Abstract
Background
Decitabine may open the chromatin structure of leukemia cells making them accessible to the calicheamicin epitope of gemtuzumab ozogamicin (GO).
Methods
110 patients (median age 70 years; range 27–89 years) were treated with decitabine and GO in a trial designed on model based futility to accommodate subject heterogeneity: Group 1: relapsed/refractory AML with complete remission duration (CRD) < 1 year (N=28, 25%); Group 2: relapsed/refractory AML with CRD ≥1 year (N=5, 5%); Group 3: untreated AML unfit for intensive chemotherapy or untreated MDS or untreated MF (N=57, 52%); and Group 4: AML evolving from MDS or relapsed/refractory MDS or MF (N=20, 18%). Treatment consisted of decitabine 20mg/m2 daily for 5 days and GO 3 mg/m2 on day 5. Post-induction therapy included 5 cycles of decitabine+GO followed by decitabine alone.
Results
CR/CRi was achieved in 39 (35%) patients; Group 1= 5/28 (17%), Group 2 = 3/5 (60%), Group 3 = 24/57 (42%), and Group 4 = 7/20 (35%). The 8-week mortality in Groups 3 and 4 was 16% and 10%, respectively. Common drug-related adverse events included nausea, mucositis and hemorrhage.
Conclusion
Decitabine and GO improved the response rate but not OS compared to historical outcomes in untreated AML ≥60 years.
Keywords: Decitabine, gemtuzumab ozogamicin, acute myeloid leukemia, MDS
INTRODUCTON
Elderly patients (≥ 60–65 years) with AML have a poor prognosis attributable to having disease inherently more resistant to current standard cytotoxic agents and/or relatively poor tolerance of these agents(1–3). Elderly patients with AML also more frequently have an antecedent hematological disorder, unfavorable cytogenetics, and poorer performance status at presentation(2, 4). Among younger patients (<65 years of age), traditional induction chemotherapy (e.g., anthracycline and cytarabine) produces complete remission (CR) in approximately 50–75% of patients (5). Unfortunately, a significant number of younger patients with AML (especially those with adverse cytogenetic features, adverse molecular mutations or antecedent hematological disorder) will be refractory to induction therapy or relapse after initial response to induction therapy. The outcomes of these patients are dismal, with low response rates and poor long-term survival with salvage therapy(6–8). The development of novel agents and/or combinations for these patient groups is warranted.
Antibody drug conjugate strategies envisage one such novel approach. CD33 is expressed by myeloid blast cells in > 80% of patients with AML suggesting that antibodies to CD33 may have specific therapeutic benefit in the treatment of AML(9, 10). Gemtuzumab ozogamycin (Mylotarg®) (GO) is a humanized anti-CD33 monoclonal antibody covalently linked to a semisynthetic derivative of a potent toxin, calicheamicin(11, 12). GO has been used in combination with induction therapy in AML with improved outcomes(11, 13–17) particularly among elderly patients and those with intermediate or good-risk cytogenetics. Decitabine (Dacogen®, 5-aza-2’-deoxycytidine) exerts its antineoplastic activity by direct incorporation into DNA with subsequent inhibition of DNA methyltransferase (DNMT)(18). DNA hypomethylation from DNMT inhibition results in re-expression of tumor suppressor genes(19, 20). In a phase III study in elderly patients (≥ 60 years) with previously untreated AML, decitabine resulted in a response rate (CR/CRp) of 18%(21).
In primary AML samples, response to GO depends on Syk expression(22). GO binds to CD33 resulting in the phosphorylation of Syk, a protein kinase that docks to the intra-cytoplasmic tail of CD33. Syk then complexes with SHP-1, a protein phosphatase(22). Activated Syk in this context acts as a tumor suppressor in hematopoietic and solid tumors. DNA hypermethylation can silence Syk expression thereby abrogating the anti-proliferative effect of GO on AML cells(23, 24). Hypomethylating agents may restore Syk expression, consequently re-establishing sensitivity of AML cells to GO. Prior exposure of AML cells to hypomethylating agent such as decitabine sensitize them to GO by reducing the expression of multi-drug resistance protein-1 (MRP-1) or by enhancing DNA intercalation by calicheamicin(25). Nand et al evaluated a combination of 5-azacitidine and GO in 133 elderly patients with newly diagnosed MDS and AML(26). They divided patients into good risk (N=79) and poor risk (N=54) groups. The good-risk group included patients who were 60–69 years or had a performance status of 0 or 1; the poor-risk group included patients who were ≥ 70 years or had a performance status of 2 or 3. Responses (CR/CRi) were seen in 44% and 35% of the good risk and poor risk patients, respectively. Median overall survival (OS) was 11 months in both risk groups. Early mortality was noted in 8% of the good risk and 13% of the poor risk patients. While these studies were ongoing, we evaluated the combination of decitabine and GO in newly diagnosed and relapsed AML and high-risk MDS patients treated at our center. Herein, the results are presented.
METHODS
Patient eligibility
Eligibility criteria included patients ≥ 18 years of age with a diagnosis of AML (other than acute promyelocytic leukemia) with refractory/relapsed disease and/or patients with newly diagnosed AML not a candidate for intensive chemotherapy in the opinion of the treating physician; previously treated, relapsed, refractory, or newly diagnosed, high-risk MDS (intermediate-2 or high by the International Prognostic Scoring System [IPSS] or ≥10% blasts)(27); and previously treated, relapsed, refractory, or newly diagnosed, with intermediate- or high-risk myelofibrosis (MF) (i.e., score ≥ 1 by the Lille scoring system)(28) or MF with symptomatic splenomegaly. Other inclusion criteria were: Eastern Cooperative Oncology Group (ECOG) performance status (PS) ≤ 3; serum creatinine ≤ 2.0 mg/dL; serum bilirubin ≤ 2.0 mg/dL; serum transaminase ≤ 2.5 times the upper limit of the normal range or or ≤ 5 times the upper limit of the normal range if the transaminase elevation was deemed related to the underlying disease. This was a single center, open-label, non-randomized study. All patients signed an informed consent form approved by the University of Texas/M. D. Anderson Cancer Center (UT/MDACC) Institutional Review Board. The study was conducted in accordance with the Declaration of Helsinki. ClinicalTrials.gov identifier: NCT00882102.
