Abstract
Background
The arterial vasculature is subjected to considerably greater biomechanical stress than the venous circulation. This is reflected in the difference in morphology between large arteries and veins, however little is known about the molecular differences that arise as a consequence of biomechanical stress. Previously, we identified a group of arterial intima-enriched (AIE) genes: sciellin, periplakin, SPRR3, envoplakin, galectin 7, and plakoglobin that are functionally related in that they contribute to the stress properties of stratified epithelium. We sought to test our hypothesis that these genes were regulated by biomechanical stress in vascular smooth muscle cells (VSMCs).
Methods
Immunofluorescence was employed to determine the expression of the AIE genes in saphenous vein coronary artery bypass grafts. Furthermore, we used a model of cyclic stress to determine if the AIE genes were regulated by biomechanical stress in VSMCs in vitro.
Results
Sciellin and periplakin were upregulated in saphenous vein coronary artery bypass grafts after arterialization, but were absent in non-arterialized saphenous veins. Sciellin, SPRR3, and periplakin transcripts were all upregulated (4.67-, 4.95-, 2.77-fold, respectively) by prolonged exposure to cyclic strain (24-72 h), but not at earlier time points.
Conclusions
These findings suggest a novel role for several human AIE genes in the VSMC response to arterialization and extended cyclic strain.
Summary
Biomechanical stress has long been implicated in vascular pathologies. We report the novel finding of a group of genes, previously studied in stratified epithelium, that were regulated by prolonged cyclic stress in vascular smooth muscle cells. This may have important implications to vascular disease.
Keywords: Biomechanical stress, Hemodynamics, CABG, Vascular SMC, Cyclic strain
1. Introduction
Blood flowing through the vasculature exerts a significant force on the cells of the arteries resulting in biomechanical stress: cyclic and static strain is produced as blood pressure pushes against the vessel wall in systole and diastole, respectively, and shear stress as the blood flows through the lumen [1,2]. However, veins exist in a low pressure environment and are less subject to such forces [3,4]. When veins are arterialized and subjected to a high-stress environment, such as when the saphenous vein is used as a coronary artery bypass graft (CABG), the primary response is extensive fibrointimal hyperplasia (FIH) followed by fibrosis and atherosclerosis that may eventually lead to complete graft failure [5,6]. Within one year of surgery, 10-15% of grafts are occluded; within 10 years 70% of grafts exhibit some FIH, and over half of those contain lesions large enough to cause occlusion [7,8]. Vascular smooth muscle cells (VSMCs) are a major constituent of the vascular wall and are key cells in neointimal lesions in grafted veins and atherosclerosis [9].
We previously identified a group of functionally related genes that we call the arterial intima-enriched (AIE) genes based on expression that is limited to the intimal VSMCs of large arteries, but not veins [10,11]. The AIE genes include sciellin, periplakin, Small Proline-Rich Protein 3 (SPRR3), galectin 7, and plakoglobin. These gene products have been previously characterized in stratified epithelia where they contribute to the ability of the tissues to withstand chemical and biomechanical stresses. Specifically, these proteins are components of the cornified envelope (CE), a 10-15 nm thick highly cross-linked structure just inside the plasma membrane of stratified epithelial cells such as keratinocytes [12]. Of particular interest to this study are sciellin, periplakin, and SPRR3, each of which has a different function in the CE. Sciellin contains a core of conserved repeats and a unique proline-rich N-terminal domain that are proposed to serve a structural role. Furthermore, it possesses a C-terminal LIM domain that is believed to be a protein-interaction domain, possibly bestowing a scaffolding function on sciellin [13–15]. Periplakin, a member of the plakin family of proteins, is enriched in desmosomes where it acts as a mediator of intermediate filament assembly [16,17]. SPRR3, is cross-linked by transglutamination to other CE members, thereby potentially serving as a flexible linker of structural proteins in the CE. SPRR3 is hypothesized to have a highly flexible core of proline-rich repeats, the most flexible of the SPRR family, that confers elasticity on the CE [18,19].
Because arteries are under considerably greater cyclic strain than veins, we hypothesized that at least a subset of the AIE genes are regulated by cyclic strain in the arteries [20]. We examined their distribution and expression by immunofluorescence in post mortem samples of arterialized saphenous CABGs and mRNA transcript levels in an in vitro model system of cyclic strain. The results of this study provide the novel finding that VSMCs up-regulate the transcripts of the structural proteins sciellin, periplakin, and SPRR3 in response to prolonged biomechanical stress. These findings shed new light on the response of VSMCs to biomechanical stress.
2. Methods
2.1. Cell culture and biomechanical stress
We isolated VSMCs from excess human aortic tissue from heart explants. Briefly, the vessels were cleaned of all connective tissue and cut longitudinally to expose the lumen, which then was scraped to remove the endothelium. The vessel wall was peeled apart to expose VSMCs on both surfaces. These were then cut into 1 cm square pieces and placed smooth muscle-side down in SmGM2 (Clonetics) and held in place by wire mesh. Outgrowths of cells derived from the explants were expanded. This resulted in a mixed population of cells from both the media and intima. After one passage, co-immunocytochemistry was performed with both anti-smooth muscle alpha actin (1:1000, Sigma), anti-smooth muscle myosin heavy chain (1:250, Abcam), and anti-vonWillebrand factor (1:200, Dako). Cells were only used for experiments when cultures were 95%−100% smooth muscle alpha actin and smooth muscle myosin heavy chain positive and vonWillebrand Factor negative. Cultures were maintained in SmGM2 and used between passages 4-9.
