Abstract
Advancement in microcatheter design and emergence of new embolic agents offer better results in endovascular treatment of brain arteriovenous malformations (AVMs). Precipitating hydrophobic injectable liquid (PHIL) (Microvention) is a newly introduced dimethyl sulfoxide-based embolic agent for endovascular use. Herein, we present three patients who underwent endovascular treatment of brain AVMs with PHIL, followed by surgical resection. Endovascular features and same-day surgical handling of the new embolic agent PHIL are presented along with histopathologic changes in the acute stage in brain AVMs are presented, and its major differences from Onyx. In our series, PHIL had moderate inflammatory reaction in the acute stage without any associated angionecrosis that is different than Onyx which cause mild inflammatory reaction with angionecrosis. Smallest vessel containing PHIL was 2.9 μm compared to 5 μm with Onyx, which suggests better penetration.
Treatment of brain arteriovenous malformations (AVMs) includes endovascular embolization, surgical resection, and stereotactic radiotherapy, alone or in combination (1). In the past, n-butyl cyanoacrylate (nBCA) was used as an adjunct to surgery; however, introduction of a dimethyl sulfoxide (DMSO)-based embolic agent, Onyx (ev3, Covidien), and advancement in microcatheter design offered better endovascular results in brain AVM treatment (2). Recently, a new DMSO-based embolic agent, precipitating hydrophobic injectable liquid (PHIL) (Microvention) has been introduced for endovascular use. PHIL is a nonadhesive co-polymer (polylactide-co-glycolide and polyhydroxyethylmethacrylate)-based liquid embolic material suspended in DMSO. An iodine (triiodophenol) component is bound to co-polymer for radiopacity. Possible advantages of PHIL are its ease of use, faster plug formation, and less computed tomography (CT) and magnetic resonance imaging (MRI) artifact during imaging follow up. PHIL was selected as the embolic agent in view of its potential advantages (3, 4). Three patients with brain AVMs had partial embolization with PHIL followed by same-day surgical resection in our institution. Here, we present our preliminary experience with PHIL in brain AVMs from an endovascular, surgical, and pathologic perspective, and outline its major differences from Onyx.
Cases
In our institution, nine cases with AVM and arteriovenous fistulae underwent PHIL embolization after obtaining informed patient consent, between August 2014 and May 2015. Herein, we focused on three of six brain AVMs who had partial embolization and same-day surgical nidus resection. Clinical, endovascular, surgical, and pathologic data were retrospectively collected and analyzed.
Case 1
A 20-year-old female patient presented with seizure and an unruptured AVM (Spetzler-Martin Grade 3) located in the right temporal lobe. Main arterial feeders were temporal branches of the right middle cerebral artery. AVM had a mixed fistulous and plexiform nidus with cortico-subcortical location. Nidus size was 4×2.5 cm. AVM had both superficial and deep venous drainage, with ectasia in veins and venous aneurysm (Fig. 1).
