Abstract
Background
Cardiorenal syndrome (CRS) encompasses conditions in which cardiac and renal disorders co-exist and are pathophysiologically related. The newest classification of CRS into seven etiologically and clinically distinct types for direct patient management purposes includes hemodynamic, uremic, vascular, neurohumoral, anemia- and/or iron metabolism-related, mineral metabolism-related and protein-energy wasting-related CRS. This classification also emphasizes the pathophysiologic pathways. The leading CRS category remains hemodynamic CRS, which is the most commonly encountered type in patient care settings and in which acute or chronic heart failure leads to renal impairment.
Summary
This review focuses on selected therapeutic strategies for the clinical management of hemodynamic CRS. This is often characterized by an exceptionally high ratio of serum urea to creatinine concentrations. Loop diuretics, positive inotropic agents including dopamine and dobutamine, vasopressin antagonists including vasopressin receptor antagonists such as tolvaptan, nesiritide and angiotensin-neprilysin inhibitors are among the pharmacologic agents used. Additional therapies include ultrafiltration (UF) via hemofiltration or dialysis. The beneficial versus unfavorable effects of these therapies on cardiac decongestion versus renal blood flow may act in opposite directions. Some of the most interesting options for the outpatient setting that deserve revisiting include portable continuous dobutamine infusion, peritoneal dialysis and outpatient UF via hemodialysis or hemofiltration.
Key Messages
The new clinically oriented CRS classification system is helpful in identifying therapeutic targets and offers a systematic approach to an optimal management algorithm with better understanding of etiologies. Most interventions including UF have not shown a favorable impact on outcomes. Outpatient portable dobutamine infusion is underutilized and not well studied. Revisiting traditional and novel strategies for outpatient management of CRS warrants clinical trials.
Key Words: Cardiorenal syndrome, Chronic kidney disease, Heart failure, Acute kidney injury, Dobutamine, Ultrafiltration
Introduction
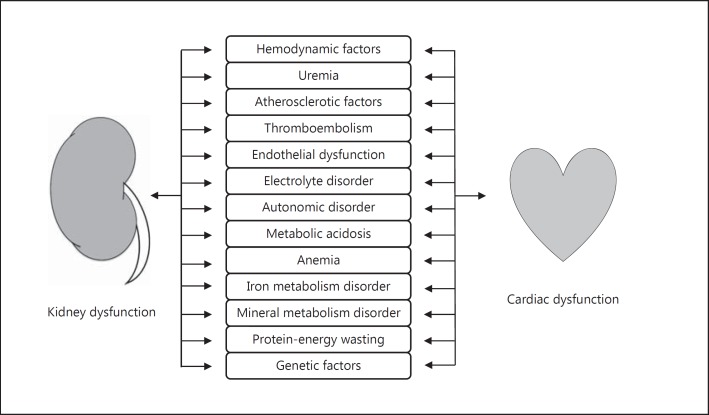
Cardiorenal syndrome (CRS) denotes conditions in which acute or chronic failure of either heart or kidney leads to the development and progression of the other organ's dysfunction [1,2]. This interaction in CRS has traditionally been explained by hemodynamic factors as manifested by low cardiac output syndrome. However, clinical presentations where concomitant cardiac and kidney dysfunction exist include heterogeneous conditions, and hence more complex bidirectional interplays between the heart and the kidney have been recognized through a number of physiologic, biochemical, structural and hormonal abnormalities in the pathogenesis of CRS (fig. 1) [3,4]. This review first highlights the recently proposed concept of newer CRS categorization based on underlying pathophysiology, which would be of help in identifying therapeutic targets and in developing treatment and management algorithms [2]. We then focus on current and potential future treatment strategies and their therapeutic targets in the management of decompensated heart failure (DHF), the management of which still remains a significant challenge in CRS [5]. The pharmacologic and non-pharmacologic regimens discussed in this review are summarized in table 1.
Fig. 1.
Putative pathophysiologic connections in CRS.
Table 1.
Pharmacologic and non-pharmacologic regimens for CRS discussed in this review
| Dose/frequency | Adverse effects | Special consideration | |
|---|---|---|---|
| Pharmacologic regimens | |||
| Loop diuretics | Intravenous: Starting 2.5 times dose of chronic oral dose followed by boluses at intervals of 6–8 h or continuous infusion. If urine output is <1 ml/kg/h, double as necessary to a maximum of 80–160 mg/h. | Electrolyte disturbances, arrhythmias, hearing impairment, tinnitus, hematologic disorders, dermatologic diseases, tubulointerstitial nephritis. | Need serial assessments and dose adjustments based on symptoms, urine output and volume status. |
| Dopamine | Intravenous: 5–15 μg/kg/min (increased systemic vascular resistance at <10 μg/kg/min). | Tachyarrhythmias, headache, nausea, cardiac ischemia, tissue necrosis. | Drug interaction with MAO-I. |
| Dobutamine | Intravenous: 2.5–20 μg/kg/min (decreased systemic vascular resistance at <5 μg/kg/min). | Hypertension, hypotension, tachyarrhythmias, headache, nausea, fever, hypersensitivity. | Drug interaction with MAO-I; contraindication for sulfite allergy. |
| Levosimendan | Intravenous: 6–24 μg/kg over 10 min followed by a continuous infusion of 0.05–0.2 μg/kg/min, adjusted according to response. | Hypotension, headache, nausea, arrhythmias. | Avoid use with other vasodilators; not currently available in the U.S. |
| Tolvaptan | Oral: 15 mg once daily; after at least 24 h, may be increased to 30 mg once daily to a maximum of 60 mg once daily titrating at 24-hour intervals. | Hepatotoxicity, hypernatremia, hypersensitivity, nausea, weakness, fever, anorexia. | Do not use for more than 30 days due to the risk of hepatotoxicity; do not use with strong CYP3A inhibitors; monitor closely for rate of serum sodium increase and neurological status. |
| Nesiritide | Intravenous: 2 μg/kg (bolus optional) followed by continuous infusion at 0.01 μg/kg/min. | Hypotension, rise in serum creatinine, headache, nausea, hypersensitivity. | Blood pressure should be closely monitored; hypotensive effects may last for several hours. |
| Sacubitril/valsartan | Oral: Start with 49/51 mg (sacubitril/valsartan) twice daily. Double the dose after 2–4 weeks, as tolerated by the patient. | Hypotension, hyperkalemia, cough, dizziness, renal failure. | Do not use with an angiotensin-converting enzyme inhibitor; do not use with aliskiren in patients with diabetes; avoid use with an angiotensin receptor blocker. |
| Non-pharmacologic regimens | |||
| RRT (UF, intermittent HD, sustained low-efficiency dialysis, continuous HD, peritoneal dialysis | Indicated when refractory to medical therapy. | Volume depletion, hypotension, hypokalemia and/or hypophosphatemia (HD). | Consultation with a nephrologist is appropriate before initiation. |
| Fluid and sodium restriction | <1.5 to 2.0 g/day of sodium, <1.5 to 2.0 l/day of water. | Hypotension, hyponatremia, RAAS activation? | Individualize based on serum sodium level and diuretic resistance. |
The New Clinically Oriented CRS Classification
Chronic kidney disease (CKD) and ‘worsening renal function’ or ‘renal impairment’ (terms used often by cardiologists as equivalent to acute kidney injury [AKI]) is highly prevalent in patients with heart failure (HF) [6,7,8]. This so-called hemodynamic CRS is usually associated with diuretic therapy resistance, high rates of cardiovascular events and recurrent AKI as well as poor outcomes, including high mortality [9]. Hemodynamic CRS involves both hemodynamic and non-hemodynamic mechanisms [10]. Several definitions and classifications of CRS have been advanced in recent years. Based on Ronco et al.'s 2008 classification [1], acute decompensated heart failure (ADHF) with worsening renal dysfunction can be categorized as ‘type 1' without clear understanding of underlying mechanisms or pathophysiology since this clinical classification system was designed based on the primarily impaired organ (heart or kidney) and the time frame of the condition (acute or chronic) [4]. However, those derangements in the heart, kidneys, neurohumoral systems and other relevant pathways are already involved as contributing pathophysiologic factors at the time of clinical presentation, and it currently seems difficult to identify the initiator and its consequence in the complex network of CRS. Therefore, these clinical descriptive types of classification systems can be challenging in terms of practicality and clinical applicability and should be considered ‘a temporary, operational expedient’ in the absence of any mechanistic alternative [11].
