Abstract
Divalent cations of two alkaline earth metals Ca2+ and Mg2+ and the transition metal Zn2+ play vital roles in the immune system, and several immune disorders are associated with disturbances of their function. Until recently, only Ca2+ was considered to serve as a second messenger. However, signaling roles for Mg2+ and Zn2+ have been recently described, leading to a reevaluation of their role as potential second messengers. Here we review the roles of these cations as second messengers in light of recent advances in Ca2+, Mg2+ and Zn2+ signaling in the immune system. Developing a better understanding of these signaling cations may lead to new therapeutic strategies for immune disorders.
Keywords: Calcium, Magnesium, Zinc, Signal transduction, Immune disorders
Cytosolic free ionic pool and cellular homeostasis
In eukaryotic cells, divalent cations exist in two principal states; one tightly bound to proteins or other negatively-charged macromolecules such as mono- or polyphosphates, and a second ionized state involved in dynamic chemical equilibria. The “bound” pool represents the majority of the intracellular cation and plays a number of roles as a vital structural and functional co-factor through strong electrostatic interactions. On the other hand, the ionized fraction (0.05% to 10%) of each cation remains “free” in the cytosol or sequestered into organelles, such as the endoplasmic reticulum (ER) or mitochondria. The signaling ability of an ion relies on the high chemical activity of the intracellular “free” pool, and its ability to be rapidly modulated without affecting the total cellular amount of the ion (Box 1: Notion of ion signaling). This modulation results from mobilization of the cation across the plasma membrane (PM) or from intracellular stores to increase the cytosolic concentration to generate transient binding complexes between the cation and proteins or other macromolecules. There are numerous technical considerations that affect the accurate measurement of intracellular concentrations divalent cations. Whereas the total amount (bound and free) can be quantified by destructive biophysical analysis, the assessment of the free pool is achieved mainly by the use of chemical indicators [1].
Box 1. Notion of ion signaling.
The second messenger concept is well defined, however its application to ion signaling requires some adjustments. Indeed, ions signal through variation of their intracellular concentrations via different transport mechanisms, which is different from the “de novo” production of a second messenger by an enzyme. Also, the size and rapidity of movement could mean that the effective “range” of signaling cations is quite broad in the cell. The concept of ion signaling relies on several fundamental features: (1) a cytosolic resting free pool present in unstimulated cells that increases in response to an extracellular stimulus, such as the engagement of a cell surface receptor to become a mobilized free pool through mechanisms supporting its (2) homeostasis and (3) mobilization of the ion from the extracellular milieu, internal stores, or a bound depot; and (4) the mobilized free pool needs to alter one or more cellular processes at physiological level.
Cytosolic free pool. In eukaryotic cells, divalent cations are mostly bound with protein or other bioactive molecules and play essential structural and functional roles. For example Zn2+ is associated with up to 10% of all cellular proteins including over 300 enzymes and more than 2,000 transcription factors. Similarly, Mg2+ is associated with more than 300 enzymes as well as nucleotides, nucleic acids, and other negatively charged macromolecules. Given the essential structural and functional roles of these ions, signaling functions require the existence of a pool that can be modulated without affecting those functions. This pool is the cytosolic free pool which is considered free because it is in ionized form and able to bind to potential effectors. The difference between the bound and free forms is that the association constant for the divalent cation is much lower for the former.
Cellular homeostasis. To fulfill its purpose without affecting the total amount of the cation, the cytosolic free pool generally represents a small fraction of the total intracellular amount. To support the signaling functions of the cytosolic free pool, homeostatic regulation maintains the cytosolic free pool low at resting state by extrusion to the outside of the cell, sequestration in intracellular pools (stores) or immobilization by binding to cytosolic binding partners (bound).
Mobilization. Extracellular, or intracellular (not represented in the figure), stimuli trigger the mobilization of the cytosolic free pool via release from intracellular stores or bound pool, or by transport from the extracellular environment.
Effectors. Finally, the mobilized pool modulates cellular functions via tipping the equilibrium to the cation-bound form of specific effector molecules generally with the comparatively high association constant that is near the concentration achieved by the mobilized free pool and above the resting free pool concentration.
The ability for a cation to be mobilized in the cell, especially across the plasma membrane, depends on two driving forces, the chemical and the electric gradient [2]. The chemical gradient corresponds to the net difference of concentrations between the extracellular (or reservoir) environment and the cytosol. For example, Ca2+ and Zn2+ intracellular free concentrations, ([Ca2+]i and [Zn2+]i, respectively, are maintained at approximately 104-fold lower than the physiological extracellular concentration, thus generating a large chemical gradient for their mobilization into the cytosol (Table 1). On the other hand, there is a much smaller difference (< 2-fold) between cytosolic free Mg2+ ([Mg2+]i) and extracellular free Mg2+ ([Mg2+]o) level leading to the conventional wisdom that it is a poor candidate for a second messenger (Table 1) [1, 2]. However, because non-excitable cells, such as immune cells, harbor a negative membrane potential ≈ −70mV, if intracellular free Mg2+ was at electrical equilibrium, its resting concentration should be 50 mM [3, 4]. Nevertheless, [Mg2+]i ranges from 0.2 to 0.5 mM, showing that intracellular free Mg2+ is regulated and maintained at a lower concentration. This creates an electrochemical gradient of 100 to 250-fold for Mg2+, which is sufficient to allow rapid mobilization across the plasma membrane (PM) (Table 1, see Regulation of cytosolic free Mg2+ section) [3, 5, 6]. Indeed, the physiological function of rapid Mg2+ fluxes in T lymphocytes has been recently demonstrated and led to interesting insights into novel immunoregulatory mechanisms [7]. Table 1 summarizes extracellular and intracellular free concentrations of Ca2+, Mg2+ and Zn2+ as well as their physicochemical properties.
Table 1.
Ca2+, Mg2+ and Zn2+ characteristics
| Ion | [x]e | % Free | Free [x]i | E/I gradienta,b |
Ion radius (pm) |
H/Ic | References |
|---|---|---|---|---|---|---|---|
| Ca2+ | 1–2 mM | 0.05% | 100 nM | 10,000 | 100 | 70 | [11] |
| Mg2+ | 1 mM | 5–10% | 0.2–0.5 mM | 2–5b | 72 | 210 | [3, 5, 6] |
| Zn2+ | 15 µM | 5–10% | 0.4 nM | 20,000 | 74 | 196 | [122, 123] |
Extracellular (E)/intracellular (I) concentration gradient,
The electrochemical E/I gradient for Mg2+ is 100–250,
H/I, hydrated to ionized volume ratio
Here, we discuss the criteria for ion signaling and report recent advances on Ca2+, Zn2+ and Mg2+ mobilization and signaling in immune cells as well as their importance in human disease pathophysiology and treatment.
Homeostasis and mobilization of divalent cations in immune cells
Mobilizing Ca2+
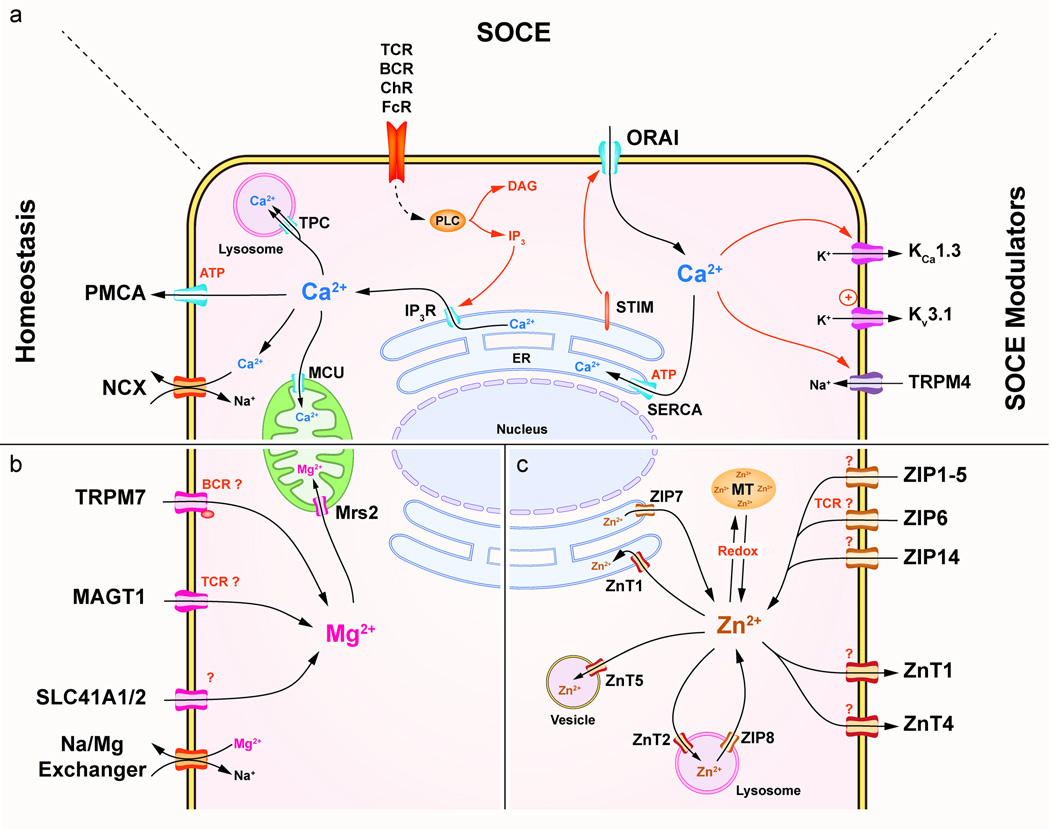
In the basal state, cytosolic free Ca2+ concentration ([Ca2+]i) is maintained at 100 nM by active extrusion of the Ca2+ from the cytosol. This occurs either through the PM by the Ca2+-ATPase (PMCA) and Ca2+/Na+ exchangers (NCX), or by deposition in ER or mitochondrial stores by the sarcoplasmic/ER Ca2+-ATPase (SERCA) or the mitochondrial Ca2+ uniporter (MCU), respectively (Figure 1a, Table 2) [8–10].
Figure 1. Divalent cations transporters.
(a) Ca2+ transport mechanisms in immune cells. Ca2+ homeostasis regulation involves the PMCA, SERCA ATPases as well as the MCU and Na+/Ca2+ exchangers (NCX). SOCE is induced by released of Ca2+ from the ER by the IP3R. STIM senses the store depletion and triggers the opening of the Ca2+ CRAC channel, ORAI. SOCE is modulated by two K+ permeable channels, KCa 1.3 and Kv 3.1 regulated by intracellular Ca2+ and depolarization respectively. These two channels help sustain SOCE generation by inducing hyperpolarization of the cell. Additionally, the Na+ permeable channel TRPM4 is gated by intracellular Ca2+ and reduces the Ca2+ mobilization. (b) Mg2+ transport mechanisms in immune cells. Mg2+ transporters include TRPM7, which have an intracellular Ser/Thr kinase domain, MAGT1, and SLC41A1/2 expressed on the PM and Mrs2 on the inner membrane of the mitochondria. (c) Zn2+ transport mechanisms in immune cells. Zn2+ transporters of the ZnT and ZIP families are expressed in various cellular compartments. Zn2+ homeostasis is completed by MT. Abbreviations: ChR, Chemokine receptor; DAG, Diacylglycerol; FcR, Fc receptor; TPC, Two pore channel.
