Abstract
PURPOSE
We aimed to evaluate the relationship between gastrectomy and the volume of liver segments II and III in patients with gastric cancer.
METHODS
Computed tomography images of 54 patients who underwent curative gastrectomy for gastric adenocarcinoma were retrospectively evaluated by two blinded observers. Volumes of the total liver and segments II and III were measured. The difference between preoperative and postoperative volume measurements was compared.
RESULTS
Total liver volumes measured by both observers in the preoperative and postoperative scans were similar (P > 0.05). High correlation was found between both observers (preoperative r=0.99; postoperative r=0.98). Total liver volumes showed a mean reduction of 13.4% after gastrectomy (P = 0.977). The mean volume of segments II and III showed similar decrease in measurements of both observers (38.4% vs. 36.4%, P = 0.363); the correlation between the observers were high (preoperative r=0.97, P < 0.001; postoperative r=0.99, P < 0.001). Volume decrease in the rest of the liver was not different between the observers (8.2% vs. 9.1%, P = 0.388). Time had poor correlation with volume change of segments II and III and the total liver for each observer (observer 1, rseg2/3=0.32, rtotal=0.13; observer 2, rseg2/3=0.37, rtotal=0.16).
CONCLUSION
Segments II and III of the liver showed significant atrophy compared with the rest of the liver and the total liver after gastrectomy. Volume reduction had poor correlation with time.
Gastric cancer is the fourth most common cancer among men and the sixth most common cancer among women. It is the third leading cause of cancer related deaths in both sexes worldwide (1). The life expectancy of the patients varies depending on the grade of the tumor, spread of the disease and the appropriate medical and surgical treatment. Many patients undergo surgery (2). In routine practice, contrast-enhanced computed tomography (CT) is used in order to understand the extent of the disease before the surgery. It is also the most important tool for follow up after the surgery (3).
The deterioration of liver function tests after laparoscopic gastrectomy has been reported in a few studies (4, 5). In addition, there were several case reports indicating ischemia and infarct of the left lobe of the liver after laparoscopic gastrectomy due to prolonged Nathanson surgical retractor compression (6–10). In this study, we aimed to evaluate the relationship between open gastrectomy and the volume of the liver segments II and III.
Methods
Study population
In this retrospective institutional review board approved study, we reviewed a database of 198 patients who underwent curative, open total or distal subtotal gastrectomy for gastric adenocarcinoma between January 2006 and December 2012. Preoperative and postoperative CT images of the patients were evaluated retrospectively through picture achieve and communication system (PACS) of our center. Patients who did not have preoperative or postoperative contrast-enhanced CT examinations in PACS, patients with liver metastasis, and patients who received neoadjuvant chemo and/or radiotherapy were excluded from the study. Totally, 54 patients who had preoperative and postoperative contrast-enhanced CT examinations were included in the analysis. None of the patients had postoperative radiotherapy. Data including age, sex, body mass index (BMI) before the surgery, tumor location, nutritional risk scores, number of harvested lymph nodes, number of metastatic lymph nodes, operation duration, intent of the surgery, TNM classification and history of alcohol consumption were obtained from records of the hospital. The nutritional risk of the patients was assessed using the Nutritional Risk Screening (NRS 2002) score suggested by the European Society of Parenteral and Enteral Nutrition (11).
CT examination
Dual-phase abdominal CT examinations were performed using 16- and 64-channel multidetector CT scanners (Brilliance 16 and Brilliance 64; Philips Medical Systems). Oral negative contrast agent and intravenous contrast agent (100 mL water-soluble nonionic contrast agent, 3mL/s) were given to all patients. Abdominal CT was performed during arterial phase for the upper abdomen (from base of the thorax until the iliac crista) and during portal venous phase for the whole abdomen (from base of the thorax to the pubic bone). Imaging was performed with 120 kVp (standard), 240–430 mA, 35–50 cm FOV in accordance with the patients, and 512×512 matrix (standard). Axial images were performed with slice thickness of 1–2 mm. Coronal reformat images were obtained from source images.
The interval of postoperative CT from the day of gastrectomy was at least three months and at most 63 months, with an average of 22 months. Postoperative CT interval was not constant among the patients and we wanted to correlate the volume change percent with postoperative time. Therefore, postoperative measurements were performed in the latest postoperative CT images that were suitable for our study during the study period. Preoperative and postoperative CT images were also examined in order to check the presence of left hepatic artery, left hepatic vein, and portal vein.
