Abstract
Endothelial nitric oxide synthase (eNOS) catalyzes the conversion of l-arginine and molecular oxygen into l-citrulline and nitric oxide (NO), a gaseous second messenger that influences cardiovascular physiology and disease. Several mechanisms regulate eNOS activity and function, including phosphorylation at Ser and Thr residues and protein-protein interactions. Combining a tandem affinity purification approach and mass spectrometry, we identified stromal cell–derived factor 2 (SDF2) as a component of the eNOS macromolecular complex in endothelial cells. SDF2 knockdown impaired agonist-stimulated NO synthesis and decreased the phosphorylation of eNOS at Ser1177, a key event required for maximal activation of eNOS. Conversely, SDF2 overexpression dose-dependently increased NO synthesis through a mechanism involving Akt and calcium (induced with ionomycin), which increased the phosphorylation of Ser1177 in eNOS. NO synthesis by iNOS (inducible NOS) and nNOS (neuronal NOS) was also enhanced upon SDF2 overexpression. We found that SDF2 was a client protein of the chaperone protein Hsp90, interacting preferentially with the M domain of Hsp90, which is the same domain that binds to eNOS. In endothelial cells exposed to vascular endothelial growth factor (VEGF), SDF2 was required for the binding of Hsp90 and calmodulin to eNOS, resulting in eNOS phosphorylation and activation. Thus, our data describe a function for SDF2 as a component of the Hsp90-eNOS complex that is critical for signal transduction in endothelial cells.
INTRODUCTION
Nitric oxide (NO) is a short-lived gaseous signaling molecule, synthesized in endothelial cells by the enzyme endothelial nitric oxide synthase (eNOS). NO plays a vital role in maintaining cardiovascular homeostasis by influencing vascular tone, smooth muscle cell proliferation and migration, leukocyte adhesion, and platelet aggregation (1). For many years, eNOS has been the focus of intense research aimed to understand its regulation under physiological and pathological conditions. Numerous studies have demonstrated that eNOS plays a protective role against pathologic vascular remodeling, hypertension, and atherosclerosis (2–4). The activity of eNOS and its ability to generate NO are regulated at the transcriptional, posttranscriptional, and posttranslational levels, and dysregulation of these mechanisms promotes the development of cardiovascular disease (1). Therefore, a deeper understanding of eNOS regulation is of crucial importance in the search for new approaches to understand its roles in health and disease.
In addition to posttranslational modifications that influence eNOS function such as protein palmitoylation, phosphorylation, glutathionylation, and S-nitrosylation, eNOS activity is modulated by protein-protein interactions. Under quiescent conditions, eNOS is anchored to plasma membrane caveolae through N-myristoylation and cysteine palmitoylation of its N terminus and is kept in an inhibited state through its interaction with caveolin-1 (5, 6). Upon stimulation with various calcium-mobilizing agonists and ionophores, including ionomycin, caveolin-1 binding is displaced by calcium-activated calmodulin (CaM), resulting in a conformational change that promotes NADPH (reduced form of nicotinamide adenine dinucleotide phosphate)–dependent electron flux to the heme moiety and overall increased NO synthesis (7–9). Another crucial regulator of eNOS activity is the molecular chaperone Hsp90. The basal binding of Hsp90 to eNOS is increased in endothelial cells by several stimuli such as vascular endothelial growth factor (VEGF), histamine, estrogen, and fluid shear stress (10). Binding of Hsp90 alone induces a conformational change that promotes eNOS activity and increases NO production (8, 10, 11). Also, Hsp90 serves as a heme chaperone for all NOS isoforms (12, 13). In addition, Hsp90 is a molecular scaffold for the recruitment of other proteins that regulate the activity of NOS, including protein kinases such as Akt. Akt phosphorylates eNOS at Ser1177 in the C-terminal reductase domain, which increases electron flow and augments calcium-CaM sensitivity of the enzyme (14–17). In addition to Hsp90, several proteins have been described as part of the eNOS protein complex with the ability to influence eNOS localization, trafficking, and catalytic activity (18).
The central goal of this study was to identify activation-state eNOS-interacting partners using a proteomic strategy of tandem affinity purification (TAP) followed by mass spectrometry (MS). Here, we identified stromal cell–derived factor-2 (SDF2) as a protein that preferentially interacted with an activated mutant form of eNOS and was required for efficient eNOS-dependent NO synthesis. We found that SDF2 was a client protein of Hsp90 that bound to its M domain. This interaction occurred in cells replete or deplete of eNOS. Upon stimulation with VEGF, SDF2 was necessary for the binding of Hsp90 and CaM to eNOS. Therefore, SDF2 is a regulator of NOS function through its binding to Hsp90. The interaction between SDF2 and Hsp90 suggests that other Hsp90-dependent processes may be influenced by SDF2.
RESULTS
TAP combined with MS identifies SDF2 as part of the eNOS activation complex
To isolate and identify activation-dependent eNOS interactors, we generated TAP-tagged versions of previously described eNOS mutants (14). The CTA tag (also known as PTP tag) is a modified TAP tag comprising a protein C epitope, a tobacco etch virus (TEV) cleavage site, and a duplicate protein A epitope, and it has been used to efficiently purify native protein complexes in yeast and mammalian cells (19).
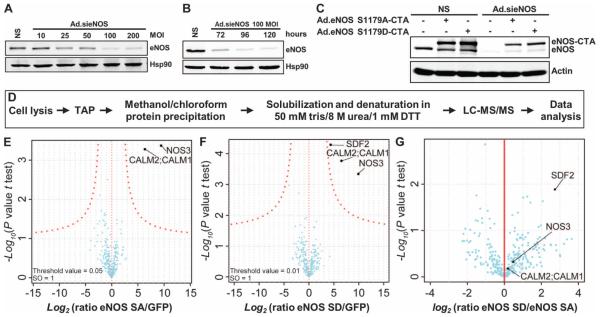
Two forms of eNOS were used to identify binding partners that specifically interact with eNOS in a particular phosphorylation state: either a less active mutant that cannot be phosphorylated by Akt or AMPK [adenosine 5′-monophosphate (AMP)–activated protein kinase] on Ser1179 (for bovine eNOS, or Ser1177 for human eNOS; eNOS S1179A) or a constitutively active phosphorylation-mimetic mutant, eNOS S1179D (20). To maintain the expression of the eNOS S1179A–CTA and eNOS S1179D–CTA fusion proteins close to physiological amounts, and to avoid competition for interacting proteins between endogenous eNOS and the fusion proteins, EA. hy926 human umbilical vein endothelial cells were first transduced with an adenoviral construct expressing a small interfering RNA against eNOS (Ad.sieNOS) (21, 22), and then were transduced with adenoviruses carrying eNOS S1179A–CTA (Ad.eNOS S1179A–CTA) or eNOS S1179D–CTA (Ad.eNOS S1179D–CTA) (Fig. 1, A to C). Infection of EA.hy926 cells with Ad.sieNOS showed that maximal knockdown (~95%) of endogenous eNOS was achieved starting at 100 multiplicity of infection (MOI) (Fig. 1A) and was sustained for several days after infection (Fig. 1B). Subsequent infection with Ad.eNOS S1179A–CTA or Ad.eNOS S1179D–CTA resulted in amounts of the eNOS fusion proteins comparable to that of the endogenous protein (Fig. 1C).
Fig. 1. TAP combined with LC-MS/MS identifies SDF2 as part of the eNOS complex.
(A and B) Representative immunoblot analyses show eNOS expression in EA.hy926 cells after transduction with increasing MOI of an adenovirus encoding a small interfering RNA (siRNA) targeting eNOS (Ad.sieNOS) (A) and the persistence of eNOS knockdown over 120 hours (B). Hsp90 was used as loading control. DTT, dithiothreitol. (C) Representative immunoblot analysis shows the expression of endogenous eNOS and adenovirally transduced eNOS S1179A–CTA and eNOS S1179D–CTA before and after knockdown of endogenous eNOS by Ad.sieNOS. n = 3 independent experiments for (A) to (C). NS, nonsilenced. (D) Experimental design from cell lysis to proteomic analysis of EA.hy926 cells expressing eGFP-CTA, eNOS S1179A–CTA, or eNOS S1179D–CTA. (E to G) Volcano plot representing results of the pull-downs of eGFP, eNOS S1179A, and eNOS S1179D. The log2 ratios of protein intensities in the eNOS SA/GFP (E), eNOS SD/GFP (F), or eNOS SD/eNOS SA (G) pull-downs were plotted against −log10 P values of the t test performed from biological triplicates. A hyperbolic curve (red dotted line) separates specific eNOS-interacting proteins from the background (blue dots).
