Abstract
AIM: To investigate estrogen receptors expression in duodenal familial adenomatous polyposis (FAP) and any relationship with epithelial proliferation/apoptosis markers.
METHODS: Twenty-two patients affected by FAP undergoing duodenal resection for malignancies were recruited. Controls were 15 healthy subjects undergoing endoscopy for dyspeptic symptoms. ER-α, ER-α, Ki-67, TUNEL and caspase 3 expression (labeling index: percentage of positive cells) were evaluated by immunohistochemistry or immunofluorescence and examined by light or confocal microscopy. Samples were assigned to four groups: normal tissue, low (LGD) and high-grade dysplasia (HGD), adenocarcinoma (AC). One-way analysis of variance, corrected by Bonferroni’s test, and Pearson’s correlation test were applied for statistical analysis.
RESULTS: ER-beta showed a progressive decline: normal tissue (23.5 ± 4.9), LGD (21.1 ± 4.8), HGD (9.3 ± 3.5), AC (7.1 ± 3.1). The normal tissue of FAP subjects expressed ER-beta like the controls (23.9 ± 6.2). Conversely, ER-α showed a progressive increase from normal tissue (24.8 ± 5.6) to AC (52.0 ± 8.2); the expression in normal tissue was similar to controls (22.5 ± 5.3). Ki67 demonstrated a statistically significant progressive increase at each disease stage up to AC. TUNEL did not reveal differences between controls and normal tissue of FAP subjects, but progressive decreases were observed in LGD, through HGD to AC. Pearson’s correlation test showed a direct relationship between ER-β and TUNEL LI (r = 0.8088, P < 0.0001). Conversely, ER-α was inversely correlated with TUNEL LI (r = - 0.7257, P < 0.0001). The co-expression of ER-β and caspase 3 declined progressively from normal to neoplastic tissue.
CONCLUSION: This study confirmed that ER-β is strongly decreased in duodenal FAP carcinomas, declining in a multiple step fashion, thereby suggesting a putative anti-carcinogenic effect. ER-α showed the opposite trend. ER-β/caspase 3 co-expression suggests this hormone’s possible involvement in apoptosis. Hormonal influences in FAP duodenal tumorigenesis, and modulation of these as a possible chemoprevention strategy, may be a promising approach.
Keywords: Familial adenomatous polyposis, Duodenal cancer, Estrogen receptors, Immunohistochemistry, Confocal microscopy, Dysplasia
Core tip: Familial adenomatous polyposis (FAP) is a genetically inherited disease featuring multiple colonic polyps, with possible duodenal involvement. No study investigating the relationship between estrogen receptors (ERs) and cellular turnover has previously been reported in duodenal FAP patient’s subset. In the present study we found that ER-β is strongly decreased in duodenal FAP carcinomas, in a multiple step fashion suggesting a putative anti-carcinogenic effect. ER-α shows the opposite trend. A possible ER-beta involvement in apoptosis is suggested by its co-expression with caspase-3. The modulation of hormonal influences in FAP duodenal tumorigenesis as a possible chemoprevention strategy may be a promising approach.
INTRODUCTION
Familial adenomatous polyposis (FAP) represents a genetic hereditary disease due to adenomatous polyposis coli (APC) gene mutation, influencing the early development of multiple (more than a hundred) colonic polyps[1]. FAP is considered to be a rare disease, with an incidence ranging from 1: 7000 to 1:8000 births per year[2,3]. The mechanism of inheritance is usually autosomal dominant, with a very high penetrance, although 25%-30% of cases arise from a de novo mutation. The APC gene acts as a tumor suppressor, being involved in the degradation of beta-catenin, a protein involved in the Wnt pathway, responsible for the genetic transcription of oncogenes such as Myc[4].
Guidelines advise a strict surveillance protocol in APC[5], starting from the age of 10-12, by annual colonoscopic examinations. The dramatically high number of adenomas is often an indication for elective prophylactic colorectal surgery[6]. The most widespread surgical options are subtotal colectomy/ileorectal anastomosis, total proctocolectomy/ileostomy, and proctocolectomy/ileal-pouch-anal anastomosis[7,8]. However, after the colonic resection, an annual surveillance protocol is essential, because the risk of a new cancer in a different site of the gastrointestinal system remains high. In particular, adenomatous polyps may arise in the duodenum, especially in the periampullary area. After colorectal carcinoma, a periampullary tumor is the most common malignancy (5%-6%), being the main cause of death in subjects who have undergone colectomy[9]. In the case of duodenal cancers, the Whipple procedure has been widely employed, but recently novel and less demolitive procedures have been successfully attempted, such as pylorus-preserving duodenectomy or pancreas-preserving duodenectomy[10].
Estrogen receptors (ERs) are a class of nuclear receptors that are closely involved in colorectal carcinogenesis[11]. In particular, the beta isoform (ER-β) has been found to be protective against cancer growth and down-regulated in colorectal cancer (CRC), whereas the alpha isoform (ER-α) promotes cell proliferation and is up-regulated in CRC[12,13]. To date, only one study has investigated the colonic expression of ERs in subjects affected by FAP, confirming this trend by demonstrating a progressive decrease of ER-β from normal tissue to CRC, through low-grade (LGD) and high-grade dysplasia[14]. The possibility of ER-beta modulation via a selective stimulation by natural products, such as phytoestrogens, has been postulated to be a possible chemoprevention strategy against CRC[11]. Indeed, some studies have demonstrated the safety and effectiveness of phytoestrogen supplementation in the prevention of CRC[15]. Recently, Calabrese et al[16] have shown that supplementation with a phytoestrogen mixture is able to decrease amount and size of duodenal adenomas in FAP patients who underwent prophylactic colectomy, thus emphasizing the role of ER in FAP, not only in the colon, but even in extra-intestinal sites.