Cells were exposed to cyclic strain as follows. Cells were plated on collagen I coated Flex I elastomer-bottom plates or Flex II solid plates, as control, (Flexcell, Int.) and exposed to cyclic strain with 15-20% elongation for 12, 24, 48 and 72 hours (60 cycles/min) [21,22]. One hour prior to starting strain, media was changed to 10% FBS/DMEM with antibiotics. Following strain application, cells were washed twice with PBS and harvested in Trizol for RNA extraction, or fixed for immunofluorescence. Cyclic strain experiments were performed a minimum of three times with cells from two independent cell isolations.
2.2. Reverse transcription and semi-quantitative PCR
RNA from VSMCs was isolated with Trizol (Invitrogen) following the manufacturer's instructions and quality confirmed by an A260/280 ratio >1.9. Semi-quantitative PCR analysis was performed as previously described [10,23]. Briefly, 1ug RNA was used for cDNA synthesis using iScript cDNA synthesis kit (Bio-Rad). The cDNA was then used for semi-quantitative PCR using SYBR-green iQ PCR supermix and run in an iCycler Real-Time PCR thermal cycler (Bio-Rad). Primer sequences and conditions for use can be found in Table 1. All transcript levels were set relative to the level of 18S RNA present in each sample. The results are presented as fold change from non-stressed samples. Results are shown from three independent experiments, each of which was analyzed in triplicate.
Table 1.
Primers used for qRT-PCR
| Tm | Product Size | Primer Sequence | |
|---|---|---|---|
| 18S Forward | 60°C | 100 bp | 5′-CGCCGCTAGAGGTGAAATTCT-3′ |
| Reverse | 5′-CGAACCTCCGACTTTCGTTCT-3′ | ||
| MMP-2 Forward | 54°C | 51 bp | 5′-ATCGCTCAGATCCGTGGTG-3′ |
| Reverse | 5 ′-CCAAATGAACCGGTCCTTGA-3′ | ||
| Elastin Forward | 54°C | 144 bp | 5′-TTCCCCGCAGTTACCTTTCC-3′ |
| Reverse | 5′-AACCACCGCACCTGCAGA-3′ | ||
| Sciellin Forward | 52°C | 145 bp | 5′-CCTGAAAACACCTGACACCA-3′ |
| Reverse | 5′-TCCTGGCATTCACTTTAGCA-3′ | ||
| Periplakin Forward | 58°C | 127 bp | 5′-ACTGGAGTGACCGCAACCTC-3′ |
| Reverse | 5 ′-TGAAATTCTCATACTGGCGCC-3′ | ||
| SPRR3 Forward | 52°C | 96 bp | 5′-ATGTCCTTCAACGGTCACTCC-3′ |
| Reverse | 5′-CTCTTCGGTTGGTGGTCTAC-3′ | ||
| Plakoglobin Forward | 60°C | 70 bp | 5 ′-CAACCAGGAGAGCAAGCTGAT-3′ |
| Reverse | 5′-GTTACGCATGATCTGCACGAG-3′ | ||
| Galectin 7 Forward | 60°C | 52 bp | 5 ′-CACGGTGCTGAGAATTCGC-3′ |
| Reverse | 5′-ATGGAACCTGCTGGCATTG-3′ | ||
| Envoplakin Forward | 60°C | 65 bp | 5 ′-CTTCCTGAACCTGTGTATCTGCC-3′ |
| Reverse | 5 ′-GGAACCGGCGGTAGTCCT-3′ |
2.3. Immunofluorescence
Sections from formalin-fixed, paraffin-embedded human saphenous vein-derived CABG grafts and control non-arterialized saphenous veins were stained as described [10]. The duration of the bypass grafts used in our studies was 12-72 months post-implantation and were obtained from both male and female patients. These grafts were from individuals who received bypass grafts for ischemic heart disease. Based on our analysis of graft morphology, grafts that demonstrated significant intimal hyperplasia and/or atherosclerosis were used for further study. Human aortic SMCs grown on elastomer membranes were fixed for 20 min at room temperature with 4% paraformaldehyde, followed by permeabilization with 0.4% Triton in PBS. Subsequently, the membranes were cut out of the dishes and cut into smaller wedges so that each membrane could be stained for several different proteins. Each membrane wedge was blocked for 1 hr with 10% goat serum and primary antibody applied in 3% goat serum overnight at 4°C. The following primary antibodies were used: polyclonal SPRR3 (1:400, Alexis Biochemicals), polyclonal sciellin (1:400, the kind gift of Dr. Howard Baden, Department of Dermatology, Cutaneous Biology Research Center, Harvard Medical School, Massachusetts General Hospital, Boston, MA [24]), polyclonal periplakin (1:100, generated against R1729-G1754. The antibody was affinity purified and recognized a single band of 195 kDa from human skin protein lysate by western blot [25,26]), monoclonal plakoglobin (1:200, Sigma), polyclonal galectin 7 (1:400, Bethyl Laboratories), monoclonal vimentin (1:100, Dako), monoclonal Golgin 97 (1:100, Molecular Probes), monoclonal Prohibitin (1:100, Calbiochem), and monoclonal protein disulfide isomerase (PDI) (1:100, Affinity BioReagents). The slides/membranes were rinsed twice with PBS and incubated in either goat anti-rabbit-Cy3, goat anti-mouse-alexa 488 (1:200, Molecular Probes); selected membranes were co-stained with FITC- or rhodamine-labeled phalloidin (1:40, Molecular Probes). Finally, the samples were counterstained with DAPI and mounted as described [10,27]. As a negative control, parallel staining was carried out with secondary antibody alone. Slides were viewed under a Zeiss Axioplan microscope (Carl Zeiss MicroImaging), and analyzed with MetaMorph Imaging system (Molecular Devices). The staining and co-localization was confirmed by fluorescent confocal microscopy using a Zeiss upright LSM510 confocal microscope and the images were analyzed using LSM Image Browser.