Treatment Regimen
The induction regimen included 5 days of decitabine at 20 mg/m2 given intravenously (IV) over 60 to 90 minutes. The day 5 decitabine dose was followed by GO at 3 mg/m2 given IV for one dose. Patients underwent a bone marrow aspiration on day 14 +/− 3 days. Patient’s whose day 14 bone marrow showed ≥ 20% cellularity with ≥ 5% blasts received an additional course of decitabine 20 mg/m2 IV daily for 5 days starting on day 15. Patients with response or with no obvious progression could receive post-induction therapy with up to 5 additional cycles of decitabine and GO as during induction, without the day 15 decitabine. Post-induction cycles were repeated every 4 to 8 weeks, depending on the recovery of neutrophil and platelet counts and toxicity. Patients who maintained clinically relevant response (CR or CRi) at the end of post-induction therapy could receive maintenance therapy with decitabine alone every 4–8 weeks for a total of up to 24 cycles of therapy.
Response Criteria and Definitions
Responses were according to established criteria(29). CR was defined by the presence of 5% blasts or less in the bone marrow, with greater than 1.0 × 109/L neutrophils and greater than 100 × 109/L platelets in the peripheral blood. CRi was as for CR, but for residual neutropenia (<1.0 × 109/L) or thrombocytopenia (<100 × 109/L)(30).
Statistical Design
The primary objective of this single-arm phase II study was to assess whether the combination of decitabine and GO could increase the CR rate by 10–15% as compared to historical response rates in similar patients treated at our center. Secondary objectives were to assess the overall survival (OS) and complete remission duration (CRD). Considering the heterogeneity in historical outcomes among patients based on the underlying disease, duration of prior remission and on whether they had new or relapsed disease, patients were up-front classified into four groups: Group 1: relapsed/refractory AML with CRD < 1 year; Group 2: relapsed/refractory AML with CRD ≥ 1 year; Group 3: untreated AML unfit for intensive chemotherapy or untreated MDS or untreated MF; and Group 4: AML evolving from MDS or relapsed/refractory MDS or MF. To account for the heterogeneity of expected remission rates in each group, a model-based Bayesian design(31) with interim stopping rules for futility was implemented. The flexibility of design allows for specific decisions to be made for each group such that enrollment in some groups may be stopped early due to futility while other groups with better remission rates continue to enroll patients. Specifically, for our study, if, within a given group, it was unlikely that decitabine in combination with GO would increase the CR rate by 10– 15% when compared to historical treatment, accrual to that group was to be terminated. The priors for group effects were calibrated to reflect a historical response rate of 15%, 50%, 44% and 28%, respectively, in Groups 1 through 4.
Data analysis
Unadjusted probabilities of OS and CRD were estimated using the method of Kaplan and Meier(32). A Bayesian logistic regression model was fit to assess the decitabine combined with GO treatment effect on CR rate within each group of patients, which included group indicators, treatment indicator within each group, as well as relevant clinical characteristics, such as age, white blood count, platelet count, bone marrow blast percentage, karyotype and ECOG PS. The priors for the group-specific decitabine combined with GO treatment effect were calibrated to reflect a similar response rate as historical controls within each group, but with much larger variability (i.e., non-informative priors for the experimental treatment effect). Similarly for OS, a Bayesian Weibull regression model was fit for each endpoint, with non-informative priors for all the model parameters as well as the scale parameter for Weibull distribution. All statistical analyses were carried out in Splus 8.2 and WinBugs1.4.
RESULTS
Study Group
A total of 110 patients were treated. Their characteristics are shown in table 1. The median age for all patients was 70 years (range, 27–89 years). Eighty-four patients had AML including 16 patients with preexisting MDS and 4 patients with preexisting myelofibrosis, 22 patients had high-risk MDS including chronic myelomonocytic leukemia in 6 patients and MDS/MPN-unclassified in 1 patient, and 4 patients had MF. Forty-four patients (40%) had high-risk cytogenetics and 11 of 95 tested patients (12%) were FLT3 mutated (including ITD and D835). Patients were enrolled into the four predefined groups as follows: Group 1 = 28 patients (25%) [age, 62 years (range, 26–83)]; Group 2 = 5 patients (5%) [age, 83 years (range, 64–88)]; Group 3 = 57 patients (52%) [age, 70 years (range, 42–87)] ; and Group 4 = 20 patients (18%) [age, 70 years (range, 32–82)].
Table 1.
