI = 33.3 |
2a | MF = 4.5b
ACI = 5.1b |
MF: better PROM at 2 years than ACI No histologic difference in specimens at second look Failure rate: MF (2.5%) vs ACI (5%) Improved PROM for younger patients (<30 y) in both groups |
| Knutsen et al24 | Femoral condyle | MF = 40 ACI = 40 |
MF = 31.1 ACI = 33.3 |
5a | MF = 4.5b
ACI = 5.1b |
No difference in PROM Failure rate: MF (23%) vs ACI (23%) 1/3 patients in each group with radiographic OA |
| Kon et al27 | Femoral condyle, trochlea | MF = 20 +ACI = 21 |
MF = 26.5 +ACI = 23.7 |
7.6 (4.0 – 11.0) | MF = 1.9b
ACI = 2.1b,c |
Return to competition: MF (80%), ACI (86%) Median RTP (mo): MF (8), ACI (12.5) ACI more stable results long term |
| OAT vs ACI | ||||||
| Horas et al23 | Femoral condyle | OAT = 20 ACI = 20 |
OAT = 35.4 ACI = 31.4 |
2a | OAT = 3.6 (3.2-5.6) ACI = 3.9 (3.2-5.6) |
No difference in PROM except for Lysholm scores higher in OAT ACI defects → fibrocartilage |
| Bentley et al1 | Femoral condyle, trochlea, patella | OAT = 42 ACI = 58 |
OAT = 31.6 ACI = 30.9 |
1.6 (1.0-2.2) | 4.7 (1.0-12.0) (not delineated between OAT and ACI) |
Excellent/good PROM: OAT (69%) vs ACI (88%) All patella OATs failed Arthroscopic good/excellent ICRS grades at 1 y: ACI (82%), OAT (34%) Proud plug placement and nonstandard rehab protocol |
| Bentley et al2 | Femoral condyle, trochlea, patella | OAT = 42 ACI = 58 |
OAT = 31.6 ACI = 30.9 |
Minimum, 10 (10-12)a | OAT = 4.0(1.0-20.0) ACI = 4.4(1.0-10.5) |
Failure rate: OAT (55%) vs ACI (17%) Steep decline in OAT outcomes after 2 y Final outcomes better in ACI |
| Comparison of the 3 techniques | ||||||
| Lim et al31 | Femoral condyle | MF = 30 OAT = 22 ACI = 18 |
MF = 32.9 OAT = 30.4 ACI = 25.1 |
MF = 6.7 OAT = 5.8 ACI = 5.2 |
MF = 2.8(1.2-3.6) OAT = 2.8(1.0-4.0) ACI = 2.8 (1.5-3.8) |
80% good or excellent ICRS results in all 3 groups at second look arthroscopy No difference in PROM or postoperative MRI grades |
ACI, autologous chondrocyte implantation; MF, microfracture; OA, osteoarthritis; OAT, osteochondral autograft transfer; PROM, patient-reported outcome measures; RTP, return to play.
Follow-up range not listed in original reference or all patients evaluated at a single time (eg, 2 years postoperative).
Lesion size range not listed in original reference.
Second generation ACI (not approved for use in the United States).
Microfracture Versus Osteochondral Autograft Transfer
In a retrospective study, patients treated with MF or OAT mosaicplasty for symptomatic articular cartilage defects of the femoral condyles or trochlea had similar clinical outcomes at intermediate-term follow-up (up to 5 years). However, patients treated with OAT mosaicplasty maintained a superior level of athletic activity compared with those treated with MF.28 The OAT group had better clinical scores, more normal-appearing cartilage on visual assessment, and a subjectively greater percentage of hyaline cartilage histologically, with more than 90% of athletes able to return to their preinjury level of sport compared with only 50% in the MF group. Clinical outcomes of MF were worse in lesions larger than 2 cm2, but there was no association between clinical outcomes and lesion size when treated with OAT.18 No significant differences at long-term follow-up were seen between patients treated with MF or OAT mosaicplasty in patient-reported outcomes, muscle strength, or radiological outcome.54
Microfracture Versus Autologous Chondrocyte Implantation
At 2 years after surgery, there was little difference between the ACI and MF groups; the Short Form (SF)–36 physical component was the only score that revealed a difference that was significantly better for the MF group, possibly related to ACI being a 2-stage procedure.25 Second-look arthroscopy performed in three-fourths of the participants 2 years after surgery showed no difference using the International Cartilage Repair Society (ICRS) grading system.5 Similarly, biopsy specimens revealed no significant difference in hyaline cartilage or fibrocartilage in the repair tissues. Treatment failure at 5 years occurred in 1 of 4 patients in both groups.24
Second- and Third-Generation Autologous Chondrocyte Implantation
Forty-one professional or semiprofessional male soccer players were treated for full-thickness osteochondral defects over a 6-year period with either second-generation arthroscopic ACI (Hyalograft C) or MF.27 More than 80% of participants in both groups returned to competition. MF allowed a faster recovery but presented a greater clinical deterioration over time, whereas ACI offered more durable clinical results.