2.4. Statistical analysis
CABG data was analyzed by chi squared analysis and the stress data using a one-way ANOVA with Newman-Keuls Comparison analysis. Error bars represent±standard deviation. <b.05 was considered statistically significant.
3. Results
3.1. Sciellin and periplakin are accumulated in coronary artery bypass grafts
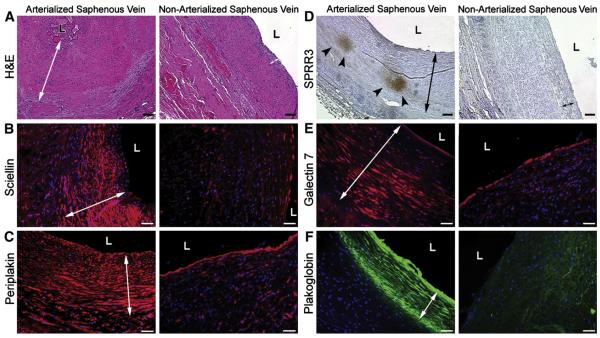
Saphenous veins are commonly used to bypass occluded coronary arteries [6]. Upon introduction into the arterial circulation, the saphenous vein graft can experience up to 10-fold increase in biomechanical forces and therefore serves as an ideal in vivo model to test the hypothesis that the AIE genes are regulated by biomechanical stress [20]. Cadaveric paraffin-embedded saphenous vein CABGs were analyzed by indirect immunofluorescence with antibodies against sciellin, periplakin, SPRR3, plakoglobin, and galectin 7. The arterialized explants all contained extensive FIH with varying degrees of thrombosis and plaque formation while the non-arterialized saphenous veins displayed only minimal intimal hyperplasia (Fig. 1A). In the absence of neointima formation, non-arterialized veins were not recognized by any of the antibodies (b-f, right panels, Table 2, n=4 non-arterialized veins). However, when arterialized veins were examined for the AIE proteins, greater than 80% and 50% were positive for sciellin and periplakin, respectively (Table 2, n=17 (sciellin) and n=26 (periplakin) and Fig. 1B, C, left panels). However, consistent with our previous observations, SPRR3 expression was restricted to atherosclerotic plaques within grafts (Fig. 1D) [10]. Furthermore, while plakoglobin and galectin 7 stained positive in some arterialized veins (but were negative in non-arterialized saphenous veins), the number of positive grafts was not statistically significant (Table 2, n=29 (plakoglobin and galectin 7), Fig. 1E, F). These findings indicate that sciellin and periplakin protein accumulated in vivo in intimal VSMCs following arterialization. Additionally, our previously described finding of atheroma-associated SPRR3 expression in arteries has been extended to atheromas present in arterialized saphenous vein grafts [10].
Fig. 1.
AIE proteins are accumulated in arterialized veins. Cadaveric saphenous vein CABGs and non-arterialized saphenous veins were examined for expression of sciellin (B), periplakin (C), SPRR3 (D, arrowheads), galectin 7 (E), and plakoglobin (F) by indirect immunofluorescence. Extensive FIH is seen in the arterialized veins (arrows), but to a lesser extent in non-arterialized veins, as seen in H&E stained sections (A). L indicates lumen. Scale bar is 80 μm (A, D) and 40 μm (B, C, E, F).
Table 2.
Quantitation of CABG staining
| Control Vein | CABG | P-Value | |
|---|---|---|---|
| Sceillin | 0/4 (0%) | 14/17 (82.4%) | <.0002 |
| Periplakin | 0/4 (0%) | 14/26 (53.8%) | <.05 |
| Galectin 7 | 0/4 (0%) | 5/29 (17.2%) | >.3 |
| Plakoglobin | 0/4 (0%) | 2/29 (6.9%) | >.3 |
Control vein is non-arterialized saphenous vein; CABG is saphenous vein coronary artery bypass graft. Each CABG counted per group represents a unique graft from different individuals.