Patient characteristics
| N=110 | |
|---|---|
| Characteristic | N (%)/ Median [Range] |
| Age | 70 [27–89] |
| Males | 63 (57) |
| Diagnosis at enrollment | |
| AML [De-novo=64, Preexisting MDS=20] | 84 (76) |
| MDS [MDS=15, CMML=6, MDS/MPN-U=1] | 22 (20) |
| MF | 4 (4) |
| ECOG performance status | 1 [0–3] |
| White Blood Cell Count × 109/L | 2.5 [0.3–121.9] |
| Hemoglobin, g/dL | 9.3 [6.9–31.9] |
| Platelets × 109/L | 37 [3–816] |
| Percentage of bone marrow blasts | 32 [0–96] |
| Cytogenetics | |
| High-risk cytogenetics | 44(40) |
| Non high-risk cytogenetics | 57(52) |
| FLT3 mutations (95 evaluated patients) | |
| FLT3 mutated | 11(10) |
| FLT3 non-mutated | 81(76) |
Abbreviations: AML, acute myeloid leukemia; MDS, myelodysplastic syndrome, CMML, chronic myelomonocytic leukemia, MDS/MPN-U, myelodysplastic/myeloproliferative neoplasm unknown, MF, myelofibrosis
Overall response and outcomes
Overall, 39 patients (35%) achieved CR/CRi with a median duration of CR/CRi of 5.8 months (range, 1 – 41). Median number of cycles for all patients was 2 (range, 1–23). Median number of cycles to CR for all patients that responded was 2 (range, 1–5). Thirty-five patients were found to have persistent blasts on the day 14 bone marrow aspirate and received an additional course of decitabine 20mg/m2 IV daily for 5 days starting day 15.
Response and outcomes by predefined groups
Responses and outcomes by predefined groups are discussed below and are shown in table 2.
Table 2.
Outcomes by predefined groups
| Group | CR/ CRi N (%) |
Median CR duration (Months) |
Median overall survival (Months) |
8-week mortality N (%) |
|---|---|---|---|---|
| 1. (R/R AML < 1 yr remission) | 5/28 (18%) | 1 | 3.5 | 3 (11%) |
| 2. R/R AML > 1 yr remission) | 3/5 (60%) | 3 | 8 | 0 (0%) |
| 3. Untreated AML | 18/40 (45%) | 7 | 7.0 | 6 (15%) |
| Untreated MDS | 5/15 (33%) | Not reached | 5.7 | 3 (20%) |
| 4. MDS evolving to AML or relapsed/refractory MDS/MF | 7/20 (35%) | 6 | 7.2 | 3 (15%) |
Abbreviations: AML; acute myeloid leukemia, yr, year; MDS; myelodysplastic syndrome; MF; myelofibrosis, CR, complete remission; CRi, complete remission with incomplete count recovery
Group 1 - relapsed/refractory AML with CRD < 1 year
Twenty-eight patients with relapsed/refractory AML who had a remission lasting ≤ 1 year were treated in this group. These patients had received a median of two (range, 1–5) prior therapies for AML with a median CRD to most recent salvage regimen of 1.5 months (range, 0–12 months). CR/CRi was achieved in 5 of 28 patients (18%) with a median OS of 3.5 months and 8-week mortality of 11%. These results were comparable to a matched cohort of 440 patients with relapsed/refractory AML and CRD < 1 year treated on protocols for relapsed/refractory AML at our institution between 2001–2010, wherein the CR/CRi rate, median OS and 8-week mortality were 15%, 4.3 months (P=0.72), and 25% (P=0.18), respectively. Based on the model-based Bayesian design interim stopping rules for futility, further accrual to this cohort was closed (supplementary table 1, 2).
Group 2 - relapsed/refractory AML with a CRD ≥ 1 year
Five patients with relapsed/refractory AML who had a complete remission lasting more than 1 year were treated in this group. CR/CRi was achieved in 3 of 5 patients (60%) with a median CRD of 3 months, a median OS of 8 months, and no 8-week mortality. The small number of patients in this group precludes meaningful comparison with matched historical cohort.
Group 3: untreated AML unfit for intensive chemotherapy or untreated MDS or untreated MF
Fifty-seven patients were enrolled in this group including 40 patients with AML and 17 patients with untreated MDS or MF (including 10 MDS, 4 CMML, 1 MDS/MPN-unclassified, and 2 MF).
CR/CRi was achieved in 18 of the 40 elderly/unfit newly diagnosed AML patients (45%) with a median OS of 7.0 months and 8-week mortality of 15%. On comparison to a matched cohort of 76 newly diagnosed AML patients who were not candidates for intensive chemotherapy and treated with hypomethylator therapy-based protocols (including single agent decitabine protocols, decitabine with or without valproate, 5-azacytidine with or without vorinostat) at our institution between 2001–2010, the CR/CRi rate, median CRD, median OS and 8-week mortality were 30% (P=0.114), 8.1 months (P=0.15) and 16% (P=0.91), respectively. Although decitabine and GO trended towards a superior response rate compared to historical data, this did not result in improved survival in this group (supplementary table 1,2). The median number of cycles administered was 3 (range, 1–23). In patients who achieved CR/CRi the median number of cycles administered was 7 (range, 1–23) and the median number of cycles to response was 2 (range, 1–5).
Among the 15 untreated high-risk MDS patients the CR/CRi rate, median OS and 8-week mortality were 33%, 5.7 months and 20%, respectively. On comparison to a matched cohort of 103 high-risk MDS patients treated at our institution with hypomethylator therapy-based protocols (including single agent decitabine, decitabine with or without valproate, 5-azacytidine with or without vorinostat) between 2001–2010, the CR/CRi rate, median OS and 8-week mortality were 59% (P=0.06), 19.9 months (P=0.03) and 7% (P=0.09), respectively. Thus, the response rate, OS and 8-week mortality were inferior among the patients who received decitabine in combination with GO (supplementary table 1,2).
Group 4: AML evolving from MDS or relapsed/refractory MDS or MF
Twenty patients with AML evolving from MDS (n=11) or with relapsed/refractory MDS or MF (n=9) (including 5 MDS, 2 CMML, and 2 MF) were included in this group. These patients had received a median of one (range, 1–2) prior therapy. The CR/CRi rate was 35% with a median OS of 7.2 months and 8-week mortality of 15%. This was compared to a matched cohort of 23 historical controls who had previously treated MDS that progressed to AML and were treated on-protocols at our center between 2001–2010, where the CR/ CRi rate, median OS and 8-week mortality were 13% (P=0.09), 6.5 months (P=0.37) and 22% (P=0.57), respectively. Decitabine in combination with GO improved the response rate compared to historical data but again did not improve the OS (supplementary table 1,2).