To the best of our knowledge, no study investigating the relationship between ERs and cellular turnover (proliferation/apoptosis) has yet been reported in the duodenal FAP patients subset. Hence, we performed an experimental study investigating the expression of ER-α and β in subjects undergoing duodenal resection for epithelial malignancy after prophylactic colectomy. Additionally, in this study ERs expression was correlated with the markers of cellular proliferation (Ki-67) and apoptosis (TUNEL). Finally, a declining co-expression of ER-beta with caspase 3, a known marker of the early stages of apoptosis, was detected in the progressive stages of FAP carcinogenesis.
MATERIALS AND METHODS
Patients
The study group included 22 patients (8 females and 14 males) affected by FAP undergoing duodenal surgical resection for epithelial malignancy. The age of enrolled patients was 44.2 ± 12.9 years (mean ± SD); median age was 44 years. The patients underwent the removal of duodenal neoplasms by different procedures in relation to the stage of the disease and according to the guidelines[5]: 4 patients underwent endoscopic polypectomy, 5 surgical polypectomy through a duodenotomy, 7 ampullectomy, 3 duodenal resection and 3 pancreatoduodenectomy. Resected lesions were histologically classified as: low grade dysplasia (LGD), high grade dysplasia (HGD) or carcinoma (AC). Formalin embedded samples were retrospectively retrieved. Collection and processing were performed according to BRISQ recommendations[17].
The control tissue consisted of duodenal biopsy samples from 15 healthy individuals (6 females and 9 males) undergoing upper endoscopy and showing a histologically normal mucosa. The mean age of the control group was similar to that of the study group (42.5 ± 11.4 years, median 43 years). Control tissues were selected from archive material. At least two biopsy samples had been taken in subjects undergoing upper endoscopy for dyspeptic symptoms from the second Duodenum. The absence of both histological and endoscopic abnormalities in this site was the main selection criterion. Further confirmation of a normal duodenal picture was based on the presence of less than 10 CD3/100 enterocytes at immuno-histochemical staining. Finally, in all patients, possible causes of “Duodenal lymphocytosis” had been excluded according to a previous report by our group[18]. Demographic and clinical characteristics of enrolled patients, including the familial polyposis history, are reported in Table 1.
Table 1.
Main features of enrolled patients
| Patient number | Age | Sex | Surgical procedure | Relatives with FAP |
| 1 | 49 | F | Surgical polypectomy/duodenotomy | Brother |
| 2 | 34 | M | Endoscopic polypectomy | Mother |
| 3 | 62 | M | Duodenal resection | Daughter |
| 4 | 29 | F | Endoscopic polypectomy | Father, sister |
| 5 | 32 | M | Ampullectomy | Mother |
| 6 | 35 | F | Duodenal resection | Father, brother |
| 7 | 46 | M | Surgical polypectomy/duodenotomy | Father |
| 8 | 59 | M | Ampullectomy | Mother, son |
| 9 | 58 | F | Duodenal resection | Father, daughter |
| 10 | 51 | F | pancreatoduodenectomy | Mother, sister |
| 11 | 46 | M | Ampullectomy | Mother, daughter, sister |
| 12 | 47 | M | Surgical polypectomy/duodenotomy | Brother |
| 13 | 33 | F | Surgical polypectomy/duodenotomy | Mother |
| 14 | 58 | M | pancreatoduodenectomy | Mother, sister |
| 15 | 24 | F | Ampullectomy | Father, aunt |
| 16 | 51 | M | Ampullectomy | Sister |
| 17 | 32 | M | Endoscopic polypectomy | Mother, brother |
| 18 | 29 | F | Endoscopic polypectomy | Mother |
| 19 | 31 | M | Ampullectomy | Father |
| 20 | 64 | M | pancreatoduodenectomy | Mother, son |
| 21 | 58 | M | Ampullectomy | Brother |
| 22 | 46 | M | Surgical polypectomy/duodenotomy | Father, brother |
Immunohistochemistry of estrogen receptors
ER-β and ER-α nuclear expression was detected by anti-ER-β (Novocastra Menarini, Milan, Italy) and anti-ER-α monoclonal (Santa Cruz, CA, United States) mouse antibodies. First, antigen was recovered (shakeup in phosphate buffered saline - PBS - with TWEEN 0.025% followed for ER-β and ER-α by enzymatic unmasking - proteolytic enzyme for auto-stainer, Dako, Copenhagen, Denmark, and microwave treatment in citric buffer at pH 6.0, respectively) and, successively, the samples underwent overnight incubation at 4 °C using primary antibodies (dilution 1:50 with PBS).
After washing in PBS, sections underwent 20 min (two steps) ambient temperature incubation with an HRP polymer-based anti-mouse kit (Biocare Medical, Concord, CA, United States). 3,3-diaminobenzidine-tetrahydrochloride (DAB, Vector laboratories) represented the chromogen, while haematoxylin (Sigma, Italy) the nuclear counterstain.
Healthy colonic mucosa as well as breast carcinoma sections represented ER-β and ER-α positive controls, respectively.
Evaluation of epithelium proliferation
Ki-67 was used as marker of epithelium proliferation and detected by monoclonal rabbit antibody (clone MIB-1, Dako, Copenhagen, Denmark). After de-waxing by xylene, samples were cleaned by absolute ethanol and water graded mixtures. Endogenous peroxidase activity was inhibited by 3% hydrogen peroxide. Antigen was unmasked by microwave oven (two steps of 5 min in citrate buffer pH 6.0) at 750 W. After non-specific antigen inhibition by goat serum (5%), primary antibody (Dako, Copenhagen, Denmark) was incubated overnight at 4 °C, at a dilution of 1: 100. Secondary peroxidase-conjugated antibody (EnVision, DakoCopenhagen, Denmark) was used (40 min, ambient temperature). Aminoethylcarbazole (Vector laboratory) was the chromogen and aqueous haematoxylin the counterstain dye.
