Abstract
Inflammatory bowel diseases are characterised by inflammation that compromises the integrity of the epithelial barrier. The intestinal epithelium is not only a static barrier but has evolved complex mechanisms to control and regulate bacterial interactions with the mucosal surface. Apical tight junction proteins are critical in the maintenance of epithelial barrier function and control of paracellular permeability. The characterisation of alterations in tight junction proteins as key players in epithelial barrier function in inflammatory bowel diseases is rapidly enhancing our understanding of critical mechanisms in disease pathogenesis as well as novel therapeutic opportunities. Here we give an overview of recent literature focusing on the role of tight junction proteins, in particular claudins, in inflammatory bowel diseases and inflammatory bowel disease associated colorectal cancer.
Keywords: Claudin, Tight junction, Ulcerative colitis, Pouchitis, Crohn’s disease
Core tip: Epithelial barrier function is compromised in inflammatory bowel diseases (IBD). Apical tight junction proteins, in particular claudins, are key players in epithelial barrier function. However, there is little consensus regarding the expression of most claudin isoforms in these conditions or whether these findings are primary or secondary to disease pathogenesis. Knowledge of tight junction protein expression and function in IBD and IBD associated colorectal cancer will enhance our understanding of critical mechanisms in disease pathogenesis as well as novel therapeutic opportunities.
INTRODUCTION
A number of routes exist for passage across the epithelial barrier between the internal and external environment. The intercellular spaces between adjacent cells, linked together by junctional complexes, are critical in regulating the mucosal barrier. Ions and solutes pass transcellulary utilising channels, carriers and transporting ATPases[1]. The paracellular pathway also regulates permeability to water, ions and low molecular weight molecules (< 600 kDa). The differences in paracellular permeability between different epithelia such as the proximal or distal nephron, has led to the concept of “leaky” or “tight” epithelia. In disease states, epithelia may become more “tight” or more “leaky” such as is seen in inflammatory bowel diseases[1].
The tight junctions (TJs) form the apical most unit, defining the boundary between the apical and basolateral membranes and are predominantly the rate-limiting factor in paracellular passage[2]. The tight junction is built up by both transmembrane proteins such as occludin, tricellulin, different claudins and junctional adhesion molecules (JAMs), as well as peripheral membrane proteins such as zona occludens (ZO)-1,-2,-3 and cingulin. They are linked to the cytoskeleton of the cell by F-actin and myosin II[3,4]. These ZO proteins have three PDZ domains that mediate binding to other transmembrane tight junction proteins such as claudins in a dynamic energy dependent manner[5]. They are also the direct targets and effectors of different signalling pathways (such as the myosin light chain kinase) thereby altering the assembly, maintenance, and barrier function of the TJ complex[6].
The expression of different TJs in the gut varies according to localization (e.g., villus vs crypt, small bowel vs colon), cell membrane localization (e.g., apical, lateral or basolateral) and the gut’s functional properties at the site[4,7,8]. For example, claudin 2 is expressed at the apical pole throughout the crypt-villus axis in the jejunum whilst in the colon expression is restricted to the crypts, whereas claudin 4 expression is throughout the crypt-villus axis in the small and large intestine. The segmental distribution of claudin expression may relate to cell differentiation, carbohydrate metabolism and transcription factors such as HNF1α, Cdx2 and GATA-4[9].
In health, the apical TJs construct a dynamic intestinal barrier that regulates the paracellular uptake of water, nutrients and electrolytes[3,5]. TJs may be size and/or charge selective and prevent contact between the proteins of the two cell poles: the basolateral and apical cell membranes[10]. While many tight junction proteins have properties of increased barrier formation, others form size and/or charge selective channels or pores[1]. Adherens junctions and desmosomes are mostly involved in communication between neighbouring epithelial cells[4,10,11]. TJ dysfunction can lead to the disruption of the intestinal barrier integrity. Changes in pH, osmotic load or cytoskeleton function all affect the barrier function of TJs[12].
There are 27 different claudin isoforms that modulate the paracellular movement of ions based on charge and size[13]. Claudin 1 and claudin 2 are able to initiate the formation of TJ strands on fibroblasts lacking TJs, suggesting that they are the major components of TJ strands[14]. Claudin 2 controls the movement of monovalent cations such as Na+ to the interstitium and reduces the paracellular trans-epithelial resistance as well as enhances trans-epithelial water flux[15,16] in contrast to other claudins (like 1, 3, 4, 5, 8) that tighten the epithelium[17-23]. Claudin 2 also directly decreases the barrier function of Claudin 1 and Claudin 4 strands[13]. Therefore the ratio of different claudins in the TJ determines its functional property as either leaky or tight. This review will focus on the role of key TJPs (Table 1) in the pathogenesis of IBD and IBD associated colorectal cancer (CRC).
Table 1.
Tight junctions of interest in inflammatory bowel diseases
| TJ protein | Function | Expression in CD | Expression in UC | Expression in pouchitis |
| Claudin 1 | Tightens the epithelium[17,23] able to initiate formation of TJ strands[14] | Active: ↑[46,65] ↔[50] | Active: ↑[65], | ↓[40] |
| Inactive: ↔[65] | ↔[45] | |||
| Inactive: ↔[65] | ||||
| Claudin 2 | Important pore-forming TJ protein[15,16,23], able to initiate formation of TJ strands[14], decreases barrier function of CLDN1 and CLDN4[13] | ↑[44,50,65,101] | ↑↑[44,50,65,101] | ↑[40] |
| Claudin 4 | Tightens the epithelium, decreases paracellular conductance through decrease in sodium permeability[19] | ↓[44] | Active: ↓[44,45] | ↔[40] |
| Active inflammation: ↑[65] | Active Inflammation: ↑[65] | |||
| ↔[50] | ||||
| Claudin 5 | Tightens the epithelium[20] | ↓[50] | ↔[40] | |
| Claudin 8 | Tightens the epithelium[21] | ↓[50] | ||
| Claudin 12 | Tightens the epithelium | ↑[48] | ||
| Claudin 18 | Uncertain function | ↑[47] | ||
| Occludin | Binds ZO-1, regulates paracellular permeability, function in cellular adhesion[102] | ↓[46,50,52] | ↓[52] | ↔[40] |
| ↔[65] | ↔[65] | |||
| ZO-1 | Mediates protein-protein interactions, link to actin cytoskeleton: “anchoring” protein[4,103] | ↓[68] | ↔[64] (Mees et al: “in patients with a history of UC”) | ↓[54] (chronic pouchitis) |
TJ: Tight junction; ZO-1: Zona occludens-1; CD: Crohn’s disease; UC: Ulcerative colitis.
EPITHELIAL BARRIER FUNCTION IN INFLAMMATORY BOWEL DISEASES
Inflammatory bowel diseases (IBD) share a multifactorial aetiology of genetic susceptibility, environmental factors and immune dysregulation[24]. These diseases are characterized by intestinal inflammation that compromises the integrity of the epithelial barrier leading to increased permeability and infiltration of pathogens[25]. Both Crohn’s disease (CD) and ulcerative colitis (UC) share common features such as epithelial breaks, a reduction in tight junction strands, and glandular atrophy[11,26,27]. Patients with clinically active CD have increased intestinal permeability[28-30]. Barrier dysfunction is likely to be caused by epithelial damage including apoptosis, erosion and ulceration that are characteristic of gut inflammation. Inflammatory cytokines associated with gut inflammation alter epithelial permeability through their effects on the junctional complexes[31-33].