Statistical analysis
Supplementary tables 1, 2 and supplementary figures 1,2 demonstrate the covariate adjusted Bayesian posterior probabilities of decitabine with GO being superior or inferior to the historical treatment for response rate and OS, respectively within each subgroup of patient. For example, in Group 1 patients, the posterior probability of decitabine+GO having a beneficial effect on response rate as compared to historical treatments is 84.6% (supplementary table 1); and the posterior probability of decitabine+GO having a harmful effect on the OS as compared to historical treatments is only 4% (supplementary table 2). Again, in Group 3 patients, the posterior probability of decitabine+GO having a beneficial effect on the response rate as compared to the historical treatment is 71.5% (supplementary table 1), however, for the same group of patients shows that the posterior probability of decitabine with GO having a harmful effect on OS is as high as 98% (supplementary table 2). As group 3 comprised of both untreated AML (n=40) and untreated MDS (n=15) and MF (n=2) patients with different expectations of response, we analyzed effect of therapy separately. Among patients with untreated AML (n=40), the posterior probability of decitabine with GO having a beneficial effect on the response rate is 93.5% and that of having a harmful effect on OS is 80%. On the other hand for untreated MDS patients (n=15), the posterior probability of decitabine with GO having a beneficial effect on the response rate is only 24% and that of having a harmful effect on OS is as high as 93%.
Toxicity
Neutropenic fever was the most common grade 3/4 toxicity (45%) (table 3). Grade 3/4 gastrointestinal and mucosal bleeding occurred in eight patients (7%). Other grade 3/4 non-hematological toxicities including nausea and mucositis were seen in five patients (5%). Cardiovascular events were observed in three patients including two cases of hypotension and one case of atrial fibrillation. Interestingly, no cases of treatment related grade 3–4 liver function abnormalities or veno-occlusive disease were identified.
Table 3.
Toxicity profile for all 110 patients
| Toxicity | Grade 2 N |
Grade 3 N |
Grade 4* N |
Hospitalizations for grade 3/4 N (%) |
|---|---|---|---|---|
| Gastrointestinal | ||||
| Nausea | 1 | 2 | 2/2 (100%) | |
| Mucositis | 2 | 1 | 1/3 (33%) | |
| Infection | ||||
| Neutropenic infection | 3 | 21 | 4 | 26/28 (93%) |
| Non-neutropenic infection | 2 | 1 | 1/3 (33%) | |
| Febrile neutropenia | 50 | 50/50 (100%) | ||
| Hemorrhage | ||||
| Gastrointestinal hemorrhage | 7 | 7/7 (100%) | ||
| Other Mucosal (Oral) | 1 | 1/1 (100%) | ||
| Cardiac | ||||
| Hypotension | 1 | 1 | 1/2 (50%) | |
| Atrial fibrillation | 1 | 1/1 (100%) | ||
| Metabolic | ||||
| Elevated alkaline phosphate | 1 | 0/1 |
Correlation of CD33 expression with response
Of the 110 patients enrolled, 87 were evaluable for CD33 within 3 months prior to initiation of decitabine and gemtuzumab therapy. CD33 positive was defined by a quantitative CD33 expression >/= 20%. 82/87 (94%) were positive. The median quantitative CD33 among the positive patients was 88% (range, 22–100). Only 5 patients were CD33 negative. Responses were noted in 28/79 (35%) of evaluable CD33+ patients and in 1/5 (20%) of evaluable CD33 negative patients. The small numbers preclude a truly meaningful comparison between CD33 expression and response.
DISCUSSION
Despite the voluntary withdrawal of GO from the US market, there have been several reports of survival benefit from the addition of GO to induction therapy in AML(13–16, 33). In younger patients the benefit seems to be more clearly accentuated among patients with intermediate and/or favourable risk cytogenetics(13, 16). In older patients, the addition of GO to cytotoxic induction regimens improved the relapse risk, event-free survival and overall survival without improving the response rate or early mortality rate(14, 15). The fractionated schedules of GO in these recent studies may have allowed safe delivery of cumulative smaller doses resulting in increased efficacy when compared to the previously approved dose of 9 mg/m2(11, 17, 34, 35). At the time we initiated this study there existed limited data regarding the use of GO in combination with lower intensity therapy such as hypomethylating agents and low-dose cytarabine.
Based on the statistical endpoints, decitabine in combination with GO improved response rate among patients with untreated AML ≥60 years of age who were unfit for chemotherapy, AML evolving from treated MDS and previously treated MDS or MF. However, this did not translate into improved survival when compared to historical data. This is similar to the recently reported MRC16 trial that showed lack of survival benefit despite improved CR rates when GO was added to low-dose cytarabine in elderly untreated AML patients(36).
An important aspect of this study is that the study design performed very well in optimally channelling accrual of patients to the arms that performed well while closing accrual to the underperforming arms. Such a design takes into consideration the patients’ heterogeneity among various prognostic subgroups in the same trial and the potential treatment-subgroup interactions. It can optimize patient allocation to effective therapy while minimizing exposure to ineffective therapy. The improved response rate among patients with newly diagnosed AML, which made up the bulk of group 3, resulted in continued accrual to this group in accordance with the flexible model-based Bayesian design. Thus, 52% of the patients accrued in our trial were in group 3. Historically, cytotoxic induction chemotherapy in elderly patients with AML and considered fit for such therapy produces a CR rate of 30–50% and an induction mortality of 25–40% (3, 6, 37, 38). The 40 treatment naïve AML patients treated with decitabine and GO in our study had an overall response rate of 45% with acceptable toxicity and lower induction mortality. These outcomes compare well to those achieved with single agent hypomethylating agents (azacytidine or decitabine) or their combinations with histone deacetylase inhibitors e.g. valproic acid or vorinostat, in comparable patients treated at our institute.