Evaluation of epithelium apoptosis
TUNEL (in situ Cell Death Detection kit, Roche, Italy) was used as apoptosis marker. Briefly, samples were de-waxed and incubated with 0.1 mol/L citrate buffer (pH 6.0) in microwave oven at 350 W for 10 min. Then, sections were treated by TUNEL probe (37 °C, 1 h). TOPRO 3 was used as counterstain (Invitrogen Molecular Probes) at a dilution of 1:5000. Confocal microscopy magnification was used for TUNEL expression evaluation.
Caspase 3 and ER-beta co-expression
Caspase-3 was investigated by a polyclonal rabbit antibody cell signaling (gene ID 836 - Novus Biologicals, Milan, Italy) as well as the co-expression with ER-beta (Novocastra Menarini, Milan, Italy). Sections were agitated in PBS buffer with TWEEN 0.025% for 10 min and incubated in microwave oven (citric buffer pH 6.0, 10 min, 750 W) for antigen unmasking. Then, they were treated (1 h, ambient temperature) in 5% BSA and FCS blocking solution. Successively, they were immersed in a combination of anti-ER-beta 1:50/anti-Caspase-3 1:50, at 4 °C overnight. Alexa 555 fluorescent-conjugated goat anti-rabbit/anti-mouse (Invitrogen, Life Technologies, Monza, Italy) at a dilution of 1:200 represented secondary antibody. Nuclear counterstain was obtained using TOPRO-3 (Invitrogen-Molecular Probes) for 10 min at ambient temperature.
Semiquantitative estimation of immunohistochemistry
Samples were examined at × 400. The count of positive cells was performed on 10 well-oriented villi/crypts. Labeling index (LI), i.e., percentage of positive cells, represented the numerical value of the estimation.
For each patient, the cell count was carried out in areas of adenocarcinoma, high-grade dysplasia, low-grade dysplasia and normal tissue. Due to the large size of the surgical resection, each sample contained all four pictures. The evaluation was performed by two blinded observers.
Statistics: Comparison among the five groups (Controls, normal mucosa in FAP, LGD, HGD, AC) of ER-beta, ER-α, ER ratio, TUNEL and Ki67 was carried out by one-way analysis of variance (ANOVA) plus post-hoc Bonferroni’s test. Correlation analysis was carried out by Pearson’s test, and the r values with 95%CI were calculated. Significance was set at P < 0.05 (two-tailed). Weighted k statistics coefficient according to Landis and Koch benchmarks was computed for diagnostic agreement of immunohistochemistry (< 0.4: poor agreement; 0.4-0.8: moderate-good agreement; > 0.8: excellent agreement). GraphPad Prism software version 5.00 for Windows (GraphPad Software, San Diego California United States) was used for computing. An expert in Biomedical Statistics evaluated the statistical methods used in this study.
RESULTS
Inter-observer agreement regarding immunohistochemistry estimation of ER-α, ER-β, TUNEL and Ki67 expression yielded k = 0.84 (95%CI: 0.72-0.94).
ER-α LI showed a progressive increase from normal tissue (24.8 ± 5.6) to AC (52.0 ± 8.2). The expression in normal tissue was similar to that in controls (22.5 ± 5.3). There was a non-significant increase (28.0 ± 7.7) in LGD, while an up-regulation of ER-α (42.2 ± 17.2) was demonstrated in HGD, similar to that in AC. Statistical analysis demonstrated Negative controls = normal tissue = LGD < HGD = AC, as reported in Figure 1. Immunohistochemical staining of ER-alpha is shown in Figure 2.
Figure 1.
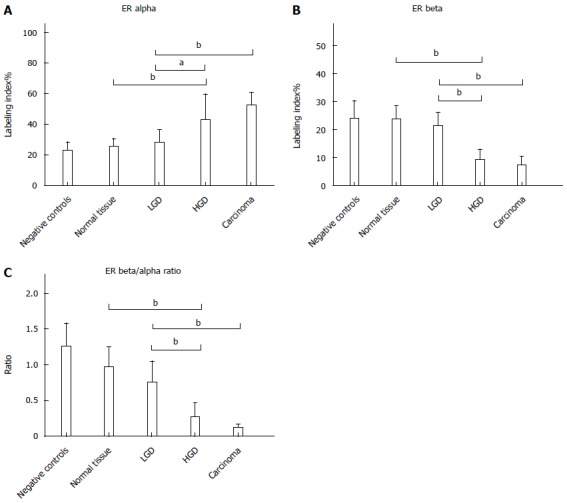
Labeling Index values of immunohistochemical analysis of ER alpha (A), ER beta (B) and ER beta/alpha ratio (C). aP < 0.05; bP < 0.001.
Figure 2.
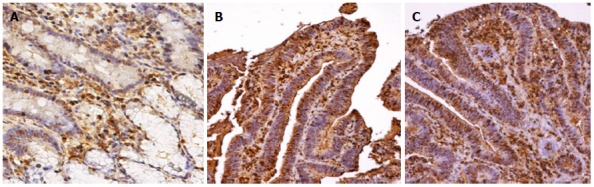
Immunohistochemical staining of ER-α. A: normal; B: HGD; C: AC.
ER-β LI revealed an opposite trend to ER-alpha, with a progressive decline from normal tissue (23.5 ± 4.9) to LGD (21.1 ± 4.8), HGD (9.3 ± 3.5) and AC (7.1 ± 3.1). The normal tissue of subjects affected by FAP expressed ER-beta like the controls (23.9 ± 6.2). Statistical analysis demonstrated Negative controls = normal tissue = LGD > HGD = AC, as reported in Figure 1. Immunohistochemical staining of ER-beta is shown in Figure 3.