For MS experiments, EA.hy926 cells were infected with Ad.sieNOS and Ad.eGFP-CTA (as a reference control), Ad.eNOS S1179A–CTA, or Ad.eNOS S1179D–CTA, and associated components were recovered from whole-cell lysates by TAP and then analyzed by liquid chromatography/ tandem mass spectrometry (LC-MS/MS) (Fig. 1D and table S1). Under these conditions, eNOS and calmodulin (CALM1, CALM2), an essential allosteric activator of eNOS, were enriched. Moreover, SDF2 was significantly enriched in eNOS S1179D pull-downs as compared to the enhanced green fluorescent protein (eGFP) or eNOS S1179A pull-downs (Fig. 1, E to G, and Tables 1 to 3), implying that SDF2 interacts with the activated eNOS complex. Infection of EA.hy926 cells with Ad.eNOS S1179A–CTA or Ad.eNOS S1179D–CTA did not alter the expression of SDF2 as compared to control (fig. S1).
Table 1.
LC-MS/MS analysis of eNOS S1179A and GFP pull-downs.
| Symbol | Protein name | Average intensity GFP ± SEM |
Average intensity eNOS S1179A ± SEM |
Unique peptides |
Log2 (ratio eNOS S1179A/GFP) |
P |
|---|---|---|---|---|---|---|
| NOS3 | Endothelial nitric oxide synthase |
25.61 ± 0.80 | 35.04 ± 0.36 | 49 | 9.43 | <0.001 |
| CALM2; CALM1 | Calmodulin | 26.08 ± 0.48 | 32.41 ± 0.40 | 4 | 6.33 | <0.001 |
Table 3.
LC-MS/MS analysis of eNOS S1179A and eNOS S1179D pull-downs.
| Symbol | Protein name | Average intensity eNOS S1179A ± SEM |
Average intensity eNOS S1179D ± SEM |
Unique peptides |
Log2 (ratio eNOS S1179D/eNOS S1179A) |
P |
|---|---|---|---|---|---|---|
| SDF2 | Stromal cell–derived factor 2 | 25.40 ± 0.61 | 28.13 ± 0.21 | 2 | 2.73 | <0.05 |
SDF2 is necessary for eNOS-dependent NO release and phosphorylation of eNOS at Ser1177
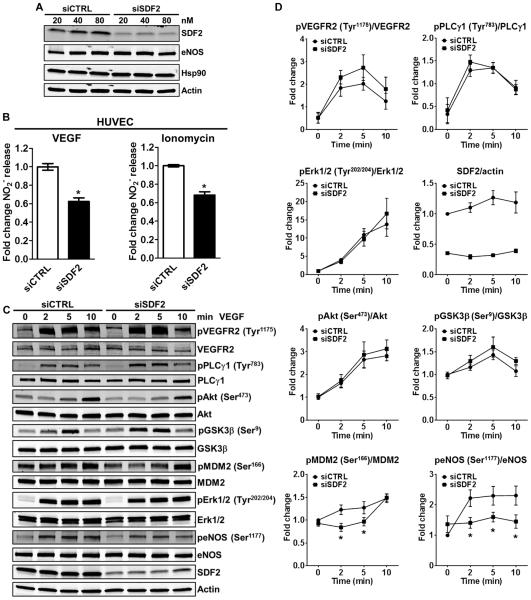
We initially sought to investigate whether SDF2 influenced eNOS-dependent NO release. SDF2 knockdown by small interfering RNA (>80%) in primary cultures of human umbilical cord endothelial cells (HUVECs) did not affect total eNOS or Hsp90 protein abundance (Fig. 2A) but reduced both VEGF- or ionomycin-stimulated NO release (Fig. 2B). To investigate whether SDF2 deficiency would influence signaling pathways downstream of vascular endothelial growth factor receptor 2 (VEGFR2) activation, we examined the phosphorylation of VEGFR2, phospholipase C–γ1 (PLCγ1), extracellular signal–regulated kinase 1/2 (Erk1/2), Akt, and some of Akt substrates including glycogen synthase kinase 3β (GSK3β), MDM2, and eNOS (Fig. 2C and quantified in Fig. 2D) after VEGF stimulation. Notably, we did not find significant differences in the phosphorylation of the proteins analyzed, except for that of MDM2 at Ser166 (decreased at 2 and 5 min, but not at 10 min after VEGF treatment) and that of eNOS at Ser1177 (at all time points examined).
Fig. 2. SDF2 is necessary for eNOS-dependent NO release and phosphorylation of eNOS at Ser1177.
(A) Immunoblot analysis shows the abundance of SDF2, eNOS, and Hsp90 in HUVECs after transfection with 20 to 80 nM control siRNA (siCTRL) or siRNA targeting SDF2 (siSDF2). Actin was used as a loading control. n = 3 independent experiments. (B) Bar graphs illustrate the averaged measurements of NO (NO2−) released by siCTRL or siSDF2 HUVECs after stimulation with VEGF (left) or ionomycin (right); n = 4 independent experiments. (C and D) Representative immunoblot analyses (C) and averaged densitometric data (D) show the effects of SDF2 knockdown on the VEGF-induced dynamic phosphorylation (p) of VEGFR2, PLCγ1, Akt, GSK3β, MDM2, Erk1/2, and eNOS over a time course of 10 min in HUVECs. Actin was used as a loading control. Data are means ± SEM. n = 5 to 10 independent experiments. *P < 0.05 compared to siCTRL.
SDF2 overexpression promotes NO release by increasing the phosphorylation of eNOS
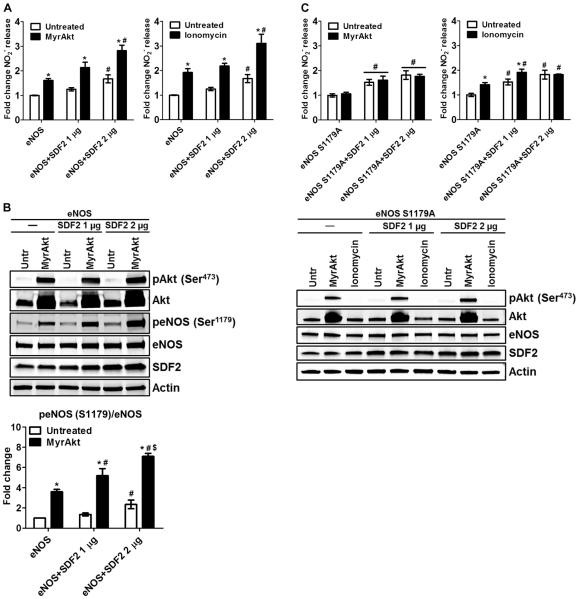
Because the loss of SDF2 reduced the phosphorylation of eNOS and NO release, we tested whether SDF2 overexpression facilitated eNOS-dependent NO production. We transiently transfected COS7 cells (which do not have endogenous eNOS) with bovine eNOS complementary DNA (cDNA) and increasing amounts of SDF2 and measured NO release in response to coexpression of a myristoylated, constitutively active form of Akt (MyrAkt) or ionomycin. SDF2 overexpression promoted basal, MyrAkt-dependent, and ionomycin-dependent NO release in a dose-dependent manner (Fig. 3A). This effect was associated with an SDF2-dependent increase in the phosphorylation of Ser1179 in eNOS both basally and after MyrAkt transfection (Fig. 3B), suggesting that SDF2 can modulate eNOS activity in the absence or presence of an exogenous stimulus by facilitating the phosphorylation and activation of eNOS. To confirm that the SDF2-mediated increase in eNOS activity was due to a direct influence on the phosphorylation of Ser1179, we transfected COS7 cells as above with eNOS S1179A cDNA and measured NO release. The eNOS S1179A mutant was not further activated by SDF2 in cells also expressing MyrAkt or stimulated with ionomycin, implying that SDF2 regulates NO release through phosphorylation of Ser1179 (Fig. 3C).
