Abstract
Currently, adult glioblastoma (GBM) patients have poor outcomes with conventional cytotoxic treatments. Because GBMs are highly angiogenic tumors, inhibitors that target tumor vasculature are considered promising therapeutic agents in these patients. Encouraging efficacy and tolerability in preliminary clinical trials suggest that targeting angiogenesis may be an effective therapeutic strategy in GBM patients. However, the survival benefits observed to date in uncontrolled trials of antiangiogenic agents have been modest, and several obstacles have limited their effectiveness. This article reviews the rationale for antiangiogenic agents in GBM, their potential mechanisms of action, and their clinical development in GBM patients. Although challenges remain with this approach, ongoing studies may improve upon the promising initial benefits already observed in GBM patients.
Keywords: Malignant glioma, Glioblastoma, Angiogenesis, Vascular endothelial growth factor, Cerebral edema
Introduction
The prognosis for patients with glioblastoma (GBM), the most common malignant glioma in adults, remains poor despite aggressive surgery, radiation, and chemotherapy. The median overall survival (OS) time for patients with GBM is <15 months [1]. Patients who progress through initial treatment fare worse, with an OS time <6 months [2], and for these patients there is no established treatment. However, new therapeutic approaches directed at the tumor vascular network have yielded encouraging results in several types of solid tumors, including GBM.
Induction of angiogenesis, the process by which tumors form new blood vessels, is required for most types of solid tumors to progress [3–6]. This insight set the stage for the development of antiangiogenic therapy [3, 7], and there are now three vascular endothelial growth factor (VEGF)-targeting drugs that have received U.S. Food and Drug Administration approval for the treatment of cancer. Based on beneficial results from phase III clinical trials, bevacizumab (Avastin®; Genentech, Inc., South San Francisco, CA), a monoclonal antibody against the VEGF-A ligand, was approved for first-line therapy in combination with chemotherapy in patients with advanced colorectal cancer, non-small cell lung cancer, and breast cancer [8–10]. In addition, two small-molecule tyrosine kinase inhibitors (TKIs) that target the VEGF receptor have shown efficacy in phase III studies as single agents. Sorafenib (Nexavar®; Bayer Pharmaceuticals Corporation, West Haven, CT) is approved for use in patients with advanced renal cell and hepatocellular carcinoma [11, 12] and sunitinib (Sutent®; Pfizer, Inc., New York) is approved for treatment of advanced renal cell carcinoma as well as for progressive gastrointestinal stromal tumors [13, 14].
Growth of malignant glioma (MG), which consists of World Health Organization (WHO) grade III (or anaplastic) astrocytoma, oligodendroglioma, and oligoastrocytoma and WHO grade IV astrocytoma (GBM), is dependent on new blood vessel formation [15–17]. Preclinical models strongly support the concept of antiangiogenic therapy for GBM [18, 19], and preliminary clinical data in GBM patients appear to validate preclinical findings. However, the clinical benefits of antiangiogenic therapies have been modest, with progression-free survival (PFS) improvements measured in months compared with historical controls, illustrating that significant challenges remain with these approaches [20]. Here, we review the rationale, mechanisms of action, and clinical development of antiangiogenic therapy in GBM and discuss strategies that may build on these encouraging initial clinical results.
Rationale for Antiangiogenic Therapy in MG
Biology of Angiogenesis in MG
The biology of brain tumor neovascularization has been recently reviewed in detail [6, 15–17], and here we briefly summarize several key features. Vasculature that is both structurally and functionally abnormal is characteristic of GBM, and microvascular proliferation is a diagnostic hallmark of GBM (Fig. 1) [21]. GBMs contain marked endothelial proliferation and highly disorganized, tortuous, large-diameter vessels with diminished pericyte coverage and increased basement membrane thickness [18, 22–24]. These vessels have increased permeability, blood flow, and transport properties and create an environment of severe hypoxia, increased interstitial pressure, acidosis, and necrosis in a spatially heterogeneous manner [25–29]. The increased permeability is associated with blood–brain barrier (BBB) disruption and results in vasogenic cerebral edema [30]. Disruption of the BBB is also the basis for the abnormal contrast leakage and enhancement observed on computed tomography (CT) and magnetic resonance imaging (MRI) scans of patients with MG.
Figure 1.
Histopathological hallmarks of glioblastomas. (A): Microvascular proliferations (arrow) appear as “glomeruloid tufts” and consist of multilayered, mitotically active endothelial cells and pericytes. (B): Pseudopalisading necrosis consists of dense arrays of radially oriented, fusiform glioma cells (arrow) that appear to palisade around a central area of fibrillary necrosis. The pseudopalisading cells have highly upregulated vascular endothelial growth factor expression and are proposed to induce microvascular proliferation in adjacent areas.
