Summary
Background
The aim of this study was to compare the efficacy of breast conservation surgery (BCS) followed by radiotherapy (RT) in triple negative breast cancer (TNBC) versus non-TNBC.
Methods
We searched the MEDLINE and EMBASE databases from inception through March 31, 2014, using search terms related to TNBC, BCS, and RT. Studies comparing the efficacy of BCS followed by RT in TNBC versus non-TNBC were reviewed.
Results
5 studies including 2,922 non-TNBC and 510 TNBC cases were selected. The overall quality of included studies was deemed moderate to high. Compared with non-TNBC, the pooled relative risk of 5-year local relapse-free survival was 1.315 (95% confidence interval (CI) 0.967-1.789; p = 0.008) for TNBC, and that of 5-year overall survival, regional relapse-free survival, and distant metastasis-free survival was 1.929 (95% CI 1.392-2.674; p = 0.000), 3.052 (95% CI 1.629-5.715; p = 0.000), and 2.407 (95% CI 1.910-3.034; p = 0.000), respectively.
Conclusion
The local control rate of TNBC treated with BCS plus RT is similar to that of non-TNBC.
Keywords: Triple negative breast cancer, TNBC, Breast conservation surgery, BCS, Radiotherapy
Introduction
Breast conservation surgery (BCS) followed by radiotherapy (RT) has been considered the standard treatment for early breast cancer patients. Previous studies demonstrated that BCS plus RT significantly reduced the risk of local recurrence in breast cancer patients [1,2,3]. Those studies treated early breast cancer as a whole group and analyzed survival outcomes. However, breast cancer represents a heterogeneous group of tumors characterized by a wide spectrum of clinical, pathological, and molecular features [4,5,6]. This wide spectrum of factors accounts for variations in response to therapy and outcomes. The subgroup of triple negative breast cancer (TNBC) is associated with a worse prognosis compared to non-TNBC. TNBC is associated with an increased risk of recurrence within the first 3 years and increased mortality during the first 3-5 years after diagnosis because of aggressive clinicopathological features and ineffectiveness of endocrine therapy or trastuzumab [7,8,9,10]. Some studies demonstrated that TNBC was worse than non-TNBC in its response to treatment with BCS followed by RT [11,12,13,14,15], while others showed no such difference [16,17,18,19,20]. Identifying the effectiveness of BCS plus RT (especially local control rate) in the treatment of TNBC would allow clinicians to offer better local treatment. Therefore, we conducted a systematic review and meta-analysis to evaluate local relapse-free survival (LFS), overall survival (OS), regional relapse-free survival (RFS), and distant metastasis-free survival (DFS) of patients with TNBC treated with BCS plus RT.
Methods
Data Sources and Searches
From inception to March 31, 2014, MEDLINE and EMBASE were searched by 2 independent investigators. The search strategy used the terms ‘triple negative breast cancer’, ‘breast conservation surgery’, and ‘radiotherapy’. Reference lists of original and review articles were double checked to identify additional relevant studies. No language restrictions were imposed.
Study Selection and Quality Assessment
For inclusion, studies had to fulfil the following criteria: i) cohort design; ii) complete data on estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2); iii) exact reporting of endpoints (relapse-free survival was defined as time from diagnosis to first relapse, local recurrence, regional recurrence, or distant metastasis, and OS as the time until death); and iv) at least 1 outcome report of LFS, OS, RFS and DFS, or sufficient information to calculate them. Study selection and data extraction were conducted independently by 2 investigators. Differences were resolved by discussion with a third investigator. Our systematic review was conducted according to the meta-analysis of observational studies in epidemiology (MOOSE) guidelines [21]. Quality assessment was performed according to the criteria recommended by Hayden et al. [22].