Volume measurement
In order to assess the volume of segments II and III, a software (Volume Tracing in Advanced Vessel Analysis; Philips Healthcare) was used in a special workstation (Extended Brilliance Workspace v3.5.0.2254; Philips Healthcare). The reliability of this software was reported in prior studies (12, 13).
All preoperative and postoperative latest CT scans were evaluated by two radiology residents who had at least one-year experience in liver volume measurement. The residents made the measurements independently and an a blinded fashion. The results were evaluated by a radiologist with more than 10 years of experience in liver imaging. Each measurement took approximately 40 minutes for both residents.
The liver segments II and III were determined according to the Coinaud classification (14). The liver parenchyma that lies medially of the ligamentum teres and left hepatic vein was accepted as segments II and III. The total volume of segments 1-4-5-6-7-8 was calculated by subtracting the volume of segments II and III from the total liver volume and referred as “the rest of the liver”.
For each observer, volume change percentage between preoperative and postoperative scans was calculated by the following formula:
Surgical method
All patients had histopathologically proven gastric adenocarcinoma. Preoperative staging included thorax and abdominal CT. Based on the location of the tumor, either open total gastrectomy with truncal vagotomy and D2 lymph node dissection (patients with proximally located tumor) or open distal subtotal gastrectomy with D2 lymph node dissection (patients with distally located tumor) was performed. Truncal vagotomy was not performed in open distal subtotal gastrectomy. Of the patients, 32 had total gastrectomy and 22 had distal subtotal gastrectomy. Unlike laparoscopic upper gastrointestinal system operations, Nathanson retractor was not used in open gastrectomy, and classical abdominal retractors were used in all operations. These retractors were used gently, approximately 5 to 10 minutes, with long intervals depending on the situation. Unfortunately, we do not have data regarding the pressure, time, and location of the applied retractors. Routinely, in patients with a replaced left hepatic artery, arteries larger than 3 mm in diameter are preserved during the operation, while smaller ones are ligated. In our study, none of the patients had a replaced left hepatic artery, and the retrospective review of the operation notes demonstrated no vascular complications. Other than gastrectomy, one patient had a splenectomy, four patients had a cholecystectomy and four patients had partial colectomy in the same operation for presence of biliarystone and desmoplastic adhesions. Postoperative time was calculated by subtracting the preoperative CT date from the postoperative CT date and measured in months.
Statistics
All measurements were evaluated by the statistics software SPSS version 20 (IBM corp.). Data distribution was evaluated with the Kolmogorov–Smirnov normality test. The difference between each observer’s preoperative and postoperative volume measurements and the difference between the two observers’ preoperative and postoperative volume measurements were compared with the paired-t test. In order to evaluate interobserver variability, correlation analysis was performed. Intraclass correlation coefficient and Pearson correlation coefficient were computed. Pearson correlation analysis was also used in order to understand the relationship of percent volume change with different pre- and postoperative time intervals between patients. For each observer, the volume loss and increase were categorized as “loss” and “increase” and Kappa statistics were used. In different operation groups, volume difference was assessed by either Mann-Whitney U test or t test. The differences between patients with atrophy of segments II and III and those without, were evaluated by chi-square and Fisher exact tests. All hypothesis testing was performed at the α = 0.05 significance level.
Results
Of 54 patients, 37 (68.5%) were men and 17 (31.5 %) were women. Mean age of the study group was 58.8±12.3 years. The mean BMI was 25.7 kg/m2. Five patients reported regular alcohol consumption. Mean tumor size was 5±2.5 cm. The mean operation time was 217±47 min. According to TNM staging, 24 patients were T1 or T2, while 30 patients were T3 or T4. Metastatic lymph nodes were found in 39 patients. None of the patients had a replaced left hepatic artery. In the postoperative CT scans, all patients had intact left hepatic artery, left hepatic vein, and left portal vein.
Minimum, maximum, and mean volume measurements of segments II and III, the rest of the liver, and total liver are summarized in Table 1. Paired t test findings are summarized in Table 2. Total liver volumes determined by both observers in preoperative and postoperative CT scans were similar, and there was no significant difference between the two observers’ results (P = 0.391 and P = 0.877, for preoperative and postoperative measurements, respectively). High correlation was found between both observers’ preoperative and postoperative total liver volumes (preoperative r=0.99, P < 0.001, postoperative r=0.98, P < 0.001). Mean volume loss of the total liver was 13.4% after the operation (P = 0.977, r=0.96).