3.2. Sciellin, Periplakin, and SPRR3 are upregulated by cyclic strain in vitro
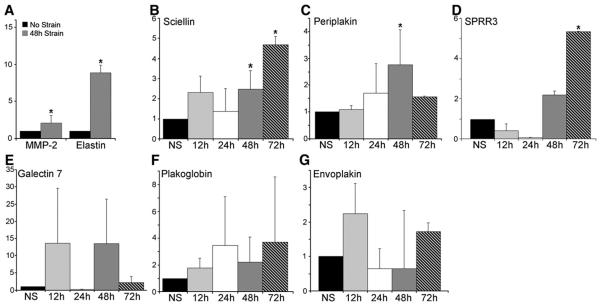
Because increased biomechanical stress during arterialization is an important stimulus for intimal hyperplasia formation, we hypothesized that expression of the AIE genes was upregulated in response to biomechanical stress [9,20,28]. To test this, VSMCs were isolated from aorta samples from two individuals. The cells were then exposed to cyclic strain by applying a computer-controlled vacuum to elastomer-bottomed plates (with 15-20% stretch). Previous studies have measured the distention of human arteries at 18% elongation, and therefore a similar level of cyclic stretch was applied to the cells in vitro [29,30]. This was followed with analysis by qRT-PCR of the mRNA transcript levels of two control genes, MMP-2 and elastin, which have been shown to be upregulated in VSMCs by biomechanical stress, and the six AIE genes [31,32]. The VSMCs were responsive to cyclic strain at 48 h as demonstrated by the upregulation of MMP-2 and elastin transcripts by 2.09±0.366-fold (P=.035, n=4) and 8.88±0.028-fold (P=.00162, n=4), respectively (Fig. 2A). Analysis of the AIE genes revealed upregulation of sciellin, periplakin, and SPRR3 at 48 and 72 h of cyclic strain (Fig. 2B, C, D). Sciellin transcripts increased by 2.47±0.926-fold (P≤.05, n=4) and 4.67±0.421-fold (P≤.05, n=3), periplakin by 2.77±1.30-fold (P≤.05, n=4) and 1.56±0.028-fold, and SPRR3 by 2.1±0.18-fold (P≤.05, n=3) and 4.95±0.029-fold (P≤.05, n=3) after cyclic strain for 48 and 72h, respectively. However, the data were not statistically significant for plakoglobin, envoplakin, and galectin 7 transcripts (Fig. 2E, F, G). These data suggest that sciellin, periplakin, and SPRR3 are all transcriptionally responsive to prolonged exposure to cyclic strain in human VSMCs.
Fig. 2.
AIE gene expression regulation by biomechanical stress. Real time RT-PCR revealed changes in transcript levels. (A) Fold change of MMP-2 and elastin after 48h. Sciellin transcripts increase significantly after prolonged cyclic strain of 48h and 72h (B), as do periplakin and SPRR3 (C, D). No statistically significant increase was seen for galectin 7, plakoglobin, or envoplakin (E, F, G). *P≤.05; NS: No Cyclic Strain.
3.3. Arterial Intima Enriched proteins had unique expression patterns in VSMCs
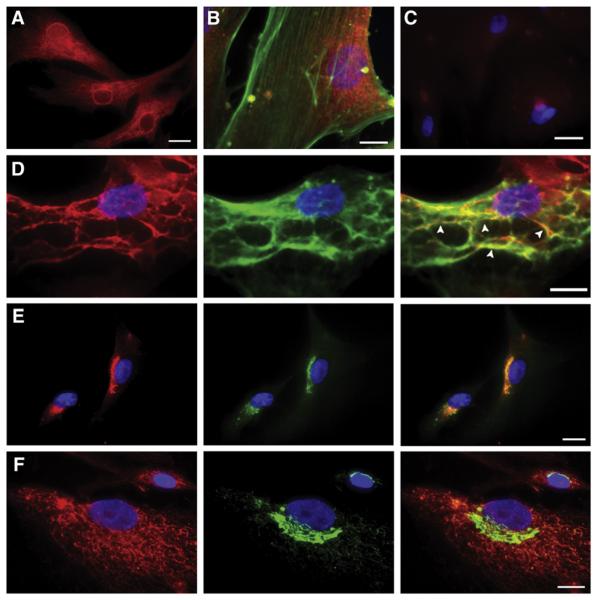
Previous studies have shown that the location of a protein in VSMCs can be modulated in response to biomechanical stress [33–36]. Therefore, subcellular localization of sciellin, periplakin, SPRR3, galectin 7, and plakoglobin was studied by indirect immunofluorescence in VSMCs with or without 72h of cyclic strain. Galectin 7 and plakoglobin were only faintly detectable and did not change cellular localization with cyclic strain when compared to unstrained controls (data not shown). Sciellin, however, displayed a diffuse cytoplasmic and peri-nuclear expression pattern (Fig. 3A) that did not change with stress. Sciellin failed to co-localize with other cellular elements, such as filamentous actin (Fig. 3B), the intermediate filament vimentin, tubulin, the golgi apparatus (as detected by an antibody against golgin 97), or the endoplasmic reticulum (as detected by an antibody against PDI) (data not shown). Therefore, sciellin distribution did not correlate with any major structural elements within the cell. A previous study demonstrated that periplakin associates with vimentin in keratinocytes, establishing its role as a structural protein [16,37]. Not surprisingly, periplakin co-localized with vimentin in VSMCs, but no change was noted with the addition of cyclic strain (Fig. 3D). Periplakin did not co-localize with actin or tubulin (data not shown). Finally, SPRR3 was detected in a peri-nuclear region in cells without strain, whereas it was more widely distributed through the cytoplasm in a reticular pattern after 72h of cyclic strain. In unstrained cells, SPRR3 co-localized with the golgi apparatus (Fig. 3E). However, after strain, the cytoplasmic distribution of SPRR3 did not co-localize with our selected golgi-, endoplasmic reticulum- or mitochondria-marker proteins. It also failed to co-localize with F-actin, vimentin, or tubulin (Fig. 3F, data not shown). For all of these experiments, the secondary antibody-only negative control was negative (Fig. 3C).