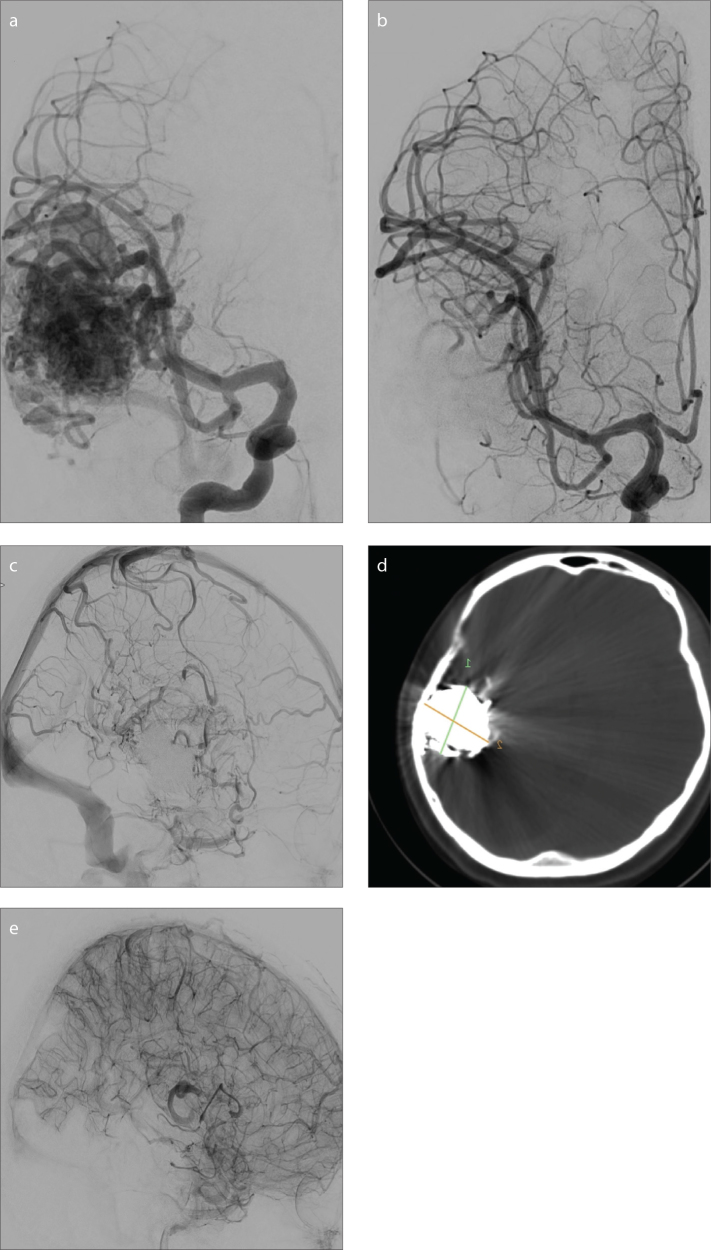
Figure 1.
a–e. A 20-year-old female with an unruptured temporal arteriovenous malformation (AVM). Anteroposterior (AP) subtracted-digital subtraction angiography (DSA) (a) shows the AVM fed by the middle cerebral artery. PHIL and Onyx were used as embolic agents. Postembolization early arterial AP subtracted-DSA (b) shows almost total obliteration; however, minimal residual nidus filling and stagnation of perinidal vascular structures were noted in venous phase (c). Coronal XperCT (d) shows PHIL and Onyx embolization of the AVM with notable artifact on CT due to Onyx. Immediately after surgery, lateral subtracted-DSA (e) shows total nidus removal without stagnated vascular structures.
Case 2
A 33-year-old female patient presented with vertigo and an unruptured AVM (Spetzler-Martin Grade 2) located in the right parieto-occipital sulcus. Main feeder was the parietal branch of the right middle cerebral artery with a partial supply by parietal branches of the right posterior cerebral artery, additionally enlargement in arterial feeders was noted. AVM had a mixed fistulous and plexiform nidus located sulcally. Nidus size was 2×2.5 cm. AVM had a single superficial venous drainage, with no ectasia in veins (Fig. 2).
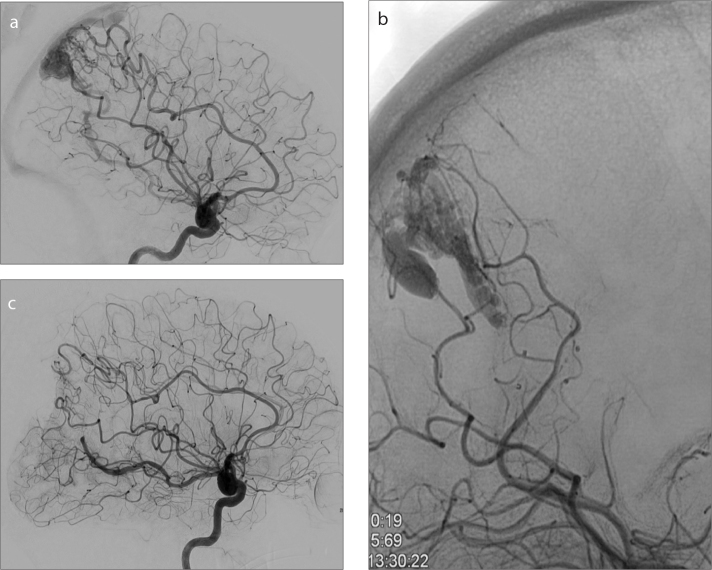
Figure 2.