Statistical Analysis
Heterogeneity tests and sensitivity analysis were performed to investigate the sources of heterogeneity in relative risk. Cochran Q test and I2 statistics were used to assess heterogeneity among studies [23]. For Q test, p < 0.05 indicates presence of heterogeneity; for I2 statistics, I2 > 50% indicates severe heterogeneity. Publication bias was evaluated using a funnel plot of a trial's effect size against the standard error [24,25]. All statistical analyses were performed using STATA version 12.0 (STATA, College Station, TX, USA).
Results
Literature Characteristics
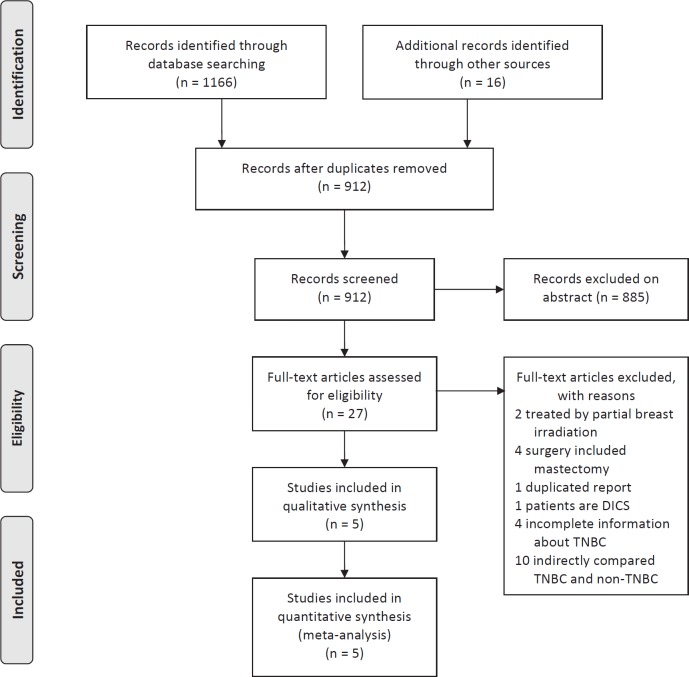
The process of evaluating articles for inclusion is depicted in figure 1. In total, 1,182 titles were reviewed, 5 studies [14,15,18,19,20] including 2,922 non-TNBC and 510 TNBC cases were selected. The characteristics of included studies are summarized in supplemental table 1 A-C (www.karger.com/?DOI=441436). When compared with non-TNBC cases, patients with TNBC were younger at diagnosis and had a larger tumor size. In addition, TNBC cases were more frequently high grade and were more likely to receive chemotherapy. However, lymph node positivity was not significantly different.
Fig. 1.
Flowchart of study selection.
Quality Assessment
On methodology and reported data, the overall quality of included studies was deemed moderate to high (supplemental table 2 A-C; www.karger.com/?DOI=441436). ER and PR status were obtained through immunohistochemistry (IHC) in all studies. For ER and PR, receptor positivity was based on more than 10% of cells testing positive according to Haffty et al. [18] and at least 1% of cells testing positive according to Barbieri et al. [19]. The remaining 3 studies did not define clear cutoffs for positivity of ER and PR. HER2 status was obtained through IHC in 2 studies [14,18], and IHC/fluorescent in situ hybridization (FISH) in 3 studies [15,19,20]. During the study period of Solin et al. [14], the currently accepted system of scoring HER2 expression as 0, 1+, 2+, or 3+ was not generally used. Rather HER2 expression was primarily reported as positive or negative. HercepTest™ (Dako, Glostrup, Denmark) scores of 2+ and 3+ were considered to indicate HER2 positivity by Haffty et al. [18] because this was the accepted classification scheme at the time of clinical treatment. HER2 was considered negative when 0 or 1+, or positive when 3+ on IHC. If tests revealed a 2+ value, FISH was conducted. However, no cutoffs were reported by Barbieri et al. [19]. A positive HER2 marker was defined as IHC identification of 3+ and/or amplified (ratio > 2.0) expression of HER2 on FISH for Gangi et al. [20] and Zaky et al. [15]. Classification of ER, PR, and HER2 status in all studies was based on IHC and/or FISH but not on genotype evaluation. Local and regional recurrences were defined as recurrent tumor developing in the ipsilateral breast (local recurrence) or regional lymph nodes (regional recurrence). Distant metastasis was defined as distant relapses. For OS, death from any cause was scored as failure. Local and regional relapses were defined as a histologically documented relapse in the ipsilateral breast or regional nodes in 2 studies [18,19]. In another 2 studies [14,15], relapse was defined as a clinically, radiologically, and/or histologically documented relapse. Distant metastasis was clinically and/or radiographically documented relapse, and death was assessed by medical record. Gangi et al. [20] did not report an exact explanation of the end points. Time of follow-up in all studies was longer than 3 years which is sufficient for TNBC due to the increased risk of recurrence within the first 3 years; however, for non-TNBC this period is not sufficient to assess recurrences and metastasis.