A randomized clinical trial comparing first-generation ACI with a periosteal patch to third-generation matrix-associated ACI (MACI) showed improvement in clinical outcome measures at 12 months, but there was no difference between the 2 techniques.55
Osteochondral Autograft Transfer Versus Autologous Chondrocyte Implantation
In a prospective trial comparing ACI with OAT, no significant difference in patient-reported outcomes at 2 years was seen, with the exception of improved Lysholm scores in the OAT group during the first 24 months.23 A larger trial of 100 patients randomized to either ACI or OAT demonstrated no significant difference between the groups at early follow-up.1 At 10 years, 17% in the ACI group had a failed repair and 55% in the mosaicplasty group failed.2 Surgical placement of a proud OAT graft explained the higher failure rate of the OAT cohort.
Microfracture Versus Osteochondral Autograft Transfer Versus Autologous Chondrocyte Implantation
In a prospective study with minimum 3-year follow-up, all 3 procedures (MF, OAT, and ACI) showed improvement in functional scores, with no differences between groups.31 Arthroscopy at 1 year showed excellent or good results in 80% of patients, regardless of technique. MF appeared to be a reasonable option as a first-line therapy given its ease and significant affordability relative to ACI or OAT.
A recent systematic review found 13 studies (917 subjects) that met the study inclusion criteria.21 Most of these were young patients undergoing cartilage repair or restoration with a long preoperative duration of knee symptoms with multiple previous surgical procedures (range, 0-13). The defects were moderately sized (range, 1.9-6.2 cm2) and primarily located on the medial femoral condyle. All surgical techniques demonstrated improvement in comparison with the preoperative status. There was no clear benefit of ACI versus MF in short-term (<5 years) outcomes; however, clinical outcomes after MF deteriorated after 1.5 to 2 years. ACI and OAT demonstrated equivalent short-term clinical outcomes, although there was more rapid improvement after OAT. ACI yielded the greatest proportion of complications, with graft hypertrophy occurring in 22% of first-generation treatments, 5% of second-generation treatment, and 7% of third-generation (MACI) treatment. Arthrofibrosis occurred in 17% of OAT cases, 2.5% of ACI cases, and 0.4% of MF cases. Younger patients with a shorter preoperative duration of symptoms and fewer prior surgical procedures had the best outcomes after both ACI and MF. A defect size of >4 cm2 was the only factor predictive of better outcomes when ACI was compared with other techniques.