The new classification system for CRS, recently proposed by Hatamizadeh et al. [2], is a functional and clinically oriented classification with direct application in patient care management [4]. It has been developed for clinical purposes to identify the main manifestation to be treated at the time of clinical evaluation, emphasizing the pathophysiologic pathways to classify CRS into seven distinct categories: (1) hemodynamic, (2) uremic, (3) vascular, (4) neurohumoral, (5) anemia- and/or iron metabolism-related, (6) mineral metabolism-related and (7) protein-energy wasting-related CRS (table 2). This classification system offers a systematic approach to an optimal treatment and management algorithm with better understanding of etiologies.
Table 2.
The new CRS classification system proposed by Hatamizadeh et al. (edited from reference [2]), and corresponding treatment strategies and potential harms
| CRS category (subclass) [subcategory] | Manifestation examples | Current strategies | Potential strategies | Potential harms |
|---|---|---|---|---|
| Hemodynamic (acute/chronic) | ||||
| kidney injury due to low cardiac output, fluid retention due to kidney dysfunction | short-term inotropic agents, diuretics, UF, vasodilators, ACE inhibitors or ARBs, aldosterone receptor blockers, digoxin, mechanical circulatory assist devices, cardiac resynchronization, heart transplantation, kidney transplantation | vasopressin receptor 2 antagonists, natriuretic peptides, neprilysin inhibition, endothelinreceptor antagonists, lusoinotropic agents, cardiac myosin activators, ryanodine-receptor stabilizers, direct renin inhibitors, recombinant relaxin, recombinant neuregulin-1, recombinant relaxin-2, angiotensin(1–9) | dual/triple RAAS blockade, antiarrhythmic drugs (except for amiodarone and dofetilide), calciumchannel blockers (except for amlodipine), NSAIDs, thiazolidinediones, long-term inotropic agents | |
| Uremic (acute/chronic) | ||||
| uremic cardiomyopathy, uremic pericarditis, uremic pleuritis | dialysis, kidney transplantation | uremic toxin absorbents | high-protein diet | |
| Vascular (acute/chronic) | ||||
| [Atherosclerotic] [Thromboembolic] [Endothelial dysfunction] | coronary artery disease, renal artery stenosis, renal artery thrombosis | statins, antiplatelets, anticoagulants, nitroglycerin, conventional atherosclerotic risk factor modifications | endothelin receptor antagonists, ACE inhibitors or ARBs, aldosterone receptor blockers, exercise training, correction of pump failure, correction of anemia | smoking |
| Neurohumoral (acute/chronic) | ||||
| [Electrolyte] [Autonomic] [Acid-base] | abnormal serum potassium level, abnormal serum calcium level, abnormal serum magnesium level, increased catecholamine, activated RAAS, metabolic acidosis | ACE inhibitors or ARBs, aldosterone receptor blockers, beta-blockers, ion exchange resins, bicarbonate, citrate | adenosine A1 receptor antagonists, direct renin inhibitors, renal denervation, hypertonic saline | long-term inotropic agents, ACE inhibitors or ARBs, aldosterone receptor blockers |
| Anemia- and/or iron metabolism-related (acute/chronic) | ||||
| [Nutrient deficiency] [Inflammation] [Renal anemia] | iron deficiency, folate deficiency, infection, renal tubular injury | iron, folic acid, erythropoiesisstimulating agents, red blood cell transfusion | anti-inflammatory interventions, anti-hepcidin therapy, activin A pathway modulation, HIF system regulation, carnitine, cyanocobalamin, vitamin C | ACE inhibitors or ARBs, aldosterone receptor blockers |
| Mineral metabolism (mostly chronic) | ||||
| hyperphosphatemia, hypercalcemia, vitamin D deficiency, elevated FGF23 | phosphate binders, calcimimetics, intense dialysis, active vitamin D compounds, dietary modification, kidney transplantation | cholecalciferol, ergocalciferol, magnesium, FGF23 pathway regulation, Klotho, Na+/Pi cotransporter inhibition | calcium, warfarin, overdosed active vitamin D | |
| Protein-energy wasting (mostly chronic) | ||||
| see reference [18] | omega-3 fatty acids, exercise training, volume overload correction | coenzyme Q10, nutritional supplementation, growth hormone, anabolic steroids, appetite stimulants, anti-inflammatory interventions, antioxidative agents, myostatin inhibitors | ||
ACE = Angiotensin-converting enzyme; ARBs = angiotensin receptor blockers; HIF = hypoxia-inducible factor; NSAIDs = non-steroidal anti-inflammatory drugs.
In the case of hemodynamic CRS, for example, the initial therapeutic strategy should mainly focus on hemodynamic abnormalities, including correction of volume overload and vasodilators as well as short-term inotropic agents if necessary. It is important to note that CKD or AKI, especially if oligo-anuric, can also lead to fluid overload and a clinical manifestation similar to ADHF, but this scenario is described under ‘uremic CRS’ under the new classification, and dialysis or ultrafiltration (UF) should be prescribed to treat patients. After that, the new classification system can also identify other potential targets, namely coronary or renal artery diseases (vascular CRS) and several neurohumoral pathways including the renin-angiotensin-aldosterone system (RAAS), vasopressin and the sympathetic nervous system (neurohumoral CRS) [12]. Neurohumoral changes are initially physiologic compensatory mechanisms, but become harmful at some point by initiating and maintaining a vicious circle [2]. Anemia is an important prognosticator in HF patients [13], and derangements in iron metabolism are common in patients with CKD and/or HF (anemia- and/or iron metabolism-related CRS) [14]. Indeed, iron supplementation to patients with symptomatic HF improves symptoms and reduces the incidence of hospitalization, which was more pronounced in patients with CKD [15]. In patients with end-stage renal disease, both hyperphosphatemia and hypercalcemia induce medial artery calcification and increase arterial stiffness, and markedly elevated fibroblast growth factor 23 (FGF23) may lead to left ventricular hypertrophy and increase the risk of HF (mineral metabolism-related CRS) [16]. Finally, multiple nutritional and catabolic alterations in patients with CKD and/or HF lead to protein-energy wasting, which is a strong risk factor for morbidity and mortality [17,18]. Established and potential treatment strategies and targets as well as potential harms corresponding to the respective category are also listed in table 2.
Established Treatment Strategies
Positive Inotropic Agents
Positive inotropic agents (i.e. dopamine, dobutamine, milrinone and levosimendan) increase cardiac contractility, but their routine use in patients with ADHF is not recommended due to concerns about their safety [19]. Indeed, use of positive inotropes is shown in some but not all studies to be associated with mortality in patients with ADHF [20,21]. Although these findings are likely the result of confounding by indication in these observational studies, a randomized clinical trial (RCT) revealed that milrinone did not improve survival or hospitalization rate, but increased sustained hypotension and arterial arrhythmia [22]. Nevertheless we advocate the short-term administration of selected inotropic agents, especially dobutamine as continuous intravenous infusion, for DHF with low cardiac output syndrome; this intervention agent may improve and maintain systemic perfusion and preserve end-organ function in ADHF patients with severe systolic dysfunction or symptomatic hypotension [19,23,24]. Worsening renal function often indicates a drop in renal perfusion from low cardiac output in the setting of hemodynamic CRS, and these positive inotropes are often used in clinical practice to manage patients with reduced cardiac output and kidney dysfunction. We suggest the use of intravenous dobutamine even as an outpatient portable infusion upon discharging the patient to a home or nursing home setting.