Figure 3.
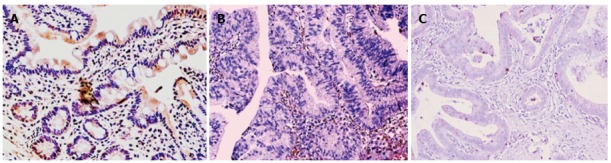
Immunohistochemical staining of ER-β. A: Normal; B: HGD; C: AC.
Finally, the ER-β/α ratio (Figure 1) showed a tendency to decline from normal tissue to AC.
Ki67 LI demonstrated a statistically significant progressive increase at each disease stage up to AC (Negative controls 20.7 ± 5.5 = Normal tissue 21.5 ± 6.3 < LGD 35.3 ± 5.2 < HGD 46.3 ± 8.5 < AC 59.3 ± 9.5). There was no difference in apoptosis (TUNEL LI) between controls and Normal tissue of FAP, but a progressive decrease in LGD, through HGD to AC (Negative controls 13.6 ± 1.9 = Normal tissue 13.1 ± 9.2 > LGD 8.0 ± 1.3 > HGD 2.3 ± 1.2 = AC 1.7 ± 0.7). Epithelium proliferation/apoptosis LI and statistics are reported in Figure 4.
Figure 4.
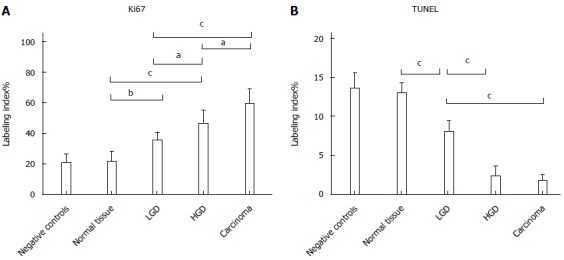
Labeling Index values of immunohistochemical analysis for proliferation - Ki67 (A) and apoptosis - TUNEL (B). aP < 0.05; bP < 0.01; cP < 0.001.
As reported in Figure 5, Pearson’s correlation test showed that ER-beta was directly correlated with TUNEL LI (r = 0.8088, 95%CI: 0.6478-0.9007, P < 0.0001), whereas ER-α was inversely correlated with TUNEL LI [r = - 0.7257, 95%CI: -0.8543 - (-0.5135), P < 0.0001]. The relationship between ER-beta and apoptosis markers was confirmed by the evidence of ER-beta/caspase 3 co-expression. This co-expression, as reported in Figure 6, was observed to decrease progressively from normal to neoplastic tissue (Negative controls 19.89 ± 5.241 = Normal tissue 21.14 ± 3.850 = LGD 18.84 ± 3.918 > HGD: 7.288 ± 3.062 = AC: 5.870 ± 2.576). In Figure 7, the co-expression of ER-beta and caspase 3 is clearly shown in normal tissue, while an evident, poor expression is observed in HGD.
Figure 5.
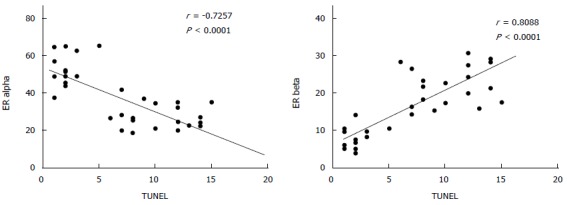
Direct correlation between ER-beta and TUNEL and inverse correlation between ER-α and TUNEL (Pearson’s test).
Figure 6.
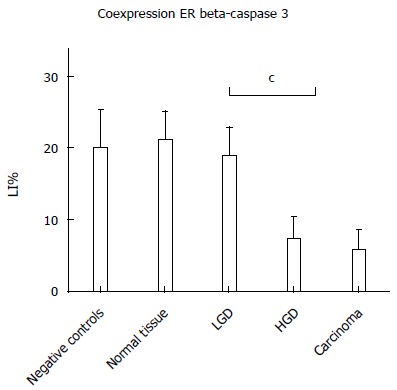
Labeling Index values of confocal microscopy analysis of ER-beta and caspase 3 co-expression. cP < 0.001.
Figure 7.
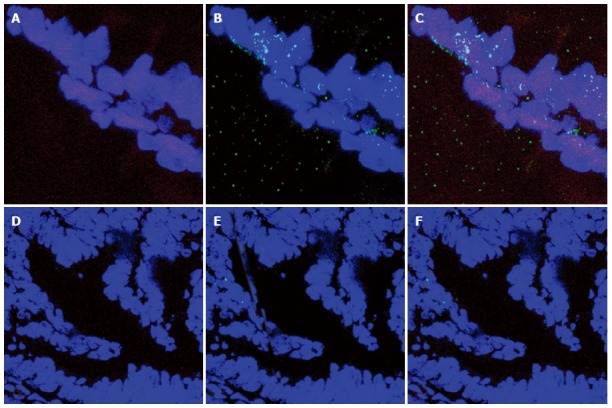
Co-expression of ER-β and caspase 3 is clearly shown in normal tissue. A: Nuclear ER-β expression in a normal villous area (red signal); B: Nuclear caspase 3 expression in a normal villous area (green signal); C: Merge of the two signals and counterstaining with TOPRO 3 (blue signal): magnification at × 1000; D: Nuclear ER-beta expression in the HGD area (red signal); E: Nuclear caspase 3 expression in the HGD area (green signal); F: Merge of the two signals and counterstaining with TOPRO 3 (blue signal): magnification at × 400.