Meta-Analysis
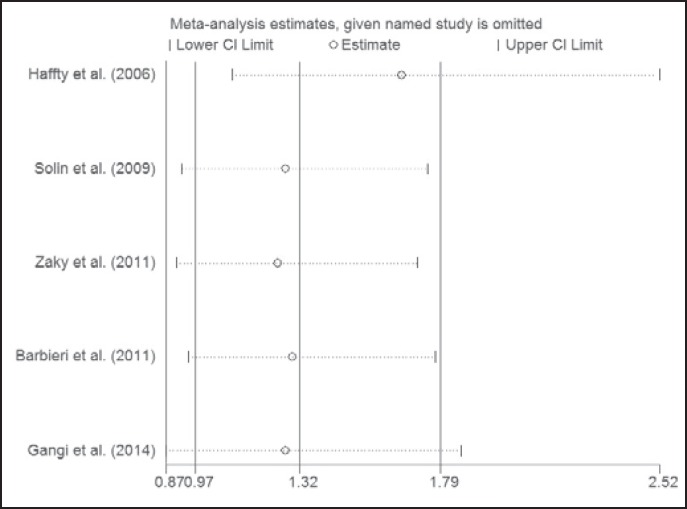
Treatment outcomes are shown in table 1. 4 studies directly provided LFS, OS, RFS, and DFS. We calculated LFS and RFS using the information provided by Barbieri et al. [19] who reported local-regional relapse-free survival only. OS could not be calculated for the study of Gangi et al. [20] due to insufficient data. Compared with non-TNBC, the pooled relative risk (RR) of 5-year LFS was 1.315 (95% confidence interval (CI) 0.967-1.789; p = 0.008) for TNBC, and that of 5-year OS, RFS, and DFS was 1.929 (95% CI 1.392-2.674; p = 0.000), 3.052 (95% CI 1.629-5.715; p = 0.000), and 2.407 (95% CI 1.910-3.034; p = 0.000), respectively (table 2 and supplemental fig. 1 A-D; www.karger.com/?DOI=441436). Sensitivity analysis indicated that the pooled RR was stable (fig. 2 and supplemental fig. 1; www.karger.com/?DOI=441436).
Table 1.
5-year outcomes of included studies
| Study [ref.] | Subgroup | OS, % | LFS, % | RFS, % | DFS, % |
|---|---|---|---|---|---|
| Haffty et al. [18] | TNBC | 80.00 | 83.00 | 94.00 | 68.00 |
| non-TNBC | 89.00 | 83.00 | 99.00 | 83.00 | |
| Solin et al. [14] | TNBC | 88.00 | 95.00 | 99.00 | 85.00 |
| non-TNBC | 94.00 | 98.00 | 100.00 | 95.00 | |
| Zaky et al. [15] | TNBC | 87.90 | 88.00 | 85.00 | 85.00 |
| non-TNBC | 95.00 | 96.00 | 96.30 | 96.00 | |
| Barbieri et al. [19] | TNBC | 80.60 | 95.60 | 100.00 | 68.40 |
| non-TNBC | 90.30 | 97.10 | 100.00 | 87.30 | |
| Gangi et al. [20] | TNBC | NC | 95.30 | 98.70 | 91.00 |
| non-TNBC | NC | 97.84 | 98.89 | 97.22 |
OS = Overall survival; LFS = local relapse-free survival; RFS = regional relapse-free survival; DFS = distant metastasis-free survival; TNBC = triple negative breast cancer; NC = not clear.