It is important to note that no specific inotropes are suggested in the 2013 American College of Cardiology Foundation/American Heart Association (ACCF/AHA) HF guidelines [19]. The synthetic catecholamine dobutamine, known as Dobutrex®, has been commonly used in clinical practice. It stimulates myocardial β1 adrenergic receptors and some α1 receptors, resulting in increased contractility and heart rate. In the vasculature, the effects of dobutamine on β2 stimulation may be equal to or slightly greater than those on α1 stimulation, which results in some vasodilation and lowered central venous and wedge pressure, particularly at lower doses (≤5 μg/kg/min). Additional points on inotropes are discussed below, including low-dose dopamine, levosimendan and the administration to outpatients with end-stage HF as a palliative measure.
Low-Dose Dopamine
Dopamine is an endogenous central neurotransmitter that has dose-dependent effects on both α- and β-adrenergic receptors as well as dopaminergic receptors. Dopamine increases cardiac output at doses in the range of 5-10 μg/kg/min primarily by increasing stroke volume, with variable effects on heart rate [25]. It also increases renal blood flow and promotes natriuresis by stimulating the dopaminergic D1 and D2 receptors in the kidneys at doses of 2-10 μg/kg/min.
The clinical significance of low-dose or so-called ‘renal-dose’ dopamine has remained controversial and without any clear consensus. Previous RCTs have suggested that low-dose dopamine may enhance diuresis and protect the kidneys in patients with DHF and reduced ejection fraction. In the Dopamine in Acute Decompensated Heart Failure (DAD-HF) trial, the combination of low-dose furosemide (5 mg/h) and low-dose dopamine (5 μg/kg/min), when compared to high-dose furosemide (20 mg/h), showed equivalent effects on urine output and decongestion during 8-hour treatment, but worsening renal function was less frequent in the combination treatment with low-dose dopamine [26]. It should be noted that participants had reduced left ventricular ejection fraction (mean 35%) but mild kidney dysfunction (mean estimated glomerular filtration rate of 58 ml/min/1.73 m2). The Renal Optimization Strategies Evaluation (ROSE) trial enrolled patients with similar characteristics (mean ejection fraction of 35% and mean glomerular filtration rate of 45 ml/min/1.73 m2) and showed consistent results in terms of diuresis and decongestion; 72-hour low-dose dopamine administration (2 μg/kg/min) did not enhance decongestion compared to placebo under the use of intravenous loop diuretics with a recommended practical dose (equal to 2.5 times the oral outpatient furosemide dose). Additionally, low-dose dopamine therapy did not show any significant renoprotective benefits evaluated by the co-primary endpoints of cumulative urine volume and change from baseline plasma cystatin C [27]. Nevertheless, there appeared to be effect modification by ejection fraction and systolic blood pressure, and dopamine therapy may offer benefit to patients with reduced ejection fraction and low systolic blood pressure just as stated in the guidelines [19,23,24]. Another important lesson from this trial is that low-dose dopamine is not renal-specific; the patients who received low-dose dopamine experienced an increased incidence of tachycardia.
Levosimendan
Levosimendan is a calcium sensitizer and possesses both positive inotropic and vasodilator effects. Several clinical trials and their meta-analyses have reported its clinical efficacy, including improved cardiac performance, rapid symptom relief, reduced hospital stays and better survival in patients with ADHF [28,29]. It also increases renal blood flow and glomerular filtration rate as other positive inotropes do [30]. When compared to dobutamine, levosimendan improved hemodynamic performance and reduced B-type natriuretic peptide more rapidly [31,32]. Although one trial also showed its survival benefit [31], this was not confirmed in the most recent large multinational trial [32]. Nevertheless, levosimendan may be more beneficial than dobutamine for treating ADHF patients with a history of prior ADHF or those on beta-blockers [33]. Its adverse effects include hypotension and arrhythmias, and it may increase mortality in patients with low systolic blood pressure [32].
Inotropes for Outpatients
Experimental data suggest that inotropic stimulation may impair the development of myocardial hibernation and precipitate myocardial infarction by both increasing ischemia severity and enhancing energy expenditure [34]. Therefore inotropes are suggested as a bridge to definitive therapy such as coronary revascularization, mechanical circulatory support and heart transplantation, and long-term inotrope use is generally not indicated due to its potential harm [35,36]. Nevertheless, portable continuous intravenous dobutamine treatment could be used as a palliative measure in patients with more severe cases of HF [19]. One of the authors (K.K.Z.) has used portable continuous intravenous infusion of dobutamine in advanced to near-terminal HF patients with severe hemodynamic CRS with serum urea to creatinine ratios >50, but well-designed studies and case series are urgently needed. One small RCT also showed that a weekly 6-hour infusion of levosimendan offers significantly better survival to end-stage HF patients than dobutamine alone or their combination, suggesting intermittent levosimendan treatment as another palliation in this population [37].
Loop Diuretics: Dose and Frequency
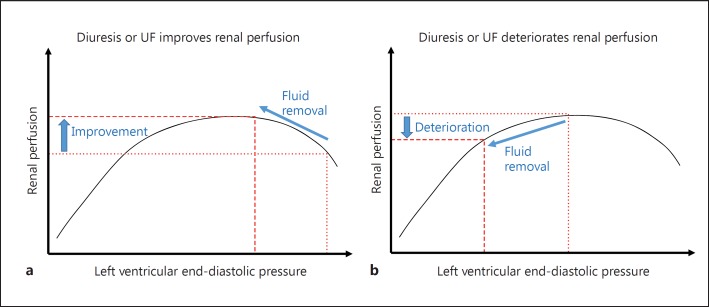
Loop diuretics (furosemide, torsemide and bumetanide) reduce sodium and chloride reabsorption in the thick ascending limb of the loop of Henle by inhibiting the apical Na+-K+-2Cl- co-transporter, leading to the excretion of up to 20-25% of filtered sodium at maximum dose. Given the contributions of increased central venous pressure and increased intra-abdominal pressure [38], correcting volume overload is of particular importance in ADHF patients with hemodynamic CRS, and these agents have been the first-line therapy of ADHF. Whereas in some cases renal function may improve by shifting the Frank-Staling curve of the DHF to the left, which will lead to improved renal perfusion (fig. 2a), in many other cases renal function may deteriorate, with worsening kidney function resulting in an even higher urea to creatinine ratio and worsening oliguria to the point of AKI as more aggressive diuretic therapy or UF ensues (fig. 2b).
Fig. 2.
Schematic representation of the modified Frank-Staling curve related to renal perfusion. a Diuretic therapy or UF can improve renal perfusion and urine output in patient A. b The same therapy in patient B may deteriorate kidney function and lead to oligo-anuria.
Patients who have developed ADHF may respond to these diuretics less than before because low cardiac output limits the diuretic delivery to the kidney and activates the RAAS and the sympathetic nervous system, which increases renal sodium reabsorption. Interstitial edema in the gastrointestinal tract, if present, may also delay the absorption of oral loop diuretics. Therefore, many expert clinicians recommend administering intravenous loop diuretics at a dose of 2.0-2.5 times the oral outpatient dose in the setting of ADHF. Since the onset and peak of diuresis is within 30 min and at 1-2 h, respectively, initial therapy should be followed by re-assessment of response. However, there is little available evidence to support a particular frequency and dose of diuretic therapy in ADHF patients, and practice patterns vary widely among physicians and centers.
Intermittent Bolus Administration
Furosemide, the most commonly used short-acting loop diuretic, promotes significant natriuresis over 6 h (hence the commercial name ‘LaSix’ was created to suggest ‘last 6 h’). However, urinary sodium excretion falls to very low levels thereafter in normal subjects [39]. This reduction of sodium excretion is attributed to the activation of the RAAS and the sympathetic nervous systems by volume depletion. Less frequent bolus injection of high-dose loop diuretics in patients with ADHF may also induce transient volume depletion and hypotension by virtue of pulse diuresis exceeding plasma refill, resulting in less net sodium excretion [40]. Moreover, a higher cumulative dose may be needed to surpass the needed threshold of glomerular filtration of furosemide into the lumen, as loop diuretics act from the luminal side of the tubule by modulating the Na+-K+-2Cl- transporter complex. Therefore, more frequent administration (i.e. three or more times per day, such as every 6-8 h) is supposed to enhance the negative sodium balance. The same is true for oral furosemide therapy in outpatients with chronic HF.