DISCUSSION
FAP is a rare disease in which a genetic mutation predisposes affected subjects to multiple colorectal cancers. Although it represents a typical monogenic Mendelian example of genetic CRC, carcinogenesis may be sustained by other pathways, including inflammatory and hormonal factors. Indeed, Giardiello et al[19] have reported a case of polyp regression in a patient with FAP after the administration of oral contraceptives. Likewise, Tonelli et al[20] reported that raloxifene, a selective ER modulator, is able to reduce desmoid tumors and mesenteric fibromatosis in patients with Gardner’s syndrome. Furthermore, engineered human colon cancer cells (HCT8) over-expressing ER-beta showed an inhibited cell proliferation and an increased cell adhesion, in a ligand-independent manner, as reported by Martineti et al[21]. The magnitude of this effect was directly correlated with the level of ER-beta expression. In this regard, our group[14] has previously demonstrated that LGD, HGD and carcinoma in FAP are characterized by a progressive decline in colonic ER-beta expression and a contemporary increase in ER-alpha expression. This finding is consistent with the results of the present study, and may provide evidence that ERs in FAP play a similar role to the one identified in both sporadic[22-24] and inflammatory CRC[25-27]. Indeed, ER-alpha acts as a pro-carcinogenic and ER-beta as an anti-carcinogenic.
The rarity of FAP is an obstacle to experimentation, but animal models mimicking the pathogenesis of FAP are useful in providing data that can be applicable to humans. Apcmin/+ mice are genetically modified rodents with the same mutation in the APC gene which occurs in human FAP. Animals develop multiple bowel adenomas evolving into carcinomas. Using this model, Cleveland et al[28] have demonstrated that the loss of ER-beta supports the growth of many large polyps, while the activation of ER-alpha triggers the Wnt/β-catenin pathway. Although the interaction between ERs and APC has not been fully investigated, a possible “key to understanding” could be deduced according to Kouzmenko et al[29]. In this study, APC was shown to be physically associated with ER-alpha in a ligand-dependent manner, thus inhibiting the transcription of ER-alpha related genes such as c-myc and cyclin D. Meanwhile, nuclear ER-beta has been demonstrated to be able to cleave beta catenin, a function that is normally played by APC[30]. In this way, ER-alpha and beta may act in reciprocally opposite ways in the regulation of the Wnt/β catenin/APC pathway.
Many attempts to obtain an effective chemoprevention have been made in human and animal models. The results support the hypothesis that selective estrogen stimulation is protective in FAP, as also in sporadic CRCs. Indeed, the promising report by Calabrese et al[16] about the beneficial effect of a selective stimulation of ER-beta on duodenal polyps offers a clue that estrogens and ERs may modulate carcinogenesis in FAP. Moreover, Barone et al[31] have shown that a phytoestrogen supplementation slows down polyp onset through a process of over-stimulation of ER-beta in Apcmin/+ mice. Additionally, Khor et al[32] demonstrated that phenethyl isothiocyanate chemoprevention is mediated by an increased expression of caspase 3 in the same model. Interestingly, some reports show that ER-beta activation induces a phosphorylation of p38/MAPK, which is involved in caspase-3 activation, driving cells into the apoptotic cycle[33,34]. Finally, caspase 3 expression is strongly reduced in Apcmin/+ mice with carcinoma[35].
All the data reported about ERs and pathophysiological mechanisms of cellular turnover have been obtained in human and animal models at the level of the large bowel in the course of FAP. As known, a subset of patients may develop adenomas and carcinomas in the duodenum. In this setting, no study about carcinogenic mechanisms and ERs involvement has yet been performed, but clinical evidence in only one study shows that ER-beta stimulation by phytoestrogens has a beneficial effect on polyp size and number[16].
In the present study, we firstly assessed the expression of ERs alpha and beta in FAP patients with duodenal carcinoma and their relationship with epithelial proliferation/apoptosis markers. Additionally, based on the evidence of a link between the ER-beta and caspase 3 pathways in anti-neoplastic activity, we studied the co-expression of these molecules. Although the small sample size may be a limit of the study, we must bear in mind the rarity of FAP, whose incidence ranges from 1:7000 to 1:8000 births per year[2,3]. Moreover, its localization in the small bowel is very uncommon, accounting for only 8.5% in a registry study of 1255 patients with FAP[36]. In any case, all studies regarding duodenal FAP have included a similar number of cases, to the best of our knowledge[37-39].
Our main results may be summarized as follows: (1) a sharp ER-beta decrease is evident in the progression from normal tissue to neoplasm; (2) ER-β expression is directly related to apoptosis expressed as TUNEL LI; and (3) the co-expression of ER-beta and caspase 3 undergoes a sharp decline from normal to neoplastic tissue. This evidence supports the possibility of a relationship between ERs and cellular turnover.
In conclusion, this report underlines the importance of ERs in FAP beyond genetic factors, underlining that hormonal carcinogenesis is a process common to both genetic and sporadic CRCs. Moreover, this study is the first to suggest a similar carcinogenic mechanism not only at colonic sites, but also in the small bowel and especially in the duodenum in the course of FAP. On these bases, FAP could be considered as an ideal target for a chemoprevention strategy based on natural products such as phytoestrogens[16,40-42], which are very selective and do not share the side effects reported for drugs such as aspirin or other cyclooxygenase inhibitors[43]. Presumably, the chemoprevention induced by ER-beta agonists may interact with a stimulation of epithelial apoptosis.
COMMENTS
Background
Familial adenomatous polyposis (FAP) is a genetically inherited disease featuring multiple colonic polyps, with a possible duodenal involvement. No study investigating the relationship between estrogen receptors (ERs) and cellular turnover has yet been reported in the subset of patients with duodenal FAP.