Table 1.
Pre- and postoperative liver volumes measured by the observers
| n=54 | Segments II and III | Rest of the liver | Total liver | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||
| Min | Max | Mean±SD | Min | Max | Mean±SD | Min | Max | Mean±SD | |
| Observer 1 | |||||||||
| Preoperative volume | 74 | 419 | 223.1±81.4 | 711 | 2306 | 1155.3±301.5 | 863 | 2557 | 1378.4±345.2 |
| Postoperative volume | 18 | 479 | 138.2±92 | 638 | 1994 | 1037.5±299.4 | 691 | 2473 | 1175.8±365.5 |
|
| |||||||||
| Observer 2 | |||||||||
| Preoperative volume | 57 | 427 | 205.6±77.6 | 731 | 2292 | 1177.1±297.9 | 858 | 2560 | 1382.8±341 |
| Postoperative volume | 12 | 471 | 130.1±92.3 | 599 | 2034 | 1047.5±299 | 681 | 2505 | 1177.7±359.5 |
Volumes are measured in mL.
Table 2.
Statistical comparison of liver volume measurements before and after operation and between observers
| Segments II and III | Rest of the liver | Total liver | ||||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| Mean±SD | P | Mean±SD | P | Mean±SD | P | |
| Observer 1–Preoperative volume | 223.1±81.4 | <0.001 | 1155.3±301.5 | 0.006 | 1378.4±345.2 | <0.001 |
| Observer 1–Postoperative volume | 138.2±92 | 1037.5±299.4 | 1175.8±365.5 | |||
|
| ||||||
| Observer 2–Preoperative volume | 205.6±77.6 | <0.001 | 1177.1±297.9 | 0.003 | 1382.8±341 | <0.001 |
| Observer 2–Postoperative volume | 130.1±92.3 | 1047.5±299 | 1177.7±359.5 | |||
|
| ||||||
| Observer 1–Preoperative volume | 223.1±81.4 | <0.001 | 1155.3±301.5 | 0.001 | 1378.4±345.2 | 0.391 |
| Observer 2–Preoperative volume | 205.6±77.6 | 1177.1±297.9 | 1382.8±341 | |||
|
| ||||||
| Observer 1–Postoperative volume | 138.2±92 | <0.001 | 1037.5±299.4 | 0.411 | 1175.8±365.5 | 0.877 |
| Observer 2–Postoperative volume | 130.1±92.3 | 1047.5±299 | 1177.7±359.5 | |||
|
| ||||||
| Observer 1–Volume change, % | 38.4 | 0.363 | 8.2 | 0.388 | 13.4 | 0.977 |
| Observer 2–Volume change, % | 36.4 | 9.1 | 13.4 | |||
All measurements are presented as mean±standard deviation (mL), except for volume change, which is presented as percentile.
SD, standard deviation.
The volumes of segments II and III showed similar decrease in both observers’ measurements after the surgery (38.4%, 36.4%, P = 0.363). There was a high correlation between the measurements of the observers (preoperative r=0.97, P < 0.001, postoperative r=0.99, P < 0.001). Although, preoperative and postoperative measurements were significantly different between the observers (P < 0.001), volume decrease was similar and measurements showed high correlation. Volume of the rest of the liver decreased by 8.2%, according to the first observer and 9.1%, according to the second observer (P = 0.388). There was a high correlation between the two observers’ preoperative and postoperative measurements (preoperative r=0.98, P < 0.001, postoperative r=0.95, P < 0.001).
The first observer found volume loss in segments II and III in 48 patients (93.8%), while the second observer found volume loss in 46 patients (85.2%). In five patients (9.2%), both observers found volume increase and in 45 patients (83.3 %) both observers found volume loss. The maximum volume loss was 87.7% in measurements of observer 1 and 88.2% in measurements of observer 2. Volume loss and increase for the rest of the liver and total liver are summarized in Table 3. Kappa test showed good agreement between two observers (κsegment 2–3=0.67, κrest of the liver=0.71, κtotal liver=0.73, P < 0.001). Examples of some patients are presented in Figs. 1 and 2.
Table 3.