Table 2.
Ca2+, Mg2+ and Zn2+ channels
| Channel | Selectivity | Loc. | Stimuli | Regulation | Expression | Functions | References |
|---|---|---|---|---|---|---|---|
| Calcium | |||||||
| SOCE and related | |||||||
| ORAI1 | Ca2+ | PM | TCR, BCR, TLR, FcR, Chemokine Rc | SOCE, inhibited by Ca2+ and ROS | Ubiquitous | Cell proliferation, Cytokines production, Degranulation, Phagocytosis, Chemotaxis, Treg development | [124] |
| ORAI3 | Ca2+ | PM | TCR | SOCE, inhibited by Ca2+ | Upregulated in activated T cells | Cell proliferation, Cytokines production | [19] |
| TRPC1/6 | Ca2+/Na+ | PM | TLR, FcR, chemokine Rc | SOCE, ARC | Mast cells, Neutro. (B, T cells ?) | Cytokine production, Degranulation | [125, 126] |
| Cav (1.2/1.3/1.4) | Ca2+ | PM | Voltage–independent | Inhibited by STIM | T cells | SOCE modulation ?, T cell homeostasis | [110, 127] |
| Voltage-dependent | SOCE (STIM independent) | DC, Macro. | Cytokine production, Chemotaxis, MHC II upregulation | [54] | |||
| SOCE modulators | |||||||
| KCa3.1 | K+ | PM | TCR, BCR | Increased [Ca2+]i | TH1, TH2, TCM, B cells | Hyperpolarization (sustains SOCE) | [128] |
| Kv1.3 | K+ | PM | TCR, BCR | Depolarization | Naïve T cells, TEM, TH17 | Hyperpolarization (sustains SOCE) | [128] |
| TRPM4 | Na+ | PM | TCR, BCR | Increased [Ca2+]i | Ubiquitous | Depolarization (inhibits SOCE) | [129] |
| Non SOCE | |||||||
| Cav (1.2/1.3/1.4) | Ca2+ | PM | Voltage–independent | Inhibited by STIM | T cells | SOCE modulation ?, T cell homeostasis | [110, 127] |
| Voltage-dependent | SOCE (STIM independent) | DC, Macro. | Cytokine production, Chemotaxis, MHC II upregulation | [54] | |||
| P2X (1/4/7) | Ca2+/Na+ | PM | Extracellular ATP/UTP | Inhibited by Mg2+ | T, B, NK cells, Neutro. | Pro-inflammatory Cytokines production, Chemotaxis, Proliferation | [117, 118, 120, 121] |
| TRPV1/2 | Ca2+/Na+ | PM | Heat (fever?), low pH, mechanical stress | Mono., Macro. | Degranulation, Phagocytosis, Cytokines production | [107, 108] | |
| TRPC3/6 | Ca2+/Na+ | PM | PLC activation (DAG), PIP2 | T, B, NK cells, Neutro. | Chemotaxis, Degranulation | [130, 131] | |
| TRPM2 | Ca2+/Na+ | PM Lys. | H2O2, NAADP, cADPR | T, B, Neutro., Mast cells, DC | Cytokine production, Degranulation | [101] | |
| Magnesium | |||||||
| TRPM6 | Mg2+>Ca2+ | PM | Inhibited by [Mg2+]i | Gut, Kidney, Hematopoietic (not T cells) | Unknown in immune cells | [3] | |
| TRPM7 | Mg2+>Ca2+ | PM | Unknown (BCR, TCR?) PIP2(?) | Inhibited by [Mg2+]i | Ubiquitous | T cell development, T and B cells proliferation, Cytokine production | [36] |
| MAGT1 | Mg2+ | PM | TCR | Unknown | Ubiquitous | CD4 T cells development, SOCE induction in T cells, NKG2D expression | [7, 32] |
| Zinc | |||||||
| Importers | |||||||
| ZIP1 | Zn2+ | PM | Unknown | [38] | |||
| ZIP2 | Zn2+ | PM | Unknown | Upregulated in asthma and sepsis | Unknown | [38, 92, 94] | |
| ZIP3 | Zn2+>others | PM | Unknown | CD34+ progenitors | T cell development | [38, 132] | |
| ZIP4 | Zn2+ | PM | Unknown | Intestine | AE | [38] | |
| ZIP6 | Zn2+ | PM | TCR (?) | Ubiquitous, T cells | INFγ production in T cells | [43–45] | |
| ZIP8 | Zn2+ | Lys. | TCR (?) | Ubiquitous, T cells, Mono. | INFγ production in T cells | [43–45] | |
| ZIP14 | Zn2+ | PM | LPS | [46, 47] | |||
| Exporters | |||||||
| ZnT1 | Zn2+ | PM | Unknown | Upregulated in T cells | Unknown | [37] | |
| ZnT4 | Zn2+ | PM | Unknown | Upregulated in T cells | Unknown | [37] | |
| ZnT5 | Zn2+ | Endo., PM (?) | FcεR stimulation | Unknown | Upregulated in activated mast cells | Recruitment of PKC to the PM (?) | [37, 68] |
Abbreviations: ARC, arachidonate-regulated Ca2+ channel, Endo., endosome; Loc., localization; Lys., lysosome; Mito., mitochondria; PA, Prader-Willi/Angelman syndrome; Ves., vesicles
Many immune functions are triggered by receptor-mediated acute elevations of cytosolic free Ca2+. The main mechanism leading to this elevation is the store-operated Ca2+ entry (SOCE), which involves mobilization across the PM triggered by the release of Ca2+ stored in the ER. SOCE is induced by a wide range of immune receptors including the T cell receptor (TCR), the B cell receptor (BCR), Fc receptors (FcR), Toll-like receptors (TLR) and chemokine receptors, among others. Receptor engagement activates various isoforms of phospholipase C (PLC), leading to the generation of the second messengers inositol 1, 4, 5-triphosphate (IP3) and diacylglycerol (DAG). IP3 activates Ca2+ release through the IP3 receptor channels (IP3R) on the ER. The depletion of Ca2+ from the ER stores is sensed by the N-terminal region of the stromal interaction molecule (STIM). This results in STIM oligomerization and translocation to a region of the ER proximal to the PM. The cytosolic C-terminal region of STIM then recruits and activates the prototypical Ca2+-release activated Ca2+ (CRAC) channel, ORAI, which promotes Ca2+ influx through the PM [11]. Our understanding of SOCE has increased greatly since the discovery of STIM and ORAI and recent updates are reviewed in [11].
Genetic deficiencies of ORAI1 and STIM1 in mice and humans lead to a drastic reductions of SOCE in immune cells including T cells, B cells, NK cells and mast cells and impaired T cell and mast cell activation (see Ca2+ and SOCE deficiency section [12–14]. In addition, there are two STIM isoforms, STIM1 and STIM2, that differ in their relative abundance and activation thresholds [15]. STIM1 activation requires higher Ca2+ store depletion than STIM2 [15]. In the mouse, deletion of both STIM isoforms in T cells profoundly decreases SOCE, greater than either one alone, and causes a lymphoproliferative disorder associated with a selective reduction of regulatory T cells (Tregs) [16]. Similarly, in B cells, the loss of both STIMs diminishes interleukin 10 (IL-10) production by regulatory B cells [17]. In both cell types, STIM2 deficiency alone leads to a milder SOCE decrease than STIM1 deficiency [15, 16]. However, STIM2 deficient T cells and B cells exhibit lower sustained Ca2+ mobilization and reduced nuclear translocation of the transcription factor, nuclear factor of activated T-cells (NFAT) [15, 16]. Additionally, recent studies suggest that both isoforms are required for optimal cytotoxic function of CD8+ T cells [15, 18]. The specific role of STIM2 in immune cells remains to be elucidated. Similarly, the three isoforms of ORAI are ubiquitously although differentially expressed in distinct cell types [15]. The ORAI1 knockdown (KD) in mouse T cells completely abrogates SOCE, whereas an ORAI2 KD has no effect implying that the latter may have no role in T cells [15]. However, other studies detected residual SOCE in ORAI1 deficient murine mast cells and T cells, suggesting ORAI2 participates in SOCE in these cells [13]. ORAI3 is induced after T cell activation and, unlike ORAI1 and ORAI2, is not inhibited by reactive oxygen species (ROS) [19]. Given that differentiation of naïve T cells into effector cells is associated with desensitization to ROS, ORAI3 might mediate SOCE desensitization following T cell activation [11, 19]. In addition to SOCE, store-independent or non-SOCE are also potentially important, although less established, mechanisms, for Ca2+ mobilization in immune cells and are discussed in Box 2.
Box 2. Non-SOCE Ca2+ mobilization: new directions and controversies.
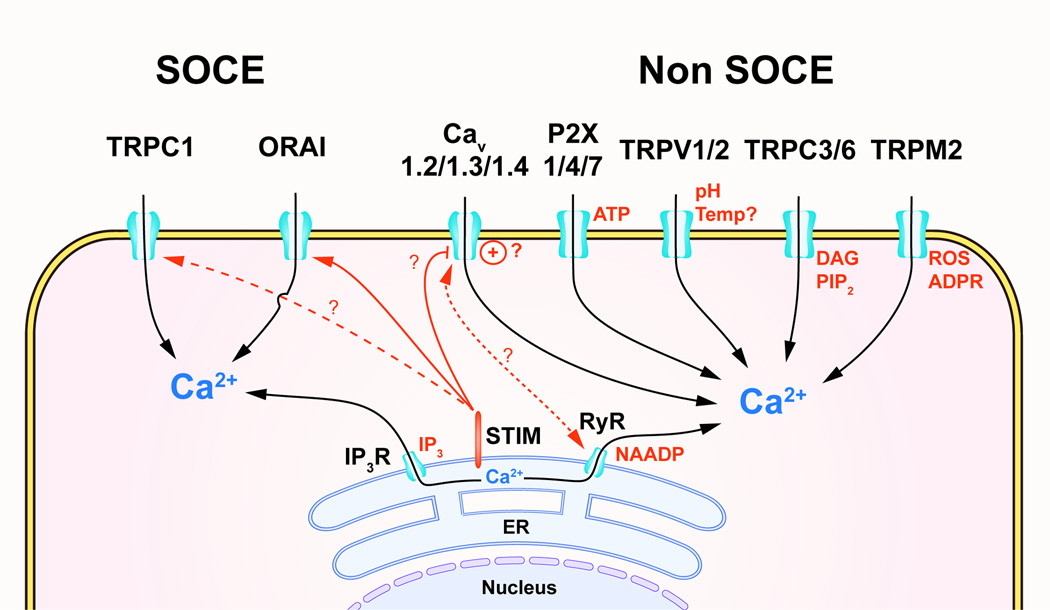
In addition to SOCE, immune cells several non-SOCE, or store-independent, Ca2+ mobilization mechanisms that are triggered by specific extracellular or intracellular ligands in immune cells (summarized in Figure 3 and Table 2) [95]. However, the physiological importance of some of these mechanisms remain to be established.