However, impaired barrier function is also evident in quiescent IBD and even in first degree relatives of patients with CD[34,35]. Genetic studies have identified new UC susceptibility loci pertaining to defects of the epithelial barrier[36,37]. Barrier properties of ileoanal pouch mucosa in both pouchitis and in ileoanal pouches where backwash ileitis was present prior to restorative proctocolectomy for UC are reduced[38-40] and increased bacterial translocation has been reported in pouches functioning for longer than 12 mo[41]. Dysregulation of the epithelial barrier with changes in paracellular permeability due to altered cell to cell junctions is likely to be significantly more selective and may be a critical primary factor in the pathogenesis of IBD.
ULCERATIVE COLITIS
Gitter et al[42] identified that in the sigmoid colon of patients with early UC where the epithelium looks intact, there are in fact already leaks from apoptotic foci. Furthermore, the higher the degree of inflammation observed, the higher the conductance of the epithelium measured[42]. A study using epithelial resistance as a measure of barrier function in samples from UC patients with inflamed sigmoid colon[43] demonstrated an 80% reduction in epithelial resistance and a decrease in epithelial (not crypt) TJ depth in inflamed samples.
Several studies have focused on expression of claudins in UC patients[43-45]. demonstrating higher expression of claudin 2 in colonic samples from patients with UC. Additionally, the increases in “pore-forming” claudin 2 correlate with disease severity on both protein and transcriptional levels. Reductions in other “tightening” tight junctions also occur concomitantly. Reduced staining intensity for claudin 3, 4 and 7 have been shown both on the surface epithelium as well as mislocation of claudin 4 extra-junctionally in UC patients[44,45].
In contrast, Poritz et al[46] found an increase in claudin 1:occludin ratios in colonic samples from UC patients compared with healthy controls and CD samples by Western blot analysis. This change in ratio was the result of both an increase in claudin 1 and a decrease in occludin. Disease severity, measured by the degree of inflammation, was directly proportional to the alterations seen in TJ structure in UC. In another study, claudin 1 was demonstrated to be upregulated in the colon of UC patients compared to healthy controls, but did not correlate to disease severity[47]. In sigmoid samples of active UC patients, a trend toward upregulation of claudin 12 (another “tightening” claudin) was observed[48]. Claudin 18 expression was found to be elevated in UC patients compared with controls, but did not correlate with severity of inflammation postulating a primary defect in barrier function[47].
CROHN’S DISEASE
In CD, intestinal permeability is considered a predictive factor for disease susceptibility and relapse[34,49]. Changes in tight junctions and epithelial apoptosis might dominate in causing barrier dysfunction[50]. Marin et al[51] studied 10 patients with CD. The epithelial cells of the terminal ileum from these patients demonstrated tight junction disorganisation which was present in minimally inflamed areas although more pronounced in cobblestone areas.
In samples from the sigmoid colon in active CD, the expression of claudins 5, 8 and 3 were decreased, whereas claudin 2 expression was moderately increased[50]. Kucharzik et al[52] demonstrated similar changes in tight junction expression in both UC and CD with global down-regulation of occludin. This finding was present both in active and quiescent UC whilst only in active CD. Das et al[53] showed claudin 2 to be strongly expressed in the ileum of approximately 50% of quiescent as well as active CD. Moreover, the distribution of claudin 2 expression was altered in colonic biopsies from CD patients and associated with disrupted tight junctions. Others have found no change in ileal expression of claudin 2 in CD, whilst in the sigmoid colon of CD patients, claudin 2 was found to be significantly down regulated compared to controls. However, claudin 12 was found to be increased in the ileum of CD patients[48].
POUCHITIS
Barrier properties of ileoanal pouch mucosa in pouchitis and in non-inflamed pouches are reduced[38] with increased bacterial translocation in long lasting pouches[41]. Merrett et al[39] showed an increase in pouch permeability in patients with pouchitis compared with those with a normally functioning pouch. Electron micrography of mucosal epithelium from non-inflamed UC pouch (Figure 1A) and pouchitis suggests membranes between cells are more loosely arranged with increased intercellular distance and perijunctional cytoskeleton condensation[54]. In addition, dendritic cells appear to penetrate the epithelial cell layer more frequently (personal communication) (Figure 1B). Analysis of claudin expression from pouch biopsies before ileal pouch anal anastomosis (IPAA), during pouchitis and at a time point over a year after ileostomy closure demonstrated an elevation in claudin 2 levels in acute pouchitis[40]. We also recently demonstrated altered expression of TJP in the ileal pouch of patients with UC. In particular increased expression of claudin 2 occurred early following ileostomy closure, prior to the development of histological inflammation. Claudin 2 was also elevated in UC patients without pouchitis compared with FAP patients and in acute, but not chronic pouchitis samples whilst epithelial expression of ZO-1 and claudin 1 were reduced in patients with chronic pouchitis. These findings suggest that increased claudin 2 expression may be an early event in the development of inflammation[54].
Figure 1.
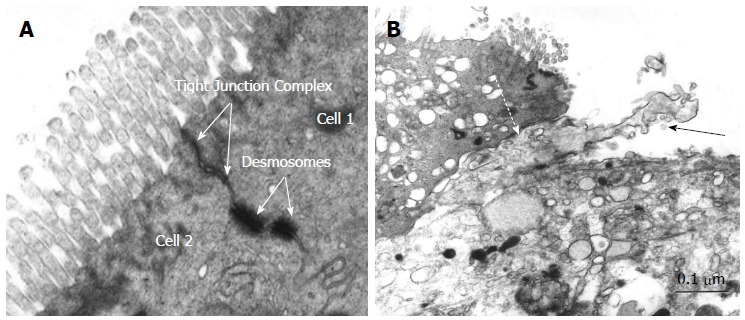
Electron microscopy of pouch epithelium tight junction complex in non-inflamed pouch (A) and electron micrograph demonstrating dendritic cell penetrating between two epithelial cells in pouchitis (B). Bar = 0.1 μm. Solid arrow: Dendritic cell; Dashed arrow: Tight junction complex between epithelial cell and dendritic cell.
TIGHT JUNCTIONS AND IBD ASSOCIATED COLORECTAL CANCER
Passage of luminal antigens or metabolites through diminished epithelial barrier is known to promote chronic inflammation and CRC[55-57]. In general, claudins are associated with different types of neoplasms, including breast, prostate, ovarian, pancreatic, gastric and colorectal carcinoma[58,59]. They may also be involved in the progression to metastasis, and provide unfavourable signalling pathways between the extra- and intracellular milieu[60-63].
Mees et al[64] investigated the expression of both adherens, and TJ proteins in patients with CRC with a history of UC. Claudins 1, 3, 4 and β-catenin were upregulated in CRC tissue compared with normal controls or intraepithelial neoplasia, whilst the expression of claudin 2, ZO-1 and occludin did not vary significantly between samples. Other studies have demonstrated elevated claudin 1 and claudin 2 levels in IBD-associated carcinoma[59,65,66]. Kinugasa et al[66] demonstrated increased staining for claudin 1 in both high-grade dysplasia and UC-associated CRC when compared with normal or UC colonic samples.