The mechanism of tumor angiogenesis was originally conceived of as the growth of new capillary vessels from pre-existing vessels; however, recent investigations have revealed a more complex and dynamic process. GBMs generate new blood vessels through several mechanisms that likely act simultaneously [17]. Classic “sprouting angiogenesis” is stimulated by hypoxia-induced secretion of growth factors, the most important being VEGF, that recruit new blood vessels [5, 31]. However, GBM may initially grow by “co-opting” pre-existing normal blood vessels. Glioma cells migrate along existing, normal blood vessels and destabilize them, causing vessel regression, reduced perfusion, hypoxia, and necrosis [32]. In response, cytokines are secreted that trigger angiogenesis [33]. Another less understood mechanism of new blood vessel growth is vasculogenesis, whereby bone marrow–derived endothelial precursor cells in the circulation are recruited to brain tumors and are directly incorporated into the tumor vasculature [34–36].
Angiogenesis is driven by a number of molecular pathways that interact in complex, redundant networks. The major proangiogenic mediator in GBM is VEGF-A (also referred to as VEGF), whose signal is transduced mainly through VEGF receptor (VEGFR)-2 (also known as KDR). VEGF is highly expressed in GBM as a consequence of diverse genetic and epigenetic cues, and its expression is associated with higher grades of astrocytoma [37–39]. Hypoxia is a principal inducer of VEGF expression from glioma cells through a mechanism mediated by the transcription factor hypoxia inducible factor-1α [37, 40–42]. Many other factors increase VEGF expression, including acidosis, nitric oxide, altered oncogenes and tumor suppressor genes (e.g., kit and TP53), cytokines (e.g., basic fibroblast growth factor [bFGF], platelet-derived growth factor [PDGF], and epidermal growth factor [EGF]), and activated intracellular signaling pathways such as phosphatidylinositol 3′ kinase (PI3K)/Akt and Ras/mitogen-activated protein kinase (MAPK) [38, 43–46].
VEGF is expressed mainly by the pseudopalisading glioma cells around zones of necrosis (Fig. 1) [47]. VEGF may also originate from host sources, including infiltrating inflammatory cells, platelets, stromal cells, and the extracellular matrix [5, 48]. Tumor-secreted VEGF functions in a paracrine manner by binding its cognate receptor VEGFR-2 on endothelial cells [49]. Stimulation of the receptor tyrosine kinase VEGFR-2 triggers a variety of signaling cascades including the PI3K/Akt and Ras/MAPK pathways. This results in increased endothelial cell proliferation, migration, and survival, as well as production of nitric oxide and increased vascular permeability [38, 50].
In addition, VEGF has a variety of effects on tumor endothelium that remain to be fully elucidated. The VEGF receptor family includes the cell surface receptors VEGFR-1, VEGFR-3, neuropilin (NRP)-1, and NRP-2. These receptors have distinct binding affinities for VEGF and its homologues (VEGF-B, VEGF-C, VEGF-D, VEGF-E, VEGF-F, and placental growth factor [PlGF]) [51], and many of these receptors are upregulated in GBM [49, 52, 53]. Each receptor is associated with relatively discrete physiological processes, and their functions in the context of tumor angiogenesis continue to be characterized [54, 55]. Furthermore, VEGF receptors are not limited to the cell surface. In nonglioma cancer models, intracellular VEGFR-1 and VEGFR-2 stimulation by VEGF promotes survival in both tumor and endothelial cells [56–58]. In addition, soluble VEGFR-2 and NRP-1 are thought to decrease angiogenesis by sequestering VEGF [59, 60].
Circulating VEGF also contributes to tumor neovasculature as a chemoattractant and possibly as an immune modulator. VEGF mobilizes and recruits bone marrow–derived myeloid cells (BMDCs), hematopoietic progenitor cells, and endothelial progenitor cells (EPCs) to GBM tumors [36, 61]. Infusion of VEGF in animal models interferes with T-cell development and the maturation of dendritic cells [62].
Many other factors promote angiogenesis in GBM through redundant or interconnected pathways with VEGF. These include the angiopoietin-1/angiopoietin-2/tie-2 signaling pathway, bFGF, insulin-like growth factor, hepatocyte growth factor/scatter factor, transforming growth factor-β, cyclo-oxygenase-2, tumor necrosis factor-α, and the interleukins [16, 17, 31, 46, 63]. Factors that mediate endothelial cell migration and invasion, such as cell surface integrins ανβ3 and ανβ5, matrix metalloproteinase (MMP)-2 and MMP-9, and tenascin-C, also promote angiogenesis in GBM [64, 65]. All of these proangiogenic mediators are balanced in vivo by endogenous inhibitors of angiogenesis such as angiostatin, endostatin, thrombospondin-1, and interferon (IFN)-γ [66].
The Notch-deltalike ligand (Dll)4 signaling pathway has emerged as a key regulator of tumor angiogenesis and a possible therapeutic target [67]. The cell surface Notch receptors are fundamental developmental regulators that transmit intercellular signals by interacting with transmembrane Dll and Jagged ligands on neighboring cells. Dll4 is expressed on endothelial cells and is essential for vascular development [68]. It is induced by VEGF, although it functions as a negative feedback regulator by inhibiting VEGF-induced endothelial activity [69, 70]. Interestingly, the administration of neutralizing antibodies to Dll4 in several tumor models, including glioma, results in reduced tumor growth but increased vessel density [71, 72]. This increased vascularity, however, is dysfunctional and inefficient, resulting in poor tumor perfusion and oxygenation.