Our results with hypomethylating agent in combination with GO among treatment naïve elderly AML patients are similar to those recently reported by Nand et al(26). The scientific premise of their study was the synergy between azacytidine and GO wherein azacytidine induced increased CD33 expression and decreased P-glycoprotein expression(37–39). Azacytidine or decitabine mediated chromatin relaxation is also expected to enhance binding of calicheamicin to DNA(25). Nand et al reported a remission rate of 44% and a median survival of 11 months in ‘good-risk’ treatment naïve elderly AML patients (good risk = age 60–88 and PS 0–1, median age=72). Patients who did not have “good-risk” features (age 70–88 and PS 2–3, median age=75) had a remission rate and median overall survival of 35% and 11 month, respectively. Our study allowed PS of up to 3 and the median age in treatment naïve AML patients was 72 years.
In contrast, the untreated MDS patients had an inferior response rate with the combination as compared to a historical cohort of 103 high-risk MDS patients treated with hypomethylating agent-based therapies at our institution. Two factors may have contributed to inferior outcomes in the MDS patients treated with decitabine and GO. In the frontline decitabine trial conducted at our institution, the median age of patients was 5 years younger than our current cohort(40). Also for various reasons including intercurrent infections the high-risk MDS patients on the decitabine and GO cohort received a median of only 3 (range, 1–13) courses of therapy as compared to a median of 7 (range, 1–49) courses of hypomethylator-based therapy in the historical cohort. Several phase III studies implementing hypomethylator-based therapy have identified a median time to best response of approximately 3–6 months with these agents(41, 42). Thus it is quite possible that the patients in our current study received a less than optimal duration of therapy resulting in inferior response rates. Irrespective of these caveats, the combination of decitabine with GO is possibly an inferior option for patients with untreated MDS.
Similar to elderly patients unfit for standard induction, patients with AML refractory to induction chemotherapy or with short remission have dismal outcomes with salvage chemotherapy(43). The response rates become progressively worse in patients failing multiple salvages. The 28 patients with relapsed/refractory AML and a CRD < 1 year (median of 1.5 months) treated on our trial had received a median of two prior salvage regimens (range, 1 to 5). CR/CRp was achieved in 18% of these patients with a median OS of 4 months. These results are similar to a matched cohort of 440 patients treated on salvage studies at our institution. Of note, the combination of GO with “3+7’ as first salvage in a small group of younger patients with AML has shown promising results(44). These results need to be confirmed in larger studies in this patient population.
In summary, decitabine in combination with GO may be a suitable regimen in newly diagnosed AML patients who are not candidates for intensive induction therapy. However, the regimen produced inferior outcomes in the small cohort of treatment naïve high-risk MDS patients evaluated. In patients with relapsed/refractory AML decitabine and GO may be a viable alternative to more intensive chemotherapy based salvage regimens.
Supplementary Material
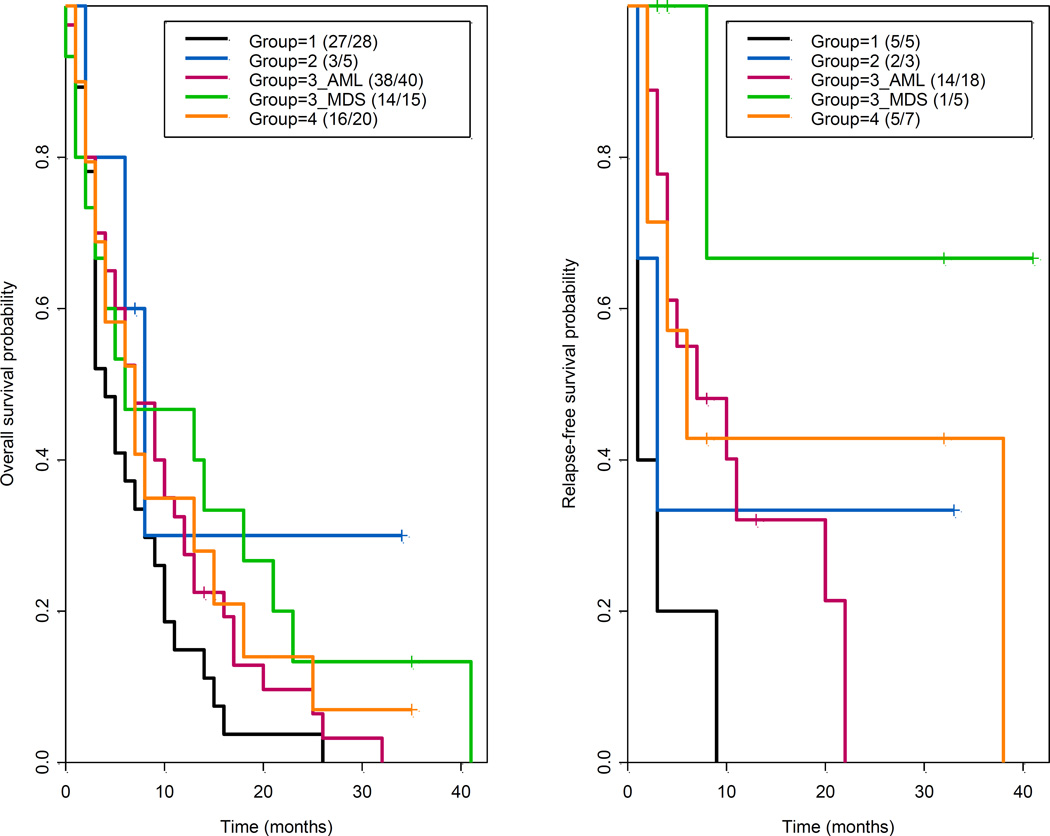
Figure 1.