In the study of Weber et al[65], the expression of claudin 1, 2, 4 and occludin were evaluated in IBD patients, IBD-associated dysplasia, acute self-limited colitis (ASLC) and sporadic adenomas. During active inflammation, claudin 1 expression was upregulated in both UC and CD patients and localization of claudin 1 was not limited to the TJ, but was expressed on the lateral membrane. Claudin 2 expression was the richest in the TJ region and the apical cytoplasm. In an identical manner to claudin 1, the expression of claudin 2 correlated with the degree of inflammation in both UC and CD, but not in ASLC[65]. Claudin 1 and claudin 2 levels were also increased in IBD-associated dysplasia and sporadic adenomas in comparison to non-dysplastic IBD. However, occludin expression and localization in both active and inactive IBD and in adenomas or carcinomas was no different compared with controls. The changes in TJ expression in IBD associated dysplasia may represent the severity and longevity of histological inflammation, a known risk factor for neoplastic progression in IBD[67].
CHICKEN OR EGG?
It is not clear whether the changes observed in tight junctions in IBD are causal, leading to abnormal epithelial barrier integrity and the aberrant immune responses, or whether the inflammation itself causes the alterations in tight junction expression and distribution.
Inflammatory cytokines and tight junction regulation
Heller et al[31] demonstrated a significant upregulation of IL-13 in patients with UC compared to CD patients and non-inflammatory controls. Following the upregulation of IL-13, there was a decrease in trans-epithelial resistance and an increase in epithelial cell apoptosis and conductance. Furthermore, IL-13 caused an upregulation of the claudin 2 gene, thus elevating claudin 2 protein production threefold. IL-13 did not have a significant effect on the expression of occludin, claudin 1 and claudin 4.
In CD, interferon gamma (IFN-γ) contributes to impairment of epithelial barrier function through disrupting tight junction complexes by causing decreased expression and increased internalization of occludin and ZO-1[68]. The effects of IFN-γ on barrier function may be mediated through 5’ adenosine monophosphate-activated protein kinase (AMPK). AMPK is key in sensing the cell’s energy levels, which decrease during inflammation and subsequently increase the demand for AMPK. Scharl et al[68] showed that inhibiting AMPK not only reverses its effect on TJs but also its negative effect on trans epithelial resistance. Watson et al[69] also showed in T84 cells (model intestinal epithelial cell line) that IFN-γ increased intestinal permeability to large molecules such as E. coli-derived lipopolysaccharide. Effects were exerted presumably by decreasing expression of occludin and increasing the expression of claudin 1 but expression of claudin 2 or 3 were not affected[69].
TNF-α can also affect tight junctions and decrease epithelial barrier function by increasing Myosin Light Chain Kinase (MLCK) phosphorylation. Inhibition of MLCK in TNF-α treated epithelial monolayers can acutely restore barrier function[32]. Furthermore, MLCK-activation promotes IL-13 expression and claudin 2 synthesis[70]. TNF-α induced MLCK expression may therefore be a critical mechanism for barrier dysfunction in UC and CD. Ileal MLCK expression is increased in ileal biopsies from patients with CD compared with controls and increased MLCK correlates with disease activity[71].
Animal models of epithelial permeability and inflammation
A recent study of transgenic mice expressing activated MLCK showed increased paracellular permeability without histological inflammation. Further analysis however, found an increase in the absolute numbers of lamina propria CD4+ lymphocytes and a significant redistribution of CD11c+ dendritic cells to the superficial lamina propria as well as polarisation to a Th1 cytokine profile[72]. Despite increased epithelial permeability these mice did not develop intestinal inflammation. However, when crossed with mice that develop spontaneous inflammation an accelerated and exaggerated inflammatory response was seen. This mouse model therefore suggests that barrier loss may not initiate inflammation but accelerate inflammatory responses.
Other studies suggest that tight junction abnormalities and epithelial permeability may precede the increase of inflammatory cytokines. Interleukin-10 (IL-10) blocks IFN-γ induced epithelial permeability and IL-10 knockout mice have increased permeability and spontaneously develop chronic intestinal inflammation[33]. Inhibition of the zonulin receptor (a key receptor in tight junction binding regulation) in IL-10 knockout mice reduced intestinal permeability and attenuated the spontaneous development of colitis[33]. However, increased intestinal permeability in IL-10 knockout mice not only preceded the onset of inflammation but also occurred significantly earlier than any differences in IFN-γ or TNF-α.
A recent study of intestine-specific claudin 7 knockout mice[73] demonstrated increased neutrophil infiltration into the lamina propria and increased mRNA expression of inflammatory cytokines without altered epithelial integrity. Further investigation showed increased epithelial permeability and paracellular absorption of small molecules and increased absorption of a bacterial derived neutrophil chemoattractant in the claudin 7 knockouts. Treatment with antibiotics or exogenous administration of the soluble bacterial derived chemoattractant, abolished and initiated the onset of inflammation respectively. This model suggests that the loss of claudin 7 enabled increased absorption of soluble bacterial products leading to the development of colonic inflammation.
Ileoanal pouch as a human model for inflammatory bowel disease
Unlike UC and CD, the ileal pouch offers a unique opportunity to study the development of inflammation before disease onset. In patients with UC, increased epithelial expression of the “pore-forming” tight junction claudin 2 was an early event after ileostomy closure and preceded increased IL-6 levels, as well as increased TLR4 and CD40 activation marker expression in patients with mucosal inflammation of the pouch at twelve months following ileostomy closure[54].
THERAPEUTIC OPPORTUNITY IN INFLAMMATORY BOWEL DISEASES
Strategies to manipulate tight junctions and intestinal permeability are likely to have an important role in the future treatment of inflammatory bowel diseases.
Anti-TNF therapy and tight junctions
Anti-TNF therapy is effective in the treatment of Crohn’s disease, ulcerative colitis and chronic pouchitis[74-76]. Barrier function is significantly restored following anti-TNF therapy for CD[77,78]. In the study by Zeissig et al[78], this was associated with a reduction in epithelial apoptosis but no significant changes in occludin, claudin 1 or claudin 4. However, other claudins including claudin 2 were not assessed. In a study of experimental colitis in mice, both etanercept and infliximab attenuated inflammation induced reductions in ZO-1 and occludin as well as reducing the upregulation of claudin 2[79]. More recently, in epithelial cell lines adalimumab prevented increased phosphorylation of myosin light chain and reversed the TNF induced down regulation of claudins 1 and 4[80].
Short chain fatty acids
In the colon, anaerobic bacterial fermentation of undigested polysaccharides leads to the production of short chain fatty acids, particularly acetic, proprionic and butyric acids. Short chain fatty acids, in particular butyrate are thought to be the principal source of energy for colonocytes and in UC patients colonocytes have demonstrated diminished oxidation of butyrate[81,82]. In vitro culture demonstrated butyrate enhanced claudin 1 transcription and enhanced barrier function[83,84]. In colonic epithelial cells treated with butyrate claudin 2 was down regulated[85]. Butyrate might be postulated to have a role in maintaining barrier function via tight junction regulation.