Osteochondral Allograft Transplantation
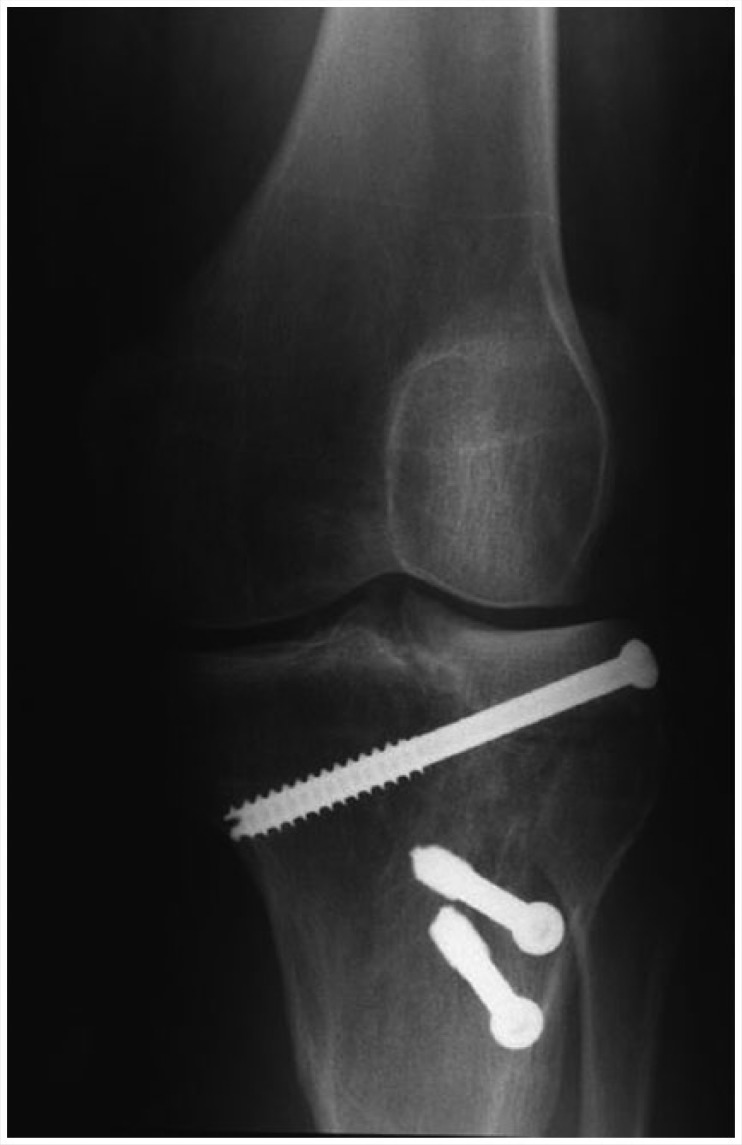
If a cartilage defect is too large for an autograft or a patient has failed a cartilage repair procedure, then a fresh OCA transplantation (mega-OAT) may be used (Figure 5).46 OCA transplantation is a single-stage technique for large osteochondral defects, in the setting of extensive subchondral bone loss.15 Chondrocyte viability directly correlates with the clinical success of OCA transplantation.39 Fresh OCAs stored at physiologic temperature have the highest level of chondrocyte viability.6
Figure 5.
Anteroposterior radiograph of a lateral unicompartmental osteochondral allograft.
One hundred twenty-two patients (129 knees) underwent OCA of the femoral condyle with graft failure defined as revision or conversion to arthroplasty.30 Survivorship was 82% at 10 years, 74% at 15 years, and 66% at 20 years. Forty-seven percent of patients underwent reoperations, and 24% failed at a mean 7.2 years. Patients older than 30 years at time of surgery and with 2 or more previous surgeries were associated with allograft failure.
A study on 60 patients with femoral condylar allografts showed 95% graft survival at 5 years and 85% at 10 years.17 Unfortunately, limited data are available on the use of OCA transplantation in an athletic population. In a recent study with 2.5-year follow-up, nearly 80% had returned to sport at the preinjury level, with all collegiate and professional athletes able to return to play.29 Risk factors for not returning to sport were older than 25 years and preoperative symptoms for longer than 12 months.
Stem Cells, Cartilage Regeneration, and Gene Therapy
The treatment options discussed thus far result in the formation of predominantly fibrocartilage. The limited capacity of damaged cartilage to regenerate and the potential morbidity associated with implanting or transferring bone and cartilage make cartilage regeneration an attractive alternative.53 The future of managing cartilage defects lies in providing biologic solutions through cartilage regeneration and tissue engineering.