a–c. A 33-year-old female with an unruptured parieto-occipital AVM. Lateral subtracted-DSA (a) shows the AVM fed mainly by the middle cerebral artery with a partial supply by the posterior cerebral artery. Postembolization AP plain radiography (b) shows partial PHIL embolization of AVM. Postsurgery lateral subtracted-DSA (c) shows total nidus removal.
Case 3
A five-year-old male patient presented with nausea, vomiting, and temporary loss of consciousness due to a ruptured AVM (Spetzler-Martin Grade 3) located in the right cerebellar hemisphere. Main feeders were the right posterior inferior cerebellar artery and the right superior cerebellar artery, with enlargement in arterial feeders. AVM had a mixed fistulous and plexiform nidus with cortico-subcortical location. Nidus size was 3.5×2.5 cm. AVM had a superficial venous drainage, with partially thrombosed aneurysm in drainage vein (Fig. 3).
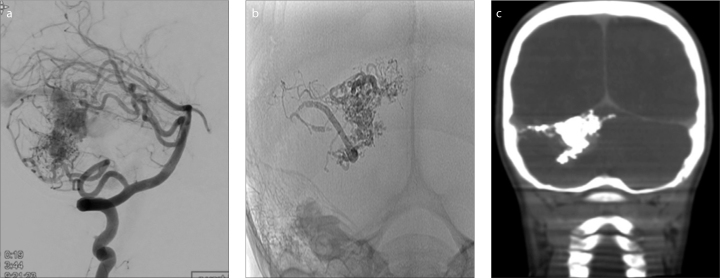
Figure 3.
a–c. A five-year-old male with a ruptured cerebellar AVM. Lateral subtracted-DSA (a) prior to embolization shows the AVM fed by posterior inferior cerebellar artery and superior cerebellar artery (SCA) branches. Postembolization AP plain radiography (b) and coronal XperCT (c) show partial PHIL embolization of AVM via SCA branches with minimal artifact noted on CT.
Technique
Embolic agents
Onyx is a nonadhesive liquid embolic agent consisting of 48 mol/L ethylene and 52 mol/L ethylene vinyl alcohol, dissolved in DMSO and mixed with tantalum powder for radiopacity. Onyx is available in ready-to-use vials of 1.5 mL at three different viscosities, Onyx 18, 20, and 34 (concentrations of ethylene vinyl alcohol: 6%, 6.5%, and 8%; respectively). The vials are shaken for at least 20 minutes for a homogeneous solution (5).
PHIL is a nonadhesive co-polymer (polylactide-co-glycolide and polyhydroxyethylmethacrylate)-based liquid embolic material suspended in DMSO. It has iodine (triiodophenol) component bound to a co-polymer for radiopacity. It comes as ready to use pre-filled syringes of 1 mL at three different concentrations; PHIL 25%, 30%, and 35% (viscosity in centistokes 16, 36, and 72, respectively) and syringes pre-filled with 1 mL DMSO. Embolic capacity of 25%, 30% and 35% PHIL is 0.85, 0.87, and 0.94 mL, respectively, per 1 mL of PHIL. Higher concentrations are preferred in the presence of a fistulous component, whereas lower concentrations are used to achieve distal access.
Endovascular treatment
In our institution, the main strategy for AVM treatment is to achieve complete nidus obliteration by endovascular route alone or in combination with same-day total microsurgical resection in incompletely embolized AVMs, if surgically accessible. Diencephalic and brain stem AVMs are outside of this strategy.