The superfamily of the transient receptor potential (TRP) channels encompass 28 members divided in six subfamilies based on their sequence homology [100]. The role of certain TRPs for Ca2+ mobilization in immune cells is relatively well established. For example, TRP melastatin 2 (TRPM2) is regulated by various intracellular ligands related to oxidative stress, such as cyclic ADP-ribose (cADPR), hydrogen peroxide (H2O2), nicotinic acid adenine dinucleotide phosphate (NAADP), and adenosine monophosphate (AMP) [101]. In vitro stimulation of T cell and B cell lines by ADPR show that TRPM2 triggers Ca2+ mobilization [101]. In neutrophils and macrophages, TRPM2 is expressed in the PM and promotes the production of various inflammatory cytokines in response to ROS, LPS and cellular acidification. Whereas, in DC, TRPM2 is expressed in the lysosomes and seems to play a role in the chemotaxis and maturation [102–104]. Another interesting example is the members of the vanilloid TRP family, TRPV1 and TRPV2 that are both expressed in immune cells [105, 106]. These channels associated with nociception are activated by noxious heat (>43°C), low pH, lipidic ligands such as prostaglandins, and mechanical stress suggesting an interesting intersection between nociception and inflammation [107, 108]. TRPV2 deficiency in macrophages is linked to impaired phagocytosis, whereas TRPV1 may have a role in sepsis but this remains unclear [107, 109]. TRPV2 has also has been associated with mast cell degranulation, however, in these studies the stimulus used were not physiological and the relevance of these findings requires further investigation [108].
In excitable cells, such as muscle cells and neurons, L-type voltage-dependent Ca2+ channels (Cav1) and ryanodine receptor channels (RyR) are responsible for SOCE after depolarization. These are also expressed in immune cells, including T cells, B cells and DC. In macrophages and DCs, Cav activation is voltage-dependent and causes SOCE by promoting Ca2+ release from the ER through a direct interaction with the channel RyR1 after LPS stimulation [54]. On the other hand, the role of Cav in T cells is more controversial [110]. Whereas, T cells expressed all Cav1 channel isoforms (Cav1.1, Cav1.2, Cav1.3 and Cav1.4) along with their regulatory subunits β3 and β4, their activation in T cells is not induced by depolarization [54, 111–113]. Recent studies established roles for Cav1 in thymic development and naïve T cell homeostasis in mouse [114]. However, incomplete characterization of the biophysical properties of Cav1 in T cells, causes their role in T cell function to be debated. In addition, two recent studies report inhibition of Cav channels by STIM in T cells, suggesting a potential reciprocal regulation of the ORAI1 and Cav channels by STIM that needs to be further investigated [115, 116].
Purinergic P2X receptors are non-selective Ca2+ channels activated by extracellular ATP/UTP. During immune responses, the release of ATP by damaged or dying cells is considered to be an important inflammatory “danger” signal. P2X4 and P2X7 strongly influence chemotaxis and pro-inflammatory cytokine production in DCs and macrophages [117, 118]. In T cells and B cells, P2X7-dependent Ca2+ influx induces proliferation and cytokine production [119]. Additionally, activation of T cells and neutrophils stimulates P2X7 via autocrine release of ATP [120, 121]. Although, all these findings are supported by in vivo studies, the biophysical characteristics of P2X in immune cells is only partly understood.
Figure Box 2: SOCE and non-SOCE Ca2+ mobilization mechanisms in immune cells. In addition to the SOCE induced by released of Ca2+ from the ER by the IP3R, ryanodine receptors (RyR) channels can also release Ca2+ from the ER. STIM senses the store depletion and triggers the opening of the Ca2+ CRAC channel, ORAI and potentially TRPC1. L-type “voltage-dependent” Ca2+ (Cav) channels induce voltage-dependent (+) and independent Ca2+ mobilization via direct interaction with RyR and are potentially inhibited by STIM. Non-SOCE mechanisms include, purinergic receptor channels (P2X), transient receptor potential vanilloid 1 and 2 (TRPV1/2) channel, TRPC3/6 and TRPM2. ADPR, ADP-ribose; NAADP, nicotinic acid adenine dinucleotide phosphate; PIP2, phosphoinositides diphosphate; ROS, radical oxygen species; Temp, temperature.
Regulation of cytosolic free Mg2+ in immune cells
The homeostatic regulation of cytosolic free Mg2+ is less well understood than Ca2+. It involves Mg2+ transporters, channels and exchangers, such as the Mg2+/Na+ exchanger (Figure 1b, Table 2). Another major participant is the melastatin transient receptor potential 7 (TRPM7) channel [3]. TRPM7 is a ubiquitously expressed, non-selective Mg2+ channel that also conducts Ca2+, Zn2+ and even Na+ in absence of divalent cations [3, 20, 21]. TRPM7 has a serine/threonine kinase domain in its cytosolic tail [22]. Whereas the kinase domain is not essential, its activity can modulate the gating of TRPM7 [22]. The critical role of TRPM7 for Mg2+ homeostasis in immune cells is illustrated by decreased free Mg2+ and cell cycle arrest in TRPM7 deficient B cell lines and impaired T cell development in T cell-specific TRPM7 conditional knockout (KO) mice [22–24]. Other regulators of free Mg2+ homeostasis in immune cells include members of the solute carrier (SLC) family SLC41A1 and SLC41A2. These transporters were identified by homology to the bacterial Mg2+ transporter, MgtE, and are expressed relatively ubiquitously [20, 25]. The role of SLC41A1/2 in free Mg2+ homeostasis was uncovered by ectopic expression in TRPM7-deficient B cell line that can rescue the decreased intracellular Mg2+ levels and defective proliferation [26, 27].
Finally, MAGT1 is a key Mg2+ transporter in immune signaling [30, 31]. Unlike TRPM7 and SLC41A1/2, MAGT1 is a uniquely Mg2+-selective channel that is ubiquitously expressed but with higher levels in immune and epithelial cells [28, 29]. Since the early 90s, elevation of intracellular free Mg2+ has been observed after stimulation of T cells with lectins in some studies, but not others [5, 30, 31]. The functional importance of Mg2+ mobilization remained unknown for twenty years until the discovery of a new primary immunodeficiency named "X-linked immunodeficiency with Mg2+ defect, Epstein Barr Virus (EBV) infection, and neoplasia" (XMEN) disease [7, 32, 33]. This disease is due to a genetic deficiency of MAGT1, which we found to be essential for a TCR-gated Mg2+ flux (see divalent cations in pathophysiology section) [7]. The MAGT1-dependent Mg2+ flux is required for the optimal activation of PLC-γ1, IP3 generation, protein kinase C theta (PKC θ) phosphorylation, and Ca2+ mobilization via SOCE (Figure 2b) [7]. The molecular mechanism of TCR gating of MAGT1 is currently unknown. MAGT1 deficiency also leads to decreased cytosolic free Mg2+ and decreased Mg2+ uptake in T cells and B cells [7, 32, 34]. Moreover, oral Mg2+ supplementation restores the level of intracellular free Mg2+ in XMEN patients [32]. The channel or transporter that restores Mg2+ homeostasis in immune cells after supplementation remains under investigation. Conversely, the Mg2+ flux cannot be restored in XMEN T cells by high external Mg2+ and apparently requires the presence of the MAGT1 protein [32]. Recently, a study using drosophila MAGT1 expressed in human neuroblastoma SH-SY5Y cell line showed MAGT1-dependent currents induced by the protein kinase C (PKC) inducer phorbol-12-myristate-13-acetate (PMA) via phosphorylation by PKC [35]. The role of this interesting observation in immunity has yet to be established. In B cells, the phosphorylation of serine 1164 of PLC-γ2 by the kinase domain of TRPM7 is dependent on extracellular Mg2+ and participates in Ca2+ mobilization (via SOCE) in response to BCR stimulation [36]. However, in this study, the authors did not assess Mg2+ mobilization in response to BCR stimulation and there is no evidence of Mg2+ mobilization by B cell activation (Figure 2c) [36].
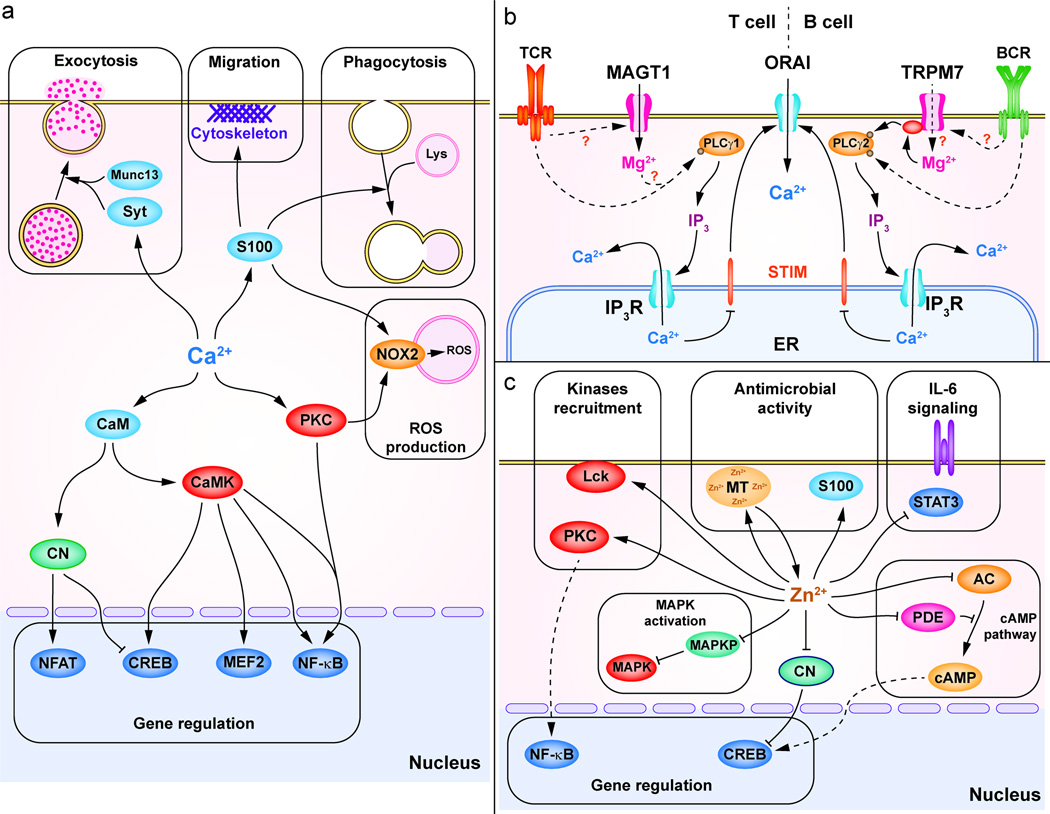
Figure 2. Divalent cations effectors and functions in immune cells.