Number of patients showing volume increase and decrease according to observers 1 and 2
| Observer 2 | |||||||
|---|---|---|---|---|---|---|---|
| Segments II and III | Rest of the liver | Total liver | |||||
|
|
|
|
|||||
| Increase | Loss | Increase | Loss | Increase | Loss | ||
| Observer 1 | Increase | 5 | 1 | 11 | 3 | 7 | 1 |
| Loss | 3 | 45 | 3 | 37 | 3 | 43 | |
| κ | 0.673 | 0.711 | 0.734 | ||||
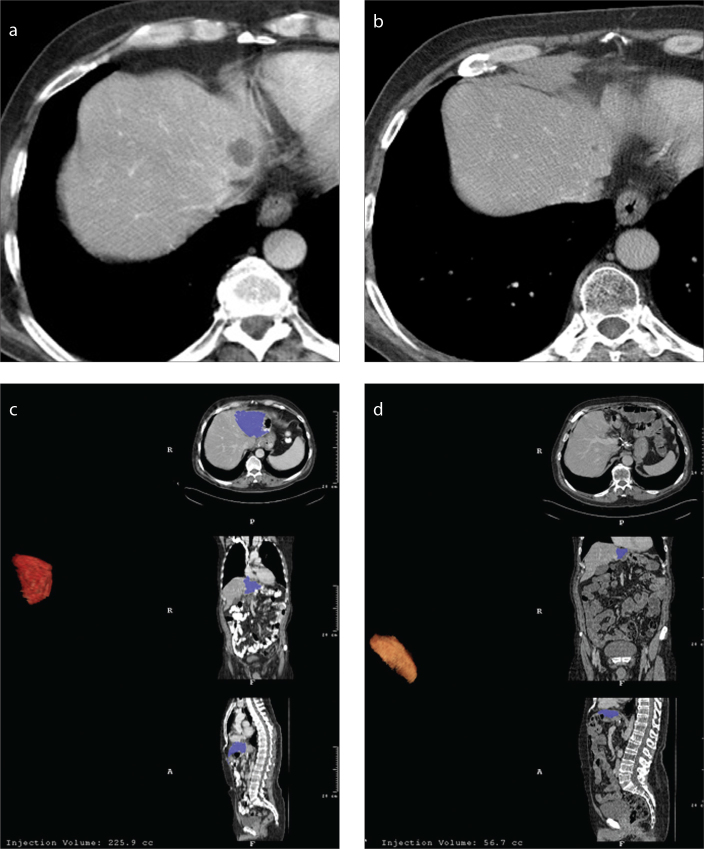
Figure 1.
a–d. A 62-year-old man with gastric cancer. Preoperative CT (a) reveals a parenchymal cyst in segment 2. In the control CT image (b) obtained four years after total gastrectomy, the parenchymal cyst shrunk as well as segments II and III. Preoperative volumetric measurement of segments II and III is 225 mL (c). Four years later, the volume of segments II and III decreased to 56 mL (d).
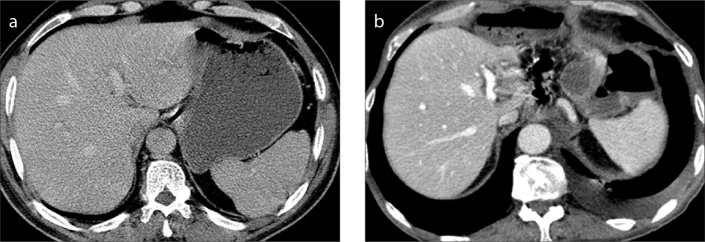
Figure 2.
a, b. A 58-year-old man with gastric cancer. Preoperative CT (a) reveals a normal liver. Two years after distal subtotal gastrectomy (b), segments II and III adjacent to the portal vein are almost invisible. Preoperative volumetric measurement of the segments II and III was 237 mL which decreased to 35.7 mL two years after the operation.
We repeated the paired t test in 45 patients, in whom both observers had found volume loss in segments II and III. After exclusion of nine patients, volume loss in segments II and III was almost 50% for both observers (observer 1, 48%; observer 2, 46.4%; P = 0.479).
Pearson correlation test showed that time had poor correlation with volume change of segments II and III and total liver for either observer (observer 1, rseg 2/3=0.32 P = 0.016, rtotal=0.13 P = 0.350; observer 2, rseg 2/3=0.37 P = 0.006, rtotal=0.16 P = 0.249). The volumes and volume change of segments II and III were not significantly different between patients with total gastrectomy and patients with distal subtotal gastrectomy (observer 1, Pvolume change = 0.460, Ppreop =0.820, Ppostop = 0.600; observer 2, Pvolume change = 0.740, Ppreop = 0.970, Ppostop = 0.690).