Vasculogenesis is increasingly being recognized as a key factor in GBM neovascularization [6, 17]. Bone marrow–derived EPCs are recruited to the site of the tumor and are directly incorporated into the walls of new vessels. Moreover, hematopoietic cell populations recruited to perivascular locations promote vasculogenesis by expressing a variety of proangiogenic factors [36, 73]. In animal models, more than half of the vessels associated with gliomas and brain tumor metastases consist of BMDCs [34, 35]. Mobilization and recruitment are principally mediated by hypoxia and VEGF, although glioma-derived stromal-derived factor (SDF)-1 and G-CSF are also chemotactic for EPCs [74, 75].
Mechanisms of Antiangiogenic Therapy
VEGF pathway–targeting agents are the most clinically developed of the antiangiogenic therapies. Although no antiangiogenic agents are approved specifically for use in GBM, several VEGF-targeting approaches are under clinical investigation in this population. These include strategies that sequester VEGF using neutralizing antibodies or decoy receptors and TKIs or other receptor antagonists that target VEGFRs (Table 1).
Table 1.
Selected antiangiogenic agents currently in clinical trials for adult malignant glioma
| Agent | Target(s) | Mechanism | Tumor type(s) | Phase | Combinations |
|---|---|---|---|---|---|
| VEGF inhibitors | |||||
| Aflibercept | VEGF-A, VEGF-B, PlGF | Decoy receptor | New GBM, recurrent or stable MG | I | TMZ and RT |
| Recurrent MG | II | ||||
| Bevacizumab | VEGF-A | Monoclonal antibody | New GBM, recurrent MG | II/III | Various combinationsa |
| VEGF receptor inhibitors | |||||
| AEE788 | VEGFR-1/VEGFR-2, EGFR | Tyrosine kinase inhibitor | Recurrent GBM | I/II | |
| Recurrent GBM | I/II | Everolimus | |||
| Cediranib | VEGFR-1 to VEGFR-3, PDGFR-β, c-Kit | Tyrosine kinase inhibitor | New GBM | I/II | TMZ and RT |
| Recurrent GBM | III | Versus lomustine (randomized) | |||
| III | |||||
| CT-322 | VEGFR-1 to VEGFR-3 | Fibronectin (adnectin)-based inhibitor | Recurrent GBM | II | Alone and with irinotecan |
| Pazopanib (GW786034) | VEGFR-1 to VEGFR-3, PDGFR-β, c-Kit | Tyrosine kinase inhibitor | Recurrent GBM | II | |
| Recurrent MG | II | Lapatinib | |||
| Sorafenib | VEGFR-2, VEGFR-3, BRAF, PDGFR-β, c-Kit, Ras, p38α | Tyrosine kinase inhibitor | New GBM, recurrent GBM | I/II | Various combinationsb |
| Sunitinib | VEGFR-2, PDGFR-β, Flt3, c-Kit | Tyrosine kinase inhibitor | Recurrent MG | I | Irinotecan |
| Recurrent MG | II | ||||
| Vandetanib (ZD6474, Zactima®) | VEGFR-2, EGFR, RET | Tyrosine kinase inhibitor | New GBM | I/II | TMZ and RT |
| Recurrent MG | I | Imatinib and hydroxyurea | |||
| Recurrent glioma | I/II | ||||
| Vatalanib (PTK787) | VEGFR-1 to VEGFR-3, PDGFR-β, c-Kit | Tyrosine kinase inhibitor | New GBM | I | TMZ and RT in patients taking EIAEDs |
| New GBM | I/II | TMZ and RT with or without vatalanib (randomized) | |||
| Recurrent MG | I | Imatinib and hydroxyurea | |||
| XL184 | VEGFR-2, Met, RET, c-Kit, Flt3, Tie-2 | Tyrosine kinase inhibitor | Recurrent GBM | II | |
| Alternative angiogenesis pathway inhibitors | |||||
| ABT-510 | CD36 receptor | Thrombospondin-1 mimetic peptide | New GBM | I | TMZ and RT |
| AMG102 | HGF/SF | Monoclonal antibody | Recurrent GBM | II | |
| Dasatinib | PDGFR-β, Src, BCR-ABL, c-Kit, EphA2 | Tyrosine kinase inhibitor | Recurrent MG | I | Erlotinib |
| Recurrent GBM | II | ||||
| Imatinib | PDGFR-β, BCR-ABL, c-Kit | Tyrosine kinase inhibitor | Recurrent MG | I | Everolimus and hydroxyurea |
| Stable or recurrent MG | I | TMZ | |||
| Tandutinib (MLN518) | PDGFR-β, Flt3, c-Kit | Tyrosine kinase inhibitor | Recurrent GBM | I/II | |
| Recurrent MG | II | Bevacizumab | |||
| Celecoxib | COX-2 | Suppress VEGF and FGF | GBM following RT | II | Multiarm study with permutations of celecoxib, thalidomide, and isotretinoin |
| Recurrent MG | II | 6-Thioguanine and capecitabine with either TMZ or lomustine | |||
| Panzem® (2ME2) | HIF-1α | Disrupt tumor microtubules and suppress HIF-1α | Recurrent GBM | II | Protracted TMZ schedule |
| Recurrent GBM | II | ||||
| Metronomic TMZ | Endothelial cells, EPCs | Induce apoptosis, inhibit recruitment of EPCs | New GBM | II | Gliadel® wafer, TMZ, and radiotherapy followed by metronomic TMZ |
| GBM following RT | II | With retinoic acid versus standard adjuvant TMZ with retinoic acid | |||
| Recurrent MG | II | Bevacizumab | |||
| Endothelial cell migration inhibitors | |||||
| ATN-161 | Integrin α5β1 | Fibronectin-derived PHSRN peptide | Recurrent MG | I/II | Carboplatin |
| Cilengitide | Integrins ανβ3 and ανβ5 | RGD synthetic peptide | New GBM | I/II | TMZ and RT |
| New GBM | III | TMZ and RT | |||
| Recurrent MG | I/II | Monotherapy (several trials) | |||
Bevacizumab is being combined in various phase II trials with TMZ and radiotherapy as well as TMZ, radiotherapy, and irinotecan for newly diagnosed GBM patients; with TMZ and erlotinib for stable GBM following radiotherapy; and with bortezomib, enzastaurin, erlotinib, etoposide, irinotecan, sorafenib, tandutinib, or metronomic TMZ for recurrent MG or GBM.