(a) A broad range of functions can be rapidly deployed through Ca2+ signals. One major Ca2+ function is the regulation of gene expression involved in cell activation, differentiation and cytokine production. Ca2+ controls the activation of NFAT via CaM-CN, CREB and myocyte enhancer factor 2 (MEF2) via CaMK and CaM and NF-κB via PKC and CaMKII. Ca2+ controls exocytosis through Ca2+-sensitive proteins such as synaptotagmins (Syt) and Munc13. S100 proteins are Ca2+-binding proteins involved in the cytoskeleton organization during migration, lysosome-phagosome fusion during phagocytosis. In neutrophils, Ca2+ controls ROS production by NOX2 via PKC and S100 proteins (S100A8/A9). (b) In T and B cells, Mg2+ is regulating Ca2+ mobilization via SOCE, by modulating PLC-γ activation. In T cells, the TCR-induced MAGT1-dependent Mg2+ flux is required to activate PLC-γ1. In B cell, TRPM7 kinase domain participates in PLC-γ2 activation together with Bruton’s tyrosine kinase (BTK) in a Mg2+-dependent manner. (c) Zn2+ is involved in the recruitment of kinases such as PKC or LCK. Zn2+ enhances MAPK activation through inhibition of the MAPKP. Zn2+ controls gene expression through NF-κB and through inhibition of CN (which promotes CREB activation). In innate immune cells, Zn2+ binding-proteins such as MT and S100 proteins (S100A8/A9) are playing anti-microbial roles by chelating Zn2+. Zn2+ inhibits IL-6 signaling by inhibiting the activation of STAT3. Finally, Zn2+ regulates the cAMP pathway through the inhibition of AC or PDE.
Different waves of Zn2+ mobilization
Cytosolic free Zn2+ is maintained at low levels by tight regulation of the expression and subcellular localization of two families of Zn2+ transporters controlling cytosolic import and export. The zinc transporter (ZnT, SLC30) family consists of 10 transporters that reduce cytosolic Zn2+ levels, whereas the fourteen importers of the Zrt1-, Irt1-like Protein (ZIP, SLC39) family increase it (Figure 1c) [37, 38]. Zn2+ homeostasis is also regulated by intracellular Zn2+-binding proteins, including metallothioneins (MT) that can sequester up to 15% of the cellular Zn2+ and release it during oxidative stress [39]. The role of MT in controlling Zn2+ homeostasis appears to be important in innate immune cells. In macrophages, MT deficiency impairs cytokine production and bactericidal activity after lipopolysaccharide (LPS) stimulation due to Zn2+-deficiency [40, 41].
Two types of Zn2+ mobilization have been described with different timing. First, in T cells, monocytes, neutrophils and mast cells, fast transcription-independent Zn2+ mobilization has been observed within minutes after the stimulation of activating receptors using fluorescent Zn2+-sensitive probes [42]. How these Zn2+ fluxes are triggered is mostly unknown. In human T cells, co-incubation with IL-2 or antigen-loaded dendritic cells (DCs) induces a rapid Zn2+ elevation that requires ZIP6 on the PM and ZIP8 on the lysosomal membrane, respectively [43–45]. In monocytes/macrophages, LPS stimulation induces quick release of Zn2+ from intracellular stores [46, 47]. The intracellular stores and the role of MT involved in Zn2+ release remain to be determined. In mast cells, FcεRI stimulation induces Zn2+ release from the ER termed “Zn2+ waves”. The mechanisms governing these “Zn2+ waves” are not well understood and their biological function remains unclear [48]. The second type of Zn2+ mobilization occurs several hours after various stimuli and requires changes in channel expression. For example, activation of T and B cells leads to a sustained cytosolic free Zn2+ elevation due to the down-regulation of several Zn2+ exporters (ZnT1, ZnT4, ZnT5, ZnT6 and ZnT7) and the up-regulation of several importers (ZIP6, ZIP10 and ZIP8) over long time course [43, 49]. Silencing ZIP6 and ZIP8 in human T cells prevents the elevation of cytosolic free Zn2+ and impairs proliferation and cytokine production during T cell activation [43–45]. The physiological role of these transporters in B cells is unknown. On the other hand, stimulation of DCs with LPS decreases intracellular Zn2+ over time via the down-regulation of ZIP6 and ZIP10 and the up-regulation of ZnT1, ZnT4 and ZnT6 [50]. Importantly, reduced cytosolic free Zn2+ is essential for major histocompatibility complex class II (MHC II) up-regulation as well as DC maturation and phagocytic function [50]. The precise molecular mechanism of these effects is unknown and it is not clear whether this is modulation of a cofactor or a signaling function per se.
Over the past few years, investigation of Ca2+, Mg2+ and Zn2+ signaling has provided many new insights in their regulatory roles in immune cells. The identification of ORAI as the CRAC channel provided the long-sought molecular validation of SOCE. However, there are still unanswered questions about the respective roles of STIM and ORAI isoforms in immune cells. Additionally, non-SOCE mechanisms may be important in immune functions even if we don’t fully understand how they work at this time (Box 2). Recently, the discovery of inducible Mg2+ and Zn2+ fluxes in immune cells has revealed the signaling function of these cations but has raised many new questions about their regulation. For example, in T cells, the gating mechanisms and compartmentalization of MAGT1 (Mg2+) and ZIP6 (Zn2+) requires further investigation in immune cells. Furthermore, new specific chelators and fluorescent probes must be developed to study Mg2+ and Zn2+ mobilization with the clarity and elegance achieved in the Ca2+ field. Mobilizing divalent cations is only the first step for ion signaling, in the next section we discuss how changes in the concentration of cytosolic free Ca2+, Mg2+ and Zn2+ control immune signaling pathways and effector functions.
Divalent cations functions in immune cells
Ca2+, a central second messenger
Ca2+ signaling triggers cell activation and differentiation principally through gene expression and the rapid deployment of effector functions (Figure 2a). Ca2+ controls the activity of several transcription factors. It stimulates NFAT via the Ca2+-sensitive protein calmodulin (CaM)-dependent activation of the phosphatase calcineurin (CN) (Figure 2a). Ca2+ also activates the cAMP response element-binding protein (CREB) and nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) through CaM kinases (CaMK) and PKC (Figure 2a) [51]. For cytolytic effector functions, Ca2+ plays an important role in immune exocytosis. In mast cells, NK cells and cytotoxic T cells (CTL), increased free Ca2+ is required for degranulation of following FcεRI and FcγRIII (CD16) and TCR stimulation, respectively [13, 52, 53]. Similarly, the up-regulation of MHC II molecules and synaptotagmin VII, a Ca2+ sensor involved in vesicle trafficking, during DC maturation depends on Ca2+ mobilization [54, 55]. Ca2+ chelation studies in mouse and human phagocytes have established that phagocytosis is mostly Ca2+-independent [56]. However, the Ca2+ requirement for the fusion of the phagosome with the lysosome is still debated [56, 57]. Moreover, in neutrophils, ROS production associated with antimicrobial functions relies on the Ca2+-dependent activation of NADPH oxidase 2 (NOX2). The mechanism of activation of NOX2 is not well defined, but apparently involves PKC, phosphatidylinositide 3-kinases (PI3K) and sphingosine kinase (SK) and the Ca2+/Zn2+-binding protein calprotectin (S100A8/A9) [56, 58, 59]. Thus, a broad range of immune functions can be rapidly deployed through the extraordinary speed of free Ca2+ moving inside and throughout the cell.
Emerging role for Mg2+ in immune cells
Beyond the essential role of Mg2+ as a cofactor for ATP, enzymes and other processes in all cells, special signaling functions have been recently documented in immune cells. Early studies documented that Mg2+ is important for T and B cell proliferation, but it was unclear whether this was due to a cofactor role, a signaling role, or both [7, 27, 60, 61]. As mention earlier, TRPM7-deficient B cell lines exhibit reduced cytosolic free Mg2+ and defective proliferation, which is restored by increasing extracellular Mg2+ and/or overexpressing other Mg2+ transporters [27, 61]. Whether TRPM7 deficiency affects total, mainly bound, Mg2+ in B cell lines was not determined [22]. Both TRPM7 and MAGT1 appear to play a role in T cell differentiation [3, 7]. Germline TRPM7 KO mice are embryonic lethal, whereas T cell-specific TRPM7 KO mice are viable but exhibit a blockade of thymic T cell development at the double negative stage [24]. XMEN patients (MAGT1-deficient), have reduced numbers of CD4 T cells especially the naïve CD4+/CD45RO−/CD27+/CD31+ cells, suggesting diminished thymic output possibly due to impaired positive selection [7, 33]. As mentioned previously, in mature T cells, TCR stimulation induces a MAGT1-dependent Mg2+ flux required for optimal activation of PLC-γ1 and Ca2+ mobilization via SOCE [7]. There appears to be no such requirement for B cell activation through BCR signaling [7].
Interestingly, we found that decreased cytosolic free Mg2+ in MAGT1 deficiency causes the loss of expression of NKG2D, an essential cytotoxicity activating receptor on NK and CD8+ T cells [32]. NKG2D recognizes specific nonclassical MHC class I-like ligands that are upregulated in virally-infected and transformed cells, including EBV-infected B cells [32]. Biochemical analysis in lymphocytes from XMEN patients revealed incompletely glycosylated NKG2D proteins that are retained intracellularly in reduced abundance [32]. It is not clear whether the failure of N-glycosylation is the cause of NKG2D deficiency. However, MAGT1 shares 60% amino acid identity with TUSC3, an ortholog of a subunit of the yeast N-glycosylation enzyme complex, raising the question of whether MAGT1 may participate in these processes [20, 29]. Strikingly, exogenous Mg2+ supplementation restores the level of cytosolic free Mg2+ and the expression of fully glycosylated NKG2D, independently of MAGT1, indicating that the full maturation and glycosylation of NKG2D is strictly dependent on the level of cellular free Mg2+ [32].