Finally, we compared the patients with atrophy of the segments II and III with the patients with no atrophy, in terms of several factors that might contribute to volume change. The number of patients with volume increase in segments II and III was very small (only six patients in the first observer and eight patients in the second observer), and there was no significant difference between the groups. Presence of nutritional risk did not have any effect on postoperative liver volumes (Table 4).
Table 4.
Comparison of patients with and without atrophy of the segments II and III
| Observer 1 | Observer 2 | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Atrophy (−) | Atrophy (+) | P | Atrophy (−) | Atrophy (+) | P | |
| Age (years) | 63.3±12.6 | 58.2±12.2 | 0.341 | 63.8±13 | 57.9±11.3 | 0.373 |
|
| ||||||
| Sex, n | 1.000 | 0.696 | ||||
| Female | 2 | 15 | 3 | 14 | ||
| Male | 4 | 33 | 5 | 32 | ||
|
| ||||||
| Tumor location, n | 0.211 | 0.248 | ||||
| Proximal | 2 | 30 | 3 | 29 | ||
| Distal | 4 | 18 | 5 | 17 | ||
|
| ||||||
| Nutritional risk, n | 1.000 | 1.000 | ||||
| (−) | 5 | 38 | 7 | 36 | ||
| (+) | 1 | 10 | 1 | 10 | ||
|
| ||||||
| Harvested lymph nodes | 26.5±13.5 | 22.9±13.8 | 0.544 | 20±13.3 | 24.5±13.7 | 0.887 |
|
| ||||||
| Metastatic lymph nodes | 7±12.2 | 9.9±10.3 | 0.540 | 9±13 | 9.6±10.3 | 0.890 |
|
| ||||||
| BMI (kg/m2) | 26.33±6.2 | 25.64±4.9 | 0.753 | 25.5±6 | 25.8±4.7 | 0.219 |
|
| ||||||
| Operative time (min) | 246.7±55 | 213.8±45.3 | 0.107 | 222.5±48.6 | 216.5±47.3 | 0.744 |
|
| ||||||
| Intent | 1.000 | 0.671 | ||||
| Radical | 4 | 35 | 5 | 34 | ||
| Palliative | 2 | 13 | 3 | 12 | ||
|
| ||||||
| Tumor size (cm) | 3.5±1.5 | 5.2±2.6 | 0.546 | 3.80±2 | 5.2±2.6 | 0.169 |
|
| ||||||
| pT | 0.389 | 0.443 | ||||
| T1–2 | 4 | 20 | 5 | 19 | ||
| T3–4 | 2 | 28 | 3 | 27 | ||
|
| ||||||
| pN | 0.882 | 0.138 | ||||
| N (−) | 4 | 11 | 4 | 11 | ||
| N (+) | 3 | 36 | 4 | 35 | ||
|
| ||||||
| Alcohol | 1.000 | 1.000 | ||||
| (−) | 6 | 43 | 8 | 41 | ||
| (+) | 0 | 5 | 0 | 5 | ||
Data are presented as mean±standard deviation or n.
BMI, body mass index.
Discussion
Our results show that in more than 80% of the patients with either total or distal subtotal open gastrectomy, segments II and III of the liver get smaller. The volume loss of segments II and III was almost four times more than that of the rest of the liver and three times more than that of the total liver (Table 2). The volume loss in segments II and III was the major contributor to the total liver volume loss compared with the rest of the liver, which showed a volume reduction of 8.2% in measurements of observer 1 and 9.1% in measurements of observer 2.
Reevaluation of the five patients in which both observers found volume increase in segments II and III revealed that two patients developed severe hepatomegaly with hepatosteatosis in postoperative images, while the remaining three patients had no significant parenchymal finding and the volume increases were less than 30 mL for both observers. In four patients, contradicting volume changes were found with one observer reporting volume increase and the other reporting volume decrease, although the volume change rates were very small. When we excluded these nine patients and repeated the statistics, volume loss in segments II and III increased to almost 50%.
We compared patients with atrophy of the segments II and III with patients without atrophy in terms of possible factors that might contribute this volume difference. As seen in Table 4, none of these variables (age, sex, BMI before the surgery, tumor location, nutritional risk scores, number of harvested lymph nodes, number of metastatic lymph nodes, operation duration, intent of the surgery, TNM classification, and history of alcohol consumption) showed significant difference.