Sorafenib is being combined in various phase I and II trials with TMZ and radiotherapy for newly diagnosed GBM and with lapatinib, erlotinib, tipifarnib, temsirolimus, bevacizumab, or a protracted schedule of TMZ for recurrent GBM.
Abbreviations: COX-2, cyclo-oxygenase 2; EGFR, epithelial growth factor receptor; EIAEDs, enzyme-inducing antiepileptic drugs; EPC, endothelial progenitor cell; FGF, fibroblast growth factor; GBM, glioblastoma; HGF/SF, hepatocyte growth factor/scatter factor; HIF-1α, hypoxia inducible factor 1α; MG, malignant glioma; PDGFR, platelet-derived growth factor; PlGF, placental growth factor; RA, retinoic acid; RT, radiotherapy; TMZ, temozolomide; VEGF, vascular endothelial growth factor.
Despite extensive preclinical evaluation, the antitumor mechanisms of anti-VEGF agents are incompletely understood. Several parallel mechanisms are thought to inhibit tumor growth in animal model systems [5, 20, 76]. Anti-VEGF agents may induce endothelial cell apoptosis and may inhibit BMDC incorporation, ultimately leading to a cytostatic effect on new blood vessel growth [77]. Effects on vascular function are more prominent, with vasoconstriction, decreased permeability, and decreased perfusion resulting in decreased delivery of oxygen and nutrients to the tumor.
When used in combination therapy, VEGF pathway inhibitors may sensitize glioma-associated endothelial cells to cytotoxic therapy [78, 79] and counteract a surge in VEGF expression and EPC recruitment induced by chemotherapy and radiation [76, 80, 81]. In addition, anti-VEGF therapy may transiently “normalize” the highly aberrant tumor vasculature and possibly improve delivery and efficacy of concurrent cytotoxic therapy [82]. Inhibition of VEGF decreases permeability and interstitial pressure, leading to more uniform blood flow and diminished tumor hypoxia [18]. In humans, vascular effects consistent with normalization were observed in colorectal cancer patients treated with bevacizumab [77], in recurrent GBM patients treated with the pan-VEGFR TKI cediranib (AZD2171, Recentin®; AstraZeneca Pharmaceuticals, Wilmington, DE) [83], and in recurrent GBM patient tissue following bevacizumab treatment [84].
With regard to specific VEGF-targeting agents, VEGF-sequestering molecules such as bevacizumab and the soluble decoy VEGFR aflibercept (VEGF-Trap®; Regeneron Pharmaceuticals, Inc., Tarrytown, NY) have the advantages of a long half-life and high specificity. Antiangiogenic TKIs were initially developed as specific competitive inhibitors of VEGFR tyrosine kinases; however, most of these TKIs have activity against other tyrosine kinases, including PDGFR, Raf, and c-Kit [85]. Although this relative lack of target specificity may result in more off-target effects, these agents may simultaneously inhibit several proangiogenic or pathogenic signaling pathways in GBM and thus confer an advantage over more specific agents [86]. Notably, intracellular VEGFR inhibition theoretically provides TKIs with an additional antitumor effect not obtained with agents that function in circulation.
Several proangiogenic pathways distinct from VEGF are also being targeted in GBM patients (summarized in Table 1). Another alternative antiangiogenic strategy is metronomic chemotherapy—the administration of low-dose conventional chemotherapy in closely spaced, regular intervals [87]. Metronomic chemotherapy schedules inhibit tumor endothelial cell proliferation and survival, prevent the recruitment of EPCs, and have antitumor activity in glioma animal models [88–90]. A recent study has shown that metronomic chemotherapy can also “normalize” tumor vessels, and thus improve delivery and efficacy of chemotherapy [91].
Furthermore, recent studies suggest that antiangiogenic agents may have activity against stem-like cells isolated from brain tumors [92]. These “brain tumor stem cells” have markedly upregulated VEGF expression, form highly angiogenic tumors in animal models [93], and reside in aberrant perivascular stem cell niches supported by endothelial cells [94]. Treatment of brain tumor stem cells with bevacizumab or metronomic chemotherapy inhibits angiogenesis and suppresses their tumorigenicity in animal models [93, 95, 96].