Fig. 3. SDF2 overexpression promotes NO release by increasing the phosphorylation of eNOS.
(A and C) Bar graphs illustrate the averaged measurements of NO (NO2−) released by COS7 cells expressing eNOS (A) or eNOS S1179A (C) and increasing amounts of SDF2 basally and after expression of constitutively active Akt (MyrAkt, left) or stimulation with ionomycin (right). (B) Representative immunoblot analyses and averaged densitometric data of the samples used in (A) show Akt and eNOS phosphorylation as well as SDF2 abundance. Actin was used as a loading control. Data are means ± SEM. n = 4 independent experiments. *P < 0.05 compared to untreated (Untr); #P < 0.05 compared to eNOS/eNOS S1179A, same treatment group; $P < 0.05 compared to eNOS + SDF2 1 μg.
SFD2 modulates the activity of other NOS isoforms
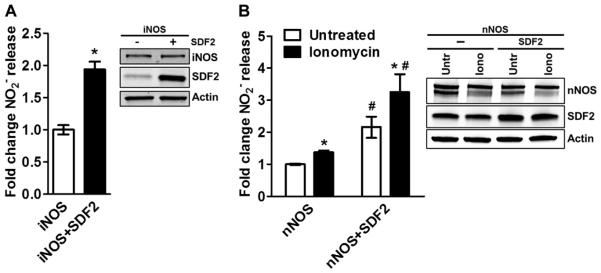
To test whether SDF2 influenced the activity of inducible NOS (iNOS) and neuronal NOS (nNOS), we transiently cotransfected COS7 cells with the cDNAs for SDF2 and iNOS or nNOS and measured basal as well as ionomycin-induced NO production (Fig. 4). Overexpression of SDF2 resulted in a significant increase in basal iNOS-dependent NO release (Fig. 4A), as well as basal and ionomycin-stimulated nNOS-dependent NO release (Fig. 4B), implying that SDF2 regulated the activity of all mammalian NOS isoforms.
Fig. 4. SFD2 modulates iNOS- and nNOS-dependent NO release.
(A and B) Bar graphs illustrate the averaged measurements of NO released by COS7 cells expressing iNOS (A, constitutive release) or nNOS (B, basal and in response to ionomycin stimulation) in the presence or absence of SDF2 (2 μg) overexpression. Representative immunoblot analyses of the samples used in the respective experiments show the abundance of iNOS or nNOS and SDF2. Actin was used as a loading control. Data are means ± SEM. n = 4 independent experiments. *P < 0.05 compared to untreated; #P < 0.05 compared to nNOS, same treatment group.
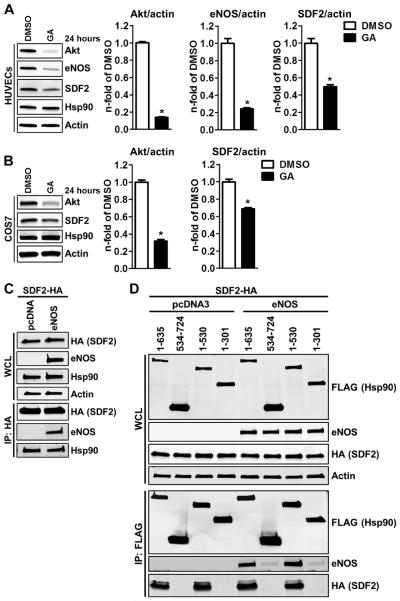
SDF2 is an Hsp90 client protein that interacts with the M domain of Hsp90
The molecular chaperone Hsp90 serves as a scaffold for the formation of a ternary activation complex with eNOS and Akt (16). Because SDF2 modulates eNOS activity both basally and following exogenous stimuli by facilitating Akt-dependent phosphorylation of Ser1179, we first determined whether SDF2 was a client protein of Hsp90, similarly to Akt and eNOS. HUVECs (Fig. 5A) or COS7 cells lacking eNOS (Fig. 5B) were treated with the Hsp90 inhibitor geldanamycin (GA). GA disrupts the adenosine triphosphatase (ATPase) function of Hsp90, triggering the ubiquitination and degradation of most Hsp90 client proteins (23). GA treatment reduced Akt, eNOS, and SDF2 protein abundance in HUVECs, and Akt and SDF2 in COS7 cells lacking eNOS, implying that SDF2 is an Hsp90 client protein and its interaction with Hsp90 can occur independently of eNOS. Coimmunoprecipitation studies in COS7 cells expressing hemagglutinin-tagged SDF2 (SDF2-HA) and empty vector or eNOS confirmed the interaction between SDF2 and endogenous Hsp90, and showed that eNOS is not required for this interaction to occur (Fig. 5C). Coimmunoprecipitation studies in human embryonic kidney (HEK) 293 cells expressing SDF2-HA and empty vector or eNOS showed that the interaction between SDF2 and Hsp90 was not altered by inhibition of either phosphoinositide 3-kinase (PI3K) or Akt or by calcium chelation (fig. S2). We also mapped the domains of Hsp90 involved in the binding of SDF2 by coexpressing SDF2-HA with a series of FLAG-tagged deletion mutants of Hsp90 in the presence or absence of eNOS in COS7 cells. Both eNOS and SDF2 coimmunoprecipitated with regions 1 to 635 and 1 to 530, but not 534 to 724 or 1 to 301 of Hsp90 (Fig. 5D). Moreover, SDF2 coimmunoprecipitated with Hsp90 independently of the presence or absence of eNOS, and the presence of each protein did not influence the interaction of the other with the M domain.
Fig. 5. SDF2 is an Hsp90 client protein and interacts with its M domain.
(A and B) Representative immunoblot analyses (left) and averaged densitometric data (right) show total Akt, eNOS, SDF2, and Hsp90 abundance in HUVECs (A) and COS7 cells (B) treated with GA or vehicle [dimethyl sulfoxide (DMSO)]. (C) Representative immunoblot analyses show the expression of HA, eNOS, and Hsp90 in whole-cell lysates (WCL) from COS7 cells cotransfected with SDF2-HA and eNOS or empty vector. HA immunoprecipitates were blotted for eNOS and Hsp90. (D) Representative immunoblot analyses show the expression of FLAG, eNOS, and HA in WCL from COS7 cells cotransfected with SDF2-HA, eNOS, or empty vector and FLAG-tagged deletion mutants of Hsp90β (as indicated). FLAG immunoprecipitates (IP: FLAG) were blotted for FLAG, eNOS, and HA. Actin was used as a loading control in (A) to (D). Data are means ± SEM. n = 3 independent experiments for (A) to (D). *P < 0.05 compared to DMSO.
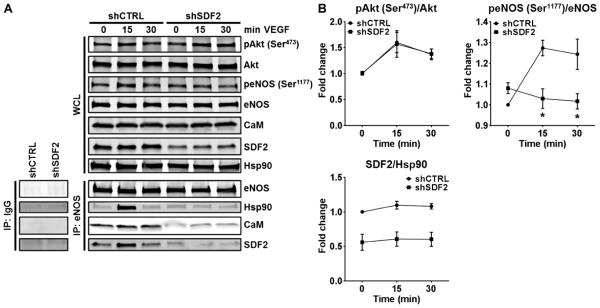
SDF2 is required for VEGF-induced recruitment of Hsp90 and calmodulin to the eNOS complex
Next, we tested whether endogenous SDF2 played a role in the formation of a ternary complex between eNOS, Hsp90, and calmodulin (CaM). To achieve stable and homogeneous SDF2 knockdown, suitable for temporal immunoprecipitation studies, stable cell lines were generated from EA. hy926 cells transduced with a lentiviral short hairpin RNA (shRNA) vector targeting the human SDF2 gene (shSDF2) or a lentiviral nonsilencing control shRNA (shCTRL). shCTRL and shSDF2 EA.hy926 cells were starved overnight and then treated with VEGF to induce complex formation (10). In line with the results from HUVECs with SDF2 knockdown, the phosphorylation of Akt at the stimulatory Ser473 was comparable between the two groups, whereas the phosphorylation of eNOS at Ser1177 was reduced in the shSDF2 cells as compared to control (Fig. 6A and quantified in Fig. 6B). Immunoprecipitation of eNOS in control cells showed that VEGF stimulated the formation of an eNOS complex including Hsp90, CaM, and SDF2. In shSDF2 cells, however, the recruitment of Hsp90 and CaM was markedly abrogated, suggesting that SDF2 is required for the physiological formation of a functional eNOS complex (Fig. 6A).