Total liver volume measurements were very similar for both observers. There was no interobserver variability in the preoperative and postoperative measurements. Total liver volume decrease was also similar for both observers with a high interobserver agreement. Although both observers’ preoperative and postoperative volume measurements of segments II and III were statistically different in the paired t test (Table 2), the difference was very small (Table 1). Segments II and III do not have definite borders unlike the total liver and it is not easy to separate these segments exactly from the whole liver. Nevertheless, both observers’ measurements of segments II and III and the rest of the liver showed high correlation in Pearson correlation tests and the calculated volume reduction rates were statistically similar. There was a good agreement between the two observers in terms of volume increase and volume loss for segments II and III, the rest of the liver and the total liver volumes.
We compared each observer’s volume measurements in patients with open total gastrectomy versus open distal subtotal gastrectomy. There was no difference between the different operation groups. This finding indicates that truncal vagotomy has no effect on volume change of segments II and III.
Based on our findings, it is not possible to clarify the underlying cause of this volume reduction of segments II and III after gastrectomy, but we can speculate for some causes. In some studies, hepatic enzymes were found to deteriorate after laparoscopic gastrectomy (4, 5). Through the literature search, we encountered some case reports that mentioned left lobe ischemia and infarct had developed after laparoscopic gastrectomy due to excessive Nathanson retractor pressure during the operation (6, 7, 10, 15). Orr and Williams found parenchymal defects and atrophy in the left lobe of the liver in postoperative CT of 27% of gastric cancer patients who had laparoscopic gastrectomy and 18% of patients who had laparoscopic bariatric surgery, and they related these findings to prolonged usage of Nathanson retractor (9). Yassa and Peters (8) reported that in 10 of 250 patients with open gastrectomy due to gastric cancer, they found focal wedge shaped, rectangular or rounded hypodensities in early postoperative CT images and related these findings to pressure necrosis secondary to surgical retractor usage.
In our study, the left lobe size change showed poor correlation with time. It may be a sign that volume change may not occur gradually but right after the operation. Therefore, the retractor pressure against the left lobe might be a possible factor. However, in our patients with open gastrectomy, Nathanson retractor was not used, instead classical abdominal retractors were used in all operations. We did not find any focal parenchymal hypodensity or a focal subcapsular scar, which might represent a possible retractor injury. For these reasons, we believe that the atrophy was not due to retractor use. Unfortunately, we did not have certain data regarding the exact pressure, time, and location of the applied retractors.
Accessory or replaced left hepatic arteries might be found in 11%–20% of patients according to angiographic studies (16, 17). None of the patients in our patient group had a replaced left hepatic artery preoperatively and no hepatic vascular complication was reported in the operation reports. Postoperative CT exams demonstrated intact common and left hepatic arteries. On the other hand, accessory arteries <1 mm, which could not be visualized on preoperative CT or during the operation, might be ligated during the operation. These arteries were not reported in operation reports. These small arteries might support feeding of segments II and III.
It is well known that there is a variable network of venous collaterals between portal system and systemic venous circulation. Paraumbilical and parabiliary venous systems are important venous networks especially on the course of portal hypertension and portal vein thrombosis (18). These small accessory veins are remnants of embryologic mesenteries, connecting hepatic circulation with the venous circulation of pancreatico-duodenal venous arcade, right gastric vein and left gastric vein which contribute venous circulation of gastric antrum (18). These veins are less often apparent in the portal phase of a CT in normal conditions and generally are well visualized on the setting of portal hypertension or acute cholecystitis (19). It was suggested that this anomalous venous drainage could be the cause of focal steatosis in certain segments of the liver due to insulin rich blood supply via pancreatico-duodenal venous arcade (19–21). In either total or distal subtotal open gastrectomy, this venous plexus is generally ligated during the operation and this situation might cause a venous ischemia causing volume decrease of the liver segments as mentioned in our study. According to our results, volume change of the liver segments II and III has a poor correlation with time, which might be a sign that the volume loss is developing right after the operation rather than gradually during the follow-up of the patients.
Impaired feeding of the patients may also be a possible factor. Especially after gastrectomy along with chemotherapy, patients have trouble to feed and they lose weight (22). Although impaired feeding and losing weight may be a possible factor, isolated atrophy of segments II and III compared to the rest of the liver is still surprising. In our study, we did not find a difference between patients with liver atrophy and those with hepatomegaly and hepatosteatosis in terms of nutritional risk factor. The low correlation between the volume decrease and the time is also not supporting this possibility. Unfortunately, we do not have the data regarding the weight and chemotherapy regime of the patients. Nevertheless, we do not think that nutritional status along with the chemotherapy is the sole factor for this volume reduction.