Fig. 3.
Localization of AIE gene products in VSMCs by indirect immunofluorescence. Sciellin (red) in a diffuse cytoplasmic and peri-nuclear pattern (A), does not co-localize with actin (FITC-phalloidin, green) (B). Secondary-only control (C). Periplakin (red) co-localized with vimentin (green) (co-localization— arrowheads) (D). SPRR3 (red) co-localizes with golgi marker (green) in the absence of cyclic strain (E) but is found in a cytoplasmic network after 72h stress (F). Nuclei are stained blue by DAPI. Scale bar is 20μm.
4. Discussion
The differences in the molecular profiles of adult arteries and veins are not well known, despite the established functional, morphological, and pathological differences [38]. We previously identified a group of genes that are expressed in arterial intimal smooth muscle cells, the arterial intimal enriched (AIE) genes [10]. This study demonstrated that expression of a subset of the AIE genes, specifically sciellin and periplakin, was increased in arterialized human veins (CABGs), and their transcripts, as well as that of SPRR3, which has an expression pattern limited to atherosclerotic plaques, were upregulated by cyclic strain. It should be noted that the VSMCs used in the in vitro studies were isolated from human aortas, whereas upregulation of protein expression in vivo was assessed in arterialized saphenous vein grafts. Nevertheless, there remains close correlation between the in vivo and in vitro work (e.g.: sciellin and periplakin expression was enhanced in the saphenous vein CABGs and in aortic VSMCs, whereas plakoglobin and envoplakin were not considerably expressed in either set of cell types).
The manner by which the AIE genes are regulated by cyclic strain remains unclear. The majority of studies that have examined signaling molecules in VSMCs in response to stress have focused on early time points (5min to 24h), whereas the effects on sciellin, periplakin, and SPRR3 were detected after 48h cyclic strain [20,21,34]. Our finding that the expression of these genes was increased following prolonged exposure to cyclic strain (48 to 72h) may provide better insight into the mechanisms of VSMC adaptation in vivo to long-term mechanical stress [28,39]. Furthermore, in light of the fact that the structural effect of cyclic strain in VSMCs is not completely understood, it is of particular interest that previous work in keratinocytes that has defined sciellin, periplakin, and SPRR3 as structural proteins is of interest [32,33]. Modulation of other structural proteins by cyclic strain in VSMCs has been previously shown. Regulation of extracellular matrix proteins such as tenascin C and elastin by cyclic strain has been demonstrated [32,33]. Zyxin, a focal adhesion protein, has been shown to translocate from focal adhesions to the nucleus where it is required for expression of certain mechanosenitive genes [33]. Thus, this study further develops the notion that cyclic strain regulates cellular function through the regulation of structural proteins, specifically sciellin, periplakin, and SPRR3. These alterations may have important implications during vascular adaptation to arterialization.
The function of the AIE genes in VSMCs remains unclear, but studies of AIE genes in stratified epithelia, where they serve as structural molecules, may yet shed light on their function in the vasculature. Sciellin was initially characterized in a screen of antigens generated from human keratinocyte CE fragments as a highly insoluble CE precursor and transglutaminase substrate [40]. Subsequently, sciellin was shown to contain a proline-rich N-terminal domain, a central 16 repeat glutamine- and lysine-rich motif—the transglutaminase substrate—and a C-terminal LIM domain [13,15]. LIM domains, which are protein-interaction motifs, have been demonstrated in other proteins associated with the cytoskeleton and biomechanical stress in VSMCs [33,41]. Notably, there was no phenotype observed in sciellin knockout mice [15]. A yeast two-hybrid screen revealed that sciellin in keratinocytes interacts with vitamin D-upregulated protein (VDUP), an endogenous inhibitor of thioredoxin [24,42]. Interestingly, VDUP expression has been shown to be regulated by biomechanical stress in cardiomyocytes and by oxidative stress in VSMCs, resulting in altered thioredoxin activity [42–44]. The relationship between VDUP and sciellin as well as VDUP and biomechanical stress implicates a possible role for sciellin and VDUP together in our system.
Periplakin has also been identified as a CE precursor. It is found in desmosomal plaques and interdesmosomal networks where it has been shown to associate with keratins and vimentin, and has been hypothesized to act as a scaffold for intermediate filament assembly [16]. Thus, our finding that periplakin co-localizes with vimentin in VSMCs is consistent with the data obtained from studies in keratinocytes. While the periplakin knockout has no obvious phenotype, deletions of other desmosomal proteins in mice, such as plakoglobin and desmoplakin, are lethal in utero with major defects in heart muscle resulting from an inability to function under biomechanical stress [17,45,46].