Finally, in animal models, antiangiogenic agents decrease brain tumor–associated vasogenic edema by decreasing tumor vessel permeability and interstitial fluid pressure [18, 30, 97]. This distinct effect on the peritumoral environment may prove to be therapeutically useful.
Clinical Trials
VEGF Ligand Sequestration
A number of retrospective and prospective studies indicated that bevacizumab, a recombinant humanized monoclonal antibody against VEGF with a half-life of up to 20 days, may have a beneficial effect in recurrent GBM patients. Several case series of recurrent GBM or MG patients have reported radiographic response rates (partial or complete responses as defined by standard criteria [98, 99]) of 35%–50% with the combination of bevacizumab and conventional chemotherapy, with responses often occurring rapidly after treatment initiation [100–103]. There was also a suggestion of clinical benefit in these reports manifested by delayed tumor progression. One study reported a 6-month (PFS6) rate of 42% for recurrent GBM patients [102], a rate higher than a historical benchmark of 15% [2]. Furthermore, an apparent antiedema effect of bevacizumab was evident in that study, because corticosteroid requirements decreased in 33% of patients.
Although treatment was generally well tolerated in these case series, thromboembolic and hemorrhagic complications were noted. In the two larger series, thromboembolic events occurred in five of 44 patients (11%) [102] and in seven of 77 patients (9%) [103]. These events included six episodes of pulmonary embolism, one superior mesenteric vein thrombosis, and one arterial event (myocardial infarction). Intratumoral hemorrhages were observed in five of 77 patients (6.5%) in one series [103], and there were two asymptomatic intracranial hemorrhages in the series of 44 patients [102]. Other notable complications included two gastrointestinal (GI) perforations [100, 102] and one case of reversible posterior leukoencephalopathy syndrome (RPLS) [103].
The first prospective study of an antiangiogenic therapy in MG patients was reported by Vredenburgh et al. [104, 105], who observed relatively high radiographic response and PFS rates using the combination of bevacizumab and irinotecan. In that phase II clinical trial, 68 recurrent MG patients were treated with bevacizumab and irinotecan in two schedules. The radiographic response rates were 57% for recurrent GBM and 61% for recurrent anaplastic glioma (AG) patients [106], which compared favorably with response rates achieved with temozolomide at first recurrence (5% for recurrent GBM and 35% for recurrent AG patients) [107, 108]. Radiographic responses were also associated with clinical improvement, because patients improved neurologically and were able to decrease or discontinue corticosteroid usage. The PFS6 rates were 43% for recurrent GBM patients and 59% for AG patients [106], considerably higher than historical references (15% and 31%, respectively). Although treatment was generally well tolerated, there was toxicity observed, with treatment-related discontinuation of therapy in 11 of 35 (31%) recurrent GBM patients [105]. Of note, there were eight (12%) thromboembolic complications, including one arterial stroke, and two (3%) central nervous system (CNS) hemorrhages [106].
Several subsequent prospective studies in recurrent MG patients showed similarly encouraging results with bevacizumab. In a study of 21 recurrent MG patients treated with the combination of bevacizumab and irinotecan, of which 17 had GBM, the MRI response rate was 36% and the OS rate at 6 months was 62% [109]. Preliminary data from an ongoing trial that randomized 167 recurrent GBM patients to receive bevacizumab alone or bevacizumab and irinotecan demonstrated a radiographic response rate of 32.9% and a PFS6 rate of 50.2% for the combination therapy [110]. The corticosteroid dose was reduced by at least 50% in the majority of enrolled patients. Notably, the median OS time of patients treated with bevacizumab alone was 9.7 months, whereas the median OS time was 8.9 months in patients treated with bevacizumab and irinotecan, suggesting that irinotecan may contribute little to the activity of bevacizumab [110]. Overall, treatment was well tolerated, with three intracranial hemorrhages noted. In a recently reported phase II study, 48 recurrent GBM patients were treated with bevacizumab alone. The PFS6 rate in that study was 29% and the 6-month OS rate was 57% [111]. Treatment was well tolerated, with six patients removed from the study for drug-related toxicities (five thromboembolic events and one bowel perforation).
Together, these prospective studies indicate that bevacizumab might have efficacy in this heavily pretreated patient population and has an acceptable toxicity profile. As a result, trials evaluating the addition of bevacizumab to standard therapy for newly diagnosed GBM patients are under way [112, 113], including a multicenter, randomized phase III trial for newly diagnosed GBM patients. In addition, a large number of clinical trials are currently evaluating bevacizumab in combination with various chemotherapeutic and molecularly targeted agents for recurrent GBM (Table 1). However, a survival advantage for bevacizumab in GBM patients has not been established in a prospective, controlled trial.
Aflibercept is a soluble VEGFR fused to an immunoglobulin constant region with a VEGF-binding affinity several hundred times greater than that of bevacizumab. It functions as a decoy receptor with the additional capacity of binding VEGF-B and PlGF [114]. Preliminary data from an ongoing phase II trial of aflibercept monotherapy in 48 recurrent MG patients showed radiographic response rates of 50% for AG and 30% for GBM patients [115], which were similar to values observed with bevacizumab and irinotecan. However, there was clinically significant toxicity, and 12 study patients (25%) had to discontinue aflibercept on average <2 months after starting therapy.