Endovascular treatment was performed under general anesthesia using a biplane flat panel digital subtraction unit (Allura Xper 20/20, Philips Healthcare). During endovascular treatments, feeding arteries were catheterized with DMSO-compatible detachable Apollo (Covidien; in cases 1, 2, and 3) and nondetachable Marathon (Covidien; in c) tip microcatheters. After proper positioning of microcatheter, its dead space was filled with DMSO. PHIL 25% was used as the embolizing agent in all three cases. After plug formation, the slow injection style used for PHIL was not different from injection of Onyx used in AVM treatment (2). In case 1, Onyx was injected into the nidus through the same microcatheter after PHIL injections. Immediately after embolization, routine Xper CT was performed to rule out possible intracranial complications. All three cases underwent same day surgery because of incomplete nidus occlusion by endovascular route.
During endovascular treatment, features of PHIL such as visibility, ease of plug formation, tendency to backflow, and behavior in arterial and venous sides were noted.
Surgical resection
After partial embolization, the patients were transferred to neurosurgery operating room under general anesthesia for total nidus resection. During surgical resection, the form PHIL takes within the vessel, its pliability for resection, the degree of blood loss and compressibility of nidus were noted. In case 1, differences between PHIL and Onyx embolized parts of the nidus were also noted.
Histopathologic examination
Partially embolized and surgically resected AVM nidi were sent to the pathology lab within 4–7 hours after embolization. Specimens were fixed in 10% buffered formalin and embedded in paraffin. Serial 2–3 μm sections were obtained and stained with hematoxylin-eosin and elastica van Gieson stain prior to review.
Specimens were evaluated for vascular and perivascular inflammation, mural angionecrosis and parenchymal hemorrhage. In order to evaluate the degree of embolic agent penetration, the smallest vessel that contained the embolic agent was identified and measured. Extravasation of the embolic agent and foreign body giant cells in the embolic cast were investigated.
Results
Partial AVM embolization using PHIL alone or in combination with Onyx was performed successfully in all three cases. Nidus obliteration rates were 90%, 80%, and 70% in cases 1, 2, and 3, respectively. Endovascular embolization was terminated in case 2, because of a small distal artery perforation with resultant subarachnoid hemorrhage during microcatheterization of an arterial feeder.
Microsurgical nidus resections were performed successfully in all three cases without any complications. During surgery, PHIL was noted within the vessels as a white chalky material that was less pliable compared to Onyx (Fig. 4).
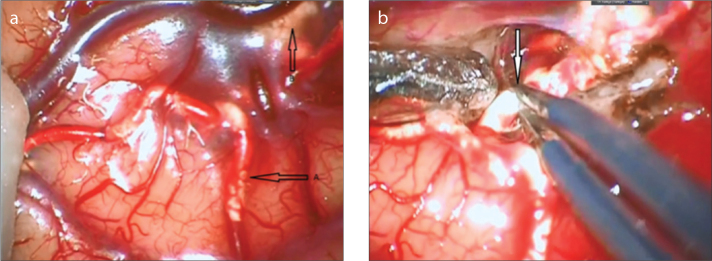
Figure 4.
a, b. Intraoperative image of PHIL. Vessel filled with the embolic agent PHIL (a, arrows) forms a rough intravascular column. Extravasation of PHIL (b, arrow) is also seen under arachnoid mater. Dissection of PHIL-filled vessel was easy, similar to Onyx (a). Meticulous handling of the vessel with low voltage bipolar cauterization is required to maintain vessel wall integrity (b).
On day 1, all cases had control digital subtraction angiography. Case 1 and 2 had total nidus resection. Partial filling was found in an unresected area of the nidus in case 3. On discharge, case 1 and 2 had modified Rankin scores of 0, and case 3 had modified Rankin score of 1.
Histopathologic exam of the surgical specimens from PHIL-embolized cases (cases 2 and 3) revealed moderate amount of vascular and acute perivascular inflammation without any evidence of chronic inflammatory response (Fig. 5). Angionecrosis was not seen in PHIL-filled vessels.
Figure 5.