Growth factors (eg, bone morphogenetic protein 2 [BMP-2] and fibroblast growth factor 2 [FGF-2]) are active molecules that can stimulate cell growth, enhance chondrogenesis, and augment the management of cartilage defects. Platelet-rich plasma (PRP) injections, which contain many of these growth factors, have shown promise as a possible solution to promote cartilage healing, improve clinical function, and decrease pain associated with cartilage lesions and osteoarthritis.7,9,48
Other cartilage repair options are based on the concept that chondrocytes from morselized or particulated cartilage can migrate to form new hyaline-like repair tissue that integrates with surrounding tissue. Particulated juvenile articular cartilage (PJAC; DeNovo NT Natural Tissue Graft; Zimmer) consists of allograft articular cartilage from donors younger than 13 years.14 A prospective study of 25 patients with 2-year follow-up (mean lesion size, 2.7 ± 0.8 cm2) demonstrated clinical outcome scores that were significantly greater than baseline scores, and there were no failures.13 Tissue samples taken at follow-up showed a mixture of hyaline and fibrocartilage with excellent integration of the transplanted tissue with the surrounding native articular cartilage.
Another treatment under investigation is gene therapy: using biologic factors to suppress cytokines (eg, tumor necrosis factors and interleukins). This has been useful in the management of rheumatoid arthritis.35 In the case of osteoarthritis, only transforming growth factor (TGF)–β1 has been studied in the clinical setting; its potential application for focal osteochondral defects has not been determined.19
Summary
In general, smaller lesions (<2 cm2) are best treated with MF or OAT. OAT shows trends toward greater longevity and durability as well as improved outcomes in high-demand patients. Intermediate-size lesions (2-4 cm2) have shown fairly equivalent treatment results using either OAT or ACI treatment options. For larger lesions (>4 cm2), ACI or OCA have shown the best results, with OCA being an option for bone defects seen with large osteochondritis dissecans lesions. Each patient’s treatment should be individualized taking into account lesion size, age, efficacy of treatment, patient preference, and cost (ie, ACI is a 2-stage procedure that is significantly more expensive and requires a longer rehabilitation period than OAT).
The “Holy Grail” for treatment of focal articular cartilage lesions has yet to be realized, as no single technique can reproduce normal hyaline cartilage.45 The variety and depth of emerging technologies have the potential to revolutionize the field of cartilage repair and regeneration over the next decade.
Footnotes
The authors report no potential conflicts of interest in the development and publication of this article.
References
- 1. Bentley G, Biant LC, Carrington RW, et al. A prospective, randomized comparison of autologous chondrocyte implantation versus mosaicplasty for osteochondral defects in the knee. J Bone Joint Surg Br. 2003;85:223-230. [DOI] [PubMed] [Google Scholar]
- 2. Bentley G, Biant LC, Vijayan S, Macmull S, Skinner JA, Carrington RW. Minimum ten-year results of a prospective randomized study of autologous chondrocyte implantation versus mosaicplasty for symptomatic articular cartilage lesions of the knee. J Bone Joint Surg Br. 2012;94:504-509. [DOI] [PubMed] [Google Scholar]
- 3. Beris AE, Lykissas MG, Kostas-Agnantis I, Manoudis GN. Treatment of full-thickness chondral defects of the knee with autologous chondrocyte implantation: a functional evaluation with long-term follow-up. Am J Sports Med. 2012;40:562-567. [DOI] [PubMed] [Google Scholar]
- 4. Brittberg M, Lindahl A, Nilsson A, Ohlsson C, Isaksson O, Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994;331:889-895. [DOI] [PubMed] [Google Scholar]