Novel compounds and probiotics
Novel compounds that alter epithelial barrier function may be available from nutritional sources. Several plant extracts have been observed to regulate TJ expression. Quercetin, a common flavanoid increase epithelial resistance in Caco-2 cell monolayers by upregulating claudin 4 expression[86]. Berberine, an isoquinolone alkaloid, prevented TNF-α induced claudin 1 disassembly and upregulation of claudin 2 in a cell culture model[87]. Polyunsaturated fatty acids can also have beneficial effects on the assembly and morphology of TJs[88]. Omega-3 and omega 6 polyunsaturated fatty acids up-regulate expression of occludin, reduce permeability and strengthen the epithelial barrier[89] Polyunsaturated fatty acids also reverse the disruptions in TJs caused by proinflammatory cytokines in Caco 2 epithelial cells[90,91] and might play a role in preventing the alteration in the epithelial barrier caused by inflammation or proinflammatory cytokines that could be exploited as a therapeutic target in the treatment of gut inflammation.
Much attention has focused on the effects probiotic bacteria and their products may have on tight junction expression and epithelial barrier function[92]. In vitro and animal models have shown Lactobacilli to attenuate epithelial permeability in experimental colitis and to upregulate tight junction expression of ZO-1, occludin and claudin-3[93-95]. VSL#3 (a mixture of eight probiotic strains) prevented the reduction and redistribution of ZO-1 and claudins -1,-3,-4 and -5 in a murine model of colitis. Furthermore, bacterial products may be a source of novel therapies affecting epithelial barrier function. Uncharacterized extracellular proteins secreted by B. longum subsp. infantis, increased the production of ZO-1 and occludin in epithelial cells[96]. Extracellular proteins derived from Lactobacillus rhamnosus GG attenuated reduction in epithelial resistance in an in vitro model, preventing the redistribution of tight junction proteins including ZO-1 and occludin in a dose dependent manner[97]. Moreover, Salmonella infection increased claudin-2 expression in epithelial cell lines facilitating its invasion. Therefore, blocking claudin-2 as a potential therapeutic target to prevent bacterial invasion has been suggested[98].
Zonulin has been shown to be a key regulator of intestinal permeability through modulation of epithelial tight junctions[99]. A synthetic peptide inhibitor of zonulin known as AT 1001 or Larazotide has undergone clinical studies in the treatment of coeliac disease[100]. In the IL-10 knockout mouse, AT 1001 reduced intestinal permeability and attenuated the development of spontaneous colitis[33]. Future studies are necessary to determine the role these proteins may have in modulating tight junctions and epithelial barrier function in inflammatory bowel diseases.
CONCLUSION
Dysregulation of TJ proteins is involved and may precede the development of IBD. It is probable that they also contribute to the development of IBD-associated CRC. Recent evidence suggests that a dysregulated expression of TJ proteins may precede the development of intestinal inflammation. However, a number of questions remain unanswered regarding the role of TJs in the aetiology of inflammatory bowel diseases. Significant differences may exist between animal models and human studies regarding TJ expression profiles and further human studies are necessary to elucidate the role of TJs in IBD aetiology or acceleration of aberrant inflammatory responses.
Claudin 2 appears to be upregulated in UC, CD and pouchitis and some studies also suggest elevated claudin 2 to be present in quiescent disease. However, there is little consensus regarding the up- or down regulation of the other claudin isoforms in these conditions. This may be explained to some extent by methodological differences and heterogeneity of patients and sampling with regard to disease activity and history. Further evaluation of the patterns of expression of claudins in active and inactive IBD patients should help to elucidate their contribution to disease, but longitudinal studies are also necessary. Future studies should evaluate therapeutic approaches that manipulate TJs, restoring epithelial barrier integrity, for the treatment of active inflammatory bowel diseases, maintenance of remission and prevention of onset of inflammation in the gut.
Footnotes
Supported by The Association for International Cancer Research (AICR, to Dr. Al-Hassi HO), Scotland; Funded by the AICR, grant No. 120234; a BBSRC Strategic Research Grant (to English N and Knight SC; WMNIP33458); the St Mark’s Hospital Foundation, United Kingdom.
Conflict-of-interest statement: None of the authors have any conflicts of interest or financial disclosures; and Dr. Hart has spoken for and is on the advisory board for MSD, Shire and Abbott.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: June 21, 2015
First decision: August 26, 2015
Article in press: December 19, 2015
P- Reviewer: Abdulnour-Nakhoul S S- Editor: Gong ZM L- Editor: A E- Editor: Wang CH
References
- 1.Krug SM, Schulzke JD, Fromm M. Tight junction, selective permeability, and related diseases. Semin Cell Dev Biol. 2014;36:166–176. doi: 10.1016/j.semcdb.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 2.Salim SY, Söderholm JD. Importance of disrupted intestinal barrier in inflammatory bowel diseases. Inflamm Bowel Dis. 2011;17:362–381. doi: 10.1002/ibd.21403. [DOI] [PubMed] [Google Scholar]
- 3.Edelblum KL, Turner JR. The tight junction in inflammatory disease: communication breakdown. Curr Opin Pharmacol. 2009;9:715–720. doi: 10.1016/j.coph.2009.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Groschwitz KR, Hogan SP. Intestinal barrier function: molecular regulation and disease pathogenesis. J Allergy Clin Immunol. 2009;124:3–20; quiz 21-22. doi: 10.1016/j.jaci.2009.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shen L, Weber CR, Turner JR. The tight junction protein complex undergoes rapid and continuous molecular remodeling at steady state. J Cell Biol. 2008;181:683–695. doi: 10.1083/jcb.200711165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schulzke JD, Ploeger S, Amasheh M, Fromm A, Zeissig S, Troeger H, Richter J, Bojarski C, Schumann M, Fromm M. Epithelial tight junctions in intestinal inflammation. Ann N Y Acad Sci. 2009;1165:294–300. doi: 10.1111/j.1749-6632.2009.04062.x. [DOI] [PubMed] [Google Scholar]