There are some limitations to our study. First, it is a retrospective study. We believe a prospective study that investigates postoperative liver volumes at different time intervals with control of the patients’ weight and the chemotherapeutic regime would better enlighten the possible reasons of volume loss in segments II and III. Second, isolated volume measurements of segments II and III were different between the two observers due to indefinite borders of these segments. Although volume measurements were statistically different, the results of both observers showed high correlation and the volume reduction rates were similar in both observers’ measurements, indicating an actual volume loss in these segments.
In conclusion, we observed that in gastric cancer patients who had either total or distal subtotal open gastrectomy, the segments II and III of the liver showed significant volume loss compared with the rest of the liver and the total liver, postoperatively. The volume loss had poor correlation with time. This might be related to venous ischemia due to disruption of the accessory veins of pancreatico-duodenal venous arcade, right gastric vein, and left gastric vein, which contribute to venous circulation of the gastric antrum.
Main points.
The volume of the liver segments II and III decreased in many patients with gastric cancer after open gastrectomy.
In more than 80% of the patients, segments II and III became smaller after gastrectomy. Volume loss in segments II and III was almost four times more than that of the rest of the liver and three times more than that of the total liver.
Volume change in segments II and III showed poor correlation with time. It may be a sign that volume change may occur right after the operation. Surgical retractor pressure against the left lobe might be a possible factor.
Accessory veins of pancreatico-duodenal arcade, right gastric vein, and left gastric vein are generally ligated during gastrectomy; this might cause a venous ischemia causing the volume decrease of segments II and III.
Footnotes
Conflict of interest disclosure
The authors declared no conflicts of interest.
References
- 1.Stomach cancer. Estimated incidence, mortality and prevalence worldwide in 2012: International Agency for Research on Cancer, World Health Organization. [Accessed May 31, 2015]. Available at http://globocan.iarc.fr/old/FactSheets/cancers/stomach-new.asp.
- 2.Demir G, Unsal D, Zengin N, et al. Analysis of resected gastric cancer in Turkish population. Hepatogastroenterology. 2014;61:259–266. [PubMed] [Google Scholar]
- 3.Hallinan JT, Venkatesh SK. Gastric carcinoma: imaging diagnosis, staging and assessment of treatment response. Cancer Imaging. 2013;13:212–227. doi: 10.1102/1470-7330.2013.0023. http://dx.doi.org/10.1102/1470-7330.2013.0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jeong GA, Cho GS, Shin EJ, Lee MS, Kim HC, Song OP. Liver function alterations after laparoscopy-assisted gastrectomy for gastric cancer and its clinical significance. World J Gastroenterol. 2011;17:372–378. doi: 10.3748/wjg.v17.i3.372. http://dx.doi.org/10.3748/wjg.v17.i3.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yoon HM, Yang HK, Lee HJ, et al. Comparison of liver function after laparoscopically assisted and open distal gastrectomies for patients with liver disease. Surg Endosc. 2011;25:1761–1765. doi: 10.1007/s00464-010-1449-4. http://dx.doi.org/10.1007/s00464-010-1449-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lyall A, Ulaner GA. False-positive FDG PET/CT due to liver parenchymal injury caused by a surgical retractor. Clin Nucl Med. 2012;37:910–911. doi: 10.1097/RLU.0b013e31825b23c0. http://dx.doi.org/10.1097/RLU.0b013e31825b23c0. [DOI] [PubMed] [Google Scholar]
- 7.Tamhankar AP, Kelty CJ, Jacob G. Retraction-related liver lobe necrosis after laparoscopic gastric surgery. J Soc Laparoendosc Surg. 2011;15:117–121. doi: 10.4293/108680811X13022985131651. http://dx.doi.org/10.4293/108680811X13022985131651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yassa NA, Peters JH. CT of focal hepatic injury due to surgical retractor. Am J Roentgenol. 1996;166:599–602. doi: 10.2214/ajr.166.3.8623634. http://dx.doi.org/10.2214/ajr.166.3.8623634. [DOI] [PubMed] [Google Scholar]