Fig. 6. SDF2 is required for VEGF-induced recruitment of Hsp90 and CaM to the eNOS complex.
(A and B) Representative immunoblot analyses (A) and averaged densitometric data (B) show the effects of SDF2 knockdown by shRNA on the VEGF-induced phosphorylation of Akt and eNOS in EA.hy926 cells. The abundance of Akt, eNOS, CaM, SDF2, and Hsp90 in whole-cell lysates (WCL) and after immunoprecipitation of eNOS (IP: eNOS) are shown. Hsp90 was used as a loading control. Control IgGs were used in control immunoprecipitation experiments (bottom left). Data are means ± SEM. n = 3 independent experiments. *P < 0.05 compared to shCTRL.
DISCUSSION
The central finding of this paper was the identification of SDF2 as an eNOS-interacting protein that complexed predominantly with the active Hsp90-bound, Ser1177/1179 phosphorylated form of eNOS. Knockdown or overexpression of SDF2 reduced or enhanced NO release, and the loss-of-function mutant eNOS S1179A was weakly activated by SDF2 overexpression, thus demonstrating the functional relevance of SDF2 as a regulator of eNOS function. Moreover, SDF2 was an Hsp90 client protein because inhibition of Hsp90 triggered its degradation (similar to many client proteins including Akt and eNOS) and its interaction occurred predominantly through SDF2 binding to the M domain, a common site for Hsp90-interacting proteins (24). Although SDF2 was detected through its preferential interaction with eNOS S1179D, eNOS was not required for its interaction with Hsp90 because this interaction occurred in cells lacking eNOS. iNOS and nNOS are also modulated by Hsp90 (12, 25–27), and their activity was enhanced upon overexpression of SDF2, thus extending the importance of the interaction between SDF2 and Hsp90 to these NOS isoforms. VEGF activation of endothelial cells stimulated the recruitment of SDF2, Hsp90, and CaM to the eNOS complex, an effect that was attenuated in cells lacking SDF2, thereby linking SDF2 with the activation complex and NO synthesis.
SDF2 was originally identified as a secreted protein using the signal sequence trap method in the mouse ST2 stromal cell line, although the actual secretion of the protein was not demonstrated (28). The amino acid sequence of SDF2 shows similarity to those of yeast dolichyl phosphate-D-mannose:protein mannosyltransferases, Pmt1p and Pmt2p; homologs of these enzymes are not present in higher eukaryotes. SDF2 has been detected also in several mouse tissues, and it localizes to the endoplasmic reticulum (ER), likely through accessory binding proteins or other amino acid sequence motifs (29). SDF2 consists of three MIR [mannosyltransferases, inositol 1,4,5-trisphosphate receptor (IP3R), and ryanodine receptor (RyR)] domains. It is speculated that MIR domains in IP3R and RyR regulate protein-protein interactions because their MIR domains interact with each other and with additional proteins such as Trp3 (30). In addition, a mammalian paralog of SDF2 named SDF2L1, which contains similar MIR domain organization, has been isolated as part of an ER chaperone complex using biochemical cross-linking approaches (31). Our data indicate that SDF2 is an Hsp90 client protein because it is destabilized by GA and interacts with Hsp90 and eNOS in a growth factor– and activation-dependent manner, implying that SDF2 may affect additional Hsp90-dependent functions such as steroid receptor maturation or chaperone activity. Whether SDF2 can serve as a client-specific co-chaperone regulating Hsp90 function, similar to cdc37 (24), or regulate its activity is not known but unlikely because the loss of SDF2 did not affect the turnover of the Hsp90 client Akt or eNOS. These data suggest that SDF2 influences Hsp90 biological functions related to the scaffolding of signaling pathways rather than its intrinsic chaperone activity. SDF2 could regulate the activator of Hsp90 ATPase 1 (AHA1) because they both bind to the M domain of Hsp90 (32) and the loss of AHA1 does not reduce the abundance of Hsp90 client proteins, but reduces the activity and phosphorylation of substrates including eNOS (33, 34). The higher rates of NO synthesis that accompany increases in the expression of SDF2 suggest that the interaction between SDF2 and Hsp90 is driven by the abundance of each protein. The interaction between Hsp90 and SDF2 was stable upon inhibition of PI3K or Akt activation or reduction of calcium concentrations. However, it is possible that other post-translational modifications of Hsp90 may play a role in modulating the interaction. Future studies will address the regulatory aspects of the interaction as well as the direct effect of SDF2 on Hsp90 activities. We cannot exclude the possibility that the complete loss of SDF2 may yield more severe effects on the function of Hsp90 in assisting protein folding or stabilizing client proteins.
Surprisingly, quantitative analysis using an optimized, TAP tag isolation procedure yielded only CaM and SDF2 as unique interactors with eNOS S1179D, implying that the high stringency of the isolation procedure or the transient nature of other protein-protein interactions hampers their detection. The TAP method has limitations, including the length of the procedure, which influences the number of interactors that are eventually identified by MS. Moreover, all labile and transient interactions, such as those with protein kinases and phosphatases, cannot be detected using this system. Combining stable isotope labeling by amino acid in cell culture (SILAC) techniques, alternative eNOS tagging approaches, and rapid purification techniques may improve the recovery and quantification of eNOS partners under various conditions in future studies. In particular, quantitative studies on the dynamic remodeling of the eNOS interactome in response to various stimuli will open interesting research avenues, which will deepen our understanding of how eNOS is regulated by protein-protein interactions and its role in cardiovascular diseases.
MATERIALS AND METHODS
Cell culture
The EA.hy926 cell line was purchased from the American Type Culture Collection (CRL-2922) and grown in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% fetal bovine serum (FBS), penicillin (100 U/ml), streptomycin (0.1 mg/ml), 2 mM glutamine, and HAT. HUVECs were obtained from the Yale University Vascular Biology and Therapeutics Core facility, plated on 0.1% gelatin-coated dishes in M199 medium supplemented with endothelial cell growth supplement (ECGS), 10% FBS, penicillin (100 U/ml), streptomycin (0.1 mg/ml), and 2 mM glutamine, and used between passages 2 and 4. AD-293, HEK293, and COS-7 cells were cultured in DMEM supplemented with 10% FBS, penicillin (100 U/ml), streptomycin (0.1 mg/ml), and 2 mM glutamine. Cultures were kept in a humidified incubator at 37°C containing 5% CO2.
Plasmid construction, generation of adenoviruses, and adenoviral transduction of cells
CTA-tagged constructs were generated as follows: polymerase chain reaction (PCR) was performed using the vector pBS1479 containing TEV_2xproteinA as a template and introducing Not-I_protein C at the N terminus of TEVand 2xStop_Hind-III at the C terminus of 2xproteinA with primers: forward (introducing Not-I_protein C), GGGGCGGCCGCTGAAGATCAGGTGGATCCTCGTCTTATTGATGGGAAAGATTATGATATTCCAACTACT; reverse (introducing 2xStop_Hind-III), CCCAAGCTTTCATCAGGTTGACTTCCCCGCGGA. The PCR fragment was then digested with restriction enzymes Not I and Hind III and inserted into the plasmid pShuttle-CMV, which had been digested with the same enzymes, generating pShuttle-CMV-CTA. Next, to generate CTA-tagged construct for adenovirus production, PCR was performed using eGFP, full-length bovine eNOS S1179A, or full-length bovine eNOS S1179D in pcDNA3 as templates using primers: forward eNOS (introducing Sal-I_Kozak sequence), GGGGTCGACGCCACCATGGGCAACTTGAAGAGTGTGGGC; reverse eNOS (introducing Not I without stop codon), CCCGCGGCCGCAGCAGCGGGGCCGGGGGTGTCTGG; forward eGFP (introducing Sal-I_Kozak sequence), GGGGTCGACGCCACCATGGTGAGCAAGGGCGAGGAG; reverse eGFP (introducing Not I without stop codon), CCCGCGGCCGCAGCCTTGTACAGCTCGTCCATGCC. Finally, to generate pShuttle-CMV vectors carrying CTA-tagged eGFP or eNOS mutants, the PCR fragments were digested with restriction enzymes Sal I and Not I and inserted into the plasmid pShuttle-CMV-CTA, which had been digested with the same enzymes.