Table 2.
Pooled relative risks (RRs) and heterogeneity of the meta-analysis
| Outcomes (5-year) | Pooled results |
Heterogeneity |
||
|---|---|---|---|---|
| RR (95% CI) | p (RR) | p (Q) | I2 (%) | |
| LFS | 1.315 | 0.080 | 0.353 | 9.400 |
| (0.967–1.789) | ||||
| IHC | 1.111 | 0.626 | 0.241 | 27.400 |
| (0.727–1.696) | ||||
| IHC/FISH | 1.600 | 0.041 | 0.444 | 0.000 |
| (1.019–2.511) | ||||
| OS | 1.929 | 0.000 | 0.965 | 0.000 |
| (1.392–2.674) | ||||
| IHC | 1.864 | 0.001 | 0.778 | 0.000 |
| (1.271–2.736) | ||||
| IHC/FISH | 2.133 | 0.017 | 0.785 | 0.000 |
| (1.148–3.965) | ||||
| RFS | 3.052 | 0.000 | 0.231 | 33.100 |
| (1.629–5.715) | ||||
| IHC | 6.179 | 0.001 | 0.584 | 0.000 |
| (2.020–18.895) | ||||
| IHC/FISH | 2.049 | 0.078 | 0.133 | 55.800 |
| (0.924–4.547) | ||||
| DFS | 2.407 | 0.000 | 0.334 | 12.600 |
| (1.910–3.034) | ||||
| IHC | 2.074 | 0.000 | 0.223 | 32.700 |
| (1.526–2.819) | ||||
| IHC/FISH | 2.978 | 0.000 | 0.612 | 0.000 |
| (2.088–4.249) | ||||
OS = Overall survival; LFS = local relapse-free survival; RFS = regional relapse-free survival; DFS = distant metastasis-free survival; CI = confidence interval; IHC = immunohistochemistry; FISH = fluorescent in situ hybridization;Q = Cochran Q test; I2 = I-squared.
Fig. 2.
Sensitivity analysis of included studies.
Heterogeneity Assessment and Publication Bias
According to the L'Abbe plot and Galbraith plot, there was little heterogeneity among the studies. We further detected p (Q) and I2, and found a small degree of heterogeneity. In subgroup analysis, slight heterogeneity was found in the IHC subgroup. Heterogeneity might result from potential misclassification of TNBC or non-TNBC by IHC-assessed HER2. Slight asymmetry was found in the funnel plot, suggesting a small publication bias. The p value for Begg's adjusted rank correlation test was 0.221, and that for Egger's regression asymmetry test was 0.067, suggesting a low probability of publication bias.
Discussion
Multiple studies have indicated that TNBC is associated with a poor prognosis. TNBC is more likely than non-TNBC to recur locally [11] and metastasize to lung and brain. The aggressive nature of TNBC may exclude such patients from BCS under the assumption that more extensive treatment would provide better effects. Our systematic review qualitatively assessed the quality of related studies, and quantitatively assessed the efficacy of BCS plus RT treatment in TNBC versus non-TNBC. Our meta-analysis showed that TNBC was associated with an equal risk with regard to 5-year local control, but worse RFS, DFS, and OS compared with non-TNBC. The pooled RR of LFS indicated that BCS plus RT is a viable option for TNBC. However, the high RR of RFS is an unexpected finding. Lacking the necessary information regarding supraclavicular, axillary, and internal mammary lymph node irradiation and lymph node dissection, it was difficult to explain this observation. The higher 5-year OS compared to 5-year DFS for TNBC was another unexpected finding. The interpretation remained debatable given the relatively small number of TNBC and the retrospective nature of the included studies.