- 5. Brittberg M, Winalski CS. Evaluation of cartilage injuries and repair. J Bone Joint Surg Am. 2003;85(suppl 2):58-69. [DOI] [PubMed] [Google Scholar]
- 6. Bugbee WD, Convery FR. Osteochondral allograft transplantation. Clin Sports Med. 1999;18:67-75. [DOI] [PubMed] [Google Scholar]
- 7. Cavallo C, Filardo G, Mariani E, et al. Comparison of platelet-rich plasma formulations for cartilage healing: an in vitro study. J Bone Joint Surg Am. 2014;96:423-429. [DOI] [PubMed] [Google Scholar]
- 8. Cole BJ, DeBerardino T, Brewster R, et al. Outcomes of autologous chondrocyte implantation in study of the treatment of articular repair (STAR) patients with osteochondritis dissecans. Am J Sports Med. 2012;40:2015-2022. [DOI] [PubMed] [Google Scholar]
- 9. Cole BJ, Seroyer ST, Filardo G, Bajaj S, Fortier LA. Platelet-rich plasma: where are we now and where are we going? Sports Health. 2010;2:203-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Craig W, David JW, Ming HZ. A current review on the biology and treatment of the articular cartilage defects (part I & part II). J Musculoskelet Res. 2003;7:157-181. [Google Scholar]
- 11. Curl WW, Krome J, Gordon ES, Rushing J, Smith BP, Poehling GG. Cartilage injuries: a review of 31,516 knee arthroscopies. Arthroscopy. 1997;13:456-460. [DOI] [PubMed] [Google Scholar]
- 12. Epstein DM, Choung E, Ashraf I, et al. Comparison of mini-open versus arthroscopic harvesting of osteochondral autografts in the knee: a cadaveric study. Arthroscopy. 2012;28:1867-1872. [DOI] [PubMed] [Google Scholar]
- 13. Farr J, Tabet S, Margerrison E, Cole BJ. Clinical, radiographic, and histological outcomes after cartilage repair with particulated juvenile articular cartilage: a 2-year prospective study. Am J Sports Med. 2014:9;42:1417-1425. [DOI] [PubMed] [Google Scholar]
- 14. Farr J, Yao JQ. Chondral defect repair with particulated juvenile cartilage allograft. Cartilage. 2011;2:346-353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gortz S, Bugbee WD. Allografts in articular cartilage repair. Instr Course Lect. 2007;56:469-480. [PubMed] [Google Scholar]
- 16. Goyal D, Keyhani S, Lee EH, Hui JH. Evidence-based status of microfracture technique: a systematic review of level I and II studies. Arthroscopy. 2013;29:1579-1588. [DOI] [PubMed] [Google Scholar]
- 17. Gross AE, Shasha N, Aubin P. Long-term follow-up of the use of fresh osteochondral allografts for posttraumatic knee defects. Clin Orthop Relat Res. 2005;(435):79-87. [DOI] [PubMed] [Google Scholar]
- 18. Gudas R, Kalesinskas RJ, Kimtys V, et al. A prospective randomized clinical study of mosaic osteochondral autologous transplant versus microfracture for the treatment of osteochondral defects in the knee joint in young athletes. Arthroscopy. 2005;21:1066-1075. [DOI] [PubMed] [Google Scholar]
- 19. Ha CW, Noh MJ, Choi KB, Lee KH. Initial phase I safety of retrovirally transduced human chondrocytes expressing transforming growth factor-beta-1 in degenerative arthritis patients. Cryotherapy. 2012;14:247-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hangody L, Fules P. Autologous osteochondral mosaicplasty for the treatment of full-thickness defects of the weight-bearing joints: ten years of experimental and clinic experience. J Bone Joint Surg Am. 2003;85-A(suppl 2):25-32. [DOI] [PubMed] [Google Scholar]
- 21. Harris JD, Siston RA, Pan X, Flanigan DC. Autologous chondrocyte implantation: a systematic review. J Bone Joint Surg Am. 2010;92:2220-2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Heir S, Nerhus TK, Røtterud JH, et al. Focal cartilage defects in the knee impair quality of life as much as severe osteoarthritis: a comparison of knee injury and osteoarthritis outcome score in 4 patient categories scheduled for knee surgery. Am J Sports Med. 2010;382:231-237. [DOI] [PubMed] [Google Scholar]