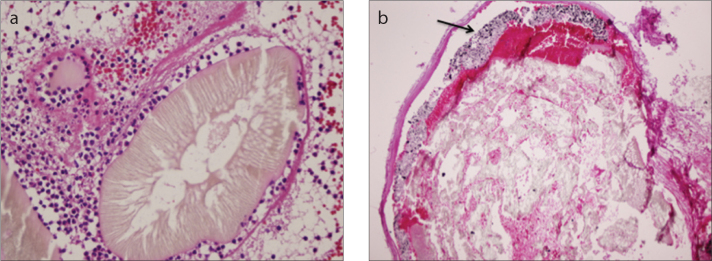
a, b. Photomicrograph (a) shows endovascular PHIL material with polymorpho nuclear leukocytes (hematoxylin eosin staining [HE], ×400). Onyx (b, arrow) is layering over the PHIL inside the vessel (HE, ×200).
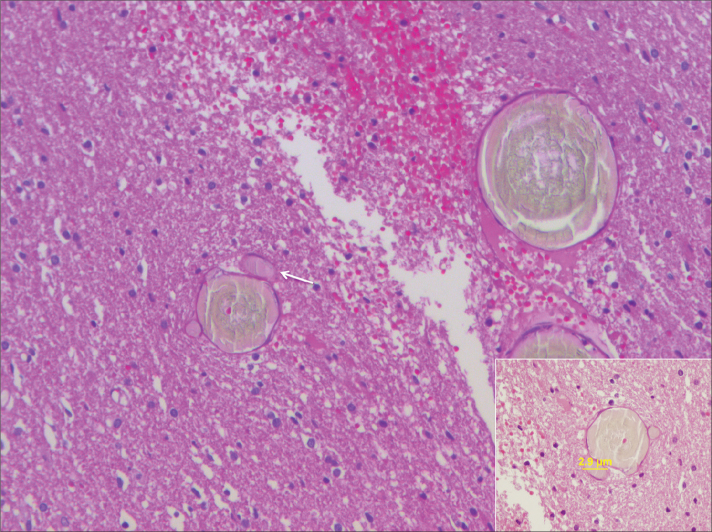
On microscopic examination, lumina of the embolized vessels appeared as hyaline eosinophilic material admixed with yellowish material that had linear and perpendicular configuration at the edge of the vessels (Fig. 5). Some parts of the embolized vessels were completely filled with the embolic material, whereas in other parts the embolic material filled a small portion of the vessel lumen, with thrombus filling the remainder of the lumen. Blood vessels ranging from 2.9 μm to 5 mm in diameter were filled with PHIL (Fig. 6).
Figure 6.
Photomicrograph showing PHIL as a hyaline eosinophilic material in lumina of embolized vessels (HE, ×200). Smallest vessel occluded (arrow) by PHIL was 2.9 μm in our specimens (inset, HE, ×400).
Foreign body giant cells were not observed and the vessel wall integrity was maintained in all embolized vessels. No evidence of perivascular extravasation of embolic material was observed in any specimen. All specimens showed evidence of hemorrhage in the surrounding perinidus brain parenchyma.
PHIL and Onyx were both used as embolic agents in case 1 (Fig. 5). Histopathologic exam showed moderate amount of vascular inflammation in PHIL embolized vessels and less vascular inflammation in Onyx-filled vessels compared with PHIL. No sign of angionecrosis was seen in PHIL embolized vessels, whereas signs of angionecrosis was evident in Onyx-filled vessels. Smallest vessels containing PHIL and Onyx were 50 μm and 32 μm, respectively.
Discussion
PHIL is a newly introduced DMSO based embolic agent that is gaining wider use in AVM treatment. In this paper, we share our preliminary radiologic and surgical experience in same-day combined treatment of AVMs, acute stage histopathologic findings of PHIL, and its major differences from Onyx.
PHIL comes as ready to use pre-filled syringes of 1 mL and does not require shaking prior to injection. Onyx is shaken for 20 minutes for a homogenous solution that requires anticipation of estimated time of usage (6).
Both PHIL and Onyx are DMSO-based liquid embolic agents that require DMSO-compatible microcatheters. As shown in case 1, Onyx can be delivered through the same catheter after PHIL injection, which offers the advantage of switch between agents during endovascular therapy without injecting additional DMSO.