Zn2+ more than a trace element in immune cells
Dietary Zn2+ deficiency decreases T cell activation, shifts TH1 responses toward TH2 responses, reduces the cytotoxic function of NK and NKT cells, and impairs cytokine production by mast cells and NK cells [62]. Interestingly, Zn2+ supplementation restores those functions, but also induces chronic inflammatory responses and suppresses TH17 development in the elderly [63–65]. TH17 cell suppression appears to be caused by the direct Zn2+-dependent inhibition of IL-6-induced phosphorylation of signal transducer and activator of transcription 3 (STAT3) by janus kinases (JAKs) [64]. At the cellular level, several studies using the metal chelator tetrakis-(2-Pyridylmethyl)ethylenediamine (TPEN) and the Zn2+ ionophore pyrithione established that Zn2+ mobilization regulates specific signaling pathways [66, 67]. Those pathways involve tyrosine kinases, PKCs, mitogen-activated protein kinases (MAPK), cAMP and JAK/STAT. In T cells, the role of Zn2+ in leukocyte C-terminal Src kinase (LCK) activation is multiple: it facilitates recruitment to the cytoplasmic tail of CD4 and CD8, stabilizes homodimerization of the enzyme, and enhances its activation by reducing the recruitment of the inhibitory phosphatase Src homology region 2 domain-containing phosphatase-1 (SHP-1) to the TCR complex [66, 67]. Reduced SHP-1 recruitment has been directly tied to Zn2+ mobilization in T cells, but whether Zn2+ plays a structural or signaling role in LCK activation is unclear [44]. In mast cells, PKC recruitment to the PM induced by FcεRI stimulation depends on Zn2+ mobilization and ZnT5 expression, but the mechanism has not been defined [68]. Zn2+ enhances mitogen-activated protein kinases (MAPK) used by phagocytes to eliminate pathogens by inhibiting MAPK phosphatases (MAPKP) [45, 46]. Also, cAMP and cyclic guanosine monophosphate (cGMP) are important second messengers produced by adenylate cyclase (AC) and guanylate cyclase (GC), respectively; and degraded by phosphodiesterases (PDE). In monocytes, the levels of cytosolic free Zn2+ modulates the cAMP/cGMP pathway by two different mechanisms. First, Zn2+ promotes cGMP accumulation by limiting the production of cAMP via direct inhibition of AC [69, 70]. This blocks monocyte differentiation without a requisite decrease of intracellular Zn2+ [69, 70]. Second, high free Zn2+ concentrations (in the low nM range) promote cAMP and cGMP accumulation by inhibiting PDEs [69, 70]. During T cell activation, inhibition of CN by Zn2+ released from the lysosomes promotes the activation of NF-κB and IFNγ production [43]. In IL-27-induced Treg cells, IL-10 production requires Zn2+ and is inhibited by MT [71]. Lastly, Zn2+ sequestration by calprotectin (S100A8/A9), expressed in monocytes and neutrophils is important to suppress Candida albicans growth [72]. Thus, Zn2+ fluctuations affect many immunological functions.
Whereas many immune functions controlled by Ca2+ are well established, our knowledge of the effectors of Zn2+ and Mg2+ is not as well developed. Given their essential roles as structural supports and co-factors in many cell functions, it is important to further characterize the effectors involved in Mg2+ and Zn2+ signaling. Additionally, Ca2+, Mg2+ and Zn2+ signaling pathways are intertwined and each cation can potentially modulate the signaling of the others.
Agonist and antagonist interactions in divalent cations signaling
Not just acting in parallel, Ca2+, Mg2+ and Zn2+ signaling pathways can intersect by direct competition for the same effectors or by cross-modulation. Classically, Mg2+ is considered to be a Ca2+ antagonist. This concept stems from their physico-chemical properties as alkali metals carrying divalent positive charges. Ca2+ has a larger atomic radius, but Mg2+ has a higher affinity for electronegative oxygen, which leads to a greater hydrated radius (Table 1). These properties allow Mg2+ to compete with Ca2+ for certain binding sites. However, the propensity of Mg2+ to be hydrated prevents the conformational changes associated with Ca2+ binding which antagonizes Ca2+ signaling [73]. For example, Mg2+ competes with Ca2+ for the binding to the EF-hand sites of CaM and causes the protein to remain in a closed, inactivated conformation [74]. Mg2+ can also antagonize Ca2+ signaling by blocking Ca2+ mobilization. For example, in vitro studies using HEK cells engineered to express various P2X channels, show that extracellular Mg2+ inhibits Ca2+ currents induced by ATP [75]. Similar observations were made on endogenous P2X7 channels using the monocyte-like cell line THP1 [76]. Other Ca2+ channels were shown to be inhibited by extracellular Mg2+, such as TRPV3 in mouse keratinocytes, Cav1.2 in mouse myocytes [77, 78].
In T cells, Mg2+-dependent activation of PLC-γ1 following TCR stimulation participates in Ca2+ mobilization, which defines a new regulatory role for Mg2+ signaling in the upstream control of Ca2+ signaling [7, 32]. Unlike the direct competition mentioned earlier, this “pro-Ca2+ signaling” role of Mg2+ does not involve common binding sites, but a convergence of two independent signaling pathways, in which Mg2+ increases PLC-γ1 activation leading to a more robust generation of IP3. This example of coordinated Mg2+ and Ca2+ signaling opens the door to further investigations and identification of the Mg2+ effector(s) that control PLC-γ1 activation [7]. In B cells, the phosphorylation of PLC-γ2 in a Mg2+-dependent manner by the kinase domain of TRPM7 might illustrate another convergence of Mg2+ and Ca2+ signaling [36].
Similar to Mg2+, Zn2+ can inhibit Ca2+ signaling via inhibition of CN or S100 proteins [43, 79, 80]. However, unlike Mg2+, Zn2+ does not compete with Ca2+ for the same binding site, but rather binds different sites on the same protein. For example, the inhibitory effect of Zn2+ on CaM does involves sites other than the Ca2+-binding EF-hand sites [74]. Zn2+ can also promote Ca2+ signaling by enhancing the recruitment of Ca2+-sensitive effectors, such as the recruitment of PKC to the membrane where the localized Ca2+ mobilization can induce activation [66, 68]. Lastly, some proteins have both Ca2+-dependent and Zn2+-dependent functions involving different binding sites, such as calprotectin (S100A8/A9 dimers) which is a Ca2+ effector for the phagocytosis as well as a Zn2+-binding protein involved in anti-bacterial function of monocytes [79, 80]. Conversely, Ca2+ signaling can promote Zn2+ mobilization in mast cells, wherein Zn2+ waves elicited in response to FcεRI stimulation are dependent on Ca2+ mobilization [48]. Moreover, Ca2+ and Zn2+ signaling coordinately regulate complex cellular processes in ways that are not completely understood. For example, during DC maturation, both Ca2+ elevation and Zn2+ diminution are required for up-regulation of MHC II and cytokine production [50, 54, 55].
Divalent cations in physiopathology
The study of human primary immunodeficiencies (PIDs) often yield novel insights into how the immune system is regulated. The understanding of the signaling roles of divalent cations is no exception. For example, the CRAC channel was identified by screening patients with severe combined immunodeficiency (SCID) associated with loss of SOCE [81]. The case is even more striking for Mg2+, because XMEN disease reveals its unexpected signaling functions [7, 32].
Ca2+ and SOCE deficiency
In humans, deleterious mutations in ORAI1 and STIM1 cause a loss of SOCE in immune cells, SCID and muscle abnormalities [81]. Both ORAI1- and STIM1-deficient patients exhibit recurrent life-threatening viral, bacterial and fungal infections, and require hematopoietic stem cell transplantation (HSCT) early in life. ORAI1 and STIM1 deficiency do not alter the numbers of innate, B or T cells, except for a reduction in the Treg population. However, defective SOCE severely compromises mature T cell activation, proliferation, and cytokine production, although it has a much milder effect on B cell function. Other features of SOCE-related immunodeficiency are lymphoproliferative disorders and autoimmunity. More comprehensive description of human SOCE deficiency is provided in [14].
Mg2+deficiency in immunodeficiency, autoimmunity and inflammation
In XMEN disease, “loss of function” mutations in MAGT1 result in essentially complete loss of the MAGT1 mRNA and protein [7, 33]. For unknown reasons, XMEN patients have modestly decreased numbers of CD4+ T cells and inversion of the CD4/CD ratio [7, 33]. As mentioned above, MAGT1 affects Mg2+ in two key respects : (1) the loss of a T cell receptor (TCR)-induced transient Mg2+ influx required for optimal T cell activation and (2) a chronic decrease of cytosolic free Mg2+ [7, 32]. The latter defect is almost entirely responsible for the deficient expression of the NKG2D receptor on NK and CD8 T cells which is an important cytolysis activating receptor against virally-infected or transformed cells (see Emerging role for Mg2+ in immune cells section) [7, 32]. The most severe and life-threatening complication of XMEN disease is uncontrolled EBV infection with increased susceptibility to EBV-driven lymphoproliferative disorders [7, 32, 33]. The fact that EBV induces the specific ligands for NKG2D might account for the severity of EBV infection when NKG2D is deficient [32]. Since increasing the cytosolic free Mg2+ to normal levels rescues the cell surface expression of NKG2D, Mg2+ supplementation may be an effective therapeutic strategy for XMEN patients and could be important for immune suppression of EBV and avoiding EBV-induced lymphoma [32].
In rodents, Mg2+ deficiency induced by low Mg2+ diet leads to decreased antibody production, thymic involution and chronic inflammation [82, 83]. Similarly, in humans, Mg2+ deficiency promotes a chronic inflammatory state that is associated with metabolic syndrome and type 2 diabetes mellitus (T2DM) [84]. Mg2+ supplementation inhibits IL-1β, TNFα and IL-6 secretion by mouse macrophages in vitro and in vivo [85, 86]. This latter observation fits with the concept of Mg2+ - Ca2+ antagonism, because reduced extracellular Mg2+ sensitizes immune cells to Ca2+ signaling leading to chronic inflammation. The precise relationship between the blood level (magnesemia) and the cytosolic level of Mg2+ is not well understood however free basal Mg2+ equilibrates more effectively with external Mg2+ then with the large amounts of intracellular bound Mg2+ [87, 88]. Thus, the full immunological impact of hypomagnesemia may be greater than we currently understand. Importantly, more than 60% of the US population does not meet the estimated daily requirement for Mg2+. The impact of the Mg2+ dietary deficiency on cytosolic free Mg2+, susceptibility to viral infections, and long-term health requires further investigation [89, 90].
Zn2+ deficiency and aging
A vital role for Zn2+ in immunity has been known for over 50 years, mostly through studies of dietary Zn2+ deficiency. The loss of expression of ZIP4 in acrodermatitis enteropathica (AE), a Zn2+ malabsorption syndrome, leads to immunodeficiency associated with lymphopenia and thymus atrophy [91]. Additionally, leukocytes from asthmatic children and pulmonary tuberculosis patients over-express ZIP2, and monocytes from septic patients up-regulate ZIP8 suggesting there is abnormal cytosolic free Zn2+ in these pathological settings [92–94].
As mentioned earlier, the study of PID has clarified signaling roles for Ca2+, Mg2+ and Zn2+. Nonetheless, these conditions also raise numerous questions about the cellular homeostasis of these ions. Mg2+ and Zn2+ deficiency can be compensated to a certain extent by external supplementation, but the mechanisms remains unknown. Unlike Zn2+ deficiency, Mg2+ deficiency can be masked by the lack of correlation between serum and cellular levels of Mg2+, which could mask the potential role of Mg2+ in pathological settings.