VEGFR TKIs
Many TKIs targeting VEGFR are under clinical development for GBM, and preliminary results are encouraging. These agents have the added clinical advantage of oral bioavailability. Cediranib, a potent pan-VEGFR TKI with activity against PDGFR and c-Kit, demonstrated promising activity in a recent phase II study. Cediranib monotherapy in patients with recurrent GBM resulted in a radiographic response rate of 56% (17 of 30 patients) and a PFS6 rate of approximately 26% [83, 116]. Furthermore, there was a steroid-sparing effect, as 15 of 16 patients who required steroids at the initiation of treatment were able to decrease or discontinue their dose. Overall, toxicity was modest. No treatment-related deaths or intracranial hemorrhages occurred, and only two of 31 patients were removed from the study as a result of toxicity. However, there was a high frequency of hypertension, and most patients required treatment with at least one antihypertensive drug. Other frequent toxicities were fatigue and diarrhea.
In that study, correlative MRI techniques, tissue samples, and blood markers were assessed to investigate mechanisms of cediranib activity. In most patients, cediranib treatment resulted in a transient reduction in permeability, tumor vessel diameter, blood volume, and blood flow by MRI criteria, suggesting a “normalization” of these vessels [83]. Additionally, specific circulating cytokines and cell populations were identified as potential biomarkers of drug resistance and tumor progression [83]. As a result of these studies, a multicenter randomized phase III trial of cediranib versus cediranib plus lomustine versus lomustine plus placebo in recurrent GBM is under way, and a phase II study of cediranib, temozolomide, and radiation in newly diagnosed GBM is also ongoing.
Vatalanib (PTK787, Novartis Pharmaceuticals Corporation, East Hanover, NJ) is a pan-VEGFR, PDGFR, and c-Kit TKI that has been clinically investigated in several advanced cancers [117]. In recurrent GBM patients, vatalanib has been studied as monotherapy [118] and in combination with temozolomide or lomustine [119] in phase I/II clinical trials. Overall, benefits were limited in these studies, with response rates of 4%–8% and modest PFS results. However, these results may have been affected by a suboptimal dosing schedule because of the relatively short half-life of the drug. More recently, vatalanib in combination with the PDGFR inhibitor imatinib (Gleevec®; Novartis Pharmaceuticals Corporation, East Hanover, NJ) and hydroxyurea produced a higher response rate (22%) in a trial of 37 recurrent GBM patients [120]. Given its favorable toxicity profile and synergism with radiotherapy in preclinical models [121], vatalanib is currently being investigated in combination with temozolomide and radiation in newly diagnosed GBM patients.
Both sorafenib and sunitinib are in early-phase clinical trials for recurrent MG. These inhibitors are active against a number of tyrosine kinases other than VEGFRs, including PDGFR, Flt-3, and c-Kit [122]. Sorafenib, which is also active against Raf, is being evaluated in separate trials as monotherapy and in combination regimens with the EGF receptor inhibitor erlotinib (Tarceva®; Genentech, Inc., South San Francisco, CA) and the mammalian target of rapamycin (mTOR) inhibitor temsirolimus (Torisel®; Wyeth Pharmaceuticals, Inc., Madison, NJ). Sorafenib seems to be moderately well tolerated in MG patients. Sunitinib is being evaluated as monotherapy and in combination with irinotecan in recurrent GBM. Several other VEGFR-targeted inhibitors are in clinical trials for recurrent GBM as single agents or in combination regimens (Table 1).
Inhibitors of Alternate Proangiogenic Signaling Pathways
The FGF pathway is thought to be an important VEGF-independent angiogenesis pathway in GBM and has recently been implicated in resistance to VEGF-targeted therapy [15, 83, 123]. Several, relatively nonspecific inhibitors of FGF-mediated angiogenesis have been extensively evaluated in GBM patients. Thalidomide, which inhibits both the bFGF and VEGF pathways [124], had little activity in phase II trials as monotherapy or in combination with carmustine or temozolomide in GBM patients [125–130]. Lenalidomide, a more potent thalidomide analog with better tolerability, appeared to have little efficacy in combination with radiation in newly diagnosed GBM patients in a recent phase I trial, although that study was not designed to assess efficacy [131]. The toxicities caused by this class of agents may be a limiting factor in their development in GBM. Other less-specific inhibitors of FGF-induced angiogenesis, including IFN-α, IFN-β, and suramin, were also found to have limited efficacy in MG patients [132–135]. Recently developed inhibitors of the FGFR tyrosine kinase have greater specificity for the FGF pathway and are potentially useful in MG, including brivanib (BMS-582664, Bristol-Myers Squibb, Princeton, NJ), TKI-258 (Novartis Pharmaceuticals Corporation, East Hanover, NJ), and XL-999 (Exelixis Inc., South San Francisco, CA).