PHIL has limited visibility during passage through the microcatheter (especially PHIL 25%). After passage through the tip of microcatheter, PHIL has a lower visibility than Onyx. PHIL is bound to iodine for radiopacity and does not lose its homogeneity during prolonged injections, which offers the advantage of consistent visibility. Onyx uses tantalum for radiopacity, which can cause inhomogeneity during prolonged injections and decrease visibility. Onyx also suffers from self-hiding effect when used in large amounts due to dense radiopaque saturation (7).
Plug formation with PHIL is faster because of less layering effect compared to Onyx, which may offer decreased duration of embolization and fluoroscopy time (2, 5, 6). Backflow into arterial side is slower with PHIL, which may limit unintended proximal arterial embolization and decrease the amount of embolic agent used.
PHIL has a column effect when it reaches the venous side, which decreases forward flow in the venous side. Decreased forward flow in venous side limits venous obliteration, which may decrease the rate of postembolization hemorrhage due to venous outflow obstruction (8).
Onyx causes CT beam hardening artifact and intense artifact in gradient recalled-echo (GRE) and susceptibility-weighted imaging (SWI) sequences which limit evaluation of perinidus parenchyma during post-treatment follow up (9, 10). PHIL has minimal CT beam hardening artifact and no artifact in GRE and SWI.
Onyx and PHIL have different gross appearances inside the embolized vessels; Onyx is dark gray, which limits its use in vascular malformations in the face due to tattoo effect. PHIL has a white color, which may offer aesthetic advantage for use in vascular malformations in the face (11).
Grossly, Onyx forms a continuous smooth surfaced gray-dark column within vessels, whereas PHIL forms a rough-surfaced whitish column interspersed by blood clots. During surgical manipulation, Onyx feels as a rubbery, pliable material, whereas PHIL is stiff and brittle (12, 13). When PHIL-filled vessel is cauterized with bipolar cautery, crunching and breaking of the intravascular column can be noted (Fig. 4). This feature of PHIL is important when handling small vessels near the distal end of PHIL column, especially when working under the nidus. However, these differences do not affect the overall surgery, and material properties are easily handled during the first few minutes of dissection.
All patients underwent embolization with PHIL followed by same-day surgical resection. PHIL was the sole embolization material in cases 2 and 3, while Onyx and PHIL were both used in case 1.
Review of the literature shows limited experience with same-day embolization and surgical resection in brain AVMs. In a case of Onyx embolization with same-day surgical resection, no evidence of associated inflammatory infiltrate was noted. In four brain AVMs surgically resected one day after embolization, mild acute inflammatory reaction was seen in two cases (14, 15). We observed moderate amount of vascular inflammation in cases 2 and 3, who were embolized with PHIL only. In case 1, parts of the nidus embolized with Onyx had less vascular inflammatory reaction compared with PHIL-embolized parts.
No evidence of chronic inflammatory response and foreign body giant cells were observed in our cases since same-day surgical nidus resection was performed.
Angionecrosis was not observed in embolization with PHIL; however, in case 1, where PHIL injection was followed by Onyx injection, angionecrosis was seen in Onyx filled vessels. Angionecrosis rate of Onyx was reported as 30.2% after an average duration of 18 days (15). Jahan et al. (14) reported angionecrosis in two of four specimens resected one day after embolization. The most likely cause of angionecrosis after Onyx embolization is the solvent DMSO (16, 17). Angionecrosis was absent in cases 2 and 3, who were embolized by PHIL only (even though PHIL is also a DMSO-based agent) and was only present in case 1, who was embolized with Onyx in addition to PHIL.
Smallest vessel occluded by PHIL was 2.9 μm in our specimens (Fig. 6). Natarjan et al. (15) reported the smallest vessel occluded by Onyx to be 5 μm with an average diameter of 81.4±103.8 μm. Jahan et al. (14) reported blood vessels ranging from 80 μm to 1 mm in diameter filled with the embolic material.