SPRR3 is a member of the small proline rich protein family that all contain glutamine- and lysinerich head and tail domains and a proline rich core. In SPRR3, the head and tail domains are substrates for transglutamination, while the proline-rich central domain is highly flexible. This structure has been correlated to a function as a crossbridging protein, such that SPRR3 is transglutaminated on either end to provide mechanical resistance, while the flexible central domain confers elasticity to the protein complex, and thus the tissue [19]. Therefore, we expected to find SPRR3 associated with structural cellular elements, such as the cytoskeleton, but were surprised to see its association with the golgi apparatus. Thus, the role of SPRR3 in isolated VSMCs remains a mystery. Interestingly, another member of the SPRR family has recently been implicated in cardiovascular physiology. Pradervand, et al., showed that SPRR1a is induced in cardiac tissue in response to ischemic and biomechanical stress and pointed to a possible cardioprotective role [47]. Furthermore, SPRR3 regulation in VSMCs by cyclic strain was recently shown to be dependent on integrin α1β1 interaction with type I collagen [11].
Future studies will focus on elucidating the function of these proteins and the mechanisms by which their expression is regulated by cyclic stress. Preliminary data from our lab suggest that sciellin, periplakin, and SPRR3 are regulated by independent pathways in VSMCs and will be pursued in future studies.
Acknowledgment
The authors wish to thank Drs. Edward J. Butterworth, Samuel A. Santoro, and Jeffery M. Davidson for their critical review of the manuscript.
Grants: This work was supported by the National Institutes of Health Training Grant 5T32HL07751 (ALP), Vanderbilt Physician Development Award, Veterans Affairs Career Development Award, and KO8HL84020 from the National Institutes of Health, Bethesda, Maryland (PPY).
Footnotes
Disclosures: This work was also supported by the Pfizer Atorvastatin Research Award (PPY).
References
- [1].Williams B. Mechanical influences on vascular smooth muscle cell function. J Hypertens. 1998;16:1921–9. doi: 10.1097/00004872-199816121-00011. [DOI] [PubMed] [Google Scholar]
- [2].Lehoux S, Tedgui A. Signal transduction of mechanical stresses in the vascular wall. Hypertension. 1998;32:338–45. doi: 10.1161/01.hyp.32.2.338. [DOI] [PubMed] [Google Scholar]
- [3].Matsushita H, Lee Kh K, Tsao PS. Cyclic strain induces reactive oxygen species production via an endothelial NAD(P)H oxidase. J Cell Biochem. 2001;81:99–106. doi: 10.1002/jcb.1094. [DOI] [PubMed] [Google Scholar]
- [4].Mayr M, Hu Y, Hainaut H, Xu Q. Mechanical stress-induced DNA damage and rac-p38mapk signal pathways mediate p53-dependent apoptosis in vascular smooth muscle cells. FASEB J. 2002;16:1423–5. doi: 10.1096/fj.02-0042fje. [DOI] [PubMed] [Google Scholar]
- [5].Purcell C, Tennant M, McGeachie J. Neo-intimal hyperplasia in vascular grafts and its implications for autologous arterial grafting. Ann R Coll Surg Engl. 1997;79:164–8. [PMC free article] [PubMed] [Google Scholar]
- [6].Motwani JG, Topol EJ. Aortocoronary saphenous vein graft disease: pathogenesis, predisposition, and prevention. Circulation. 1998;97:916–31. doi: 10.1161/01.cir.97.9.916. [DOI] [PubMed] [Google Scholar]
- [7].Shuhaiber JH, Evans AN, Massad MG, Geha AS. Mechanisms and future directions for prevention of vein graft failure in coronary bypass surgery. Eur J Cardiothorac Surg. 2002;22:387–96. doi: 10.1016/s1010-7940(02)00253-1. [DOI] [PubMed] [Google Scholar]
- [8].Wallner K, Li C, Fishbein MC, Shah PK, Sharifi BG. Arterialization of human vein grafts is associated with tenascin-c expression. J Am Coll Cardiol. 1999;34:871–5. doi: 10.1016/s0735-1097(99)00272-7. [DOI] [PubMed] [Google Scholar]
- [9].Sidawy AN, Sumpio BE, DePalma RG. The basic science of vascular disease. Futura Pub. Co.; Armonk (NY): 1997. [Google Scholar]
- [10].Young PP, Modur V, Teleron AA, Ladenson JH. Enrichment of genes in the aortic intima that are associated with stratified epithelium: implications of underlying biomechanical and barrier properties of the arterial intima. Circulation. 2005;111:2382–90. doi: 10.1161/01.CIR.0000164235.26339.78. [DOI] [PubMed] [Google Scholar]