Continuous Administration
Continuous administration of intravenous loop diuretics is an alternative to intravenous bolus injection that may induce greater and more sustained diuresis over time, allowing for more steady-state fluid removal [40]. Additionally, the risk of hearing loss or tinnitus, an important adverse effect of high-dose loop diuretics, may be lower with continuous administration than with bolus injection [41].
The Diuretic Optimization Strategies Evaluation (DOSE) trial simultaneously evaluated the effect of continuous (versus every 12 h bolus) furosemide administration and the effect of high-dose (vs. low-dose) furosemide therapy in patients with ADHF [42]. Low and high dose were defined as equivalent to and 2.5 times the patient's previous oral dose, respectively, but dose adjustments were allowed after 48 h. Compared to the continuous infusion strategy, the bolus injection strategy required a higher total furosemide dose over the course of 72 h, resulting in similar diuresis and clinical outcomes. Meanwhile, the high-dose strategy was associated with greater diuresis and better secondary outcomes than the low-dose strategy. There was also a trend toward better patients' global assessment of symptoms with the high-dose strategy, but this also appeared to be associated with a transient increase in serum creatinine which almost disappeared after day 7.
There are two other recent small RCTs comparing the continuous and bolus intravenous furosemide administration strategy. One study showed that continuous infusion induced greater diuresis but was associated with subsequent worsening in renal function, high rates of re-hospitalization and poor survival after 6 months [43]. The other study showed no significant difference in study outcomes [44]. A meta-analysis adding the DOSE study and these two small RCTs showed an association of continuous infusion with greater weight loss but not with other efficacy endpoints [45].
Currently available studies do not identify differences in strategies, but several methodological concerns should be noted in the previous RCTs. An initial bolus furosemide injection before starting continuous infusion is important to determine the diuretic responsiveness and to quickly achieve therapeutic drug concentrations, especially in highly diuretic-resistant patients [46]. Indeed, a subanalysis of the DOSE study suggested that the bolus injection strategy may increase diuresis in patients who have received high-dose loop diuretics before admission [47]. The importance of initial high-dose bolus furosemide injection was also recently acknowledged as a ‘furosemide stress test’ which discriminates patients with progressive AKI from the others [48]. However, this initial bolus injection was not implemented along the continuous infusion strategy in most studies. The study dose of furosemide was also often fixed during a certain period, which may have brought about poor patient management. For example, the DOSE study fixed furosemide doses until after 48 h, but this may be insufficient given the high prevalence (85%) of persistent congestion on day 3 [49]. These study procedures were inconsistent with clinical practice.
The ultimate goal of diuretic treatment in patients with ADHF is to achieve an optimal volume status individualized to patients with various degrees of cardiac dysfunction, without inducing significant adverse effects such as hypotension, arrhythmias due to electrolyte disturbances and ototoxicity. In this regard, the findings of previous RCTs remind us that serial assessments and individualized adjustments of dose and frequency should be implemented as necessary after the initial intravenous bolus loop diuretic injection [19].
Fluid Removal via Renal Replacement Therapy
Ultrafiltration
UF is ‘indicated’ in patients with diuretic-resistant volume overload due to ADHF and kidney injury. Compared to pharmacologic diuretic therapy, UF offers definite, adjustable isotonic fluid removal through convection (via hemofiltration) or diffusion (via reverse osmosis during dialysis) of plasma across a semipermeable membrane, and thus maintains electrolyte levels especially in the case of convection. Most previous studies have used UF rates of up to 500 ml/h, in which case a central venous catheter is not necessary because a peripheral catheter placed in the antecubital fossa often offers an adequate blood flow rate of 40 ml/min [50,51]. This makes UF implementable outside the intensive care unit setting without specialized nursing. Nevertheless, compulsory fluid removal by UF may lead to volume depletion and hypotension, resulting in ischemic organ damage including worsening renal perfusion (fig. 2).
The clinical efficacy of early initiation of UF in ADHF has been evaluated in four RCTs where patients exhibited mild kidney dysfunction at baseline (mean serum creatinine levels of 1.5-2.0 mg/dl) [51,52,53,54]. Compared to ‘usual’ or ‘standard’ therapy, UF up to 500 ml/h offered a greater amount of fluid removal in two studies [51,52], resulting in better improvement in dyspnea and HF symptoms at 48 h in one study [52] and less re-hospitalizations for HF and unscheduled visits at 90 days in the other [52]. Both studies showed no significant difference in serum creatinine levels between groups. In contrast, in the Cardiorenal Rescue Study in Acute Decompensated Heart Failure (CARRESS-HF), which compared slow continuous UF to ‘stepped pharmacologic therapy’ in patients with persistent congestion and worsening renal function, fluid and weight loss was similar between the two groups, and the UF groups experienced a higher increase in serum creatinine levels at 96 h and more adverse events [53]. These results apparently conflict with previous studies, but it should be noted that the maximum UF rate was lower in previous studies (200 ml/h vs. 500 ml/h) and that the pharmacologic therapy group showed a mean urinary volume of approximately 3,000 ml/day throughout the intervention period, which led to equivalent fluid removal between groups. Additionally, the stepped pharmacologic therapy algorithm included not only loop and thiazide diuretics but also vasodilator and inotropic agents, all of which were prohibited in the UF group. Furthermore, serum cystatin C levels, a sensitive marker of early kidney dysfunction, showed no significant difference between the intervention arms, questioning whether UF indeed worsened kidney function in this study. In contrast, the Continuous Ultrafiltration for Congestive Heart Failure (CUORE) trial showed that patients who received UF as first-line therapy and not as rescue therapy as in the other trials experienced less worsening of renal function and a decreased incidence of re-hospitalization during 1 year after discharge despite similar body weight reduction compared to patients who received standard therapy [54]. However, the underlying mechanisms for these results remained unclear. These favorable long-term effects of UF as a first-line therapy should be validated by further trials because the small sample size of that trial was determined without any evidence or data to support their assumption (8% re-hospitalization rate in the UF group).
Given the risk of adverse events and RAAS activation [55], the available evidence does not appear to support the implementation of a mechanical fluid removal strategy for ADHF or CRS when an adequate amount of urine volume is anticipated with available pharmacologic therapy. This is consistent with the statement in the 2013 ACCF/AHA HF guidelines [19].
Dialysis
In case of more advanced AKI, the Kidney Disease: Improving Global Outcomes (KDIGO) clinical practice guidelines state that dialysis, not UF alone, should be initiated to treat or prevent life-threatening signs such as uremic symptoms (i.e. pericarditis, pleuritis and encephalopathy), hyperkalemia and severe metabolic acidosis as well as fluid overload [56]. The broader clinical context, the presence of conditions modifiable with dialysis and trends of laboratory tests, rather than single urea or creatinine thresholds alone, should be considered in making this decision. Additionally, we believe that trends in urinary volume, especially in the first few hours of treatment, are of particular importance in the settings of ADHF with kidney dysfunction.
Despite initial enthusiasm about the use of ‘peritoneal dialysis’ as a gentler fluid removal strategy [57], to date no specific form of renal replacement therapy (RRT), be it peritoneal dialysis or hemodialysis (HD), has been shown to increase overall or subsequent dialysis-free survival among critically ill patients with AKI [58,59]. Therefore, selection of the RRT modality primarily depends on local expertise and availability of staff and equipment for a specific treatment as well as the patient's hemodynamic status, and intermittent and continuous RRT should be implemented as a complementary therapy in patients with AKI or decompensated CKD [56]. Meanwhile, a recent systematic review demonstrated the association of continuous RRT with lower incidence of developing end-stage renal disease in AKI survivors [60]. Given that slower solute clearance and fluid removal with continuous therapy reduces the risk of systemic hypotension during treatment and permits superior management of body fluid volume [61], continuous RRT may lead to better clinical outcome in patients at risk of unstable hemodynamic conditions such as ADHF.