- 7.Amasheh S, Fromm M, Günzel D. Claudins of intestine and nephron - a correlation of molecular tight junction structure and barrier function. Acta Physiol (Oxf) 2011;201:133–140. doi: 10.1111/j.1748-1716.2010.02148.x. [DOI] [PubMed] [Google Scholar]
- 8.Rahner C, Mitic LL, Anderson JM. Heterogeneity in expression and subcellular localization of claudins 2, 3, 4, and 5 in the rat liver, pancreas, and gut. Gastroenterology. 2001;120:411–422. doi: 10.1053/gast.2001.21736. [DOI] [PubMed] [Google Scholar]
- 9.Escaffit F, Boudreau F, Beaulieu JF. Differential expression of claudin-2 along the human intestine: Implication of GATA-4 in the maintenance of claudin-2 in differentiating cells. J Cell Physiol. 2005;203:15–26. doi: 10.1002/jcp.20189. [DOI] [PubMed] [Google Scholar]
- 10.Hartsock A, Nelson WJ. Adherens and tight junctions: structure, function and connections to the actin cytoskeleton. Biochim Biophys Acta. 2008;1778:660–669. doi: 10.1016/j.bbamem.2007.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bruewer M, Samarin S, Nusrat A. Inflammatory bowel disease and the apical junctional complex. Ann N Y Acad Sci. 2006;1072:242–252. doi: 10.1196/annals.1326.017. [DOI] [PubMed] [Google Scholar]
- 12.Schneeberger EE, Lynch RD. Structure, function, and regulation of cellular tight junctions. Am J Physiol. 1992;262:L647–L661. doi: 10.1152/ajplung.1992.262.6.L647. [DOI] [PubMed] [Google Scholar]
- 13.Turksen K, Troy TC. Barriers built on claudins. J Cell Sci. 2004;117:2435–2447. doi: 10.1242/jcs.01235. [DOI] [PubMed] [Google Scholar]
- 14.Morita K, Furuse M, Fujimoto K, Tsukita S. Claudin multigene family encoding four-transmembrane domain protein components of tight junction strands. Proc Natl Acad Sci USA. 1999;96:511–516. doi: 10.1073/pnas.96.2.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Amasheh S, Meiri N, Gitter AH, Schöneberg T, Mankertz J, Schulzke JD, Fromm M. Claudin-2 expression induces cation-selective channels in tight junctions of epithelial cells. J Cell Sci. 2002;115:4969–4976. doi: 10.1242/jcs.00165. [DOI] [PubMed] [Google Scholar]
- 16.Rosenthal R, Milatz S, Krug SM, Oelrich B, Schulzke JD, Amasheh S, Günzel D, Fromm M. Claudin-2, a component of the tight junction, forms a paracellular water channel. J Cell Sci. 2010;123:1913–1921. doi: 10.1242/jcs.060665. [DOI] [PubMed] [Google Scholar]
- 17.Furuse M, Hata M, Furuse K, Yoshida Y, Haratake A, Sugitani Y, Noda T, Kubo A, Tsukita S. Claudin-based tight junctions are crucial for the mammalian epidermal barrier: a lesson from claudin-1-deficient mice. J Cell Biol. 2002;156:1099–1111. doi: 10.1083/jcb.200110122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Milatz S, Krug SM, Rosenthal R, Günzel D, Müller D, Schulzke JD, Amasheh S, Fromm M. Claudin-3 acts as a sealing component of the tight junction for ions of either charge and uncharged solutes. Biochim Biophys Acta. 2010;1798:2048–2057. doi: 10.1016/j.bbamem.2010.07.014. [DOI] [PubMed] [Google Scholar]
- 19.Van Itallie C, Rahner C, Anderson JM. Regulated expression of claudin-4 decreases paracellular conductance through a selective decrease in sodium permeability. J Clin Invest. 2001;107:1319–1327. doi: 10.1172/JCI12464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Amasheh S, Schmidt T, Mahn M, Florian P, Mankertz J, Tavalali S, Gitter AH, Schulzke JD, Fromm M. Contribution of claudin-5 to barrier properties in tight junctions of epithelial cells. Cell Tissue Res. 2005;321:89–96. doi: 10.1007/s00441-005-1101-0. [DOI] [PubMed] [Google Scholar]
- 21.Angelow S, Kim KJ, Yu AS. Claudin-8 modulates paracellular permeability to acidic and basic ions in MDCK II cells. J Physiol. 2006;571:15–26. doi: 10.1113/jphysiol.2005.099135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Angelow S, Yu AS. Claudins and paracellular transport: an update. Curr Opin Nephrol Hypertens. 2007;16:459–464. doi: 10.1097/MNH.0b013e32820ac97d. [DOI] [PubMed] [Google Scholar]
- 23.Angelow S, Ahlstrom R, Yu AS. Biology of claudins. Am J Physiol Renal Physiol. 2008;295:F867–F876. doi: 10.1152/ajprenal.90264.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baumgart DC, Carding SR. Inflammatory bowel disease: cause and immunobiology. Lancet. 2007;369:1627–1640. doi: 10.1016/S0140-6736(07)60750-8. [DOI] [PubMed] [Google Scholar]
- 25.Teshima CW, Dieleman LA, Meddings JB. Abnormal intestinal permeability in Crohn’s disease pathogenesis. Ann N Y Acad Sci. 2012;1258:159–165. doi: 10.1111/j.1749-6632.2012.06612.x. [DOI] [PubMed] [Google Scholar]
- 26.Weber CR, Turner JR. Inflammatory bowel disease: is it really just another break in the wall? Gut. 2007;56:6–8. doi: 10.1136/gut.2006.104182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Clayburgh DR, Shen L, Turner JR. A porous defense: the leaky epithelial barrier in intestinal disease. Lab Invest. 2004;84:282–291. doi: 10.1038/labinvest.3700050. [DOI] [PubMed] [Google Scholar]
- 28.Hollander D. Crohn’s disease--a permeability disorder of the tight junction? Gut. 1988;29:1621–1624. doi: 10.1136/gut.29.12.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hollander D, Vadheim CM, Brettholz E, Petersen GM, Delahunty T, Rotter JI. Increased intestinal permeability in patients with Crohn‘s disease and their relatives. A possible etiologic factor. Ann Intern Med. 1986;105:883–885. doi: 10.7326/0003-4819-105-6-883. [DOI] [PubMed] [Google Scholar]
- 30.Munkholm P, Langholz E, Hollander D, Thornberg K, Orholm M, Katz KD, Binder V. Intestinal permeability in patients with Crohn’s disease and ulcerative colitis and their first degree relatives. Gut. 1994;35:68–72. doi: 10.1136/gut.35.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heller F, Florian P, Bojarski C, Richter J, Christ M, Hillenbrand B, Mankertz J, Gitter AH, Bürgel N, Fromm M, et al. Interleukin-13 is the key effector Th2 cytokine in ulcerative colitis that affects epithelial tight junctions, apoptosis, and cell restitution. Gastroenterology. 2005;129:550–564. doi: 10.1016/j.gastro.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 32.Zolotarevsky Y, Hecht G, Koutsouris A, Gonzalez DE, Quan C, Tom J, Mrsny RJ, Turner JR. A membrane-permeant peptide that inhibits MLC kinase restores barrier function in in vitro models of intestinal disease. Gastroenterology. 2002;123:163–172. doi: 10.1053/gast.2002.34235. [DOI] [PubMed] [Google Scholar]
- 33.Arrieta MC, Madsen K, Doyle J, Meddings J. Reducing small intestinal permeability attenuates colitis in the IL10 gene-deficient mouse. Gut. 2009;58:41–48. doi: 10.1136/gut.2008.150888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.D’Incà R, Di Leo V, Corrao G, Martines D, D’Odorico A, Mestriner C, Venturi C, Longo G, Sturniolo GC. Intestinal permeability test as a predictor of clinical course in Crohn’s disease. Am J Gastroenterol. 1999;94:2956–2960. doi: 10.1111/j.1572-0241.1999.01444.x. [DOI] [PubMed] [Google Scholar]