Research frontiers
The authors performed an experimental study investigating the expression of ER-α and beta in subjects who had undergone duodenal resection for epithelial malignancy after prophylactic colectomy. Additionally, in this study ERs expression was correlated with the markers of cellular proliferation (Ki-67) and apoptosis (TUNEL). Finally, a co-expression of ER-beta with caspase 3, a known marker of the early stages of apoptosis, was detected in the progressive stages of FAP carcinogenesis.
Innovations and breakthroughs
ER-beta showed a progressive decline from normal tissue to high grade dysplasia (HGD) and adenocarcinoma (AC). The normal tissue of FAP subjects expressed ER-beta like the controls. TUNEL did not reveal a difference between controls and normal tissue of FAP, but decreasing levels in low grade dysplasia, through HGD to AC. ER-α showed a progressive increase from normal tissue to AC; the expression in FAP normal tissue was similar to that in controls. Ki-67 demonstrated a statistically significant progressive increase at each disease stage up to AC. A decreasing co-expression of ER-β and caspase 3 was observed from normal to neoplastic tissue.
Applications
FAP could be considered as an ideal target for a chemoprevention strategy based on natural products such as phytoestrogens, which are very selective and do not share the side effects reported for drugs such as aspirin or other cyclooxygenase inhibitors. Presumably, the chemoprevention induced by ER-beta agonists may interact with a stimulation of epithelial apoptosis.
Peer-review
This paper is well written and the work was well done. The results are very interesting and bring new important knowledges in adenomatous polyposis.
Footnotes
Institutional review board statement: The study was reviewed and approved after two meetings of all the authors before and after immune-histochemistry and confocal microscopy.
Informed consent statement: All study participants provided informed written consent prior to the endoscopic investigation or surgery; additional oral consent to perform immunohistochemistry or confocal microscopy was obtained.
Conflict-of-interest statement: No author received fees for this study. There is no conflict of interest.
Data sharing statement: No additional data are available. Moreover, the presented data are anonymized and risk of identification is low.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: September 15, 2015
First decision: November 27, 2015
Article in press: January 9, 2016
P- Reviewer: Guan YS, Jang IS, Yang T S- Editor: Qi Y L- Editor: A E- Editor: Liu XM
References
- 1.Half E, Bercovich D, Rozen P. Familial adenomatous polyposis. Orphanet J Rare Dis. 2009;4:22. doi: 10.1186/1750-1172-4-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.REED TE, NEEL JV. A genetic study of multiple polyposis of the colon with an appendix deriving a method of estimating relative fitness. Am J Hum Genet. 1955;7:236–263. [PMC free article] [PubMed] [Google Scholar]
- 3.Alm T. Surgical treatment of hereditary adenomatosis of the colon and rectum in Sweden during the last 20 years. Part II. Patients with prophylactic operations, primary and late results. Discussion and summary. Acta Chir Scand. 1975;141:228–237. [PubMed] [Google Scholar]
- 4.Nelson S, Näthke IS. Interactions and functions of the adenomatous polyposis coli (APC) protein at a glance. J Cell Sci. 2013;126:873–877. doi: 10.1242/jcs.100479. [DOI] [PubMed] [Google Scholar]
- 5.Vasen HF, Möslein G, Alonso A, Aretz S, Bernstein I, Bertario L, Blanco I, Bülow S, Burn J, Capella G, et al. Guidelines for the clinical management of familial adenomatous polyposis (FAP) Gut. 2008;57:704–713. doi: 10.1136/gut.2007.136127. [DOI] [PubMed] [Google Scholar]
- 6.Tudyka VN, Clark SK. Surgical treatment in familial adenomatous polyposis. Ann Gastroenterol. 2012;25:201–206. [PMC free article] [PubMed] [Google Scholar]
- 7.Aziz O, Athanasiou T, Fazio VW, Nicholls RJ, Darzi AW, Church J, Phillips RK, Tekkis PP. Meta-analysis of observational studies of ileorectal versus ileal pouch-anal anastomosis for familial adenomatous polyposis. Br J Surg. 2006;93:407–417. doi: 10.1002/bjs.5276. [DOI] [PubMed] [Google Scholar]
- 8.Setti-Carraro P, Nicholls RJ. Choice of prophylactic surgery for the large bowel component of familial adenomatous polyposis. Br J Surg. 1996;83:885–892. doi: 10.1002/bjs.1800830704. [DOI] [PubMed] [Google Scholar]
- 9.Serrano PE, Grant RC, Berk TC, Kim D, Al-Ali H, Cohen Z, Pollett A, Riddell R, Silverberg MS, Kortan P, et al. Progression and Management of Duodenal Neoplasia in Familial Adenomatous Polyposis: A Cohort Study. Ann Surg. 2015;261:1138–1144. doi: 10.1097/SLA.0000000000000734. [DOI] [PubMed] [Google Scholar]