In conclusion, we acknowledge the limited patient number in this case series. Main possible advantages of PHIL compared with the widely used Onyx from an endovascular perspective are its ease of use, faster plug formation, and less CT and MRI artifact during follow up. Although PHIL has decreased visibility, it has constant homogeneity. From a surgical and histopathologic perspective, in comparison with Onyx, PHIL is brittle during vessel dissection and less pliable during surgery, and causes moderate vascular inflammatory effect and no angionecrosis on same-day resected specimens. Histopathologic analysis showed a higher degree of penetration with PHIL than with Onyx. This may be an advantage during the embolization of complex malformations, but may also be an a disadvantage due to small perforator occlusion. Larger prospective multicenter series are needed to confirm the safety and effectiveness of this new embolic material.
Main points.
Onyx is a widely used nonadhesive liquid DMSO-based embolic agent mixed with tantalum powder for radiopacity.
Precipitating hydrophobic injectable liquid (PHIL) is a newly introduced nonadhesive DMSO-based liquid embolic agent with an iodine component for radiopacity.
Main advantages of PHIL compared to Onyx are its ease of use, faster plug formation, and less CT and MRI artifact at follow-up imaging.
PHIL is more brittle during vessel dissection and less pliable during surgery in comparison to Onyx.
PHIL causes moderate vascular inflammatory effect and no angionecrosis on same-day resected specimens.
Footnotes
Conflict of interest disclosure
N.K. has a proctoring and consulting agreement with MicroVention, Inc. and proctoring agreement Covidien, Inc. C.I. has a proctoring agreement with MicroVention, Inc. and Covidien, Inc. The other authors have no conflict to report.
References
- 1.Barr JC, Ogilvy CS. Selection of treatment modalities or observation of arteriovenous malformations. Neurosurg Clin N Am. 2012;23:63–75. doi: 10.1016/j.nec.2011.09.010. http://dx.doi.org/10.1016/j.nec.2011.09.010. [DOI] [PubMed] [Google Scholar]
- 2.Saatci I, Geyik S, Yavuz K, Cekirge HS. Endovascular treatment of brain arteriovenous malformations with prolonged intranidal Onyx injection technique: long-term results in 350 consecutive patients with completed endovascular treatment course. J Neurosurg. 2011;115:78–88. doi: 10.3171/2011.2.JNS09830. http://dx.doi.org/10.3171/2011.2.JNS09830. [DOI] [PubMed] [Google Scholar]
- 3.Leyon JJ, Chavda S, Thomas A, Lamin S. Preliminary experience with the liquid embolic material agent PHIL (Precipitating Hydrophobic Injectable Liquid) in treating cranial and spinal dural arteriovenous fistulas: technical note. J Neurointerv Surg. 2015 doi: 10.1136/neurintsurg-2015-011684. [Epub ahead of print] http://dx.doi.org/10.1136/neurintsurg-2015-011684. [DOI] [PubMed] [Google Scholar]
- 4.Gentric JC, Raymond J, Batista A, Salazkin I, Gevry G, Darsaut TE. Dual-lumen balloon catheters may improve liquid embolization of vascular malformations: an experimental study in Swine. AJNR Am J Neuroradiol. 2015;36:977–981. doi: 10.3174/ajnr.A4211. http://dx.doi.org/10.3174/ajnr.A4211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Rooij WJ, Jacobs S, Sluzewski M, van der Pol B, Beute GN, Sprengers ME. Curative embolization of brain arteriovenous malformations with onyx: patient selection, embolization technique, and results. AJNR Am J Neuroradiol. 2012;33:1299–1304. doi: 10.3174/ajnr.A2947. http://dx.doi.org/10.3174/ajnr.A2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Panagiotopoulos V, Gizewski E, Asgari S, Regel J, Forsting M, Wanke I. Embolization of intracranial arteriovenous malformations with ethylene-vinyl alcohol copolymer (Onyx) AJNR Am J Neuroradiol. 2009;30:99–106. doi: 10.3174/ajnr.A1314. http://dx.doi.org/10.3174/ajnr.A1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Katsaridis V, Papagiannaki C, Aimar E. Curative embolization of cerebral arteriovenous malformations (AVMs) with Onyx in 101 patients. Neuroradiology. 2008;50:589–597. doi: 10.1007/s00234-008-0382-x. http://dx.doi.org/10.1007/s00234-008-0382-x. [DOI] [PubMed] [Google Scholar]