- 23. Horas U, Pelinkovic D, Herr G, Aigner T, Schnettler R. Autologous chondrocyte implantation and osteochondral cylinder transplantation in cartilage repair of the knee joint: a prospective, comparative trial. J Bone Joint Surg Am. 2003;85-A:185-192. [DOI] [PubMed] [Google Scholar]
- 24. Knutsen G, Drogset JO, Engebretsen L, et al. A randomized trial comparing autologous chondrocyte implantation with microfracture: findings at five years. J Bone Joint Surg Am. 2007;89:2105-2112. [DOI] [PubMed] [Google Scholar]
- 25. Knutsen G, Engebretsen L, Ludvigsen TC, et al. Autologous chondrocyte implantation compared with microfracture in the knee: a randomized trial. J Bone Joint Surg Am. 2004;86-A:455-464. [DOI] [PubMed] [Google Scholar]
- 26. Koh JL, Kowalski A, Lautenschlager E. The effect of angled osteochondral grafting on contact pressure: a biomechanical study. Am J Sports Med. 2006;34:116-119. [DOI] [PubMed] [Google Scholar]
- 27. Kon E, Filardo G, Berruto M, et al. Articular cartilage treatment in high-level male soccer players: a prospective comparative study of arthroscopic second-generation autologous chondrocyte implantation versus microfracture. Am J Sports Med. 2011;39:2549-2557. [DOI] [PubMed] [Google Scholar]
- 28. Krych AJ, Harnly HW, Rodeo SA, Williams RJ., 3rd Activity levels are higher after osteochondral autograft transfer mosaicplasty than after microfracture for articular cartilage defects of the knee: a retrospective comparative study. J Bone Joint Surg Am. 2012;94:971-978. [DOI] [PubMed] [Google Scholar]
- 29. Krych AJ, Robertson CM, Williams RJ., 3rd Cartilage study group: return to athletic activity after osteochondral allograft transplantation in the knee. Am J Sports Med. 2012;40:1053-1059. [DOI] [PubMed] [Google Scholar]
- 30. Levy YD, Görtz S, Pulido PA, McCauley JC, Bugbee WD. Do fresh osteochondral allografts successfully treat femoral condyle lesions? Clin Orthop Relat Res. 2013;471:231-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lim HC, Bae JH, Song SH, Park YE, Kim SJ. Current treatments of isolated articular cartilage lesions of the knee achieve similar outcomes. Clin Orthop Relat Res. 2012;470:2261-2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Marlovits S, Striessnig G, Resinger CT, et al. Definition of pertinent parameters for the evaluation of articular cartilage repair tissue with high-resolution magnetic resonance imaging. Eur J Radiol. 2004;52:310-319. [DOI] [PubMed] [Google Scholar]
- 33. Matsusue Y, Yamamuro T, Hama H. Arthroscopic multiple osteochondral transplantation to the chondral defect in the knee associated with anterior cruciate ligament disruption. Arthroscopy. 1993;9:318-321. [DOI] [PubMed] [Google Scholar]
- 34. McNickle AG, Provencher MT, Cole BJ. Overview of existing cartilage repair technology. Sports Med Arthrosc. 2008;16:196-201. [DOI] [PubMed] [Google Scholar]
- 35. Mewar D, Wilson AG. Treatment of rheumatoid arthritis with tumour necrosis factor inhibitors. Br J Pharmacol. 2011;162:785-791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Minas T, Von Keudell A, Bryant T, Gomoll AH. The John Insall Award: a minimum 10-year outcome study of autologous chondrocyte implantation. Clin Orthop Relat Res. 2014;472:41-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mithoefer K, McAdams T, Williams RJ, Kreuz PC, Mandelbaum BR. Clinical efficacy of the microfracture technique for articular cartilage repair in the knee: an evidence-based systematic analysis. Am J Sports Med. 2009;37:2053-2063. [DOI] [PubMed] [Google Scholar]
- 38. Moradi B, Schönit E, Nierhoff C, et al. First-generation autologous chondrocyte implantation in patients with cartilage defects of the knee: 7 to 14 years’ clinical and magnetic resonance imaging follow-up evaluation. Arthroscopy. 2012;28:1851-1861. [DOI] [PubMed] [Google Scholar]