Recent studies also support this hypothesis. In a multicenter RCT, patients in an intensive care unit with kidney dysfunction (serum creatinine >2 mg/dl) were randomly allocated to intermittent or continuous hemodiafiltration. Although continuous treatment showed no significant benefit overall, a pre-specified subgroup analysis showed a trend toward lower mortality in patients with HF [62]. Another study retrospectively examined critically ill patients who initiated RRT for AKI and subsequently survived 90 days, and found that the risk of developing end-stage renal failure was lower among continuous treatment recipients than propensity-matched intermittent HD recipients [63]. Moreover, the association of continuous treatment with lower risk of end-stage renal disease was pronounced in patients with prior HF but lost significance in those without prior HF.
Prolonged intermittent HD, often termed sustained low-efficiency dialysis, has been proposed as another alternative modality for AKI [64,65]. A RCT performed in surgical intensive care units showed that prolonged intermittent HD for patients with AKI, compared to continuous hemofiltration, provided similar hemodynamic stability and 90-day mortality and reduced medical cost and nursing time [66]. These results were consistent with previous observational studies including non-surgical critically ill patients [67,68]. Nevertheless, there is still little evidence for this modality in patients with severe ADHF. Given its economic advantage, well-designed, adequately powered RCTs are necessary to evaluate the clinical efficacy of prolonged intermittent HD in this population.
Salt and Fluid Restriction: Beneficial or Harmful?
The neurohumoral changes in HF limit both sodium and water excretion. Arginine vasopressin directly enhances water reabsorption in the collecting duct, whereas angiotensin II and norepinephrine increase proximal sodium and water reabsorption and thereby decrease sodium delivery to the distal tubule. Increased angiotensin II levels also stimulate thirst, leading to enhanced water intake. Therefore, fluid restriction in conjunction with sodium restriction contributes to better volume management with diuretics [69], and fluid restriction also counteracts hyponatremia, which is relatively common in severe HF and associated with poor outcomes [70].
Several guidelines for the management of HF proposed daily sodium and fluid intake restriction (<1.5 to 2.0 g/day and <1.5 to 2.0 l/day, respectively) in symptomatic or hospitalized HF patients [19,23,24]. However, there are insufficient data to endorse these specific restriction levels. The efficacy of severe fluid restriction (<1 l/day) with or without sodium restriction was evaluated in two RCTs, but neither study showed any benefit [71,72]. Another systematic review evaluating the effect of sodium restriction on renal outcomes in diverse populations also showed that there is reasonable evidence to support an association between high sodium intake (>4.6 g/day) and adverse renal outcomes, but not between low sodium intake and better renal outcomes [73]. It should be noted that sodium restriction appears to have competing effects on both the heart and the kidney, lowering blood pressure and activating the RAAS. Sodium and fluid restriction may also interact with other dietary factors, especially in CKD patients who have other dietary restrictions. Thus, those restrictions should not be routinely implemented but individualized based on serum sodium levels, the extent of diuretic resistance and other clinical conditions.
Potential Therapeutic Strategies
Vasopressin Receptor 2 Antagonists
Tolvaptan, a selective vasopressin receptor 2 antagonist, promotes urinary free water excretion (aquaresis) without affecting urinary electrolyte excretion by binding to the vasopressin receptor 2 of the distal nephron [74]. Tolvaptan appeared to decrease body weight and edema and to increase serum sodium concentrations in HF patients without worsening vital signs or renal function [75]. However, the Efficacy of Vasopressin Antagonism in Heart Failure Outcome Study with Tolvaptan (EVEREST) trial showed no significant benefit of oral tolvaptan in terms of cardiovascular death, hospitalization for HF or their composite outcome over 2 years in 4,133 ADHF patients [76]. Nevertheless, tolvaptan significantly improved patient-assessed dyspnea and hyponatremia, but slightly increased serum creatinine levels [77]. Its clinical efficacy in advanced CKD should be evaluated in future clinical trials.
Nesiritide
Nesiritide is a recombinant B-type natriuretic peptide approved for the management of ADHF. The recommended administration is a 2 μg/kg bolus injection followed by continuous infusion at a dose of 0.010 μg/kg/min. Although nesiritide produces modest improvement in dyspnea, a meta-analysis suggested that it may worsen renal function and hasten death, potentially due to its hypotensive effects [78]. Meanwhile, small studies using low-dose nesiritide (0.005 μg/kg/min without bolus) in ADHF and cardiac surgery patients have shown increased urine output and preserved kidney function [79,80].
Two RCTs have been conducted so far to address these issues. In the Acute Study of Clinical Effectiveness of Nesiritide in Decompensated Heart Failure (ASCEND-HF) trial, the use of normal-dose nesiritide on top of standard care in patients with ADHF neither increased nor decreased the incidence of death or re-hospitalization for HF, although it showed a small improvement in self-reported dyspnea [81]. Nesiritide was also associated with an increased incidence of hypotension, but not with worsening renal function or increased urine output [82]. Meanwhile, the above-mentioned ROSE trial showed that low-dose nesiritide added to standard diuretic therapy did not enhance decongestion or improve kidney function in patients with ADHF and kidney dysfunction, but there were trends toward higher urinary volume and lower serum cystatin C levels in patients who had low ejection fraction or low systolic blood pressure [27]. Thus, low-dose nesiritide may be indicated for patients with ADHF and reduced ejection fraction.
Angiotensin-Neprilysin Inhibitor
Neprilysin is an enzyme that contributes to the breakdown of several endogenous vasoactive peptides (natriuretic peptides, bradykinin and adrenomedullin) which counteract the neurohormonal activation leading to vasoconstriction, sodium retention and maladaptive remodeling [83]. Inhibition of neprilysin is a potential therapeutic strategy to delay the development or progression of cardiovascular disease and kidney disease. However, a study of combined inhibition of angiotensin-converting enzyme and neprilysin failed due to a high incidence of serious angioedema. To minimize the risk of this adverse effect, LCZ696 was developed, combining a neprilysin inhibitor prodrug (sacubitril) with an angiotensin receptor blocker (valsartan).
The Prospective Comparison of ARNI with ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure (PARADIGM-HF) trial is a phase III double-blind RCT evaluating the efficacy and safety profile of LCZ696 versus the angiotensin-converting enzyme inhibitor enalapril (a standard treatment) in 8,436 patients with chronic HF and reduced ejection fraction [84]. The trial was terminated early after a median follow-up of 27 months because of an overwhelming benefit of LCZ696 in terms of hospitalization for HF, death from cardiovascular causes and their composite as well as all-cause mortality (estimated hazard ratio ∼0.8, p < 0.001 for all). LCZ696, compared to enalapril, increased the incidence of symptomatic hypotension (14.0 vs. 9.2%) and non-serious angioedema (0.5 vs. 0.2%) but significantly reduced the risk of developing kidney dysfunction defined as serum creatinine ≥2.5 mg/dl (3.3 vs. 4.5%). These findings are consistent across subgroups and seem to provide convincing evidence to support LCZ696 as a new cornerstone in the management of chronic HF.
Conclusion
Although the definition of CRS has evolved over time with the understanding of bidirectional interplays between the heart and the kidneys, there is limited evidence to provide a specific treatment strategy using positive inotropes, diuretics, RRT as well as salt and fluid restriction for patients with ADHF and kidney dysfunction. Nevertheless, available evidence appears to support the 2013 ACCF/AHA guidelines for the management of heart failure and highlights the importance of individualized treatment. The new CRS classification system by Hatamizadeh et al. [2] would be helpful in identifying therapeutic targets and offers a systematic approach to optimal treatment as well as a management algorithm with better understanding of etiologies. It may eventually alter outcomes and enhance patient care and future investigations.
Disclosure Statement
K.K.Z. has received honoraria and/or support from Abbott, Abbvie, Alexion, Amgen, ASN, Astra-Zeneca, AVEO, Chugai, DaVita, Fresenius, Genetech, Haymarket Media, Hospira, Kabi, Keryx, NIH, NKF, Relypsa, Resverlogix, Sanofi, Shire, Vifor and ZS Pharma. C.P.K. has received honoraria from Sanofi-Aventis, Relypsa and ZS Pharma.
Acknowledgements
K.K.Z. is supported by National Institute of Diabetes, Digestive and Kidney Disease grants R01-DK096920 and K24-DK091419 as well as philanthropist grants from Mr. Harold Simmons, Mr. Louis Chang, Mr. Joseph Lee and AVEO. C.P.K. is supported by National Institute of Diabetes, Digestive and Kidney Disease grants R01-DK096920 and U01-DK102163. Y.O. is supported by a Shinya Foundation for International Exchange of Osaka University Graduate School of Medicine grant.