- 9.Orr KE, Williams MP. MDCT of retractor-related hepatic injury following laparoscopic surgery: appearances, incidence, and follow-up. Clin Radiol. 2014;69:606–610. doi: 10.1016/j.crad.2014.01.008. http://dx.doi.org/10.1016/j.crad.2014.01.008. [DOI] [PubMed] [Google Scholar]
- 10.Harikrishnan J, Jackson P, Patel R, Najmaldin A. Segmental liver atrophy: a complication of the Nathanson retractor. JSM Clin Case Rep. 2014;2:1012–1013. [Google Scholar]
- 11.Kondrup J, Rasmussen HH, Hamberg O, Stanga Z. Nutritional risk screening (NRS 2002): a new method based on an analysis of controlled clinical trials. Clin Nutr. 2003;22:321–336. doi: 10.1016/s0261-5614(02)00214-5. http://dx.doi.org/10.1016/S0261-5614(02)00214-5. [DOI] [PubMed] [Google Scholar]
- 12.Hermoye L, Laamari-Azjal I, Cao Z, et al. Liver segmentation in living liver transplant donors: comparison of semiautomatic and manual methods. Radiology. 2005;234:171–178. doi: 10.1148/radiol.2341031801. http://dx.doi.org/10.1148/radiol.2341031801. [DOI] [PubMed] [Google Scholar]
- 13.Suzuki K, Epstein ML, Kohlbrenner R, et al. Quantitative radiology: automated CT liver volumetry compared with interactive volumetry and manual volumetry. Am J Roentgenol. 2011;197:706–712. doi: 10.2214/AJR.10.5958. http://dx.doi.org/10.2214/AJR.10.5958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Couinaud C. Liver anatomy: portal (and suprahepatic) or biliary segmentation. Digest Surg. 1999;16:459–467. doi: 10.1159/000018770. http://dx.doi.org/10.1159/000018770. [DOI] [PubMed] [Google Scholar]
- 15.Kitagawa T, Iriyama K. Hepatic infarction as a complication of gastric cancer surgery: report of four cases. Surg Today. 1998;28:542–546. doi: 10.1007/s005950050180. http://dx.doi.org/10.1007/s005950050180. [DOI] [PubMed] [Google Scholar]
- 16.Ugurel MS, Battal B, Bozlar U, et al. Anatomical variations of hepatic arterial system, coeliac trunk and renal arteries: an analysis with multidetector CT angiography. Brit J Radiol. 2010;83:661–667. doi: 10.1259/bjr/21236482. http://dx.doi.org/10.1259/bjr/21236482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Covey AM, Brody LA, Maluccio MA, Getrajdman GI, Brown KT. Variant hepatic arterial anatomy revisited: digital subtraction angiography performed in 600 patients. Radiology. 2002;224:542–547. doi: 10.1148/radiol.2242011283. http://dx.doi.org/10.1148/radiol.2242011283. [DOI] [PubMed] [Google Scholar]
- 18.Breen DJ, Rutherford EE, Stedman B, Lee-Elliott C, Hacking CN. Intrahepatic arterioportal shunting and anomalous venous drainage: understanding the CT features in the liver. Eur Radiol. 2004;14:2249–2260. doi: 10.1007/s00330-004-2334-0. http://dx.doi.org/10.1007/s00330-004-2334-0. [DOI] [PubMed] [Google Scholar]
- 19.Unal E, Ozmen MN, Akata D, Karcaaltincaba M. Imaging of aberrant left gastric vein and associated pseudolesions of segments II and III of the liver and mimickers. Diagn Interv Radiol. 2015;21:105–110. doi: 10.5152/dir.2014.14360. http://dx.doi.org/10.5152/dir.2014.14360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nakayama T, Yoshimitsu K, Masuda K. Pseudolesion in segment IV of the liver with focal fatty deposition caused by the parabiliary venous drainage. Comput Med Imaging Graph. 2000;24:259–263. doi: 10.1016/s0895-6111(00)00021-5. http://dx.doi.org/10.1016/S0895-6111(00)00021-5. [DOI] [PubMed] [Google Scholar]
- 21.Battaglia DM, Wanless IR, Brady AP, Mackenzie RL. Intrahepatic sequestered segment of liver presenting as focal fatty change. Am J Gastroenterol. 1995;90:2238–2239. [PubMed] [Google Scholar]
- 22.Gavazzi C, Colatruglio S, Sironi A, Mazzaferro V, Miceli R. Importance of early nutritional screening in patients with gastric cancer. Brit J Nutr. 2011;106:1773–1778. doi: 10.1017/S0007114511002509. http://dx.doi.org/10.1017/S0007114511002509. [DOI] [PubMed] [Google Scholar]