- 10.Parc Y, Mabrut JY, Shields C. Surgical management of the duodenal manifestations of familial adenomatous polyposis. Br J Surg. 2011;98:480–484. doi: 10.1002/bjs.7374. [DOI] [PubMed] [Google Scholar]
- 11.Barone M, Tanzi S, Lofano K, Scavo MP, Guido R, Demarinis L, Principi MB, Bucci A, Di Leo A. Estrogens, phytoestrogens and colorectal neoproliferative lesions. Genes Nutr. 2008;3:7–13. doi: 10.1007/s12263-008-0081-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Francavilla A, Di Leo A, Polimeno L, Conte D, Barone M, Fanizza G, Chiumarulo C, Rizzo G, Rubino M. Nuclear and cytosolic estrogen receptors in human colon carcinoma and in surrounding noncancerous colonic tissue. Gastroenterology. 1987;93:1301–1306. doi: 10.1016/0016-5085(87)90259-9. [DOI] [PubMed] [Google Scholar]
- 13.Di Leo A, Barone M, Maiorano E, Tanzi S, Piscitelli D, Marangi S, Lofano K, Ierardi E, Principi M, Francavilla A. ER-beta expression in large bowel adenomas: implications in colon carcinogenesis. Dig Liver Dis. 2008;40:260–266. doi: 10.1016/j.dld.2007.10.018. [DOI] [PubMed] [Google Scholar]
- 14.Barone M, Scavo MP, Papagni S, Piscitelli D, Guido R, Di Lena M, Comelli MC, Di Leo A. ERβ expression in normal, adenomatous and carcinomatous tissues of patients with familial adenomatous polyposis. Scand J Gastroenterol. 2010;45:1320–1328. doi: 10.3109/00365521.2010.487915. [DOI] [PubMed] [Google Scholar]
- 15.Principi M, Di Leo A, Pricci M, Scavo MP, Guido R, Tanzi S, Piscitelli D, Pisani A, Ierardi E, Comelli MC, et al. Phytoestrogens/insoluble fibers and colonic estrogen receptor β: randomized, double-blind, placebo-controlled study. World J Gastroenterol. 2013;19:4325–4333. doi: 10.3748/wjg.v19.i27.4325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Calabrese C, Praticò C, Calafiore A, Coscia M, Gentilini L, Poggioli G, Gionchetti P, Campieri M, Rizzello F. Eviendep® reduces number and size of duodenal polyps in familial adenomatous polyposis patients with ileal pouch-anal anastomosis. World J Gastroenterol. 2013;19:5671–5677. doi: 10.3748/wjg.v19.i34.5671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moore HM, Kelly AB, Jewell SD, McShane LM, Clark DP, Greenspan R, Hayes DF, Hainaut P, Kim P, Mansfield EA, et al. Biospecimen reporting for improved study quality (BRISQ) Cancer Cytopathol. 2011;119:92–101. doi: 10.1002/cncy.20147. [DOI] [PubMed] [Google Scholar]
- 18.Losurdo G, Piscitelli D, Giangaspero A, Principi M, Buffelli F, Giorgio F, Montenegro L, Sorrentino C, Amoruso A, Ierardi E, et al. Evolution of nonspecific duodenal lymphocytosis over 2 years of follow-up. World J Gastroenterol. 2015;21:7545–7552. doi: 10.3748/wjg.v21.i24.7545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Giardiello FM, Hylind LM, Trimbath JD, Hamilton SR, Romans KE, Cruz-Correa M, Corretti MC, Offerhaus GJ, Yang VW. Oral contraceptives and polyp regression in familial adenomatous polyposis. Gastroenterology. 2005;128:1077–1080. doi: 10.1053/j.gastro.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 20.Tonelli F, Ficari F, Valanzano R, Brandi ML. Treatment of desmoids and mesenteric fibromatosis in familial adenomatous polyposis with raloxifene. Tumori. 2003;89:391–396. doi: 10.1177/030089160308900408. [DOI] [PubMed] [Google Scholar]
- 21.Martineti V, Picariello L, Tognarini I, Carbonell Sala S, Gozzini A, Azzari C, Mavilia C, Tanini A, Falchetti A, Fiorelli G, et al. ERbeta is a potent inhibitor of cell proliferation in the HCT8 human colon cancer cell line through regulation of cell cycle components. Endocr Relat Cancer. 2005;12:455–469. doi: 10.1677/erc.1.00861. [DOI] [PubMed] [Google Scholar]
- 22.Cavallini A, Messa C, Pricci M, Caruso ML, Barone M, Di Leo A. Distribution of estrogen receptor subtypes, expression of their variant forms, and clinicopathological characteristics of human colorectal cancer. Dig Dis Sci. 2002;47:2720–2728. doi: 10.1023/a:1021053105096. [DOI] [PubMed] [Google Scholar]
- 23.Di Leo A, Messa C, Russo F, Misciagna G, Guerra V, Taveri R, Leo S. Prognostic value of cytosolic estrogen receptors in human colorectal carcinoma and surrounding mucosa. Preliminary results. Dig Dis Sci. 1994;39:2038–2042. doi: 10.1007/BF02088144. [DOI] [PubMed] [Google Scholar]
- 24.Barzi A, Lenz AM, Labonte MJ, Lenz HJ. Molecular pathways: Estrogen pathway in colorectal cancer. Clin Cancer Res. 2013;19:5842–5848. doi: 10.1158/1078-0432.CCR-13-0325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Principi M, De Tullio N, Scavo MP, Piscitelli D, Marzullo A, Russo S, Albano F, Lofano K, Papagni S, Barone M, et al. Estrogen receptors expression in long-lasting ulcerative pancolitis with and without dysplasia: a preliminary report. Scand J Gastroenterol. 2012;47:1253–1254. doi: 10.3109/00365521.2012.685757. [DOI] [PubMed] [Google Scholar]