References
- 1.Ronco C, Haapio M, House AA, et al. Cardiorenal syndrome. J Am Coll Cardiol. 2008;52:1527–1539. doi: 10.1016/j.jacc.2008.07.051. [DOI] [PubMed] [Google Scholar]
- 2.Hatamizadeh P, Fonarow GC, Budoff MJ, et al. Cardiorenal syndrome: pathophysiology and potential targets for clinical management. Nat Rev Nephrol. 2013;9:99–111. doi: 10.1038/nrneph.2012.279. [DOI] [PubMed] [Google Scholar]
- 3.McCullough PA, Kellum JA, Haase M, et al. Pathophysiology of the cardiorenal syndromes: executive summary from the eleventh consensus conference of the Acute Dialysis Quality Initiative (ADQI) Contrib Nephrol. 2013;182:82–98. doi: 10.1159/000349969. [DOI] [PubMed] [Google Scholar]
- 4.Braam B, Joles JA, Danishwar AH, et al. Cardiorenal syndrome - current understanding and future perspectives. Nat Rev Nephrol. 2014;10:48–55. doi: 10.1038/nrneph.2013.250. [DOI] [PubMed] [Google Scholar]
- 5.Verbrugge FH, Grieten L, Mullens W. Management of the cardiorenal syndrome in decompensated heart failure. Cardiorenal Med. 2014;4:176–188. doi: 10.1159/000366168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith GL, Lichtman JH, Bracken MB, et al. Renal impairment and outcomes in heart failure: systematic review and meta-analysis. J Am Coll Cardiol. 2006;47:1987–1996. doi: 10.1016/j.jacc.2005.11.084. [DOI] [PubMed] [Google Scholar]
- 7.Gigante A, Liberatori M, Gasperini ML, et al. Prevalence and clinical features of patients with the cardiorenal syndrome admitted to an internal medicine ward. Cardiorenal Med. 2014;4:88–94. doi: 10.1159/000362566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clementi A, Virzi GM, Goh CY, et al. Cardiorenal syndrome type 4: a review. Cardiorenal Med. 2013;3:63–70. doi: 10.1159/000350397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Damman K, Navis G, Voors AA, et al. Worsening renal function and prognosis in heart failure: systematic review and meta-analysis. J Card Fail. 2007;13:599–608. doi: 10.1016/j.cardfail.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 10.Virzi GM, Clementi A, Brocca A, et al. The hemodynamic and nonhemodynamic crosstalk in cardiorenal syndrome type 1. Cardiorenal Med. 2014;4:103–112. doi: 10.1159/000362650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scadding JG. Diagnosis: the clinician and the computer. Lancet. 1967;2:877–882. doi: 10.1016/s0140-6736(67)92608-6. [DOI] [PubMed] [Google Scholar]
- 12.Palazzuoli A, Ruocco G, Pellegrini M, et al. Patients with cardiorenal syndrome revealed increased neurohormonal activity, tubular and myocardial damage compared to heart failure patients with preserved renal function. Cardiorenal Med. 2014;4:257–268. doi: 10.1159/000368375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Groenveld HF, Januzzi JL, Damman K, et al. Anemia and mortality in heart failure patients: a systematic review and meta-analysis. J Am Coll Cardiol. 2008;52:818–827. doi: 10.1016/j.jacc.2008.04.061. [DOI] [PubMed] [Google Scholar]
- 14.Okonko DO, Mandal AK, Missouris CG, et al. Disordered iron homeostasis in chronic heart failure: prevalence, predictors, and relation to anemia, exercise capacity, and survival. J Am Coll Cardiol. 2011;58:1241–1251. doi: 10.1016/j.jacc.2011.04.040. [DOI] [PubMed] [Google Scholar]
- 15.Ponikowski P, van Veldhuisen DJ, Comin-Colet J, et al. Beneficial effects of long-term intravenous iron therapy with ferric carboxymaltose in patients with symptomatic heart failure and iron deficiency. Eur Heart J. 2015;36:657–668. doi: 10.1093/eurheartj/ehu385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heine GH. Mineral metabolism in heart disease. Curr Opin Nephrol Hypertens. 2015;24:310–316. doi: 10.1097/MNH.0000000000000129. [DOI] [PubMed] [Google Scholar]
- 17.Anker SD, Sharma R. The syndrome of cardiac cachexia. Int J Cardiol. 2002;85:51–66. doi: 10.1016/s0167-5273(02)00233-4. [DOI] [PubMed] [Google Scholar]
- 18.Fouque D, Kalantar-Zadeh K, Kopple J, et al. A proposed nomenclature and diagnostic criteria for protein-energy wasting in acute and chronic kidney disease. Kidney Int. 2008;73:391–398. doi: 10.1038/sj.ki.5002585. [DOI] [PubMed] [Google Scholar]
- 19.Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62:e147–e239. doi: 10.1016/j.jacc.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 20.Abraham WT, Adams KF, Fonarow GC, et al. In-hospital mortality in patients with acute decompensated heart failure requiring intravenous vasoactive medications: an analysis from the Acute Decompensated Heart Failure National Registry (ADHERE) J Am Coll Cardiol. 2005;46:57–64. doi: 10.1016/j.jacc.2005.03.051. [DOI] [PubMed] [Google Scholar]
- 21.Elkayam U, Tasissa G, Binanay C, et al. Use and impact of inotropes and vasodilator therapy in hospitalized patients with severe heart failure. Am Heart J. 2007;153:98–104. doi: 10.1016/j.ahj.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 22.Cuffe MS, Califf RM, Adams KF, Jr, et al. Short-term intravenous milrinone for acute exacerbation of chronic heart failure: a randomized controlled trial. JAMA. 2002;287:1541–1547. doi: 10.1001/jama.287.12.1541. [DOI] [PubMed] [Google Scholar]
- 23.McMurray JJ, Adamopoulos S, Anker SD, et al. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2012;14:803–869. doi: 10.1093/eurjhf/hfs105. [DOI] [PubMed] [Google Scholar]
- 24.Lindenfeld J, Albert NM, Boehmer JP, et al. HFSA 2010 Comprehensive Heart Failure Practice Guideline. J Card Fail. 2010;16:e1–e194. doi: 10.1016/j.cardfail.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 25.Elkayam U, Ng TM, Hatamizadeh P, et al. Renal vasodilatory action of dopamine in patients with heart failure: magnitude of effect and site of action. Circulation. 2008;117:200–205. doi: 10.1161/CIRCULATIONAHA.107.737106. [DOI] [PubMed] [Google Scholar]
- 26.Giamouzis G, Butler J, Starling RC, et al. Impact of dopamine infusion on renal function in hospitalized heart failure patients: results of the Dopamine in Acute Decompensated Heart Failure (DAD-HF) Trial. J Card Fail. 2010;16:922–930. doi: 10.1016/j.cardfail.2010.07.246. [DOI] [PubMed] [Google Scholar]
- 27.Chen HH, Anstrom KJ, Givertz MM, et al. Low-dose dopamine or low-dose nesiritide in acute heart failure with renal dysfunction: the ROSE acute heart failure randomized trial. JAMA. 2013;310:2533–2543. doi: 10.1001/jama.2013.282190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Husebye T, Eritsland J, Muller C, et al. Levosimendan in acute heart failure following primary percutaneous coronary intervention-treated acute ST-elevation myocardial infarction. Results from the LEAF trial: a randomized, placebo-controlled study. Eur J Heart Fail. 2013;15:565–572. doi: 10.1093/eurjhf/hfs215. [DOI] [PubMed] [Google Scholar]
- 29.Koster G, Wetterslev J, Gluud C, et al. Effects of levosimendan for low cardiac output syndrome in critically ill patients: systematic review with meta-analysis and trial sequential analysis. Intensive Care Med. 2015;41:203–221. doi: 10.1007/s00134-014-3604-1. [DOI] [PubMed] [Google Scholar]