All constructs were verified by sequencing and immunoblotting. Replication-deficient adenoviruses expressing eGFP-CTA, eNOS S1179A–CTA, or eNOS S1179D–CTA were generated by the AdEasy Adenoviral Vector System (Stratagene) (35). Briefly, all pShuttle-CMV vectors carrying eGFP-CTA, full-length bovine eNOS S1179A–CTA or eNOS S1179D–CTA were linearized with Pme I and subsequently cotransformed into Escherichia coli BJ5183 cells with an adenoviral backbone plasmid, pAdEasy-1. Recombinants were selected by kanamycin resistance and verified by restriction enzyme digestion. The conf irmed recombinant plasmids were then transfected into the adenoviral packaging AD-293 cell line. Viral production was monitored over 7 to 10 days by visualization of GFP expression and cytopathic effect (CPE). After 7 to 10 days, viral particles were harvested and purified by banding on a cesium chloride gradient. The purified viruses were then dialyzed and stored at −80°C. Infection of EA.hy926 cells with 25 MOI of viruses resulted in close to 100% of the cells expressing the gene of interest with no signs of toxicity.
Replication-deficient adenoviruses encoding small interfering RNA n°. 3122 targeting eNOS (Ad.sieNOS) were generated using the Block-iT U6 RNAi Entry Vector system as previously described (21, 22). EA.hy926 cells were plated in 150-mm dishes until 60% confluent and then transduced with 10 to 200 MOI of Ad.sieNOS. After 20 hours, cells were transduced with 25 MOI of Ad.eGFP-CTA, eNOS S1179A, or eNOS S1179D, and after an additional 20 hours, culture media were replaced with fresh culture media and cells were allowed to grow for 72 hours until confluent.
Cell lysis and TAP
Confluent EA.hy926 cells transduced with adenoviruses as described above and expressing either eGFP-CTA, eNOS S1179A–CTA, or eNOS S1179D–CTA (triplicates of six 150-mm plates per group) were washed twice with ice-cold phosphate-buffered saline (PBS) and then collected in TAP lysis buffer [1% NP-40, 20 mM tris (pH 8), 150 mM NaCl, 10% glycerol (v/v), 1 mM DTT, 1 mM CaCl2, 10 mM NaF, 0.25 mM Na3VO4, 5 nM calyculin A, 50 mM β-glycerophosphate, and EDTA-free Complete Protease Inhibitors (Roche)] with the aid of cell scrapers and incubated for 30 to 45 min at 4°C on an end-over-end rocker. For each experiment, 5 mg of whole-cell lysates was subjected to TAP. All steps were performed at 4°C. First, whole-cell lysates (5 μl/mg) of packed immunoglobulin G (IgG) Sepharose (GE Healthcare) were washed twice with TAP lysis buffer and then incubated for 2 hours with each whole-cell lysate with gentle rocking on an end-over-end rocker. The IgG Sepharose beads binding the CTA-tagged proteins were recovered by centrifugation at 1500 rpm for 2 min, followed by three washes with TAP lysis buffer without NP-40 and two washes with TEV buffer (Invitrogen) to eliminate unbound proteins and detergents. The IgG Sepharose beads were then resuspended in 150 μl of TEV buffer containing 25 U of AcTEV Protease (Invitrogen) and incubated overnight on an end-over-end rocker, allowing for an effective and complete elution of the native protein complexes from the IgG beads. Samples were then centrifuged at 5000 rpm for 2 min to separate the IgG Sepharose beads from the supernatant containing the eluted protein complexes, which were then transferred to a fresh tube and precipitated by methanol/chloroform, dried, and resuspended in freshly prepared 50 mM tris containing 8 M urea and 1 mM DTT before processing for MS.
Mass spectrometry
Proteins were reduced with 1 mM DTT for 30 min, alkylated with 5.5 mM iodoacetamide for 20 min in the dark, and digested for 3 hours at room temperature with the endoproteinase LysC. The samples were diluted four times with ABC buffer (50 mM ammonium bicarbonate in H2O, pH 8.0) and digested overnight at 37°C with trypsin. The resulting peptide mixture was acidified by the addition of trifluoroacetic acid. Peptides were desalted following the protocol for StageTip purification (36). Samples were eluted with 60 μl of buffer B (80% ACN, 0.1% formic acid in H2O) and reduced in a Vacufuge plus (Eppendorf) to a final volume of 3 μl. Buffer A (2 μl) (0.1% formic acid in H2O) was added, and the resulting 5 μl was injected through high-performance liquid chromatography.
Analysis of the peptide mixture was performed as described previously (37). Briefly, peptides were separated on 15-cm columns (New Objectives) with a 75-μm inner diameter, packed in-house with 1.9 μm C18 resin (Dr. Maisch GmbH). Peptides were eluted at a constant flow rate of 250 nl for 95 min with a linear acetonitrile gradient from 5 to 30%. Eluted peptides were directly sprayed into a Q Exactive mass spectrometer (Thermo). The mass spectrometer was operated in a data-dependent mode to automatically switch between full-scan MS and up to 10 data-dependent MS/MS scans. Maximum injection time for MS scans was 20 ms with a target value of 3,000,000 at a resolution of 70,000 at mass/charge ratio (m/z) of 200. The 10 most intense multiple charged ions (z ≥ 2) from the survey scan were selected for MS/MS scans. Peptides were fragmented with higher-energy collision dissociation (38) with normalized collision energies of 25. Target values for MS/MS were set to 1,000,000 with a maximum injection time of 120 ms at a resolution of 17,500 at m/z of 200. Dynamic exclusion of sequenced peptides was set to 25 s. Resulting MS and MS/MS spectra were analyzed using MaxQuant (version1.3.0.5), using its integrated ANDROMEDA search algorithms (39, 40). Peak lists were searched against local databases for human proteins concatenated with reversed copies of all sequences. Carbamidomethylation of cysteine was set as fixed modification, and variable modifications were methionine oxidation and N-terminal acetylation. Maximum mass deviation was 6 ppm for MS peaks and 20 ppm for MS/MS peaks with a maximum of two missed cleavages allowed and a minimum peptide length of six amino acids. Label-free quantitation was performed using the QUBIC software package as described previously (41). All calculations and plots were done with the R software package (http://r-project.org/).
Transient SDF2 knockdown by small interfering RNA and generation of EA.hy926 cell line with stable SDF2 knockdown
HUVECs between passages 2 and 4 were transfected with 20 to 80 nM small interfering RNA targeting human SDF2 (Santa Cruz Biotechnology, sc-94163) or a control small interfering RNA (Qiagen, 1027281), using Oligofectamine (Invitrogen). After 5 hours, M199 supplemented with 2× ECGS, penicillin-streptomycin and glutamine, and 20% FBS was added to the same volume of transfection media. The following day, media were changed to regular growth media and cells were allowed to grow to confluency for 72 hours for further experiments.
To generate an endothelial cell line with stable SDF2 knockdown, EA.hy926 cells were transduced with lentiviral particles expressing an shRNA targeting human SDF2, generated as follows. pLKO.1-lentiviral shRNA vectors targeting human SDF2 gene (shSDF2) and nonsilencing pLKO.1 control vector (shCTRL) were purchased from Sigma-Aldrich (MISSION shRNAs; for SDF2: TRCN0000292455). Lentiviral particles carrying shSDF2 or shCTRL were produced by cotransfecting lentiviral packaging vectors (psPAX2 and pMD2.G, Addgene) with the shRNA lentiviral vector in HEK293T cells. Supernatants containing the lentivirus expressing either shSDF2 or shCTRL were harvested 48 hours after transfection and filtered with 0.45-mm filter. EA.hy926 cells were incubated with the supernatant containing virus particles with polybrene (8 μg/ml) overnight, and then viral media were replaced with fresh media. After 48 hours, transduced cells were selected by puromycin (5 μg/ml) for over 7 days. The knockdown of SDF2 was evaluated by Western blot analysis.