- 35.Peeters M, Geypens B, Claus D, Nevens H, Ghoos Y, Verbeke G, Baert F, Vermeire S, Vlietinck R, Rutgeerts P. Clustering of increased small intestinal permeability in families with Crohn’s disease. Gastroenterology. 1997;113:802–807. doi: 10.1016/s0016-5085(97)70174-4. [DOI] [PubMed] [Google Scholar]
- 36.Anderson CA, Boucher G, Lees CW, Franke A, D’Amato M, Taylor KD, Lee JC, Goyette P, Imielinski M, Latiano A, et al. Meta-analysis identifies 29 additional ulcerative colitis risk loci, increasing the number of confirmed associations to 47. Nat Genet. 2011;43:246–252. doi: 10.1038/ng.764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barrett JC, Lee JC, Lees CW, Prescott NJ, Anderson CA, Phillips A, Wesley E, Parnell K, Zhang H, Drummond H, et al. Genome-wide association study of ulcerative colitis identifies three new susceptibility loci, including the HNF4A region. Nat Genet. 2009;41:1330–1334. doi: 10.1038/ng.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kroesen AJ, Dullat S, Schulzke JD, Fromm M, Buhr HJ. Permanently increased mucosal permeability in patients with backwash ileitis after ileoanal pouch for ulcerative colitis. Scand J Gastroenterol. 2008;43:704–711. doi: 10.1080/00365520701873206. [DOI] [PubMed] [Google Scholar]
- 39.Merrett MN, Soper N, Mortensen N, Jewell DP. Intestinal permeability in the ileal pouch. Gut. 1996;39:226–230. doi: 10.1136/gut.39.2.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Amasheh S, Dullat S, Fromm M, Schulzke JD, Buhr HJ, Kroesen AJ. Inflamed pouch mucosa possesses altered tight junctions indicating recurrence of inflammatory bowel disease. Int J Colorectal Dis. 2009;24:1149–1156. doi: 10.1007/s00384-009-0737-8. [DOI] [PubMed] [Google Scholar]
- 41.Kroesen AJ, Leistenschneider P, Lehmann K, Ransco C, Dullat S, Blaut M, Schulzke JD, Fromm M, Buhr HJ. Increased bacterial permeation in long-lasting ileoanal pouches. Inflamm Bowel Dis. 2006;12:736–744. doi: 10.1097/00054725-200608000-00010. [DOI] [PubMed] [Google Scholar]
- 42.Gitter AH, Wullstein F, Fromm M, Schulzke JD. Epithelial barrier defects in ulcerative colitis: characterization and quantification by electrophysiological imaging. Gastroenterology. 2001;121:1320–1328. doi: 10.1053/gast.2001.29694. [DOI] [PubMed] [Google Scholar]
- 43.Schmitz H, Barmeyer C, Fromm M, Runkel N, Foss HD, Bentzel CJ, Riecken EO, Schulzke JD. Altered tight junction structure contributes to the impaired epithelial barrier function in ulcerative colitis. Gastroenterology. 1999;116:301–309. doi: 10.1016/s0016-5085(99)70126-5. [DOI] [PubMed] [Google Scholar]
- 44.Prasad S, Mingrino R, Kaukinen K, Hayes KL, Powell RM, MacDonald TT, Collins JE. Inflammatory processes have differential effects on claudins 2, 3 and 4 in colonic epithelial cells. Lab Invest. 2005;85:1139–1162. doi: 10.1038/labinvest.3700316. [DOI] [PubMed] [Google Scholar]
- 45.Oshima T, Miwa H, Joh T. Changes in the expression of claudins in active ulcerative colitis. J Gastroenterol Hepatol. 2008;23 Suppl 2:S146–S150. doi: 10.1111/j.1440-1746.2008.05405.x. [DOI] [PubMed] [Google Scholar]
- 46.Poritz LS, Harris LR, Kelly AA, Koltun WA. Increase in the tight junction protein claudin-1 in intestinal inflammation. Dig Dis Sci. 2011;56:2802–2809. doi: 10.1007/s10620-011-1688-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zwiers A, Fuss IJ, Leijen S, Mulder CJ, Kraal G, Bouma G. Increased expression of the tight junction molecule claudin-18 A1 in both experimental colitis and ulcerative colitis. Inflamm Bowel Dis. 2008;14:1652–1659. doi: 10.1002/ibd.20695. [DOI] [PubMed] [Google Scholar]
- 48.Lameris AL, Huybers S, Kaukinen K, Mäkelä TH, Bindels RJ, Hoenderop JG, Nevalainen PI. Expression profiling of claudins in the human gastrointestinal tract in health and during inflammatory bowel disease. Scand J Gastroenterol. 2013;48:58–69. doi: 10.3109/00365521.2012.741616. [DOI] [PubMed] [Google Scholar]
- 49.Wyatt J, Vogelsang H, Hübl W, Waldhöer T, Lochs H. Intestinal permeability and the prediction of relapse in Crohn’s disease. Lancet. 1993;341:1437–1439. doi: 10.1016/0140-6736(93)90882-h. [DOI] [PubMed] [Google Scholar]
- 50.Zeissig S, Bürgel N, Günzel D, Richter J, Mankertz J, Wahnschaffe U, Kroesen AJ, Zeitz M, Fromm M, Schulzke JD. Changes in expression and distribution of claudin 2, 5 and 8 lead to discontinuous tight junctions and barrier dysfunction in active Crohn’s disease. Gut. 2007;56:61–72. doi: 10.1136/gut.2006.094375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Marin ML, Greenstein AJ, Geller SA, Gordon RE, Aufses AH. A freeze fracture study of Crohn‘s disease of the terminal ileum: changes in epithelial tight junction organization. Am J Gastroenterol. 1983;78:537–547. [PubMed] [Google Scholar]
- 52.Kucharzik T, Walsh SV, Chen J, Parkos CA, Nusrat A. Neutrophil transmigration in inflammatory bowel disease is associated with differential expression of epithelial intercellular junction proteins. Am J Pathol. 2001;159:2001–2009. doi: 10.1016/S0002-9440(10)63051-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Das P, Goswami P, Das TK, Nag T, Sreenivas V, Ahuja V, Panda SK, Gupta SD, Makharia GK. Comparative tight junction protein expressions in colonic Crohn’s disease, ulcerative colitis, and tuberculosis: a new perspective. Virchows Arch. 2012;460:261–270. doi: 10.1007/s00428-012-1195-1. [DOI] [PubMed] [Google Scholar]
- 54.Landy J, Al-Hassi HO, Ronde E, English NR, Mann ER, Bernardo D, Ciclitira PJ, Clark SK, Knight SC, Hart AL. Innate immune factors in the development and maintenance of pouchitis. Inflamm Bowel Dis. 2014;20:1942–1949. doi: 10.1097/MIB.0000000000000182. [DOI] [PubMed] [Google Scholar]