- 30.Bragadottir G, Redfors B, Ricksten SE. Effects of levosimendan on glomerular filtration rate, renal blood flow, and renal oxygenation after cardiac surgery with cardiopulmonary bypass: a randomized placebo-controlled study. Crit Care Med. 2013;41:2328–2335. doi: 10.1097/CCM.0b013e31828e946a. [DOI] [PubMed] [Google Scholar]
- 31.Follath F, Cleland JG, Just H, et al. Efficacy and safety of intravenous levosimendan compared with dobutamine in severe low-output heart failure (the LIDO study): a randomised double-blind trial. Lancet. 2002;360:196–202. doi: 10.1016/s0140-6736(02)09455-2. [DOI] [PubMed] [Google Scholar]
- 32.Mebazaa A, Nieminen MS, Packer M, et al. Levosimendan vs dobutamine for patients with acute decompensated heart failure: the SURVIVE Randomized Trial. JAMA. 2007;297:1883–1891. doi: 10.1001/jama.297.17.1883. [DOI] [PubMed] [Google Scholar]
- 33.Mebazaa A, Nieminen MS, Filippatos GS, et al. Levosimendan vs. dobutamine: outcomes for acute heart failure patients on beta-blockers in SURVIVE. Eur J Heart Fail. 2009;11:304–311. doi: 10.1093/eurjhf/hfn045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schulz R, Rose J, Martin C, et al. Development of short-term myocardial hibernation. Its limitation by the severity of ischemia and inotropic stimulation. Circulation. 1993;88:684–695. doi: 10.1161/01.cir.88.2.684. [DOI] [PubMed] [Google Scholar]
- 35.Cohn JN, Goldstein SO, Greenberg BH, et al. A dose-dependent increase in mortality with vesnarinone among patients with severe heart failure. Vesnarinone Trial Investigators. N Engl J Med. 1998;339:1810–1816. doi: 10.1056/NEJM199812173392503. [DOI] [PubMed] [Google Scholar]
- 36.Hampton JR, van Veldhuisen DJ, Kleber FX, et al. Randomised study of effect of ibopamine on survival in patients with advanced severe heart failure. Second Prospective Randomised Study of Ibopamine on Mortality and Efficacy (PRIME II) Investigators. Lancet. 1997;349:971–977. doi: 10.1016/s0140-6736(96)10488-8. [DOI] [PubMed] [Google Scholar]
- 37.Bonios MJ, Terrovitis JV, Drakos SG, et al. Comparison of three different regimens of intermittent inotrope infusions for end stage heart failure. Int J Cardiol. 2012;159:225–229. doi: 10.1016/j.ijcard.2011.03.013. [DOI] [PubMed] [Google Scholar]
- 38.Bock JS, Gottlieb SS. Cardiorenal syndrome: new perspectives. Circulation. 2010;121:2592–2600. doi: 10.1161/CIRCULATIONAHA.109.886473. [DOI] [PubMed] [Google Scholar]
- 39.Francis GS, Siegel RM, Goldsmith SR, et al. Acute vasoconstrictor response to intravenous furosemide in patients with chronic congestive heart failure. Activation of the neurohumoral axis. Ann Intern Med. 1985;103:1–6. doi: 10.7326/0003-4819-103-1-1. [DOI] [PubMed] [Google Scholar]
- 40.Rudy DW, Voelker JR, Greene PK, et al. Loop diuretics for chronic renal insufficiency: a continuous infusion is more efficacious than bolus therapy. Ann Intern Med. 1991;115:360–366. doi: 10.7326/0003-4819-115-5-360. [DOI] [PubMed] [Google Scholar]
- 41.Salvador DR, Rey NR, Ramos GC, et al. Continuous infusion versus bolus injection of loop diuretics in congestive heart failure. Cochrane Database Syst Rev. 2005;3:CD003178. doi: 10.1002/14651858.CD003178.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Felker GM, Lee KL, Bull DA, et al. Diuretic strategies in patients with acute decompensated heart failure. N Engl J Med. 2011;364:797–805. doi: 10.1056/NEJMoa1005419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Palazzuoli A, Pellegrini M, Ruocco G, et al. Continuous versus bolus intermittent loop diuretic infusion in acutely decompensated heart failure: a prospective randomized trial. Crit Care. 2014;18:R134. doi: 10.1186/cc13952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Allen LA, Turer AT, Dewald T, et al. Continuous versus bolus dosing of furosemide for patients hospitalized for heart failure. Am J Cardiol. 2010;105:1794–1797. doi: 10.1016/j.amjcard.2010.01.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wu MY, Chang NC, Su CL, et al. Loop diuretic strategies in patients with acute decompensated heart failure: a meta-analysis of randomized controlled trials. J Crit Care. 2014;29:2–9. doi: 10.1016/j.jcrc.2013.10.009. [DOI] [PubMed] [Google Scholar]
- 46.Brater DC. Diuretic therapy. N Engl J Med. 1998;339:387–395. doi: 10.1056/NEJM199808063390607. [DOI] [PubMed] [Google Scholar]
- 47.Shah RV, McNulty S, O'Connor CM, et al. Effect of admission oral diuretic dose on response to continuous versus bolus intravenous diuretics in acute heart failure: an analysis from diuretic optimization strategies in acute heart failure. Am Heart J. 2012;164:862–868. doi: 10.1016/j.ahj.2012.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chawla LS, Davison DL, Brasha-Mitchell E, et al. Development and standardization of a furosemide stress test to predict the severity of acute kidney injury. Crit Care. 2013;17:R207. doi: 10.1186/cc13015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jenkins PG. Diuretic strategies in patients with acute heart failure. N Engl J Med. 2011;364:2066. doi: 10.1056/NEJMc1103708. author reply 2069. [DOI] [PubMed] [Google Scholar]
- 50.Jaski BE, Ha J, Denys BG, et al. Peripherally inserted veno-venous ultrafiltration for rapid treatment of volume overloaded patients. J Card Fail. 2003;9:227–231. doi: 10.1054/jcaf.2003.28. [DOI] [PubMed] [Google Scholar]
- 51.Costanzo MR, Guglin ME, Saltzberg MT, et al. Ultrafiltration versus intravenous diuretics for patients hospitalized for acute decompensated heart failure. J Am Coll Cardiol. 2007;49:675–683. doi: 10.1016/j.jacc.2006.07.073. [DOI] [PubMed] [Google Scholar]
- 52.Bart BA, Boyle A, Bank AJ, et al. Ultrafiltration versus usual care for hospitalized patients with heart failure: the Relief for Acutely Fluid-Overloaded Patients With Decompensated Congestive Heart Failure (RAPID-CHF) trial. J Am Coll Cardiol. 2005;46:2043–2046. doi: 10.1016/j.jacc.2005.05.098. [DOI] [PubMed] [Google Scholar]
- 53.Bart BA, Goldsmith SR, Lee KL, et al. Ultrafiltration in decompensated heart failure with cardiorenal syndrome. N Engl J Med. 2012;367:2296–2304. doi: 10.1056/NEJMoa1210357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Marenzi G, Muratori M, Cosentino ER, et al. Continuous ultrafiltration for congestive heart failure: the CUORE trial. J Card Fail. 2014;20:9–17. doi: 10.1016/j.cardfail.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 55.Mentz RJ, Stevens SR, DeVore AD, et al. Decongestion strategies and renin-angiotensin-aldosterone system activation in acute heart failure. JACC Heart Fail. 2015;3:97–107. doi: 10.1016/j.jchf.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kidney Disease Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group: KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney Int Suppl. 2012;2 [Google Scholar]
- 57.Lu R, Muciño-Bermejo MJ, Ribeiro LC, et al. Peritoneal dialysis in patients with refractory congestive heart failure: a systematic review. Cardiorenal Med. 2015;5:145–156. doi: 10.1159/000380915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pannu N, Klarenbach S, Wiebe N, et al. Renal replacement therapy in patients with acute renal failure: a systematic review. JAMA. 2008;299:793–805. doi: 10.1001/jama.299.7.793. [DOI] [PubMed] [Google Scholar]