Transfection of cells
COS7 and HEK293 cells were transfected using Lipofectamine 2000 (Invitrogen). After 5 hours of incubation, media were replaced with regular complete growth media and cells were grown to confluency for an additional 48 hours. The following plasmids, alone or in combination, were used for transfection: pcDNA3 empty plasmid (1 μg), bovine eNOS in pcDNA3 (1 μg), bovine eNOS S1179A in pcDNA3 (1 μg), human iNOS in pcDNA3 (1.5 μg), human nNOS in pcDNA3 (1.5 μg), myristoylated Akt1 (MyrAkt, 0.5 μg) (14), human SDF2 in pCMV6-XL5 (1 or 2 μg, OriGene SC115785, accession number NM_006923.2), Hsp90β deletion mutants (amino acids 1 to 635, 534 to 724, 1 to 530, and 1 to 301) in pcDNA5FLAG (0.5 μg) (16), and human HA-tagged SDF2 in pcDNA3 (SDF2-HA, 0.5 μg). SDF2-HA plasmid was generated as follows: PCR was performed using the human SDF2 in the pCMV6-XL5 plasmid (OriGene, SC115785) as a template with primers (forward: CAGGATCCGCCACCATGGCTGTAGTACCTC; reverse: TAGAATTCCTAAGCGTAGTCTGGGACGTCGTATGGGTACAGCTCTGCATGGTG) to produce a human SDF2-HA, which was flanked with site for restriction enzymes Bam HI and Eco RI. The PCR fragment was digested with these restriction enzymes and inserted into the plasmid pcDNA3, which had been digested with the same enzymes. The construct was verified by DNA sequencing and immunoblotting.
Measurement of NO release in cell culture media
Nitrite (NO2−), the major oxidation product of NO in the absence of oxyhemoglobin or superoxide anion, is formed when NO reacts with dissolved oxygen. Therefore, as a readout of the amount of NO released in the cell culture media, the amount of nitrite was measured usinga Nitric Oxide Analyzer (Sievers 270B) after reaction with iodide and acetic acid at room temperature. Confluent HUVEC monolayers (passages 2 and 3) were serum-starved for 2 hours in M199 containing neither FBS nor ECGS and supplemented with 10 μM sepiapterin (Sigma-Aldrich), followed by treatment with VEGF (50 ng/ml) (Genentech) for 30 min or 1 μM ionomycin for 15 min before collection of an aliquot of the culture media. Confluent COS7 cell monolayers, including those transfected with nNOS, were serum-starved overnight in DMEM containing 0.5% FBS, followed by treatment with 10 μM ionomycin for 15 min before collection of an aliquot of the culture media, as indicated in the figure legends. COS7 cells transfected with MyrAkt or iNOS were also starved as described above and then incubated with fresh media for 1 hour before collection of an aliquot of the culture media. In all experiments, net NO release was calculated by NO-specific chemiluminescence after subtracting unstimulated basal release as described previously (14). Whole-cell lysates from each well were used for subsequent immunoblot analyses. Moreover, protein concentrations were used to normalize the amounts of NO measured for each sample.
Immunoblot analysis
HUVECs were serum-starved (no FBS, no ECGS) for 2 hours, followed by stimulation with VEGF (50 ng/ml) for 2, 5, and 10 min. In separate experiments, HUVECs or COS7 cells were treated for 24 hours with GA (1 μM; Sigma-Aldrich). Cells were then washed twice with ice-cold PBS and immediately resuspended in lysis buffer (50 mM tris-HCl, 1% NP-40, 0.1% SDS, 0.1% deoxycholic acid, 0.1 mM EDTA, 0.1 mM EGTA, protease and phosphatase inhibitors) with the aid of cell scrapers, and incubated for 30 to 45 min on ice. For all samples, including those used for coimmunoprecipitation studies (see below), protein extracts (20 μg) were separated by SDS–polyacrylamide gel electrophoresis (SDS-PAGE) and then transferred to 0.45-mm nitrocellulose membranes (Bio-Rad). After 1 hour of incubation with 5% milk/TBS-T to block unspecific binding of primary antibodies, membranes were probed with primary antibodies (all from Cell Signaling Technology, unless specified otherwise) against phospho-Tyr1175 (clone 19A10, catalog no. 2478) and total VEGFR2 (Santa Cruz Biotechnology, clone A-3, catalog no. 6251), phospho-Tyr783 (clone D6M9S, catalog no. 14008) and total PLCγ1 (catalog no. 21822), phospho-Ser473 (clone D9E, catalog no. 4060) and total Akt1 (catalog no. 2938), phospho-Ser9 (catalog no. 9336) and total GSK3β (clone 27C10, catalog no. 9315), phospho-Ser166 MDM2 (catalog no. 3521) and total MDM2 (Santa Cruz Biotechnology, clone C-18, catalog no. 812), phospho-Thr202/Tyr204 (catalog no. 9101) and total Erk1/2 (clone L34F12, catalog no. 4696), phospho-Ser1177 (catalog no. 9571) and total eNOS (Santa Cruz Biotechnology, clone C-20, catalog no. 654), calmodulin (Millipore, catalog no. 05-173), SDF2 (Santa Cruz Biotechnology, clone J22, catalog no. 100660), HA (Roche, clone 3F10, catalog no. 11867423001), FLAG (Sigma-Aldrich, clone M2, catalog no. F1804), actin (Sigma-Aldrich, catalog no. A5441), and Hsp90 (BD, catalog no. 610419), followed by species-specific secondary antibodies anti-IgG conjugated with either Alexa Fluor 680 (Invitrogen) or IRDye800 (Rockland). Blots were washed and visualized using a LI-COR Odyssey imager. The number of biological replicates used for each immunoblot analysis is specified in the figure legend. Densitometric analyses were performed using the ImageJ software.
Immunoprecipitation
COS7 transfected with SDF2-HA, FLAG-tagged deletion mutants of Hsp90, and pcDNA or eNOS for 24 hours were grown until confluent in DMEM containing 10% FBS and then used for coimmunoprecipitation studies. HEK293 cells transfected with SDF2-HA and pcDNA or eNOS for 24 hours were starved overnight in DMEM containing 0.5% FBS. Before stimulation, cells were pretreated with the PI3K inhibitor LY294002 (15 μM, for 30 min; Calbiochem) or the Akt inhibitor MK-2206 (5 μM, for 30 min; Selleckchem) or the intracellular calcium chelator BAPTA-AM (25 μM, for 5 min; Sigma-Aldrich) or vehicle as control. After 15 min of stimulation with 10% FBS, SDF2-HA was pulled down from whole-cell lysates as described below. shCTRL and shSDF2 EA.hy926 monolayers were starved overnight in DMEM containing 0.5% FBS and then treated with VEGF (50 ng/ml) for 15 and 30 min to induce complex formation. COS7, HEK293, or EA.hy926 cells (shCTRL/shSDF2) were washed twice with ice-cold PBS and immediately resuspended in TAP lysis buffer with the aid of cell scrapers, and incubated for 30 to 45 min at 4°C on an end-over-end rocker. All steps were performed at 4°C. SDF2-HA and FLAG-tagged Hsp90 deletion mutants were immunoprecipitated from 500 μg of whole-cell lysate using 20 μl of packed HA epitope tag antibody, agarose conjugate (Pierce), or Anti-FLAG M2 Affinity Gel (Sigma-Aldrich), respectively, which were washed twice with TAP lysis buffer before incubation with the whole-cell lysate overnight on an end-over-end rocker. Before eNOS immunoprecipitation, 1 mg of whole-cell lysate per group was precleared for 1 hour by incubation with 10 μl of packed rec-Protein G–Sepharose 4B Conjugate (Invitrogen), followed by an overnight incubation with 2 μg of eNOS antibody (Santa Cruz Biotechnology, clone C-20, catalog no. 654) or control rabbit IgG (Santa Cruz Biotechnology, catalog no. 2027) per 500 μg of whole-cell lysates. Samples were then incubated for 2 hours with 20 μl of packed rec-Protein G–Sepharose 4B Conjugate (Invitrogen), which were washed twice with TAP lysis buffer before incubation with the whole-cell lysate. All immunoprecipitated proteins were recovered by centrifugation of the resins at 2000 rpm for 2 min at 4°C, followed by one wash with TAP lysis buffer and three washes with TAP lysis buffer without NP-40. Proteins bound to SDF2-HA or eNOS were eluted by boiling samples for 10 min in SDS sample buffer, whereas proteins bound to FLAG-tagged Hsp90 deletion mutants were eluted by incubating samples for 1 hour at room temperature with 3X FLAG Peptide (0.5 mg/ml) (Sigma-Aldrich), followed by centrifugation at 2000 rpm for 2 min at 4°C to recover the supernatants. Samples were then analyzed by SDS-PAGE and immunoblotting.