- 39. Pallante AL, Chen AC, Ball ST, et al. The in vivo performance of osteochondral allografts in the goat is diminished with extended storage and decreased cartilage cellularity. Am J Sports Med. 2012;40:1814-1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Pearce SC, Hurtig MB, Clarnette R, Kalra M, Cowan B, Miniaci A. An investigation of 2 techniques for optimizing joint surface congruity using multiple cylindrical osteochondral autografts. Arthroscopy. 2001;17:50-55. [DOI] [PubMed] [Google Scholar]
- 41. Pestka JM, Bode G, Salzmann G, Sudkamp NP, Niemeyer P. Clinical outcome of autologous chondrocyte implantation for failed microfracture treatment of full-thickness cartilage defects of the knee joint. Am J Sports Med. 2012;40:325-331. [DOI] [PubMed] [Google Scholar]
- 42. Peterson L, Brittberg M, Kiviranta I, Akerlund EL, Lindahl A. Autologous chondrocyte transplantation: biomechanics and long-term durability. Am J Sports Med. 2002;30:2-12. [DOI] [PubMed] [Google Scholar]
- 43. Peterson L, Minas T, Brittberg M, Lindahl A. Treatment of osteochondritis dissecans of the knee with autologous chondrocyte transplantation: results at two to ten years. J Bone Joint Surg Am. 2003;85-A(suppl 2):17-24. [DOI] [PubMed] [Google Scholar]
- 44. Roberts S, McCall IW, Darby AJ, et al. Autologous chondrocyte implantation for cartilage repair: monitoring its success by magnetic resonance imaging and histology. Arthritis Res Ther. 2003;5:60-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sgaglione NA. Decision-making and approach to articular cartilage surgery. Sports Med Arthrosc Rev. 2003;11:192-201. [Google Scholar]
- 46. Sherman SL, Garrity J, Bauer K, Cook J, Stannard J, Bugbee W. Fresh osteochondral allograft transplantation for the knee: current concepts. J Am Acad Orthop Surg. 2014;22:121-133. [DOI] [PubMed] [Google Scholar]
- 47. Solheim E1, Hegna J, Øyen J, Harlem T, Strand T. Results at 10 to 14 years after osteochondral autografting (mosaicplasty) in articular cartilage defects in the knee. Knee. 2013;20:287-290. [DOI] [PubMed] [Google Scholar]
- 48. Spakova T, Rosocha J, Lacko M, Harvanova D, Gharaibeh A. Treatment of knee joint osteoarthritis with autologous platelet-rich plasma in comparison with hyaluronic acid. Am J Phys Med Rehabil. 2012;91:411-417. [DOI] [PubMed] [Google Scholar]
- 49. Stanish WD, McCormack R, Forriol F, et al. Novel scaffold-based BST-CarGel treatment results in superior cartilage repair compared with microfracture in a randomized controlled trial. J Bone Joint Surg Am. 2013;95:1640-1650. [DOI] [PubMed] [Google Scholar]
- 50. Steadman JR, Briggs KK, Rodrigo JJ, Kocher MS, Gill TJ, Rodkey WG. Outcomes of microfracture for traumatic chondral defects of the knee: average 11-year follow-up. Arthroscopy. 2003;19:477-484. [DOI] [PubMed] [Google Scholar]
- 51. Steadman JR, Miller BS, Karas SG, Schlegel TF, Briggs KK, Hawkins RJ. The microfracture technique in the treatment of full-thickness chondral lesions of the knee in National Football League players. J Knee Surg. 2003;16:83-86. [PubMed] [Google Scholar]
- 52. Steadman JR, Rodkey WG, Singleton SB, et al. Microfracture technique for full-thickness chondral defects: technique and clinical results. Oper Tech Orthop. 1997;7:300-304. [Google Scholar]
- 53. Tuan RS, Chen AF, Klatt BA. Cartilage regeneration. J Am Acad Orthop Surg. 2013;21:303-311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ulstein S, Årøen A, Røtterud JH, Løken S, Engebretsen L, Heir S. Microfracture technique versus osteochondral autologous transplantation mosaicplasty in patients with articular chondral lesions of the knee: a prospective randomized trial with long-term follow-up. Knee Surg Sports Traumatol Arthrosc. 2014;22:1207-1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Zeifang F, Oberle D, Nierhoff C, Richter W, Moradi B, Schmitt H. Autologous chondrocyte implantation using the original periosteum-cover technique versus matrix-associated autologous chondrocyte implantation: a randomized clinical trial. Am J Sports Med. 2010;38:924-933. [DOI] [PubMed] [Google Scholar]