Kaplan-Meier estimates for overall survival and relapse-free survival by study group
Acknowledgments
Funding source: This study was conducted following the guidelines of The University of Texas MD Anderson Cancer Centre after local IRB approval. It was supported in part by the MD Anderson Cancer Centre Support Grant (CCSG) CA016672, and Eisai Corporation.
GB; Research funding from Eisai corporation.
We would like to thank Eisai corporation for supporting the clinical trial.
Footnotes
Trial Registration: clinicaltrials.gov identifier: NCT01375140
Conflicts of Interest Disclosure: The other authors have no relevant conflicts of interest.
AUTHORSHIP CONTRIBUTIONS
ND and GB wrote the paper; GB and HK designed and coordinated the study; ZV, MK, JB, SO, AF, SV, TK, EJ, SF, NP, CD, JC, GB, HK enrolled the patients and conducted the research; and ND, GB, XW, SP, MD analysed the data and performed the statistics. All of the authors participated in the discussion, have reviewed and approved the current version of the manuscript.
SUPPLEMENTARY INFORMATION is available at Leukemia's website.
REFERENCES
- 1.Kantarjian HM. Therapy for elderly patients with acute myeloid leukemia: a problem in search of solutions. Cancer. 2007 Mar 15;109(6):1007–1010. doi: 10.1002/cncr.22502. [DOI] [PubMed] [Google Scholar]
- 2.Kantarjian H, O'Brien S, Cortes J, Giles F, Faderl S, Jabbour E, et al. Results of intensive chemotherapy in 998 patients age 65 years or older with acute myeloid leukemia or high-risk myelodysplastic syndrome: predictive prognostic models for outcome. Cancer. 2006 Mar 1;106(5):1090–1098. doi: 10.1002/cncr.21723. [DOI] [PubMed] [Google Scholar]
- 3.Nazha A, Ravandi F. Acute myeloid leukemia in the elderly: do we know who should be treated and how? Leukemia & lymphoma. 2014 May;55(5):979–987. doi: 10.3109/10428194.2013.828348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Appelbaum FR, Gundacker H, Head DR, Slovak ML, Willman CL, Godwin JE, et al. Age and acute myeloid leukemia. Blood. 2006 May 1;107(9):3481–3485. doi: 10.1182/blood-2005-09-3724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tallman MS, Gilliland DG, Rowe JM. Drug therapy for acute myeloid leukemia. Blood. 2005 Aug 15;106(4):1154–1163. doi: 10.1182/blood-2005-01-0178. [DOI] [PubMed] [Google Scholar]
- 6.Estey EH. Treatment of relapsed and refractory acute myelogenous leukemia. Leukemia. 2000 Mar;14(3):476–479. doi: 10.1038/sj.leu.2401568. [DOI] [PubMed] [Google Scholar]
- 7.Ravandi F, Cortes J, Faderl S, O'Brien S, Garcia-Manero G, Verstovsek S, et al. Characteristics and outcome of patients with acute myeloid leukemia refractory to 1 cycle of high-dose cytarabine-based induction chemotherapy. Blood. 2010 Dec 23;116(26):5818–5823. doi: 10.1182/blood-2010-07-296392. quiz 6153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stanisic S, Kalaycio M. Treatment of refractory and relapsed acute myelogenous leukemia. Expert Rev Anticancer Ther. 2002 Jun;2(3):287–295. doi: 10.1586/14737140.2.3.287. [DOI] [PubMed] [Google Scholar]
- 9.Scheinberg DA, Lovett D, Divgi CR, Graham MC, Berman E, Pentlow K, et al. A phase I trial of monoclonal antibody M195 in acute myelogenous leukemia: specific bone marrow targeting and internalization of radionuclide. Journal of Clinical Oncology. 1991 Mar;9(3):478–490. doi: 10.1200/JCO.1991.9.3.478. [DOI] [PubMed] [Google Scholar]
- 10.Appelbaum FR, Matthews DC, Eary JF, Badger CC, Kellogg M, Press OW, et al. The use of radiolabeled anti-CD33 antibody to augment marrow irradiation prior to marrow transplantation for acute myelogenous leukemia. Transplantation. 1992 Nov;54(5):829–833. doi: 10.1097/00007890-199211000-00012. [DOI] [PubMed] [Google Scholar]
- 11.Sievers EL, Larson RA, Stadtmauer EA, Estey E, Lowenberg B, Dombret H, et al. Efficacy and safety of gemtuzumab ozogamicin in patients with CD33-positive acute myeloid leukemia in first relapse. Journal of Clinical Oncology. 2001 Jul 1;19(13):3244–3254. doi: 10.1200/JCO.2001.19.13.3244. [DOI] [PubMed] [Google Scholar]
- 12.Zein N, Poncin M, Nilakantan R, Ellestad GA. Calicheamicin gamma 1I and DNA: molecular recognition process responsible for site-specificity. Science. 1989 May 12;244(4905):697–699. doi: 10.1126/science.2717946. [DOI] [PubMed] [Google Scholar]
- 13.Burnett AK, Hills RK, Milligan D, Kjeldsen L, Kell J, Russell NH, et al. Identification of patients with acute myeloblastic leukemia who benefit from the addition of gemtuzumab ozogamicin: results of the MRC AML15 trial. Journal of Clinical Oncology. 2011 Feb 1;29(4):369–377. doi: 10.1200/JCO.2010.31.4310. [DOI] [PubMed] [Google Scholar]