- 8.Rangel-Castilla L, Spetzler RF, Nakaji P. Normal perfusion pressure breakthrough theory: a reappraisal after 35 years. Neurosurg Rev. 2014;38:399–405. doi: 10.1007/s10143-014-0600-4. http://dx.doi.org/10.1007/s10143-014-0600-4. [DOI] [PubMed] [Google Scholar]
- 9.Pierot L, Kadziolka K, Litre F, Rousseaux P. Combined treatment of brain AVMs with use of Onyx embolization followed by radiosurgery. AJNR Am J Neuroradiol. 2013;34:1395–1400. doi: 10.3174/ajnr.A3409. http://dx.doi.org/10.3174/ajnr.A3409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saatci I, Cekirge HS, Ciceri EF, Mawad ME, Pamuk AG, Besim A. CT and MR imaging findings and their implications in the follow-up of patients with intracranial aneurysms treated with endosaccular occlusion with onyx. AJNR Am J Neuroradiol. 2003;24:567–578. [PMC free article] [PubMed] [Google Scholar]
- 11.Arat A, Cil BE, Vargel I, et al. Embolization of high-flow craniofacial vascular malformations with onyx. AJNR Am J Neuroradiol. 2007;28:1409–1414. doi: 10.3174/ajnr.A0547. http://dx.doi.org/10.3174/ajnr.A0547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Akin ED, Perkins E, Ross IB. Surgical handling characteristics of an ethylene vinyl alcohol copolymer compared with N-butyl cyanoacrylate used for embolization of vessels in an arteriovenous malformation resection model in swine. J Neurosurg. 2003;98:366–370. doi: 10.3171/jns.2003.98.2.0366. http://dx.doi.org/10.3171/jns.2003.98.2.0366. [DOI] [PubMed] [Google Scholar]
- 13.Hamada J, Kai Y, Morioka M, et al. A nonadhesive liquid embolic agent composed of ethylene vinyl alcohol copolymer and ethanol mixture for the treatment of cerebral arteriovenous malformations: experimental study. J Neurosurg. 2002;97:889–895. doi: 10.3171/jns.2002.97.4.0889. http://dx.doi.org/10.3171/jns.2002.97.4.0889. [DOI] [PubMed] [Google Scholar]
- 14.Jahan R, Murayama Y, Gobin YP, Duckwiler GR, Vinters HV, Vinuela F. Embolization of arteriovenous malformations with Onyx: clinicopathological experience in 23 patients. Neurosurgery. 2001;48:984–995. doi: 10.1097/00006123-200105000-00003. http://dx.doi.org/10.1097/00006123-200105000-00003. [DOI] [PubMed] [Google Scholar]
- 15.Natarajan SK, Born D, Ghodke B, Britz GW, Sekhar LN. Histopathological changes in brain arteriovenous malformations after embolization using Onyx or N-butyl cyanoacrylate. Laboratory investigation. J Neurosurg. 2009;111:105–113. doi: 10.3171/2008.12.JNS08441. http://dx.doi.org/10.3171/2008.12.JNS08441. [DOI] [PubMed] [Google Scholar]
- 16.Chaloupka JC, Vinuela F, Vinters HV, Robert J. Technical feasibility and histopathologic studies of ethylene vinyl copolymer (EVAL) using a swine endovascular embolization model. AJNR Am J Neuroradiol. 1994;15:1107–1115. [PMC free article] [PubMed] [Google Scholar]
- 17.Murayama Y, Vinuela F, Ulhoa A, et al. Nonadhesive liquid embolic agent for cerebral arteriovenous malformations: preliminary histopathological studies in swine rete mirabile. Neurosurgery. 1998;43:1164–1175. doi: 10.1097/00006123-199811000-00081. http://dx.doi.org/10.1097/00006123-199811000-00081. [DOI] [PubMed] [Google Scholar]