Concluding remarks: ion signaling as therapeutic target
Recent advances in understanding the role of divalent cations in immune cells have uncovered new signaling functions for Mg2+ and Zn2+ and have unveiled new vistas for future investigation. Many questions about Mg2+ and Zn2+ signaling are still unanswered. In fact, there are a plethora of apparent Mg2+ transport and channel proteins whose role in human physiology has not yet been determined [20]. For example, amongst the twenty-two Mg2+ channels known, sixteen are expressed in T cells, but except the few mentioned is this review, little is known about their potential role in immunity. Moreover, Mg2+ and Zn2+ mobilization has been observed in a variety of cell types, however the molecular mechanisms regulating these cation changes and the molecular consequences remain elusive [95]. Technical limitations such as probe sensitivity, low electrogenicity, and other considerations prevent the precise study of the regulated mobilization of these cations. Another aspect that begs additional experimentation is the understanding of the various effectors of divalent cations signaling and the network(s) that they generate. Beyond enhancing our fundamental scientific understanding, answering these questions has important medical implications especially, but not only, for immune disorders associated with divalent cations signaling deficiency, such as XMEN disease, SOCE deficiency or even dietary Zn2+ deficiency. Thirty years ago, the discovery of the immunosuppressant abilities of the Ca2+ signaling inhibitors such as the CN inhibitor cyclosporine A, revolutionized organ transplantation [96]. Thus, uncovering new Mg2+ and Zn2+ sensitive targets or functions could identify new therapeutic targets for immunomodulation or affecting the pathophysiology of other diseases. Moreover, the promising results of Mg2+ and Zn2+ supplementation in various pathological settings should encourage the development of new ion supplementation strategies [32, 86, 97–99].
Highlights.
Ca2+, Mg2+ and Zn2+ are signaling cations with interconnected signal networks
Cation signaling is essential for immunity and defects underlie immune diseases
Mechanisms of Mg2+ and Zn2+ mobilization and effectors require further definition
Modulation of ion signaling may lead to novel therapies for immune diseases
Acknowledgments
This work was supported by the Division of Intramural Research, NIAID, NIH. We thank past and present members of the Lenardo laboratory for their intellectual and experimental contributions to this review. We thank our colleagues at Merck for generous collaboration support. We apologize to the many investigators whose work could not be cited due to space limitations.
References
- 1.Tsien RY. Intracellular measurements of ion activities. Annual review of biophysics and bioengineering. 1983;12:91–116. doi: 10.1146/annurev.bb.12.060183.000515. [DOI] [PubMed] [Google Scholar]
- 2.Wolf FI, et al. Cell physiology of magnesium. Molecular aspects of medicine. 2003;24:11–26. doi: 10.1016/s0098-2997(02)00088-2. [DOI] [PubMed] [Google Scholar]
- 3.Romani AM. Magnesium homeostasis in Mammalian cells. Metal ions in life sciences. 2013;12:69–118. doi: 10.1007/978-94-007-5561-1_4. [DOI] [PubMed] [Google Scholar]
- 4.Flatman PW. Mechanisms of magnesium transport. Annu Rev Physiol. 1991;53:259–271. doi: 10.1146/annurev.ph.53.030191.001355. [DOI] [PubMed] [Google Scholar]
- 5.Ng LL, et al. Intracellular free magnesium in human lymphocytes and the response to lectins. Clinical science. 1991;80:539–547. doi: 10.1042/cs0800539. [DOI] [PubMed] [Google Scholar]
- 6.Takaya J, et al. Can magnesium act as a second messenger? Current data on translocation induced by various biologically active substances. Magnesium research : official organ of the International Society for the Development of Research on Magnesium. 2000;13:139–146. [PubMed] [Google Scholar]
- 7.Li FY, et al. Second messenger role for Mg2+ revealed by human T-cell immunodeficiency. Nature. 2011;475:471–476. doi: 10.1038/nature10246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baughman JM. Integrative genomics identifies MCU as an essential component of the mitochondrial calcium uniporter. Nature. 2011:341–345. doi: 10.1038/nature10234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Stefani D. A forty-kilodalton protein of the inner membrane is the mitochondrial calcium uniporter. Nature. 2011;476:336–340. doi: 10.1038/nature10230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zampese E, Pizzo P. Intracellular organelles in the saga of Ca2+ homeostasis: different molecules for different purposes? Cellular and molecular life sciences : CMLS. 2012;69:1077–1104. doi: 10.1007/s00018-011-0845-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shaw PJ, et al. Molecular regulation of CRAC channels and their role in lymphocyte function. Cellular and molecular life sciences : CMLS. 2013;70:2637–2656. doi: 10.1007/s00018-012-1175-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baba Y, et al. Essential function for the calcium sensor STIM1 in mast cell activation and anaphylactic responses. Nat Immunol. 2008;9:81–88. doi: 10.1038/ni1546. [DOI] [PubMed] [Google Scholar]
- 13.Vig M, et al. Defective mast cell effector functions in mice lacking the CRACM1 pore subunit of store-operated calcium release-activated calcium channels. Nat Immunol. 2008;9:89–96. doi: 10.1038/ni1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feske S. CRAC channelopathies. Pflugers Archiv : European journal of physiology. 2010;460:417–435. doi: 10.1007/s00424-009-0777-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoth M, Niemeyer BA. The neglected CRAC proteins: Orai2, Orai3, and STIM2. Current topics in membranes. 2013;71:237–271. doi: 10.1016/B978-0-12-407870-3.00010-X. [DOI] [PubMed] [Google Scholar]
- 16.Oh-Hora M, et al. Dual functions for the endoplasmic reticulum calcium sensors STIM1 and STIM2 in T cell activation and tolerance. Nat Immunol. 2008;9:432–443. doi: 10.1038/ni1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matsumoto M. The calcium sensors STIM1 and STIM2 control B cell regulatory function through interleukin-10 production. Immunity. 2011;34:703–714. doi: 10.1016/j.immuni.2011.03.016. [DOI] [PubMed] [Google Scholar]
- 18.Weidinger C, et al. STIM1 and STIM2-mediated Ca(2+) influx regulates antitumour immunity by CD8(+) T cells. EMBO molecular medicine. 2013;5:1311–1321. doi: 10.1002/emmm.201302989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bogeski I, et al. Differential redox regulation of ORAI ion channels: a mechanism to tune cellular calcium signaling. Sci Signal. 2010;3:ra24. doi: 10.1126/scisignal.2000672. [DOI] [PubMed] [Google Scholar]
- 20.Quamme GA. Molecular identification of ancient and modern mammalian magnesium transporters. Am J Physiol Cell Physiol. 2010;298:C407–C429. doi: 10.1152/ajpcell.00124.2009. [DOI] [PubMed] [Google Scholar]
- 21.Wenning AS, et al. TRP expression pattern and the functional importance of TRPC3 in primary human T-cells. Biochimica et biophysica acta. 2011;1813:412–423. doi: 10.1016/j.bbamcr.2010.12.022. [DOI] [PubMed] [Google Scholar]
- 22.Schmitz C, et al. Regulation of vertebrate cellular Mg2+ homeostasis by TRPM7. Cell. 2003;114:191–200. doi: 10.1016/s0092-8674(03)00556-7. [DOI] [PubMed] [Google Scholar]
- 23.Ryazanova LV, et al. TRPM7 is essential for Mg(2+) homeostasis in mammals. Nature communications. 2010;1:109. doi: 10.1038/ncomms1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jin J. Deletion of Trpm7 disrupts embryonic development and thymopoiesis without altering Mg2+ homeostasis. Science. 2008;322:756–760. doi: 10.1126/science.1163493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brandao K, et al. The role of Mg2+ in immune cells. Immunol Res. 2013;55:261–269. doi: 10.1007/s12026-012-8371-x. [DOI] [PubMed] [Google Scholar]
- 26.Sahni J, et al. SLC41A2 encodes a plasma-membrane Mg2+ transporter. The Biochemical journal. 2007;401:505–513. doi: 10.1042/BJ20060673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mandt T, et al. SLC41A1 Mg(2+) transport is regulated via Mg(2+)-dependent endosomal recycling through its N-terminal cytoplasmic domain. The Biochemical journal. 2011;439:129–139. doi: 10.1042/BJ20110807. [DOI] [PubMed] [Google Scholar]
- 28.Goytain A, Quamme GA. Identification and characterization of a novel mammalian Mg2+ transporter with channel-like properties. BMC Genomics. 2005;6:48. doi: 10.1186/1471-2164-6-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou H, Clapham DE. Mammalian MagT1 and TUSC3 are required for cellular magnesium uptake and vertebrate embryonic development. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:15750–15755. doi: 10.1073/pnas.0908332106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rink TJ, et al. Cytoplasmic pH and free Mg2+ in lymphocytes. The Journal of cell biology. 1982;95:189–196. doi: 10.1083/jcb.95.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rijkers GT, Griffioen AW. Changes in free cytoplasmic magnesium following activation of human lymphocytes. The Biochemical journal. 1993;289(Pt 2):373–377. doi: 10.1042/bj2890373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chaigne-Delalande B, et al. Mg2+ regulates cytotoxic functions of NK and CD8 T cells in chronic EBV infection through NKG2D. Science. 2013;341:186–191. doi: 10.1126/science.1240094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li FY, et al. XMEN disease: a new primary immunodeficiency affecting Mg2+ regulation of immunity against Epstein-Barr virus. Blood. 2014;123:2148–2152. doi: 10.1182/blood-2013-11-538686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Qiao Y, et al. Variant ATRX syndrome with dysfunction of ATRX and MAGT1 genes. Human mutation. 2014;35:58–62. doi: 10.1002/humu.22465. [DOI] [PubMed] [Google Scholar]
- 35.Hu Y. Drosophila MagT1 is upregulated by PKC activation. Biochem Biophys Res Commun. 2013;436:140–144. doi: 10.1016/j.bbrc.2013.05.048. [DOI] [PubMed] [Google Scholar]
- 36.Deason-Towne F, et al. Identification of Ser/Thr phosphorylation sites in the C2-domain of phospholipase C gamma2 (PLCgamma2) using TRPM7-kinase. Cell Signal. 2012;24:2070–2075. doi: 10.1016/j.cellsig.2012.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huang L, Tepaamorndech S. The SLC30 family of zinc transporters - a review of current understanding of their biological and pathophysiological roles. Molecular aspects of medicine. 2013;34:548–560. doi: 10.1016/j.mam.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 38.Jeong J, Eide DJ. The SLC39 family of zinc transporters. Molecular aspects of medicine. 2013;34:612–619. doi: 10.1016/j.mam.2012.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maret W. Redox biochemistry of mammalian metallothioneins. Journal of biological inorganic chemistry : JBIC : a publication of the Society of Biological Inorganic Chemistry. 2011;16:1079–1086. doi: 10.1007/s00775-011-0800-0. [DOI] [PubMed] [Google Scholar]