- 14.Castaigne S, Pautas C, Terre C, Raffoux E, Bordessoule D, Bastie JN, et al. Effect of gemtuzumab ozogamicin on survival of adult patients with de-novo acute myeloid leukaemia (ALFA-0701): a randomised, open-label, phase 3 study. Lancet. 2012 Apr 21;379(9825):1508–1516. doi: 10.1016/S0140-6736(12)60485-1. [DOI] [PubMed] [Google Scholar]
- 15.Burnett AK, Russell NH, Hills RK, Kell J, Freeman S, Kjeldsen L, et al. Addition of gemtuzumab ozogamicin to induction chemotherapy improves survival in older patients with acute myeloid leukemia. Journal of Clinical Oncology. 2012 Nov 10;30(32):3924–3931. doi: 10.1200/JCO.2012.42.2964. [DOI] [PubMed] [Google Scholar]
- 16.Delaunay J, Recher C, Pigneux A, Witz F, Vey N, Blanchet O, et al. Addition of Gemtuzumab Ozogamycin to Chemotherapy Improves Event-Free Survival but Not Overall Survival of AML Patients with Intermediate Cytogenetics Not Eligible for Allogeneic Transplantation. Results of the GOELAMS AML 2006 IR Study. Blood. 2011 Nov 18;118(21):37–38. [Google Scholar]
- 17.Bross PF, Beitz J, Chen G, Chen XH, Duffy E, Kieffer L, et al. Approval summary: gemtuzumab ozogamicin in relapsed acute myeloid leukemia. Clinical cancer research : an official journal of the American Association for Cancer Research. 2001 Jun;7(6):1490–1496. [PubMed] [Google Scholar]
- 18.Christman JK. 5-Azacytidine and 5-aza-2 '-deoxycytidine as inhibitors of DNA methylation: mechanistic studies and their implications for cancer therapy. Oncogene. 2002 Aug 12;21(35):5483–5495. doi: 10.1038/sj.onc.1205699. [DOI] [PubMed] [Google Scholar]
- 19.Karpf AR, Jones DA. Reactivating the expression of methylation silenced genes in human cancer. Oncogene. 2002 Aug 12;21(35):5496–5503. doi: 10.1038/sj.onc.1205602. [DOI] [PubMed] [Google Scholar]
- 20.Jones PA, Taylor SM. Cellular differentiation, cytidine analogs and DNA methylation. Cell. 1980 May;20(1):85–93. doi: 10.1016/0092-8674(80)90237-8. [DOI] [PubMed] [Google Scholar]
- 21.Kantarjian HM, Thomas XG, Dmoszynska A, Wierzbowska A, Mazur G, Mayer J, et al. Multicenter, randomized, open-label, phase III trial of decitabine versus patient choice, with physician advice, of either supportive care or low-dose cytarabine for the treatment of older patients with newly diagnosed acute myeloid leukemia. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012 Jul 20;30(21):2670–2677. doi: 10.1200/JCO.2011.38.9429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Balaian L, Ball ED. Cytotoxic activity of gemtuzumab ozogamicin (Mylotarg) in acute myeloid leukemia correlates with the expression of protein kinase Syk. Leukemia : official journal of the Leukemia Society of America, Leukemia Research Fund, UK. 2006 Dec;20(12):2093–2101. doi: 10.1038/sj.leu.2404437. [DOI] [PubMed] [Google Scholar]
- 23.Goodman PA, Burkhardt N, Juran B, Tibbles HE, Uckun FM. Hypermethylation of the spleen tyrosine kinase promoter in T-lineage acute lymphoblastic leukemia. Oncogene. 2003 Apr 24;22(16):2504–2514. doi: 10.1038/sj.onc.1206313. [DOI] [PubMed] [Google Scholar]
- 24.Yuan Y, Mendez R, Sahin A, Dai JL. Hypermethylation leads to silencing of the SYK gene in human breast cancer. Cancer research. 2001 Jul 15;61(14):5558–5561. [PubMed] [Google Scholar]
- 25.Kurimoto M, Matsuoka H, Hanaoka N, Uneda S, Murayama T, Sonoki T, et al. Pretreatment of leukemic cells with low-dose decitabine markedly enhances the cytotoxicity of gemtuzumab ozogamicin. Leukemia. 2013 Jan;27(1):233–235. doi: 10.1038/leu.2012.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nand S, Othus M, Godwin JE, Willman CL, Norwood TH, Howard DS, et al. A phase 2 trial of azacitidine and gemtuzumab ozogamicin therapy in older patients with acute myeloid leukemia. Blood. 2013 Nov 14;122(20):3432–3439. doi: 10.1182/blood-2013-06-506592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Greenberg P, Cox C, LeBeau MM, Fenaux P, Morel P, Sanz G, et al. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood. 1997 Mar 15;89(6):2079–2088. [PubMed] [Google Scholar]
- 28.Passamonti F, Cervantes F, Vannucchi AM, Morra E, Rumi E, Pereira A, et al. A dynamic prognostic model to predict survival in primary myelofibrosis: a study by the IWG-MRT (International Working Group for Myeloproliferative Neoplasms Research and Treatment) Blood. 2010 Mar 4;115(9):1703–1708. doi: 10.1182/blood-2009-09-245837. [DOI] [PubMed] [Google Scholar]
- 29.Cheson BD, Bennett JM, Kopecky KJ, Buchner T, Willman CL, Estey EH, et al. Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2003 Dec 15;21(24):4642–4649. doi: 10.1200/JCO.2003.04.036. [DOI] [PubMed] [Google Scholar]