- [11].Pyle AL, Atkinson JB, Pozzi A, Reese J, Eckes B, Davidson JM, Crimmins DL, Young PP. Regulation of the atheroma-enriched protein, sprr3, in vascular smooth muscle cells through cyclic strain is dependent on integrin {alpha}1{beta}1/collagen interaction. Am J Pathol. 2008 doi: 10.2353/ajpath.2008.080042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Nemes Z, Steinert PM. Bricks and mortar of the epidermal barrier. Exp Mol Med. 1999;31:5–19. doi: 10.1038/emm.1999.2. [DOI] [PubMed] [Google Scholar]
- [13].Champliaud MF, Burgeson RE, Jin W, Baden HP, Olson PF. Cdna cloning and characterization of sciellin, a LIM domain protein of the keratinocyte cornified envelope. J Biol Chem. 1998;273:31547–54. doi: 10.1074/jbc.273.47.31547. [DOI] [PubMed] [Google Scholar]
- [14].Kalinin AE, Kajava AV, Steinert PM. Epithelial barrier function: Assembly and structural features of the cornified cell envelope. Bioessays. 2002;24:789–800. doi: 10.1002/bies.10144. [DOI] [PubMed] [Google Scholar]
- [15].Baden HP, Champliaud MF, Sundberg JP, Viel A. Targeted deletion of the sciellin gene resulted in normal development and maturation. Genesis. 2005;42:219–28. doi: 10.1002/gene.20133. [DOI] [PubMed] [Google Scholar]
- [16].Kazerounian S, Uitto J, Aho S. Unique role for the periplakin tail in intermediate filament association: specific binding to keratin 8 and vimentin. Exp Dermatol. 2002;11:428–38. doi: 10.1034/j.1600-0625.2002.110506.x. [DOI] [PubMed] [Google Scholar]
- [17].Aho S, Li K, Ryoo Y, McGee C, Ishida-Yamamoto A, Uitto J, Klement JF. Periplakin gene targeting reveals a constituent of the cornified cell envelope dispensable for normal mouse development. Mol Cell Biol. 2004;24:6410–8. doi: 10.1128/MCB.24.14.6410-6418.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Cabral A, Voskamp P, Cleton-Jansen AM, South A, Nizetic D, Backendorf C. Structural organization and regulation of the small proline-rich family of cornified envelope precursors suggest a role in adaptive barrier function. J Biol Chem. 2001;276:19231–7. doi: 10.1074/jbc.M100336200. [DOI] [PubMed] [Google Scholar]
- [19].Candi E, Schmidt R, Melino G. The cornified envelope: A model of cell death in the skin. Nat Rev Mol Cell Biol. 2005;6:328–40. doi: 10.1038/nrm1619. [DOI] [PubMed] [Google Scholar]
- [20].Zampetaki A, Zhang Z, Hu Y, Xu Q. Biomechanical stress induces IL-6 expression in smooth muscle cells via ras/rac1-p38 mapk-nf-kappab signaling pathways. Am J Physiol Heart Circ Physiol. 2005;288:H2946–54. doi: 10.1152/ajpheart.00919.2004. [DOI] [PubMed] [Google Scholar]
- [21].Mayr M, Li C, Zou Y, Huemer U, Hu Y, Xu Q. Biomechanical stress-induced apoptosis in vein grafts involves p38 mitogen-activated protein kinases. Faseb J. 2000;14:261–70. doi: 10.1096/fasebj.14.2.261. [DOI] [PubMed] [Google Scholar]
- [22].Jiang MJ, Yu YJ, Chen YL, Lee YM, Hung LS. Cyclic strain stimulates monocyte chemotactic protein-1 mRNA expression in smooth muscle cells. J Cell Biochem. 1999;76:303–10. doi: 10.1002/(sici)1097-4644(20000201)76:2<303::aid-jcb13>3.3.co;2-5. [DOI] [PubMed] [Google Scholar]
- [23].Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Champliaud MF, Viel A, Baden HP. The expression of vitamin D-upregulated protein 1 in skin and its interaction with sciellin in cultured keratinocytes. J Invest Dermatol. 2003;121:781–5. doi: 10.1046/j.1523-1747.2003.12539.x. [DOI] [PubMed] [Google Scholar]
- [25].Ruhrberg C, Hajibagheri MA, Parry DA, Watt FM. Periplakin, a novel component of cornified envelopes and desmosomes that belongs to the plakin family and forms complexes with envoplakin. J Cell Biol. 1997;139:1835–49. doi: 10.1083/jcb.139.7.1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].DiColandrea T, Karashima T, Maatta A, Watt FM. Subcellular distribution of envoplakin and periplakin: insights into their role as precursors of the epidermal cornified envelope. J Cell Biol. 2000;151:573–86. doi: 10.1083/jcb.151.3.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Hamilton DW, Maul TM, Vorp DA. Characterization of the response of bone marrow-derived progenitor cells to cyclic strain: implications for vascular tissue-engineering applications. Tissue Eng. 2004;10:361–9. doi: 10.1089/107632704323061726. [DOI] [PubMed] [Google Scholar]
- [28].Xu Q. Biomechanical-stress-induced signaling and gene expression in the development of arteriosclerosis. Trends Cardiovasc Med. 2000;10:35–41. doi: 10.1016/s1050-1738(00)00042-6. [DOI] [PubMed] [Google Scholar]
- [29].Dobrin PB. Mechanical properties of arteries. Physiol Rev. 1978;58:397–460. doi: 10.1152/physrev.1978.58.2.397. [DOI] [PubMed] [Google Scholar]