- 40.Sugiura T, et al. Dysfunction of macrophages in metallothionein-knock out mice. Journal of UOEH. 2004;26:193–205. doi: 10.7888/juoeh.26.193. [DOI] [PubMed] [Google Scholar]
- 41.Itoh N, et al. Reduced bactericidal activity and nitric oxide production in metallothionein-deficient macrophages in response to lipopolysaccharide stimulation. Toxicology. 2005;216:188–196. doi: 10.1016/j.tox.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 42.Haase H, Rink L. Zinc signals and immune function. Biofactors. 2014;40:27–40. doi: 10.1002/biof.1114. [DOI] [PubMed] [Google Scholar]
- 43.Aydemir TB, et al. Zinc transporter ZIP8 (SLC39A8) and zinc influence IFN-gamma expression in activated human T cells. J Leukoc Biol. 2009;86:337–348. doi: 10.1189/jlb.1208759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yu M. Regulation of T cell receptor signaling by activation-induced zinc influx. The Journal of experimental medicine. 2011;208:775–785. doi: 10.1084/jem.20100031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kaltenberg J, et al. Zinc signals promote IL-2-dependent proliferation of T cells. Eur J Immunol. 2010;40:1496–1503. doi: 10.1002/eji.200939574. [DOI] [PubMed] [Google Scholar]
- 46.Haase H, et al. Zinc signals are essential for lipopolysaccharide-induced signal transduction in monocytes. J Immunol. 2008;181:6491–6502. doi: 10.4049/jimmunol.181.9.6491. [DOI] [PubMed] [Google Scholar]
- 47.Sayadi A, et al. Zip14 expression induced by lipopolysaccharides in macrophages attenuates inflammatory response. Inflammation research : official journal of the European Histamine Research Society … [et al.] 2013;62:133–143. doi: 10.1007/s00011-012-0559-y. [DOI] [PubMed] [Google Scholar]
- 48.Hirano T. Roles of zinc and zinc signaling in immunity: zinc as an intracellular signaling molecule. Adv. Immunol. 2008;97:149–176. doi: 10.1016/S0065-2776(08)00003-5. [DOI] [PubMed] [Google Scholar]
- 49.Overbeck S, et al. Intracellular zinc homeostasis in leukocyte subsets is regulated by different expression of zinc exporters ZnT-1 to ZnT-9. J. Leukoc. Biol. 2008;83:368–380. doi: 10.1189/jlb.0307148. [DOI] [PubMed] [Google Scholar]
- 50.Kitamura H, et al. Toll-like receptor-mediated regulation of zinc homeostasis influences dendritic cell function. Nat Immunol. 2006;7:971–977. doi: 10.1038/ni1373. [DOI] [PubMed] [Google Scholar]
- 51.Bootman MD. Calcium signaling. Cold Spring Harbor perspectives in biology. 2012;4:a011171. doi: 10.1101/cshperspect.a011171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Maul-Pavicic A. ORAI1-mediated calcium influx is required for human cytotoxic lymphocyte degranulation and target cell lysis. Proc. Natl Acad. Sci. USA. 2011;108:3324–3329. doi: 10.1073/pnas.1013285108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Baba Y, et al. Calcium signaling in B cells: Regulation of cytosolic Ca increase and its sensor molecules, STIM1 and STIM2. Mol Immunol. 2013 doi: 10.1016/j.molimm.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 54.Vukcevic M, et al. Ryanodine receptor activation by Ca v 1.2 is involved in dendritic cell major histocompatibility complex class II surface expression. J Biol Chem. 2008;283:34913–34922. doi: 10.1074/jbc.M804472200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Becker SM, et al. Differential role of the Ca(2+) sensor synaptotagmin VII in macrophages and dendritic cells. Immunobiology. 2009;214:495–505. doi: 10.1016/j.imbio.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nunes P, Demaurex N. The role of calcium signaling in phagocytosis. J Leukoc Biol. 2010;88:57–68. doi: 10.1189/jlb.0110028. [DOI] [PubMed] [Google Scholar]
- 57.Braun A, et al. STIM1 is essential for Fcgamma receptor activation and autoimmune inflammation. Blood. 2009;113:1097–1104. doi: 10.1182/blood-2008-05-158477. [DOI] [PubMed] [Google Scholar]
- 58.Brechard S, et al. STIM1 but not STIM2 is an essential regulator of Ca2+ influx-mediated NADPH oxidase activity in neutrophil-like HL-60 cells. Biochem Pharmacol. 2009;78:504–513. doi: 10.1016/j.bcp.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 59.Schenten V, et al. Sphingosine kinases regulate NOX2 activity via p38 MAPK-dependent translocation of S100A8/A9. J Leukoc Biol. 2011;89:587–596. doi: 10.1189/jlb.0510304. [DOI] [PubMed] [Google Scholar]
- 60.Abboud CN, et al. The requirements for ionized calcium and magnesium in lymphocyte proliferation. J Cell Physiol. 1985;122:64–72. doi: 10.1002/jcp.1041220111. [DOI] [PubMed] [Google Scholar]
- 61.Deason-Towne F, et al. The Mg2+ transporter MagT1 partially rescues cell growth and Mg2+ uptake in cells lacking the channel-kinase TRPM7. FEBS Lett. 2011;585:2275–2278. doi: 10.1016/j.febslet.2011.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ibs KH, Rink L. Zinc-altered immune function. The Journal of nutrition. 2003;133:1452S–1456S. doi: 10.1093/jn/133.5.1452S. [DOI] [PubMed] [Google Scholar]
- 63.Haase H, Rink L. Functional significance of zinc-related signaling pathways in immune cells. Annu Rev Nutr. 2009;29:133–152. doi: 10.1146/annurev-nutr-080508-141119. [DOI] [PubMed] [Google Scholar]
- 64.Kitabayashi C, et al. Zinc suppresses Th17 development via inhibition of STAT3 activation. International immunology. 2010;22:375–386. doi: 10.1093/intimm/dxq017. [DOI] [PubMed] [Google Scholar]
- 65.Wong CP, Ho E. Zinc and its role in age-related inflammation and immune dysfunction. Molecular nutrition & food research. 2012;56:77–87. doi: 10.1002/mnfr.201100511. [DOI] [PubMed] [Google Scholar]
- 66.Lin RS, et al. Zinc is essential for binding of p56(lck) to CD4 and CD8alpha. J. Biol. Chem. 1998;273:32878. doi: 10.1074/jbc.273.49.32878. [DOI] [PubMed] [Google Scholar]
- 67.Romir J, et al. Crystal structure analysis and solution studies of human Lck-SH3; zinc-induced homodimerization competes with the binding of proline-rich motifs. Journal of molecular biology. 2007;365:1417–1428. doi: 10.1016/j.jmb.2006.10.058. [DOI] [PubMed] [Google Scholar]
- 68.Nishida K, et al. Zinc transporter Znt5/Slc30a5 is required for the mast cell-mediated delayed-type allergic reaction but not the immediate-type reaction. The Journal of experimental medicine. 2009;206:1351–1364. doi: 10.1084/jem.20082533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.von Bulow V, et al. Zinc-dependent suppression of TNF-alpha production is mediated by protein kinase A-induced inhibition of Raf-1, I kappa B kinase beta, and NF-kappa B. J Immunol. 2007;179:4180–4186. doi: 10.4049/jimmunol.179.6.4180. [DOI] [PubMed] [Google Scholar]
- 70.Dubben S, et al. Cellular zinc homeostasis is a regulator in monocyte differentiation of HL-60 cells by 1 alpha, 25-dihydroxyvitamin D3. J Leukoc Biol. 2010;87:833–844. doi: 10.1189/jlb.0409241. [DOI] [PubMed] [Google Scholar]
- 71.Wu C. Metallothioneins negatively regulate IL-27-induced type 1 regulatory T-cell differentiation. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:7802–7807. doi: 10.1073/pnas.1211776110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Striz I, Trebichavsky I. Calprotectin - a pleiotropic molecule in acute and chronic inflammation. Physiological research / Academia Scientiarum Bohemoslovaca. 2004;53:245–253. [PubMed] [Google Scholar]
- 73.Gifford JL, et al. Structures and metal-ion-binding properties of the Ca2+ -binding helix-loop-helix EF-hand motifs. The Biochemical journal. 2007;405:199–221. doi: 10.1042/BJ20070255. [DOI] [PubMed] [Google Scholar]
- 74.Senguen FT, Grabarek Z. X-ray structures of magnesium and manganese complexes with the N-terminal domain of calmodulin: insights into the mechanism and specificity of metal ion binding to an EF-hand. Biochemistry. 2012;51:6182–6194. doi: 10.1021/bi300698h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Li M. Subtype-specific control of P2X receptor channel signaling by ATP and Mg2+ Proceedings of the National Academy of Sciences of the United States of America. 2013;110:E3455–E3463. doi: 10.1073/pnas.1308088110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lee M, et al. Mg2+ ions reduce microglial and THP-1 cell neurotoxicity by inhibiting Ca2+ entry through purinergic channels. Brain Res. 2011;1369:21–35. doi: 10.1016/j.brainres.2010.10.084. [DOI] [PubMed] [Google Scholar]
- 77.Luo J, et al. Tonic inhibition of TRPV3 by Mg2+ in mouse epidermal keratinocytes. The Journal of investigative dermatology. 2012;132:2158–2165. doi: 10.1038/jid.2012.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhang J, et al. Role of Cav1.2 L-type Ca2+ channels in vascular tone: effects of nifedipine and Mg2+ American journal of physiology. Heart and circulatory physiology. 2007;292:H415–H425. doi: 10.1152/ajpheart.01214.2005. [DOI] [PubMed] [Google Scholar]
- 79.Moroz OV, et al. The role of zinc in the S100 proteins: insights from the X-ray structures. Amino acids. 2011;41:761–772. doi: 10.1007/s00726-010-0540-4. [DOI] [PubMed] [Google Scholar]
- 80.Donato R, et al. Functions of S100 proteins. Current molecular medicine. 2013;13:24–57. [PMC free article] [PubMed] [Google Scholar]
- 81.Feske S, et al. A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature. 2006;441:179–185. doi: 10.1038/nature04702. [DOI] [PubMed] [Google Scholar]
- 82.Malpuech-Brugere C, et al. Accelerated thymus involution in magnesium-deficient rats is related to enhanced apoptosis and sensitivity to oxidative stress. The British journal of nutrition. 1999;81:405–411. [PubMed] [Google Scholar]
- 83.Zimowska W, et al. Morphological and immune response alterations in the intestinal mucosa of the mouse after short periods on a low-magnesium diet. The British journal of nutrition. 2002;88:515–522. doi: 10.1079/BJN2002696. [DOI] [PubMed] [Google Scholar]
- 84.Rayssiguier Y, et al. Magnesium deficiency and metabolic syndrome: stress and inflammation may reflect calcium activation. Magnesium research : official organ of the International Society for the Development of Research on Magnesium. 2010;23:73–80. doi: 10.1684/mrh.2010.0208. [DOI] [PubMed] [Google Scholar]
- 85.Lin CY, et al. L-type calcium channels are involved in mediating the anti-inflammatory effects of magnesium sulphate. Br J Anaesth. 2010;104:44–51. doi: 10.1093/bja/aep336. [DOI] [PubMed] [Google Scholar]