The current systematic review had some advantages. First, the retrospective cohort studies included were of moderate to high quality with all articles adjusting for key confounding variables. Thus inherent weakness due to potential confounding factors was minimized in all observational studies and meta-analyses. Second, little heterogeneity and only slight publication biases were observed in the studies included in our meta-analysis.
However, possible limitations of our meta-analysis must be considered. First, in retrospective cohort studies, exclusion of potential biases was difficult. Studies included in our systematic review varied in ascertainment of HER2 status, study population, age, and chemotherapy. Hence, confounding factors could be inherent in the included studies. We attempted to manage this heterogeneity with appropriate meta-analytic techniques but failed due to insufficient data. Second, only 5 studies were included in our systematic review, and the sample of TNBC was substantial. These aspects may significantly reduce the statistical power of the analysis. Third, funnel plot and parameters of the Begg's/Egger's test may be inappropriate to estimate publication bias among 5 included studies. The methodologies of publication bias assessment used in the current study may therefore have brought about inaccurate results.
Conclusion
The current meta-analysis indicates that TNBC treated with BCS followed by RT showed a similar local control rate but worse RFS, DFS, and OS compared to non-TNBC. Hence, TNBC should not be considered for non-conservative surgery. Further large prospective cohort-designed or randomized clinical trials on this issue should be performed to verify the results.
Disclosure Statement
The authors declare that they have no competing interests.
Supplemental Material
Supplemental Table 1. A Basic study characteristics; B Study treatment; C Surgery and radiotherapy in the studies
Supplemental Table 2. A, B Study quality; C Quality assessment
Supplemental Fig. 1. A Pooled relative risks (RRs) of 5-year local relapse-free survival (LFS) of triple negative breast cancer (TNBC) versus non-TNBC; B Pooled RRs of 5-year overall survival (OS) of TNBC versus non-TNBC; C Pooled RRs of 5-year regional relapse-free survival (RFS) of TNBC versus non-TNBC; D Pooled RRs of 5-year distant metastasis-free survival (DFS) of TNBC versus non-TNBC.
Supplementary Material
Supplementary data
References
- 1.No authors listed Favourable and unfavourable effects on long-term survival of radiotherapy for early breast cancer: an overview of the randomised trials. Early Breast Cancer Trialists' Collaborative Group. Lancet. 2000;355:1757–1770. [PubMed] [Google Scholar]
- 2.Clarke M, Collins R, Darby S, et al. Early Breast Cancer Trialists' Collaborative Group (EBCTCG) Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;366:2087–2106. doi: 10.1016/S0140-6736(05)67887-7. [DOI] [PubMed] [Google Scholar]
- 3.Darby S, McGale P, Correa C, et al. Early Breast Cancer Trialists' Collaborative Group (EBCTCG) Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet. 2011;378:1707–1716. doi: 10.1016/S0140-6736(11)61629-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Perou CM, Sørlie T, Eisen MB, et al. Molecular portraits of human breast tumours. Nature. 2000;406:747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 5.Sørlie T. Molecular portraits of breast cancer: tumour subtypes as distinct disease entities. Eur J Cancer. 2004;40:2667–2675. doi: 10.1016/j.ejca.2004.08.021. [DOI] [PubMed] [Google Scholar]