- [30].Wagner CT, Durante W, Christodoulides N, Hellums JD, Schafer AI. Hemodynamic forces induce the expression of heme oxygenase in cultured vascular smooth muscle cells. J Clin Invest. 1997;100:589–96. doi: 10.1172/JCI119569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Asanuma K, Magid R, Johnson C, Nerem RM, Galis ZS. Uniaxial strain upregulates matrix-degrading enzymes produced by human vascular smooth muscle cells. Am J Physiol Heart Circ Physiol. 2003;284:H1778–84. doi: 10.1152/ajpheart.00494.2002. [DOI] [PubMed] [Google Scholar]
- [32].Sutcliffe MC, Davidson JM. Effect of static stretching on elastin production by porcine aortic smooth muscle cells. Matrix. 1990;10:148–53. doi: 10.1016/s0934-8832(11)80163-0. [DOI] [PubMed] [Google Scholar]
- [33].Cattaruzza M, Lattrich C, Hecker M. Focal adhesion protein zyxin is a mechanosensitive modulator of gene expression in vascular smooth muscle cells. Hypertension. 2004;43:726–30. doi: 10.1161/01.HYP.0000119189.82659.52. [DOI] [PubMed] [Google Scholar]
- [34].Sedding DG, Hermsen J, Seay U, Eickelberg O, Kummer W, Schwencke C, Strasser RH, Tillmanns H, Braun-Dullaeus RC. Caveolin-1 facilitates mechanosensitive protein kinase b (akt) signaling in vitro and in vivo. Circ Res. 2005;96:635–42. doi: 10.1161/01.RES.0000160610.61306.0f. [DOI] [PubMed] [Google Scholar]
- [35].Li C, Wernig F, Leitges M, Hu Y, Xu Q. Mechanical stress-activated pkcdelta regulates smooth muscle cell migration. FASEB J. 2003;17:2106–8. doi: 10.1096/fj.03-0150fje. [DOI] [PubMed] [Google Scholar]
- [36].Morawietz H, Ma YH, Vives F, Wilson E, Sukhatme VP, Holtz J, Ives HE. Rapid induction and translocation of egr-1 in response to mechanical strain in vascular smooth muscle cells. Circ Res. 1999;84:678–87. doi: 10.1161/01.res.84.6.678. [DOI] [PubMed] [Google Scholar]
- [37].Karashima T, Watt FM. Interaction of periplakin and envoplakin with intermediate filaments. J Cell Sci. 2002;115:5027–37. doi: 10.1242/jcs.00191. [DOI] [PubMed] [Google Scholar]
- [38].Lawson ND, Weinstein BM. Arteries and veins: making a difference with zebrafish. Nat Rev Genet. 2002;3:674–82. doi: 10.1038/nrg888. [DOI] [PubMed] [Google Scholar]
- [39].O'Rourke M. Mechanical principles in arterial disease. Hypertension. 1995;26:2–9. doi: 10.1161/01.hyp.26.1.2. [DOI] [PubMed] [Google Scholar]
- [40].Kvedar JC, Manabe M, Phillips SB, Ross BS, Baden HP. Characterization of sciellin, a precursor to the cornified envelope of human keratinocytes. Differentiation. 1992;49:195–204. doi: 10.1111/j.1432-0436.1992.tb00667.x. [DOI] [PubMed] [Google Scholar]
- [41].Wei J, Gorman TE, Liu X, Ith B, Tseng A, Chen Z, Simon DI, Layne MD, Yet SF. Increased neointima formation in cysteine-rich protein 2-deficient mice in response to vascular injury. Circ Res. 2005;97:1323–31. doi: 10.1161/01.RES.0000194331.76925.5c. [DOI] [PubMed] [Google Scholar]
- [42].Yoshioka J, Schulze PC, Cupesi M, Sylvan JD, MacGillivray C, Gannon J, Huang H, Lee RT. Thioredoxin-interacting protein controls cardiac hypertrophy through regulation of thioredoxin activity. Circulation. 2004;109:2581–6. doi: 10.1161/01.CIR.0000129771.32215.44. [DOI] [PubMed] [Google Scholar]
- [43].Wang Y, De Keulenaer GW, Lee RT. Vitamin D(3)-up-regulated protein-1 is a stress-responsive gene that regulates cardiomyocyte viability through interaction with thioredoxin. J Biol Chem. 2002;277:26496–500. doi: 10.1074/jbc.M202133200. [DOI] [PubMed] [Google Scholar]
- [44].Schulze PC, De Keulenaer GW, Yoshioka J, Kassik KA, Lee RT. Vitamin d3-upregulated protein-1 (vdup-1) regulates redox-dependent vascular smooth muscle cell proliferation through interaction with thioredoxin. Circ Res. 2002;91:689–95. doi: 10.1161/01.res.0000037982.55074.f6. [DOI] [PubMed] [Google Scholar]
- [45].Bierkamp C, McLaughlin KJ, Schwarz H, Huber O, Kemler R. Embryonic heart and skin defects in mice lacking plakoglobin. Dev Biol. 1996;180:780–5. doi: 10.1006/dbio.1996.0346. [DOI] [PubMed] [Google Scholar]
- [46].Gallicano GI, Bauer C, Fuchs E. Rescuing desmoplakin function in extra-embryonic ectoderm reveals the importance of this protein in embryonic heart, neuroepithelium, skin and vasculature. Development. 2001;128:929–41. doi: 10.1242/dev.128.6.929. [DOI] [PubMed] [Google Scholar]
- [47].Pradervand S, Yasukawa H, Muller OG, Kjekshus H, Nakamura T, St Amand TR, Yajima T, Matsumura K, Duplain H, Iwatate M, Woodard S, Pedrazzini T, Ross J, Firsov D, Rossier BC, Hoshijima M, Chien KR. Small proline-rich protein 1a is a gp130 pathway- and stress-inducible cardioprotective protein. EMBO J. 2004;23:4517–25. doi: 10.1038/sj.emboj.7600454. [DOI] [PMC free article] [PubMed] [Google Scholar]