- 86.Tam Tam HB, et al. Magnesium sulfate ameliorates maternal and fetal inflammation in a rat model of maternal infection. Am J Obstet Gynecol. 2011;204:364, e361–e368. doi: 10.1016/j.ajog.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 87.Barbagallo M, et al. Magnesium homeostasis and aging. Magnesium research : official organ of the International Society for the Development of Research on Magnesium. 2009;22:235–246. doi: 10.1684/mrh.2009.0187. [DOI] [PubMed] [Google Scholar]
- 88.Ulger Z, et al. Intra-erythrocyte magnesium levels and their clinical implications in geriatric outpatients. The journal of nutrition, health & aging. 2010;14:810–814. doi: 10.1007/s12603-010-0121-y. [DOI] [PubMed] [Google Scholar]
- 89.Castiglioni S, Maier JA. Magnesium and cancer: a dangerous liason. Magnesium research : official organ of the International Society for the Development of Research on Magnesium. 2011;24:S92–S100. doi: 10.1684/mrh.2011.0285. [DOI] [PubMed] [Google Scholar]
- 90.Rosanoff A, et al. Suboptimal magnesium status in the United States: are the health consequences underestimated? Nutr Rev. 2012;70:153–164. doi: 10.1111/j.1753-4887.2011.00465.x. [DOI] [PubMed] [Google Scholar]
- 91.Ackland ML, Michalczyk A. Zinc deficiency and its inherited disorders -a review. Genes & nutrition. 2006;1:41–49. doi: 10.1007/BF02829935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Xu TF, et al. Overexpression of Zip-2 mRNA in the leukocytes of asthmatic infants. Pediatric pulmonology. 2009;44:763–767. doi: 10.1002/ppul.21052. [DOI] [PubMed] [Google Scholar]
- 93.Besecker BY, et al. A comparison of zinc metabolism, inflammation, and disease severity in critically ill infected and noninfected adults early after intensive care unit admission. Am J Clin Nutr. 2011;93:1356–1364. doi: 10.3945/ajcn.110.008417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tao YT, et al. Up-regulation of Slc39A2(Zip2) mRNA in peripheral blood mononuclear cells from patients with pulmonary tuberculosis. Molecular biology reports. 2013;40:4979–4984. doi: 10.1007/s11033-013-2598-z. [DOI] [PubMed] [Google Scholar]
- 95.Feske S, et al. Ion channels and transporters in lymphocyte function and immunity. Nat Rev Immunol. 2012;12:532–547. doi: 10.1038/nri3233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Starzl TE, et al. Liver transplantation with use of cyclosporin a and prednisone. The New England journal of medicine. 1981;305:266–269. doi: 10.1056/NEJM198107303050507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kahmann L, et al. Zinc supplementation in the elderly reduces spontaneous inflammatory cytokine release and restores T cell functions. Rejuvenation research. 2008;11:227–237. doi: 10.1089/rej.2007.0613. [DOI] [PubMed] [Google Scholar]
- 98.Sugimoto J, et al. Magnesium decreases inflammatory cytokine production: a novel innate immunomodulatory mechanism. J Immunol. 2012;188:6338–6346. doi: 10.4049/jimmunol.1101765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Consolo LZ, et al. Zinc supplementation in children and adolescents with acute leukemia. Eur J Clin Nutr. 2013;67:1056–1059. doi: 10.1038/ejcn.2013.146. [DOI] [PubMed] [Google Scholar]
- 100.Gees M, et al. The role of transient receptor potential cation channels in Ca2+ signaling. Cold Spring Harbor perspectives in biology. 2010;2:a003962. doi: 10.1101/cshperspect.a003962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Knowles H, et al. The TRPM2 ion channel, an oxidative stress and metabolic sensor regulating innate immunity and inflammation. Immunol Res. 2013;55:241–248. doi: 10.1007/s12026-012-8373-8. [DOI] [PubMed] [Google Scholar]
- 102.Sumoza-Toledo A, et al. Dendritic cell maturation and chemotaxis is regulated by TRPM2-mediated lysosomal Ca2+ release. FASEB J. 2011;25:3529–3542. doi: 10.1096/fj.10-178483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Di A. The redox-sensitive cation channel TRPM2 modulates phagocyte ROS production and inflammation. Nature Immunol. 2012;13:29–34. doi: 10.1038/ni.2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yamamoto S. TRPM2-mediated Ca2+ influx induces chemokine production in monocytes that aggravates inflammatory neutrophil infiltration. Nature Med. 2008;14:738–747. doi: 10.1038/nm1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Peralvarez-Marin A, et al. What do we know about the transient receptor potential vanilloid 2 (TRPV2) ion channel? FEBS J. 2013;280:5471–5487. doi: 10.1111/febs.12302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Saunders CI, et al. Expression of transient receptor potential vanilloid 1 (TRPV1) and 2 (TRPV2) in human peripheral blood. Mol Immunol. 2007;44:1429–1435. doi: 10.1016/j.molimm.2006.04.027. [DOI] [PubMed] [Google Scholar]
- 107.Fernandes ES, et al. TRPV1 deletion enhances local inflammation and accelerates the onset of systemic inflammatory response syndrome. J Immunol. 2012;188:5741–5751. doi: 10.4049/jimmunol.1102147. [DOI] [PubMed] [Google Scholar]
- 108.Zhang D, et al. Mast-cell degranulation induced by physical stimuli involves the activation of transient-receptor-potential channel TRPV2. Physiological research / Academia Scientiarum Bohemoslovaca. 2012;61:113–124. doi: 10.33549/physiolres.932053. [DOI] [PubMed] [Google Scholar]
- 109.Link TM, et al. TRPV2 has a pivotal role in macrophage particle binding and phagocytosis. Nat Immunol. 2010;11:232–239. doi: 10.1038/ni.1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Niemeyer BA, Hoth M. Excitable T cells: Ca(v)1.4 channel contributions and controversies. Immunity. 2011;35:315–317. doi: 10.1016/j.immuni.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 111.Kotturi MF, Jefferies WA. Molecular characterization of L-type calcium channel splice variants expressed in human T lymphocytes. Mol Immunol. 2005;42:1461–1474. doi: 10.1016/j.molimm.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 112.Badou A, et al. Critical role for the beta regulatory subunits of Cav channels in T lymphocyte function. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:15529–15534. doi: 10.1073/pnas.0607262103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Grafton G, et al. A non-voltage-gated calcium channel with L-type characteristics activated by B cell receptor ligation. Biochem Pharmacol. 2003;66:2001–2009. doi: 10.1016/j.bcp.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 114.Omilusik K, et al. The Ca(v)1.4 calcium channel is a critical regulator of T cell receptor signaling and naive T cell homeostasis. Immunity. 2011;35:349–360. doi: 10.1016/j.immuni.2011.07.011. [DOI] [PubMed] [Google Scholar]
- 115.Park CY, et al. The CRAC channel activator STIM1 binds and inhibits L-type voltage-gated calcium channels. Science. 2010;330:101–105. doi: 10.1126/science.1191027. [DOI] [PubMed] [Google Scholar]
- 116.Wang Y, et al. The calcium store sensor, STIM1, reciprocally controls Orai and CaV1.2 channels. Science. 2010;330:105–109. doi: 10.1126/science.1191086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kawano A, et al. Regulation of P2X7-dependent inflammatory functions by P2X4 receptor in mouse macrophages. Biochem Biophys Res Commun. 2012;420:102–107. doi: 10.1016/j.bbrc.2012.02.122. [DOI] [PubMed] [Google Scholar]
- 118.Sakaki H, et al. P2X4 receptor regulates P2X7 receptor-dependent IL-1beta and IL-18 release in mouse bone marrow-derived dendritic cells. Biochem Biophys Res Commun. 2013;432:406–411. doi: 10.1016/j.bbrc.2013.01.135. [DOI] [PubMed] [Google Scholar]
- 119.Junger WG. Immune cell regulation by autocrine purinergic signalling. Nat Rev Immunol. 2011;11:201–212. doi: 10.1038/nri2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Yip L, et al. Autocrine regulation of T-cell activation by ATP release and P2X7 receptors. FASEB J. 2009;23:1685–1693. doi: 10.1096/fj.08-126458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Chen Y, et al. Purinergic signaling: a fundamental mechanism in neutrophil activation. Sci Signal. 2010;3:ra45. doi: 10.1126/scisignal.2000549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Rukgauer M, et al. Reference values for the trace elements copper, manganese, selenium, and zinc in the serum/plasma of children, adolescents, and adults. Journal of trace elements in medicine and biology : organ of the Society for Minerals and Trace Elements. 1997;11:92–98. doi: 10.1016/S0946-672X(97)80032-6. [DOI] [PubMed] [Google Scholar]
- 123.Haase H, et al. Flow cytometric measurement of labile zinc in peripheral blood mononuclear cells. Analytical biochemistry. 2006;352:222–230. doi: 10.1016/j.ab.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 124.Shaw PJ, Feske S. Physiological and pathophysiological functions of SOCE in the immune system. Front Biosci (Elite Ed) 2012;4:2253–2268. doi: 10.2741/540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Suzuki R, et al. Loss of TRPC1-mediated Ca2+ influx contributes to impaired degranulation in Fyn-deficient mouse bone marrow-derived mast cells. J Leukoc Biol. 2010;88:863–875. doi: 10.1189/jlb.0510253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Cheng KT, et al. Contribution and regulation of TRPC channels in store-operated Ca2+ entry. Current topics in membranes. 2013;71:149–179. doi: 10.1016/B978-0-12-407870-3.00007-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Omilusik K. The CaV1.4 calcium channel is a critical regulator of T cell receptor signaling and naive T cell homeostasis. Immunity. 2011;35:349–360. doi: 10.1016/j.immuni.2011.07.011. [DOI] [PubMed] [Google Scholar]
- 128.Di L. Inhibition of the K+ channel KCa3.1 ameliorates T cell-mediated colitis. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:1541–1546. doi: 10.1073/pnas.0910133107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Vennekens R, Nilius B. Insights into TRPM4 function, regulation and physiological role. Handb. Exp. Pharmacol. 2007;179:269–285. doi: 10.1007/978-3-540-34891-7_16. [DOI] [PubMed] [Google Scholar]
- 130.Numaga T, et al. Ca2+ influx and protein scaffolding via TRPC3 sustain PKCbeta and ERK activation in B cells. J Cell Sci. 2010;123:927–938. doi: 10.1242/jcs.061051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Carrillo C, et al. Diacylglycerol-containing oleic acid induces increases in [Ca(2+)](i) via TRPC3/6 channels in human T-cells. Biochimica et biophysica acta. 2012;1821:618–626. doi: 10.1016/j.bbalip.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 132.M M. Zinc improves the development of human CD34+ cell progenitors towards natural killer cells and induces the expression of GATA-3 transcription factor. Int. J. Biochem. Cell Biol. 2007;39:955. doi: 10.1016/j.biocel.2007.01.011. [DOI] [PubMed] [Google Scholar]