- 30.Sievers EL, Larson RA, Stadtmauer EA, Estey E, Lowenberg B, Dombret H, et al. Efficacy and safety of gemtuzumab ozogamicin in patients with CD33-positive acute myeloid leukemia in first relapse. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2001 Jul 1;19(13):3244–3254. doi: 10.1200/JCO.2001.19.13.3244. [DOI] [PubMed] [Google Scholar]
- 31.Wathen JK, Thall PF, Cook JD, Estey EH. Accounting for patient heterogeneity in phase II clinical trials. Statistics in medicine. 2008 Jul 10;27(15):2802–2815. doi: 10.1002/sim.3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaplan EL, Meier P. Nonparametric-Estimation from Incomplete Observations. J Am Stat Assoc. 1958;53(282):457–481. [Google Scholar]
- 33.Petersdorf SH, Kopecky KJ, Slovak M, Willman C, Nevill T, Brandwein J, et al. A phase 3 study of gemtuzumab ozogamicin during induction and postconsolidation therapy in younger patients with acute myeloid leukemia. Blood. 2013 Jun 13;121(24):4854–4860. doi: 10.1182/blood-2013-01-466706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Larson RA, Boogaerts M, Estey E, Karanes C, Stadtmauer EA, Sievers EL, et al. Antibody-targeted chemotherapy of older patients with acute myeloid leukemia in first relapse using Mylotarg (gemtuzumab ozogamicin) Leukemia. 2002 Sep;16(9):1627–1636. doi: 10.1038/sj.leu.2402677. [DOI] [PubMed] [Google Scholar]
- 35.Larson RA, Sievers EL, Stadtmauer EA, Lowenberg B, Estey EH, Dombret H, et al. Final report of the efficacy and safety of gemtuzumab ozogamicin (Mylotarg) in patients with CD33-positive acute myeloid leukemia in first recurrence. Cancer. 2005 Oct 1;104(7):1442–1452. doi: 10.1002/cncr.21326. [DOI] [PubMed] [Google Scholar]
- 36.Burnett AK, Hills RK, Hunter AE, Milligan D, Kell WJ, Wheatley K, et al. The addition of gemtuzumab ozogamicin to low-dose Ara-C improves remission rate but does not significantly prolong survival in older patients with acute myeloid leukaemia: results from the LRF AML14 and NCRI AML16 pick-a-winner comparison. Leukemia. 2013 Jan;27(1):75–81. doi: 10.1038/leu.2012.229. [DOI] [PubMed] [Google Scholar]
- 37.Nand S, Godwin J, Smith S, Barton K, Michaelis L, Alkan S, et al. Hydroxyurea, azacitidine and gemtuzumab ozogamicin therapy in patients with previously untreated non-M3 acute myeloid leukemia and high-risk myelodysplastic syndromes in the elderly: results from a pilot trial. Leuk Lymphoma. 2008 Nov;49(11):2141–2147. doi: 10.1080/10428190802451254. [DOI] [PubMed] [Google Scholar]
- 38.Walter RB, Raden BW, Hong TC, Flowers DA, Bernstein ID, Linenberger ML. Multidrug resistance protein attenuates gemtuzumab ozogamicin-induced cytotoxicity in acute myeloid leukemia cells. Blood. 2003 Aug 15;102(4):1466–1473. doi: 10.1182/blood-2003-02-0396. [DOI] [PubMed] [Google Scholar]
- 39.Walter RB, Gooley TA, van der Velden VH, Loken MR, van Dongen JJ, Flowers DA, et al. CD33 expression and P-glycoprotein-mediated drug efflux inversely correlate and predict clinical outcome in patients with acute myeloid leukemia treated with gemtuzumab ozogamicin monotherapy. Blood. 2007 May 15;109(10):4168–4170. doi: 10.1182/blood-2006-09-047399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kantarjian H, Oki Y, Garcia-Manero G, Huang X, O'Brien S, Cortes J, et al. Results of a randomized study of 3 schedules of low-dose decitabine in higher-risk myelodysplastic syndrome and chronic myelomonocytic leukemia. Blood. 2007 Jan 1;109(1):52–57. doi: 10.1182/blood-2006-05-021162. [DOI] [PubMed] [Google Scholar]
- 41.Kantarjian H, Issa JP, Rosenfeld CS, Bennett JM, Albitar M, DiPersio J, et al. Decitabine improves patient outcomes in myelodysplastic syndromes: results of a phase III randomized study. Cancer. 2006 Apr 15;106(8):1794–1803. doi: 10.1002/cncr.21792. [DOI] [PubMed] [Google Scholar]
- 42.Silverman LR, Demakos EP, Peterson BL, Kornblith AB, Holland JC, Odchimar-Reissig R, et al. Randomized controlled trial of azacitidine in patients with the myelodysplastic syndrome: a study of the cancer and leukemia group B. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2002 May 15;20(10):2429–2440. doi: 10.1200/JCO.2002.04.117. [DOI] [PubMed] [Google Scholar]
- 43.Estey E, Kornblau S, Pierce S, Kantarjian H, Beran M, Keating M. A stratification system for evaluating and selecting therapies in patients with relapsed or primary refractory acute myelogenous leukemia. Blood. 1996 Jul 15;88(2):756. [PubMed] [Google Scholar]
- 44.Malfuson JV, Konopacki J, Thepenier C, Eddou H, Foissaud V, de Revel T. Fractionated doses of gemtuzumab ozogamicin combined with 3 + 7 induction chemotherapy as salvage treatment for young patients with acute myeloid leukemia in first relapse. Annals of hematology. 2012 Dec;91(12):1871–1877. doi: 10.1007/s00277-012-1528-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.