The PDGF pathway plays a direct role in GBM angiogenesis in addition to its role in glioma transformation [136]. However, clinical trials with the PDGFR inhibitor imatinib produced disappointing results in recurrent MG patients [137, 138]. Several trials of imatinib and hydroxyurea combination therapy have been completed with promising PFS6 rates of 24%–32% in recurrent GBM [137, 139, 140]. A follow-up study of imatinib, hydroxyurea, and the mTOR inhibitor everolimus (RAD001, Novartis Pharmaceuticals Corporation, East Hanover, NJ) for recurrent MG is ongoing. The newer PDGFR TKIs tandutinib (MLN518, Millennium Pharmaceuticals, Cambridge, MA) and dasatinib (Sprycel®; Bristol-Myers Squibb, Princeton, NJ) have better CNS penetration than imatinib and thus potentially better efficacy, and are in clinical trials for recurrent GBM.
Inhibitors of other alternative angiogenic pathways have been evaluated in GBM with limited success. Enzastaurin (LY317615, Eli Lilly and Company, Indianapolis, IN), a selective inhibitor of protein kinase C-β, was studied in a phase III randomized trial against lomustine for recurrent GBM; however, the study was stopped after an interim analysis because of the failure to achieve efficacy milestones [141]. The selective cyclo-oxygenase-2 inhibitor celecoxib (Celebrex®; Pfizer, Inc., New York) inhibited angiogenesis in preclinical GBM models [142]; however, only modest PFS6 rates of 19% and 25% were observed when celecoxib was combined with either irinotecan or 13-cis-retinoic acid, respectively, in recurrent MG patients [143, 144].
Inhibitors of Endothelial Cell Migration
Cilengitide (EMD121974; Merck & Co., Inc., Whitehouse Station, NJ) is a cyclic RGD peptide competitive inhibitor of the cell surface ανβ3 and ανβ5 integrins, and mediates migration and survival of endothelial cells [145]. Several clinical trials have shown cilengitide to have minimal toxicity and marginal activity as a single agent in recurrent MG patients. No maximum-tolerated dose was defined in two phase I studies [146, 147], and no reproducible toxicities were observed in a separate phase II trial [148]. Furthermore, a trial evaluating intratumoral cilengitide after i.v. drug administration demonstrated good tumor penetration [149]. Promising efficacy was observed in a phase II trial of newly diagnosed GBM patients [150]. In that study, 81 patients were randomized to receive standard radiotherapy and temozolomide with or without cilengitide. The primary endpoint of the study was reached because patients treated with cilengitide had a PFS6 rate of 65.4%, significantly higher than the rate achieved with standard therapy alone (53.6%) [1]. Toxicity was similar in the cilengitide-containing and control arms. Based on these encouraging results, a multicenter, randomized phase III trial is under way for newly diagnosed GBM with methylated O-6-methylguanine-DNA methyltransferase gene promoter.
Metronomic Chemotherapy
Several chemotherapeutic agents have been tested in metronomic schedules for recurrent GBM patients [151–153]. Although these regimens were generally well tolerated, no significant survival benefits were observed in these early studies. More recently, two trials using low-dose, frequently administered temozolomide in recurrent GBM patients showed modest gains in terms of the PFS6 rate (35% in a GBM cohort of one study and 39% in another study [154, 155]) over historical controls. Ongoing clinical trials are evaluating the addition of bevacizumab to metronomic chemotherapy because superior efficacy was observed in preclinical models [91, 156].
Antiedema Effects
Several reports have noted reduced steroid requirements in patients treated with anti-VEGF agents, and one study specifically quantified the reduction in vasogenic edema using MRI techniques [83]. These studies indicate that anti- VEGF therapy may be useful in treating tumor-associated vasogenic cerebral edema. Additionally, a small retrospective series suggested that bevacizumab may also reduce edema and the mass effect associated with cerebral radiation necrosis [157]. Further development for this indication may be warranted because studies suggest that concurrent chemotherapy and radiation may increase the risk for tumor necrosis and edema [158, 159].
Current Obstacles and Future Directions
Although preliminary data from several prospective studies have demonstrated encouraging improvements in radiographic response and PFS rates, a definitive survival benefit has not been demonstrated in GBM patients. Moreover, future studies of antiangiogenic agents in the MG patient population must address a number of challenges, outlined below.