- 26.Principi M, Scavo MP, Piscitelli D, Villanacci V, Lovero R, Losurdo G, Girardi B, Ierardi E, Di Leo A. The sharp decline of beta estrogen receptors expression in long-lasting ulcerative-associated carcinoma. Scand J Gastroenterol. 2015;50:1002–1010. doi: 10.3109/00365521.2014.978817. [DOI] [PubMed] [Google Scholar]
- 27.Principi M, Barone M, Pricci M, De Tullio N, Losurdo G, Ierardi E, Di Leo A. Ulcerative colitis: from inflammation to cancer. Do estrogen receptors have a role? World J Gastroenterol. 2014;20:11496–11504. doi: 10.3748/wjg.v20.i33.11496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cleveland AG, Oikarinen SI, Bynoté KK, Marttinen M, Rafter JJ, Gustafsson JA, Roy SK, Pitot HC, Korach KS, Lubahn DB, et al. Disruption of estrogen receptor signaling enhances intestinal neoplasia in Apc(Min/+) mice. Carcinogenesis. 2009;30:1581–1590. doi: 10.1093/carcin/bgp132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kouzmenko AP, Takeyama K, Kawasaki Y, Akiyama T, Kato S. Ligand-dependent interaction between estrogen receptor alpha and adenomatous polyposis coli. Genes Cells. 2008;13:723–730. doi: 10.1111/j.1365-2443.2008.01200.x. [DOI] [PubMed] [Google Scholar]
- 30.Chaudhary SC, Singh T, Talwelkar SS, Srivastava RK, Arumugam A, Weng Z, Elmets CA, Afaq F, Kopelovich L, Athar M. Erb-041, an estrogen receptor-β agonist, inhibits skin photocarcinogenesis in SKH-1 hairless mice by downregulating the WNT signaling pathway. Cancer Prev Res (Phila) 2014;7:186–198. doi: 10.1158/1940-6207.CAPR-13-0276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barone M, Tanzi S, Lofano K, Scavo MP, Pricci M, Demarinis L, Papagni S, Guido R, Maiorano E, Ingravallo G, et al. Dietary-induced ERbeta upregulation counteracts intestinal neoplasia development in intact male ApcMin/+ mice. Carcinogenesis. 2010;31:269–274. doi: 10.1093/carcin/bgp275. [DOI] [PubMed] [Google Scholar]
- 32.Khor TO, Cheung WK, Prawan A, Reddy BS, Kong AN. Chemoprevention of familial adenomatous polyposis in Apc(Min/+) mice by phenethyl isothiocyanate (PEITC) Mol Carcinog. 2008;47:321–325. doi: 10.1002/mc.20390. [DOI] [PubMed] [Google Scholar]
- 33.Acconcia F, Totta P, Ogawa S, Cardillo I, Inoue S, Leone S, Trentalance A, Muramatsu M, Marino M. Survival versus apoptotic 17beta-estradiol effect: role of ER alpha and ER beta activated non-genomic signaling. J Cell Physiol. 2005;203:193–201. doi: 10.1002/jcp.20219. [DOI] [PubMed] [Google Scholar]
- 34.Galluzzo P, Caiazza F, Moreno S, Marino M. Role of ERbeta palmitoylation in the inhibition of human colon cancer cell proliferation. Endocr Relat Cancer. 2007;14:153–167. doi: 10.1677/ERC-06-0020. [DOI] [PubMed] [Google Scholar]
- 35.Chen T, Yang I, Irby R, Shain KH, Wang HG, Quackenbush J, Coppola D, Cheng JQ, Yeatman TJ. Regulation of caspase expression and apoptosis by adenomatous polyposis coli. Cancer Res. 2003;63:4368–4374. [PubMed] [Google Scholar]
- 36.Jagelman DG, DeCosse JJ, Bussey HJ. Upper gastrointestinal cancer in familial adenomatous polyposis. Lancet. 1988;1:1149–1151. doi: 10.1016/s0140-6736(88)91962-9. [DOI] [PubMed] [Google Scholar]
- 37.Goldstein AL, Kariv R, Klausner JM, Tulchinsky H. Patterns of Adenoma Recurrence in Familial Adenomatous Polyposis Patients after Ileal Pouch-Anal Anastomosis. Dig Surg. 2015;32:421–425. doi: 10.1159/000439143. [DOI] [PubMed] [Google Scholar]
- 38.Rubio CA, Kaufeldt A, Kohan R, Ushoida M, Ezelius E, Björk S, Lindahl J. β-catenin Helices in the cytoplasm of sporadic and FAP duodenal adenomas. Anticancer Res. 2015;35:1433–1436. [PubMed] [Google Scholar]
- 39.Papagni S, Rizzi S, Principi M, Albano F, Contaldo A, Iannone A, Losurdo G, Genco E, Lastella P, Ierardi E, et al. Familial adenomatous polyposis small bowel surveillance: could indicators for video-capsule endoscopy be ascertained? Minerva Gastroenterol Dietol. 2015:In press. [PubMed] [Google Scholar]
- 40.Kim B, Giardiello FM. Chemoprevention in familial adenomatous polyposis. Best Pract Res Clin Gastroenterol. 2011;25:607–622. doi: 10.1016/j.bpg.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Principi M, De Censi A. Prevention of colorectal adenomas. Colorectal Dis. 2015;17 Suppl 1:20–24. doi: 10.1111/codi.12817. [DOI] [PubMed] [Google Scholar]
- 42.Bringiotti R, Ierardi E, De Tullio N, Fracella MR, Brindicci D, Marmo R, Albano F, Papagni S, Di Leo A, Principi M. Education and imaging. Gastroenterology: video capsule endoscopy disclosure of unprecedented therapeutic effect of Eviendep on small bowel polyposis in Lynch syndrome. J Gastroenterol Hepatol. 2015;30:801. doi: 10.1111/jgh.12912. [DOI] [PubMed] [Google Scholar]
- 43.Ishikawa H, Wakabayashi K, Suzuki S, Mutoh M, Hirata K, Nakamura T, Takeyama I, Kawano A, Gondo N, Abe T, et al. Preventive effects of low-dose aspirin on colorectal adenoma growth in patients with familial adenomatous polyposis: double-blind, randomized clinical trial. Cancer Med. 2013;2:50–56. doi: 10.1002/cam4.46. [DOI] [PMC free article] [PubMed] [Google Scholar]