- 55.Wu P, Wu D, Ni C, Ye J, Chen W, Hu G, Wang Z, Wang C, Zhang Z, Xia W, et al. γδT17 cells promote the accumulation and expansion of myeloid-derived suppressor cells in human colorectal cancer. Immunity. 2014;40:785–800. doi: 10.1016/j.immuni.2014.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Grivennikov SI, Wang K, Mucida D, Stewart CA, Schnabl B, Jauch D, Taniguchi K, Yu GY, Osterreicher CH, Hung KE, et al. Adenoma-linked barrier defects and microbial products drive IL-23/IL-17-mediated tumour growth. Nature. 2012;491:254–258. doi: 10.1038/nature11465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dejea CM, Wick EC, Hechenbleikner EM, White JR, Mark Welch JL, Rossetti BJ, Peterson SN, Snesrud EC, Borisy GG, Lazarev M, et al. Microbiota organization is a distinct feature of proximal colorectal cancers. Proc Natl Acad Sci USA. 2014;111:18321–18326. doi: 10.1073/pnas.1406199111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Turksen K, Troy TC. Junctions gone bad: claudins and loss of the barrier in cancer. Biochim Biophys Acta. 2011;1816:73–79. doi: 10.1016/j.bbcan.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 59.Dhawan P, Ahmad R, Chaturvedi R, Smith JJ, Midha R, Mittal MK, Krishnan M, Chen X, Eschrich S, Yeatman TJ, et al. Claudin-2 expression increases tumorigenicity of colon cancer cells: role of epidermal growth factor receptor activation. Oncogene. 2011;30:3234–3247. doi: 10.1038/onc.2011.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mullin JM. Potential interplay between luminal growth factors and increased tight junction permeability in epithelial carcinogenesis. J Exp Zool. 1997;279:484–489. doi: 10.1002/(sici)1097-010x(19971201)279:5<484::aid-jez11>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 61.Ren J, Hamada J, Takeichi N, Fujikawa S, Kobayashi H. Ultrastructural differences in junctional intercellular communication between highly and weakly metastatic clones derived from rat mammary carcinoma. Cancer Res. 1990;50:358–362. [PubMed] [Google Scholar]
- 62.Ersoz S, Mungan S, Cobanoglu U, Turgutalp H, Ozoran Y. Prognostic importance of Claudin-1 and Claudin-4 expression in colon carcinomas. Pathol Res Pract. 2011;207:285–289. doi: 10.1016/j.prp.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 63.Webb PG, Spillman MA, Baumgartner HK. Claudins play a role in normal and tumor cell motility. BMC Cell Biol. 2013;14:19. doi: 10.1186/1471-2121-14-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mees ST, Mennigen R, Spieker T, Rijcken E, Senninger N, Haier J, Bruewer M. Expression of tight and adherens junction proteins in ulcerative colitis associated colorectal carcinoma: upregulation of claudin-1, claudin-3, claudin-4, and beta-catenin. Int J Colorectal Dis. 2009;24:361–368. doi: 10.1007/s00384-009-0653-y. [DOI] [PubMed] [Google Scholar]
- 65.Weber CR, Nalle SC, Tretiakova M, Rubin DT, Turner JR. Claudin-1 and claudin-2 expression is elevated in inflammatory bowel disease and may contribute to early neoplastic transformation. Lab Invest. 2008;88:1110–1120. doi: 10.1038/labinvest.2008.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kinugasa T, Akagi Y, Yoshida T, Ryu Y, Shiratuchi I, Ishibashi N, Shirouzu K. Increased claudin-1 protein expression contributes to tumorigenesis in ulcerative colitis-associated colorectal cancer. Anticancer Res. 2010;30:3181–3186. [PubMed] [Google Scholar]
- 67.Gupta RB, Harpaz N, Itzkowitz S, Hossain S, Matula S, Kornbluth A, Bodian C, Ullman T. Histologic inflammation is a risk factor for progression to colorectal neoplasia in ulcerative colitis: a cohort study. Gastroenterology. 2007;133:1099–1105; quiz 1340-1341. doi: 10.1053/j.gastro.2007.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Scharl M, Paul G, Barrett KE, McCole DF. AMP-activated protein kinase mediates the interferon-gamma-induced decrease in intestinal epithelial barrier function. J Biol Chem. 2009;284:27952–27963. doi: 10.1074/jbc.M109.046292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Watson CJ, Hoare CJ, Garrod DR, Carlson GL, Warhurst G. Interferon-gamma selectively increases epithelial permeability to large molecules by activating different populations of paracellular pores. J Cell Sci. 2005;118:5221–5230. doi: 10.1242/jcs.02630. [DOI] [PubMed] [Google Scholar]
- 70.Weber CR, Raleigh DR, Su L, Shen L, Sullivan EA, Wang Y, Turner JR. Epithelial myosin light chain kinase activation induces mucosal interleukin-13 expression to alter tight junction ion selectivity. J Biol Chem. 2010;285:12037–12046. doi: 10.1074/jbc.M109.064808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Blair SA, Kane SV, Clayburgh DR, Turner JR. Epithelial myosin light chain kinase expression and activity are upregulated in inflammatory bowel disease. Lab Invest. 2006;86:191–201. doi: 10.1038/labinvest.3700373. [DOI] [PubMed] [Google Scholar]
- 72.Su L, Shen L, Clayburgh DR, Nalle SC, Sullivan EA, Meddings JB, Abraham C, Turner JR. Targeted epithelial tight junction dysfunction causes immune activation and contributes to development of experimental colitis. Gastroenterology. 2009;136:551–563. doi: 10.1053/j.gastro.2008.10.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tanaka H, Takechi M, Kiyonari H, Shioi G, Tamura A, Tsukita S. Intestinal deletion of Claudin-7 enhances paracellular organic solute flux and initiates colonic inflammation in mice. Gut. 2015;64:1529–1538. doi: 10.1136/gutjnl-2014-308419. [DOI] [PubMed] [Google Scholar]
- 74.Hanauer SB, Feagan BG, Lichtenstein GR, Mayer LF, Schreiber S, Colombel JF, Rachmilewitz D, Wolf DC, Olson A, Bao W, et al. Maintenance infliximab for Crohn’s disease: the ACCENT I randomised trial. Lancet. 2002;359:1541–1549. doi: 10.1016/S0140-6736(02)08512-4. [DOI] [PubMed] [Google Scholar]
- 75.Rutgeerts P, Sandborn WJ, Feagan BG, Reinisch W, Olson A, Johanns J, Travers S, Rachmilewitz D, Hanauer SB, Lichtenstein GR, et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2005;353:2462–2476. doi: 10.1056/NEJMoa050516. [DOI] [PubMed] [Google Scholar]
- 76.Barreiro-de Acosta M, García-Bosch O, Souto R, Mañosa M, Miranda J, García-Sanchez V, Gordillo J, Chacon S, Loras C, Carpio D, et al. Efficacy of infliximab rescue therapy in patients with chronic refractory pouchitis: a multicenter study. Inflamm Bowel Dis. 2012;18:812–817. doi: 10.1002/ibd.21821. [DOI] [PubMed] [Google Scholar]
- 77.Suenaert P, Bulteel V, Lemmens L, Noman M, Geypens B, Van Assche G, Geboes K, Ceuppens JL, Rutgeerts P. Anti-tumor necrosis factor treatment restores the gut barrier in Crohn’s disease. Am J Gastroenterol. 2002;97:2000–2004. doi: 10.1111/j.1572-0241.2002.05914.x. [DOI] [PubMed] [Google Scholar]
- 78.Zeissig S, Bojarski C, Buergel N, Mankertz J, Zeitz M, Fromm M, Schulzke JD. Downregulation of epithelial apoptosis and barrier repair in active Crohn’s disease by tumour necrosis factor alpha antibody treatment. Gut. 2004;53:1295–1302. doi: 10.1136/gut.2003.036632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fries W, Muja C, Crisafulli C, Cuzzocrea S, Mazzon E. Dynamics of enterocyte tight junctions: effect of experimental colitis and two different anti-TNF strategies. Am J Physiol Gastrointest Liver Physiol. 2008;294:G938–G947. doi: 10.1152/ajpgi.00469.2007. [DOI] [PubMed] [Google Scholar]