Statistical analysis
Results are presented as means ± SEM. All experiments in which the effects of two variables were tested were analyzed by two-way analysis of variance (ANOVA) followed by Bonferroni post hoc test. Differences between two groups were compared by unpaired Student’s t test. P ≤ 0.05 was considered significant.
Supplementary Material
Table 2.
LC-MS/MS analysis of eNOS S1179D and GFP pull-downs.
| Symbol | Protein name | Average intensity GFP ± SEM |
Average intensity eNOS S1179D ± SEM |
Unique peptides |
Log2 (ratio eNOS S1179D/GFP) |
P |
|---|---|---|---|---|---|---|
| NOS3 | Endothelial nitric oxide synthase | 25.61 ± 0.80 | 35.52 ± 0.49 | 49 | 9.91 | <0.001 |
| CALM2; CALM1 | Calmodulin | 26.08 ± 0.48 | 32.60 ± 0.05 | 4 | 6.52 | <0.001 |
| SDF2 | Stromal cell–derived factor 2 | 23.78 ± 0.12 | 28.13 ± 0.21 | 2 | 4.35 | <0.001 |
Acknowledgments
We would like to thank D. Fulton for the eNOS shRNA adenovirus, G. Davis-Arrington for assistance with HUVEC isolation, and R. Babbitt for excellent technical support. Funding: This work was supported by grants R01 HL64793, R01 HL61371, R01 HL081190, and P01 HL70295 from the NIH to W.C.S. Author contributions: M.S. and W.C.S. designed the research; M.S., F.F., E.J.P., and M.S. performed the research; F.F. and T.C.W. performed MS analyses; M.S., F.F., and E.J.P. analyzed data; and M.S. and W.C.S. wrote the manuscript. Competing interests: The authors declare that they have no competing interests. Data and materials availability: The MS proteomics data have been deposited to the ProteomeXchange Consortium through the PRIDE partner repository with the data set identifier PXD002598.
Footnotes
SUPPLEMENTARY MATERIALS
www.sciencesignaling.org/cgi/content/full/8/390/ra81/DC1
Fig. S1. Overexpression of eNOS S1179A or S1179D does not affect SDF2 abundance.
Fig. S2. The SDF2-Hsp90 interaction is independent of PI3K or Akt activation and intracellular calcium concentration.
Table S1. MS analysis of label-free eNOS pull-downs.
REFERENCES AND NOTES
- 1.Förstermann U, Sessa WC. Nitric oxide synthases: Regulation and function. Eur. Heart J. 2012;33:829–837. doi: 10.1093/eurheartj/ehr304. 837a–837d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rudic RD, Shesely EG, Maeda N, Smithies O, Segal SS, Sessa WC. Direct evidence for the importance of endothelium-derived nitric oxide in vascular remodeling. J. Clin. Invest. 1998;101:731–736. doi: 10.1172/JCI1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shesely EG, Maeda N, Kim H-S, Desai KM, Krege JH, Laubach VE, Sherman PA, Sessa WC, Smithies O. Elevated blood pressures in mice lacking endothelial nitric oxide synthase. Proc. Natl. Acad. Sci. U.S.A. 1996;93:13176–13181. doi: 10.1073/pnas.93.23.13176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kuhlencordt PJ, Gyurko R, Han F, Scherrer-Crosbie M, Aretz TH, Hajjar R, Picard MH, Huang PL. Accelerated atherosclerosis, aortic aneurysm formation, and ischemic heart disease in apolipoprotein E/endothelial nitric oxide synthase double-knockout mice. Circulation. 2001;104:448–454. doi: 10.1161/hc2901.091399. [DOI] [PubMed] [Google Scholar]
- 5.García-Cardeña G, Fan R, Stern DF, Liu J, Sessa WC. Endothelial nitric oxide synthase is regulated by tyrosine phosphorylation and interacts with caveolin-1. J. Biol. Chem. 1996;271:27237–27240. doi: 10.1074/jbc.271.44.27237. [DOI] [PubMed] [Google Scholar]
- 6.Ju H, Zou R, Venema VJ, Venema RC. Direct interaction of endothelial nitric-oxide synthase and caveolin-1 inhibits synthase activity. J. Biol. Chem. 1997;272:18522–18525. doi: 10.1074/jbc.272.30.18522. [DOI] [PubMed] [Google Scholar]
- 7.Fulton D, Gratton J-P, Sessa WC. Post-translational control of endothelial nitric oxide synthase: Why isn’t calcium/calmodulin enough? J. Pharmacol. Exp. Ther. 2001;299:818–824. [PubMed] [Google Scholar]
- 8.Gratton J-P, Fontana J, O’Connor DS, García-Cardeña G, McCabe TJ, Sessa WC. Reconstitution of an endothelial nitric-oxide synthase (eNOS), hsp90, and caveolin-1 complex in vitro: Evidence that hsp90 facilitates calmodulin stimulated displacement of eNOS from caveolin-1. J. Biol. Chem. 2000;275:22268–22272. doi: 10.1074/jbc.M001644200. [DOI] [PubMed] [Google Scholar]
- 9.Michel JB, Feron O, Sase K, Prabhakar P, Michel T. Caveolin versus calmodulin. Counterbalancing allosteric modulators of endothelial nitric oxide synthase. J. Biol. Chem. 1997;272:25907–25912. doi: 10.1074/jbc.272.41.25907. [DOI] [PubMed] [Google Scholar]
- 10.García-Cardeña G, Fan R, Shah V, Sorrentino R, Cirino G, Papapetropoulos A, Sessa WC. Dynamic activation of endothelial nitric oxide synthase by Hsp90. Nature. 1998;392:821–824. doi: 10.1038/33934. [DOI] [PubMed] [Google Scholar]
- 11.Takahashi S, Mendelsohn ME. Synergistic activation of endothelial nitric-oxide synthase (eNOS) by HSP90 and Akt: Calcium-independent eNOS activation involves formation of an HSP90-Akt-CaM-bound eNOS complex. J. Biol. Chem. 2003;278:30821–30827. doi: 10.1074/jbc.M304471200. [DOI] [PubMed] [Google Scholar]
- 12.Bender AT, Silverstein AM, Demady DR, Kanelakis KC, Noguchi S, Pratt WB, Osawa Y. Neuronal nitric-oxide synthase is regulated by the Hsp90-based chaperone system in vivo. J. Biol. Chem. 1999;274:1472–1478. doi: 10.1074/jbc.274.3.1472. [DOI] [PubMed] [Google Scholar]
- 13.Ghosh A, Chawla-Sarkar M, Stuehr DJ. Hsp90 interacts with inducible NO synthase client protein in its heme-free state and then drives heme insertion by an ATP-dependent process. FASEB J. 2011;25:2049–2060. doi: 10.1096/fj.10-180554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fulton D, Gratton J-P, McCabe TJ, Fontana J, Fujio Y, Walsh K, Franke TF, Papapetropoulos A, Sessa WC. Regulation of endothelium-derived nitric oxide production by the protein kinase Akt. Nature. 1999;399:597–601. doi: 10.1038/21218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dimmeler S, Fleming I, Fisslthaler B, Hermann C, Busse R, Zeiher AM. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature. 1999;399:601–605. doi: 10.1038/21224. [DOI] [PubMed] [Google Scholar]
- 16.Fontana J, Fulton D, Chen Y, Fairchild TA, McCabe TJ, Fujita N, Tsuruo T, Sessa WC. Domain mapping studies reveal that the M domain of hsp90 serves as a molecular scaffold to regulate Akt-dependent phosphorylation of endothelial nitric oxide synthase and NO release. Circ. Res. 2002;90:866–873. doi: 10.1161/01.res.0000016837.26733.be. [DOI] [PubMed] [Google Scholar]