- 59.Bagshaw SM, Berthiaume LR, Delaney A, et al. Continuous versus intermittent renal replacement therapy for critically ill patients with acute kidney injury: a meta-analysis. Crit Care Med. 2008;36:610–617. doi: 10.1097/01.CCM.0B013E3181611F552. [DOI] [PubMed] [Google Scholar]
- 60.Schneider AG, Bellomo R, Bagshaw SM, et al. Choice of renal replacement therapy modality and dialysis dependence after acute kidney injury: a systematic review and meta-analysis. Intensive Care Med. 2013;39:987–997. doi: 10.1007/s00134-013-2864-5. [DOI] [PubMed] [Google Scholar]
- 61.Tolwani A. Continuous renal-replacement therapy for acute kidney injury. N Engl J Med. 2012;367:2505–2514. doi: 10.1056/NEJMct1206045. [DOI] [PubMed] [Google Scholar]
- 62.Lins RL, Elseviers MM, Van der Niepen P, et al. Intermittent versus continuous renal replacement therapy for acute kidney injury patients admitted to the intensive care unit: results of a randomized clinical trial. Nephrol Dial Transplant. 2009;24:512–518. doi: 10.1093/ndt/gfn560. [DOI] [PubMed] [Google Scholar]
- 63.Wald R, Shariff SZ, Adhikari NK, et al. The association between renal replacement therapy modality and long-term outcomes among critically ill adults with acute kidney injury: a retrospective cohort study. Crit Care Med. 2014;42:868–877. doi: 10.1097/CCM.0000000000000042. [DOI] [PubMed] [Google Scholar]
- 64.Lonnemann G, Floege J, Kliem V, et al. Extended daily veno-venous high-flux haemodialysis in patients with acute renal failure and multiple organ dysfunction syndrome using a single path batch dialysis system. Nephrol Dial Transplant. 2000;15:1189–1193. doi: 10.1093/ndt/15.8.1189. [DOI] [PubMed] [Google Scholar]
- 65.Kielstein JT, Kretschmer U, Ernst T, et al. Efficacy and cardiovascular tolerability of extended dialysis in critically ill patients: a randomized controlled study. Am J Kidney Dis. 2004;43:342–349. doi: 10.1053/j.ajkd.2003.10.021. [DOI] [PubMed] [Google Scholar]
- 66.Schwenger V, Weigand MA, Hoffmann O, et al. Sustained low efficiency dialysis using a single-pass batch system in acute kidney injury - a randomized interventional trial: the REnal Replacement Therapy Study in Intensive Care Unit PatiEnts. Crit Care. 2012;16:R140. doi: 10.1186/cc11445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Marshall MR, Creamer JM, Foster M, et al. Mortality rate comparison after switching from continuous to prolonged intermittent renal replacement for acute kidney injury in three intensive care units from different countries. Nephrol Dial Transplant. 2011;26:2169–2175. doi: 10.1093/ndt/gfq694. [DOI] [PubMed] [Google Scholar]
- 68.Srisawat N, Lawsin L, Uchino S, et al. Cost of acute renal replacement therapy in the intensive care unit: results from The Beginning and Ending Supportive Therapy for the Kidney (BEST Kidney) study. Crit Care. 2010;14:R46. doi: 10.1186/cc8933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wilcox CS, Mitch WE, Kelly RA, et al. Response of the kidney to furosemide. I. Effects of salt intake and renal compensation. J Lab Clin Med. 1983;102:450–458. [PubMed] [Google Scholar]
- 70.Elhassan EA, Schrier RW. Hyponatremia: diagnosis, complications, and management including V2 receptor antagonists. Curr Opin Nephrol Hypertens. 2011;20:161–168. doi: 10.1097/MNH.0b013e3283436f14. [DOI] [PubMed] [Google Scholar]
- 71.Travers B, O'Loughlin C, Murphy NF, et al. Fluid restriction in the management of decompensated heart failure: no impact on time to clinical stability. J Card Fail. 2007;13:128–132. doi: 10.1016/j.cardfail.2006.10.012. [DOI] [PubMed] [Google Scholar]
- 72.Aliti GB, Rabelo ER, Clausell N, et al. Aggressive fluid and sodium restriction in acute decompensated heart failure: a randomized clinical trial. JAMA Intern Med. 2013;173:1058–1064. doi: 10.1001/jamainternmed.2013.552. [DOI] [PubMed] [Google Scholar]
- 73.Smyth A, O'Donnell MJ, Yusuf S, et al. Sodium intake and renal outcomes: a systematic review. Am J Hypertens. 2014;27:1277–1284. doi: 10.1093/ajh/hpt294. [DOI] [PubMed] [Google Scholar]
- 74.Schrier RW, Gross P, Gheorghiade M, et al. Tolvaptan, a selective oral vasopressin V2-receptor antagonist, for hyponatremia. N Engl J Med. 2006;355:2099–2112. doi: 10.1056/NEJMoa065181. [DOI] [PubMed] [Google Scholar]
- 75.Gheorghiade M, Gattis WA, O'Connor CM, et al. Effects of tolvaptan, a vasopressin antagonist, in patients hospitalized with worsening heart failure: a randomized controlled trial. JAMA. 2004;291:1963–1971. doi: 10.1001/jama.291.16.1963. [DOI] [PubMed] [Google Scholar]
- 76.Konstam MA, Gheorghiade M, Burnett JC, Jr, et al. Effects of oral tolvaptan in patients hospitalized for worsening heart failure: the EVEREST Outcome Trial. JAMA. 2007;297:1319–1331. doi: 10.1001/jama.297.12.1319. [DOI] [PubMed] [Google Scholar]
- 77.Gheorghiade M, Konstam MA, Burnett JC, Jr, et al. Short-term clinical effects of tolvaptan, an oral vasopressin antagonist, in patients hospitalized for heart failure: the EVEREST Clinical Status Trials. JAMA. 2007;297:1332–1343. doi: 10.1001/jama.297.12.1332. [DOI] [PubMed] [Google Scholar]
- 78.Sackner-Bernstein JD, Kowalski M, Fox M, et al. Short-term risk of death after treatment with nesiritide for decompensated heart failure: a pooled analysis of randomized controlled trials. JAMA. 2005;293:1900–1905. doi: 10.1001/jama.293.15.1900. [DOI] [PubMed] [Google Scholar]
- 79.Chen HH, Sundt TM, Cook DJ, et al. Low dose nesiritide and the preservation of renal function in patients with renal dysfunction undergoing cardiopulmonary-bypass surgery: a double-blind placebo-controlled pilot study. Circulation. 2007;116:I134–I138. doi: 10.1161/CIRCULATIONAHA.106.697250. [DOI] [PubMed] [Google Scholar]
- 80.Riter HG, Redfield MM, Burnett JC, et al. Nonhypotensive low-dose nesiritide has differential renal effects compared with standard-dose nesiritide in patients with acute decompensated heart failure and renal dysfunction. J Am Coll Cardiol. 2006;47:2334–2335. doi: 10.1016/j.jacc.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 81.O'Connor CM, Starling RC, Hernandez AF, et al. Effect of nesiritide in patients with acute decompensated heart failure. N Engl J Med. 2011;365:32–43. doi: 10.1056/NEJMoa1100171. [DOI] [PubMed] [Google Scholar]
- 82.Gottlieb SS, Stebbins A, Voors AA, et al. Effects of nesiritide and predictors of urine output in acute decompensated heart failure: results from ASCEND-HF (acute study of clinical effectiveness of nesiritide and decompensated heart failure) J Am Coll Cardiol. 2013;62:1177–1183. doi: 10.1016/j.jacc.2013.04.073. [DOI] [PubMed] [Google Scholar]
- 83.Voors AA, Dorhout B, van der Meer P. The potential role of valsartan + AHU377 (LCZ696) in the treatment of heart failure. Expert Opin Investig Drugs. 2013;22:1041–1047. doi: 10.1517/13543784.2013.797963. [DOI] [PubMed] [Google Scholar]
- 84.McMurray JJ, Packer M, Desai AS, et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371:993–1004. doi: 10.1056/NEJMoa1409077. [DOI] [PubMed] [Google Scholar]