- 80.Fischer A, Gluth M, Pape UF, Wiedenmann B, Theuring F, Baumgart DC. Adalimumab prevents barrier dysfunction and antagonizes distinct effects of TNF-α on tight junction proteins and signaling pathways in intestinal epithelial cells. Am J Physiol Gastrointest Liver Physiol. 2013;304:G970–G979. doi: 10.1152/ajpgi.00183.2012. [DOI] [PubMed] [Google Scholar]
- 81.Roediger WE. The colonic epithelium in ulcerative colitis: an energy-deficiency disease? Lancet. 1980;2:712–715. doi: 10.1016/s0140-6736(80)91934-0. [DOI] [PubMed] [Google Scholar]
- 82.Thibault R, Blachier F, Darcy-Vrillon B, de Coppet P, Bourreille A, Segain JP. Butyrate utilization by the colonic mucosa in inflammatory bowel diseases: a transport deficiency. Inflamm Bowel Dis. 2010;16:684–695. doi: 10.1002/ibd.21108. [DOI] [PubMed] [Google Scholar]
- 83.Chapman MA, Grahn MF, Hutton M, Williams NS. Butyrate metabolism in the terminal ileal mucosa of patients with ulcerative colitis. Br J Surg. 1995;82:36–38. doi: 10.1002/bjs.1800820115. [DOI] [PubMed] [Google Scholar]
- 84.Wang HB, Wang PY, Wang X, Wan YL, Liu YC. Butyrate enhances intestinal epithelial barrier function via up-regulation of tight junction protein Claudin-1 transcription. Dig Dis Sci. 2012;57:3126–3135. doi: 10.1007/s10620-012-2259-4. [DOI] [PubMed] [Google Scholar]
- 85.Daly K, Shirazi-Beechey SP. Microarray analysis of butyrate regulated genes in colonic epithelial cells. DNA Cell Biol. 2006;25:49–62. doi: 10.1089/dna.2006.25.49. [DOI] [PubMed] [Google Scholar]
- 86.Amasheh M, Schlichter S, Amasheh S, Mankertz J, Zeitz M, Fromm M, Schulzke JD. Quercetin enhances epithelial barrier function and increases claudin-4 expression in Caco-2 cells. J Nutr. 2008;138:1067–1073. doi: 10.1093/jn/138.6.1067. [DOI] [PubMed] [Google Scholar]
- 87.Amasheh M, Fromm A, Krug SM, Amasheh S, Andres S, Zeitz M, Fromm M, Schulzke JD. TNFalpha-induced and berberine-antagonized tight junction barrier impairment via tyrosine kinase, Akt and NFkappaB signaling. J Cell Sci. 2010;123:4145–4155. doi: 10.1242/jcs.070896. [DOI] [PubMed] [Google Scholar]
- 88.Ulluwishewa D, Anderson RC, McNabb WC, Moughan PJ, Wells JM, Roy NC. Regulation of tight junction permeability by intestinal bacteria and dietary components. J Nutr. 2011;141:769–776. doi: 10.3945/jn.110.135657. [DOI] [PubMed] [Google Scholar]
- 89.Li Q, Zhang Q, Zhang M, Wang C, Zhu Z, Li N, Li J. Effect of n-3 polyunsaturated fatty acids on membrane microdomain localization of tight junction proteins in experimental colitis. FEBS J. 2008;275:411–420. doi: 10.1111/j.1742-4658.2007.06210.x. [DOI] [PubMed] [Google Scholar]
- 90.Li Q, Zhang Q, Wang M, Zhao S, Xu G, Li J. n-3 polyunsaturated fatty acids prevent disruption of epithelial barrier function induced by proinflammatory cytokines. Mol Immunol. 2008;45:1356–1365. doi: 10.1016/j.molimm.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 91.Amasheh M, Andres S, Amasheh S, Fromm M, Schulzke JD. Barrier effects of nutritional factors. Ann N Y Acad Sci. 2009;1165:267–273. doi: 10.1111/j.1749-6632.2009.04063.x. [DOI] [PubMed] [Google Scholar]
- 92.Bergmann KR, Liu SX, Tian R, Kushnir A, Turner JR, Li HL, Chou PM, Weber CR, De Plaen IG. Bifidobacteria stabilize claudins at tight junctions and prevent intestinal barrier dysfunction in mouse necrotizing enterocolitis. Am J Pathol. 2013;182:1595–1606. doi: 10.1016/j.ajpath.2013.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Anderson RC, Cookson AL, McNabb WC, Park Z, McCann MJ, Kelly WJ, Roy NC. Lactobacillus plantarum MB452 enhances the function of the intestinal barrier by increasing the expression levels of genes involved in tight junction formation. BMC Microbiol. 2010;10:316. doi: 10.1186/1471-2180-10-316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Liu ZH, Shen TY, Zhang P, Ma YL, Moyer MP, Qin HL. Protective effects of Lactobacillus plantarum against epithelial barrier dysfunction of human colon cell line NCM460. World J Gastroenterol. 2010;16:5759–5765. doi: 10.3748/wjg.v16.i45.5759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Patel RM, Myers LS, Kurundkar AR, Maheshwari A, Nusrat A, Lin PW. Probiotic bacteria induce maturation of intestinal claudin 3 expression and barrier function. Am J Pathol. 2012;180:626–635. doi: 10.1016/j.ajpath.2011.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ewaschuk JB, Diaz H, Meddings L, Diederichs B, Dmytrash A, Backer J, Looijer-van Langen M, Madsen KL. Secreted bioactive factors from Bifidobacterium infantis enhance epithelial cell barrier function. Am J Physiol Gastrointest Liver Physiol. 2008;295:G1025–G1034. doi: 10.1152/ajpgi.90227.2008. [DOI] [PubMed] [Google Scholar]
- 97.Seth A, Yan F, Polk DB, Rao RK. Probiotics ameliorate the hydrogen peroxide-induced epithelial barrier disruption by a PKC- and MAP kinase-dependent mechanism. Am J Physiol Gastrointest Liver Physiol. 2008;294:G1060–G1069. doi: 10.1152/ajpgi.00202.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhang YG, Wu S, Xia Y, Sun J. Salmonella infection upregulates the leaky protein claudin-2 in intestinal epithelial cells. PLoS One. 2013;8:e58606. doi: 10.1371/journal.pone.0058606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Fasano A. Zonulin, regulation of tight junctions, and autoimmune diseases. Ann N Y Acad Sci. 2012;1258:25–33. doi: 10.1111/j.1749-6632.2012.06538.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Paterson BM, Lammers KM, Arrieta MC, Fasano A, Meddings JB. The safety, tolerance, pharmacokinetic and pharmacodynamic effects of single doses of AT-1001 in coeliac disease subjects: a proof of concept study. Aliment Pharmacol Ther. 2007;26:757–766. doi: 10.1111/j.1365-2036.2007.03413.x. [DOI] [PubMed] [Google Scholar]
- 101.Denizot J, Sivignon A, Barreau F, Darcha C, Chan HF, Stanners CP, Hofman P, Darfeuille-Michaud A, Barnich N. Adherent-invasive Escherichia coli induce claudin-2 expression and barrier defect in CEABAC10 mice and Crohn’s disease patients. Inflamm Bowel Dis. 2012;18:294–304. doi: 10.1002/ibd.21787. [DOI] [PubMed] [Google Scholar]
- 102.Feldman GJ, Mullin JM, Ryan MP. Occludin: structure, function and regulation. Adv Drug Deliv Rev. 2005;57:883–917. doi: 10.1016/j.addr.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 103.Umeda K, Matsui T, Nakayama M, Furuse K, Sasaki H, Furuse M, Tsukita S. Establishment and characterization of cultured epithelial cells lacking expression of ZO-1. J Biol Chem. 2004;279:44785–44794. doi: 10.1074/jbc.M406563200. [DOI] [PubMed] [Google Scholar]