- 17.Schleicher M, Yu J, Murata T, Derakhshan B, Atochin D, Qian L, Kashiwagi S, Di Lorenzo A, Harrison KD, Huang PL, Sessa WC. The Akt1-eNOS axis illustrates the specificity of kinase-substrate relationships in vivo. Sci. Signal. 2009;2:ra41. doi: 10.1126/scisignal.2000343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qian J, Fulton D. Post-translational regulation of endothelial nitric oxide synthase in vascular endothelium. Front. Physiol. 2013;4:347. doi: 10.3389/fphys.2013.00347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schimanski B, Nguyen TN, Günzl A. Highly efficient tandem affinity purification of trypanosome protein complexes based on a novel epitope combination. Eukaryot. Cell. 2005;4:1942–1950. doi: 10.1128/EC.4.11.1942-1950.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McCabe TJ, Fulton D, Roman LJ, Sessa WC. Enhanced electron flux and reduced calmodulin dissociation may explain “calcium-independent” eNOS activation by phosphorylation. J. Biol. Chem. 2000;275:6123–6128. doi: 10.1074/jbc.275.9.6123. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Q, Church JE, Jagnandan D, Catravas JD, Sessa WC, Fulton D. Functional relevance of Golgi- and plasma membrane-localized endothelial NO synthase in reconstituted endothelial cells. Arterioscler. Thromb. Vasc. Biol. 2006;26:1015–1021. doi: 10.1161/01.ATV.0000216044.49494.c4. [DOI] [PubMed] [Google Scholar]
- 22.Qian J, Zhang Q, Church JE, Stepp DW, Rudic RD, Fulton DJ. Role of local production of endothelium-derived nitric oxide on cGMP signaling and S-nitrosylation. Am. J. Physiol. Heart Circ. Physiol. 2010;298:H112–H118. doi: 10.1152/ajpheart.00614.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Whitesell L, Lindquist SL. HSP90 and the chaperoning of cancer. Nat. Rev. Cancer. 2005;5:761–772. doi: 10.1038/nrc1716. [DOI] [PubMed] [Google Scholar]
- 24.Taipale M, Krykbaeva I, Koeva M, Kayatekin C, Westover KD, Karras GI, Lindquist S. Quantitative analysis of Hsp90-client interactions reveals principles of substrate recognition. Cell. 2012;150:987–1001. doi: 10.1016/j.cell.2012.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Song Y, Zweier JL, Xia Y. Heat-shock protein 90 augments neuronal nitric oxide synthase activity by enhancing Ca2+/calmodulin binding. Biochem. J. 2001;355:357–360. doi: 10.1042/0264-6021:3550357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peng H-M, Morishima Y, Clapp KM, Lau M, Pratt WB, Osawa Y. Dynamic cycling with Hsp90 stabilizes neuronal nitric oxide synthase through calmodulin-dependent inhibition of ubiquitination. Biochemistry. 2009;48:8483–8490. doi: 10.1021/bi901058g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yoshida M, Xia Y. Heat shock protein 90 as an endogenous protein enhancer of inducible nitric-oxide synthase. J. Biol. Chem. 2003;278:36953–36958. doi: 10.1074/jbc.M305214200. [DOI] [PubMed] [Google Scholar]
- 28.Hamada T, Tashiro K, Tada H, Inazawa J, Shirozu M, Shibahara K, Nakamura T, Martina N, Nakano T, Honjo T. Isolation and characterization of a novel secretory protein, stromal cell-derived factor-2 (SDF-2) using the signal sequence trap method. Gene. 1996;176:211–214. doi: 10.1016/0378-1119(96)00251-x. [DOI] [PubMed] [Google Scholar]
- 29.Lorenzon-Ojea AR, Caldeira W, Ribeiro AF, Fisher SJ, Guzzo CR, Bevilacqua E. Stromal cell derived factor-2 (Sdf2): A novel protein expressed in mouse. Int. J. Biochem. Cell Biol. 2014;53:262–270. doi: 10.1016/j.biocel.2014.05.024. [DOI] [PubMed] [Google Scholar]
- 30.Ponting CP. Novel repeats in ryanodine and IP3 receptors and protein O-mannosyltransferases. Trends Biochem. Sci. 2000;25:48–50. doi: 10.1016/s0968-0004(99)01513-3. [DOI] [PubMed] [Google Scholar]
- 31.Meunier L, Usherwood Y-K, Chung KT, Hendershot LM. A subset of chaperones and folding enzymes form multiprotein complexes in endoplasmic reticulum to bind nascent proteins. Mol. Biol. Cell. 2002;13:4456–4469. doi: 10.1091/mbc.E02-05-0311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Röhl A, Rohrberg J, Buchner J. The chaperone Hsp90: Changing partners for demanding clients. Trends Biochem. Sci. 2013;38:253–262. doi: 10.1016/j.tibs.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 33.Desjardins F, Delisle C, Gratton JP. Modulation of the cochaperone AHA1 regulates heat-shock protein 90 and endothelial NO synthase activation by vascular endothelial growth factor. Arterioscler. Thromb. Vasc. Biol. 2012;32:2484–2492. doi: 10.1161/ATVBAHA.112.256008. [DOI] [PubMed] [Google Scholar]
- 34.Holmes JL, Sharp SY, Hobbs S, Workman P. Silencing of HSP90 cochaperone AHA1 expression decreases client protein activation and increases cellular sensitivity to the HSP90 inhibitor 17-allylamino-17-demethoxygeldanamycin. Cancer Res. 2008;68:1188–1197. doi: 10.1158/0008-5472.CAN-07-3268. [DOI] [PubMed] [Google Scholar]
- 35.He T-C, Zhou S, da Costa LT, Yu J, Kinzler KW, Vogelstein B. A simplified system for generating recombinant adenoviruses. Proc. Natl. Acad. Sci. U.S.A. 1998;95:2509–2514. doi: 10.1073/pnas.95.5.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rappsilber J, Mann M, Ishihama Y. Protocol for micro-purification, enrichment, pre-fractionation and storage of peptides for proteomics using StageTips. Nat. Protoc. 2007;2:1896–1906. doi: 10.1038/nprot.2007.261. [DOI] [PubMed] [Google Scholar]
- 37.Fröhlich F, Christiano R, Walther TC. Native SILAC: Metabolic labeling of proteins in prototroph microorganisms based on lysine synthesis regulation. Mol. Cell. Proteomics. 2013;12:1995–2005. doi: 10.1074/mcp.M112.025742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Olsen JV, Macek B, Lange O, Makarov A, Horning S, Mann M. Higher-energy C-trap dissociation for peptide modification analysis. Nat. Methods. 2007;4:709–712. doi: 10.1038/nmeth1060. [DOI] [PubMed] [Google Scholar]
- 39.Cox J, Mann M. MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nat. Biotechnol. 2008;26:1367–1372. doi: 10.1038/nbt.1511. [DOI] [PubMed] [Google Scholar]
- 40.Cox J, Neuhauser N, Michalski A, Scheltema RA, Olsen JV, Mann M. Andromeda: A peptide search engine integrated into the MaxQuant environment. J. Proteome Res. 2011;10:1794–1805. doi: 10.1021/pr101065j. [DOI] [PubMed] [Google Scholar]
- 41.Hubner NC, Bird AW, Cox J, Splettstoesser B, Bandilla P, Poser I, Hyman A, Mann M. Quantitative proteomics combined with BAC TransgeneOmics reveals in vivo protein interactions. J. Cell Biol. 2010;189:739–754. doi: 10.1083/jcb.200911091. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.