- 6.Sørlie T, Perou CM, Tibshirani R, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A. 2001;98:10869–10874. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tischkowitz M, Brunet JS, Bégin LR, et al. Use of immunohistochemical markers can refine prognosis in triple negative breast cancer. BMC Cancer. 2007;7:134. doi: 10.1186/1471-2407-7-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheang MC, Voduc D, Bajdik C, et al. Basal-like breast cancer defined by five biomarkers has superior prognostic value than triple-negative phenotype. Clin Cancer Res. 2008;14:1368–1376. doi: 10.1158/1078-0432.CCR-07-1658. [DOI] [PubMed] [Google Scholar]
- 9.Dent R, Trudeau M, Pritchard KI, et al. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res. 2007;13:4429–4434. doi: 10.1158/1078-0432.CCR-06-3045. [DOI] [PubMed] [Google Scholar]
- 10.Liedtke C, Mazouni C, Hess KR, et al. Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J Clin Oncol. 2008;26:1275–1281. doi: 10.1200/JCO.2007.14.4147. [DOI] [PubMed] [Google Scholar]
- 11.Voduc KD, Cheang MC, Tyldesley S, et al. Breast cancer subtypes and the risk of local and regional relapse. J Clin Oncol. 2010;28:1684–1691. doi: 10.1200/JCO.2009.24.9284. [DOI] [PubMed] [Google Scholar]
- 12.Panoff JE, Hurley J, Takita C, et al. Risk of locoregional recurrence by receptor status in breast cancer patients receiving modern systemic therapy and post-mastectomy radiation. Breast Cancer Res Treat. 2011;128:899–906. doi: 10.1007/s10549-011-1495-1. [DOI] [PubMed] [Google Scholar]
- 13.Nguyen PL, Taghian AG, Katz MS, et al. Breast cancer subtype approximated by estrogen receptor, progesterone receptor, and HER-2 is associated with local and distant recurrence after breast-conserving therapy. J Clin Oncol. 2008;26:2373–2378. doi: 10.1200/JCO.2007.14.4287. [DOI] [PubMed] [Google Scholar]
- 14.Solin LJ, Hwang WT, Vapiwala N. Outcome after breast conservation treatment with radiation for women with triple-negative early-stage invasive breast carcinoma. Clin Breast Cancer. 2009;9:96–100. doi: 10.3816/CBC.2009.n.018. [DOI] [PubMed] [Google Scholar]
- 15.Zaky SS, Lund M, May KA, et al. The negative effect of triple-negative breast cancer on outcome after breast-conserving therapy. Ann Surg Oncol. 2011;18:2858–2865. doi: 10.1245/s10434-011-1669-4. [DOI] [PubMed] [Google Scholar]
- 16.Millar EK, Graham PH, O'Toole SA, et al. Prediction of local recurrence, distant metastases, and death after breast-conserving therapy in early-stage invasive breast cancer using a five-biomarker panel. J Clin Oncol. 2009;27:4701–4708. doi: 10.1200/JCO.2008.21.7075. [DOI] [PubMed] [Google Scholar]
- 17.Freedman GM, Anderson PR, Li T, Nicolaou N. Locoregional recurrence of triple-negative breast cancer after breast-conserving surgery and radiation. Cancer. 2009;115:946–951. doi: 10.1002/cncr.24094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haffty BG, Yang Q, Reiss M, et al. Locoregional relapse and distant metastasis in conservatively managed triple negative early-stage breast cancer. J Clin Oncol. 2006;24:5652–5657. doi: 10.1200/JCO.2006.06.5664. [DOI] [PubMed] [Google Scholar]
- 19.Barbieri V, Sanpaolo P, Genovesi D. Prognostic impact of triple negative phenotype in conservatively treated breast cancer. Breast J. 2011;17:377–382. doi: 10.1111/j.1524-4741.2011.01100.x. [DOI] [PubMed] [Google Scholar]
- 20.Gangi A, Chung A, Mirocha J, et al. Breast-conserving therapy for triple-negative breast cancer. JAMA Surg. 2014;149:252–258. doi: 10.1001/jamasurg.2013.3037. [DOI] [PubMed] [Google Scholar]
- 21.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 22.Hayden JA, Cote P, Bombardier C. Evaluation of the quality of prognosis studies in systematic reviews. Ann Intern Med. 2006;144:427–437. doi: 10.7326/0003-4819-144-6-200603210-00010. [DOI] [PubMed] [Google Scholar]
- 23.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 24.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. [PubMed] [Google Scholar]
- 25.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data