Assessing Tumor Response
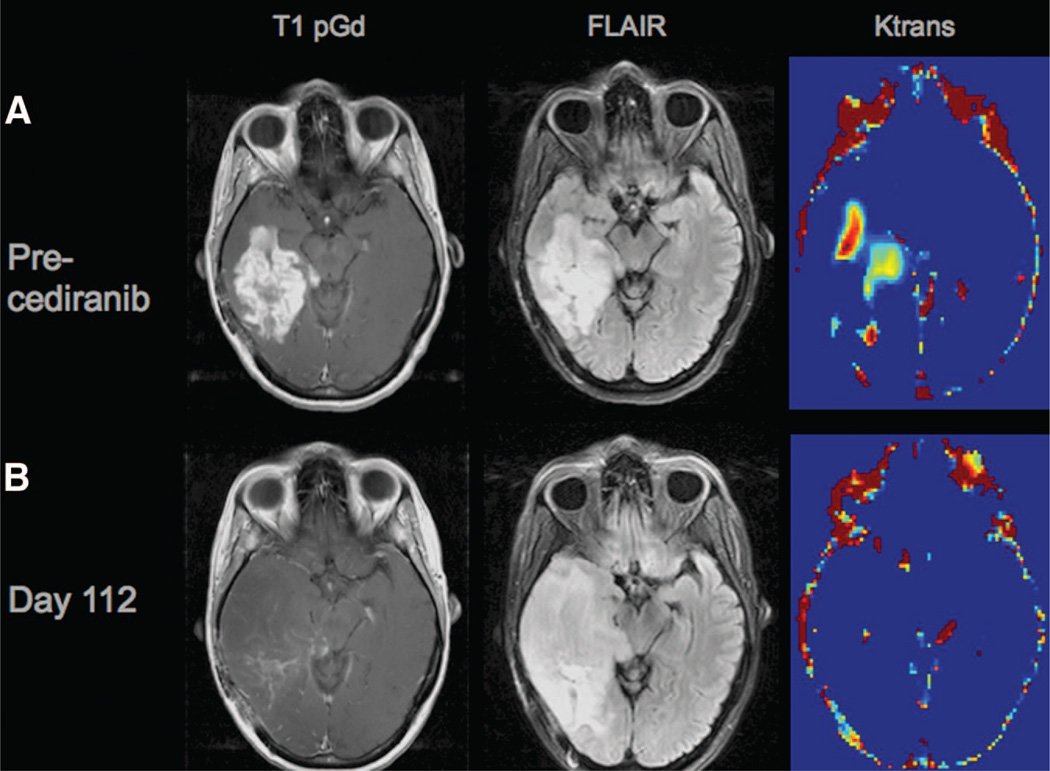
Accurate assessment of treatment response and progression in GBM patients treated with VEGF inhibitors is a critical objective. Contrast enhancement on CT and MRI scans is a reflection of VEGF-mediated BBB dysfunction and may not represent the contours of the underlying tumor. However, the criteria currently in use for assessment of brain tumor response are dependent on contrast enhancement [98] (Fig. 2). Anti-VEGF agents decrease permeability of cerebral vessels and diminish contrast enhancement on standard MRI [83], making interpretation of these alterations challenging. Newer imaging techniques that provide functional information may confer greater reliability in measuring tumor activity during antiangiogenic treatment, although none of these techniques has been validated. Among these, dynamic contrast-enhanced MRI and diffusion-weighted MRI are promising candidates [160, 161]. In addition, positron emission tomography (PET) [109, 162] and MRI-PET fusion techniques demonstrate potential utility in this setting [163]. In a small prospective study, the [18F] fluorothymidine-PET response at 1–2 weeks after initiating treatment with bevacizumab and irinotecan was reported to predict OS in recurrent MG patients [109]; however, this observation requires validation in larger prospective trials. There is an urgent need to define more reliable response criteria for GBM patients treated with antiangiogenic agents.
Figure 2.
Disease progression without contrast-enhancement on magnetic resonance imaging (MRI). (A): The top row depicts a glioblastoma prior to treatment with cediranib using different MRI techniques: a T1-weighted anatomic image after i.v. administration of a contrast agent (gadolinium-DTPA) (T1 pGd, left panel), demonstrating a region of bright signal corresponding to the recurrent brain tumor in the right temporal lobe; a T2-weighted image acquired with a fluid-attenuated inversion recovery (FLAIR) sequence (middle panel), where bright signal is present in the area of the contrast enhancement evident in the left panel as well as in a small surrounding margin; and a map of Ktrans, a measure of blood–brain barrier permeability, demonstrating regions of bright signal corresponding to areas of high permeability. (B): The same tumor at day 112 of cediranib therapy (bottom row) has marked improvements in contrast enhancement (left panel) and permeability (right panel); however, a significant increase in the area of FLAIR signal abnormality (middle panel) represents noncontrast-enhancing disease progression. Note also the mass effect on the right midbrain.
Resistance to Anti-VEGF Therapies
It is increasingly being recognized that responding GBM patients ultimately progress despite anti-VEGF treatment, with a time period typically on the order of months. When GBM patients fail treatment with bevacizumab and chemotherapy, there is usually rapid tumor progression [164]. Several distinct mechanisms of treatment resistance have been hypothesized [123]. In GBM, evidence suggests that initially responsive tumors may activate alternative proangiogenic signaling pathways in response to VEGF inhibition [165]. Recurrent GBM patients who progressed during treatment with cediranib had elevated circulating bFGF, SDF-1α, and Tie-2 levels, suggesting that these alternative proangiogenic pathways may provide a means of escape from therapy [83].
Increased tumor invasion by “co-option” of pre-existing blood vessels after anti-VEGF treatment is well described in preclinical glioma models [166]. Consistent with these preclinical findings, an enhanced infiltrative phenotype by MRI characteristics (Fig. 2) was recently described in recurrent GBM patients following anti-VEGF therapy [102]. A recent study suggested that areas of very low apparent diffusion coefficient (ADC) values on MRI may represent infiltrative tumor. The authors showed that the area of very low ADC increased during treatment with cediranib [167]. Another mechanism implicated in resistance to anti-VEGF therapy includes increased vasculogenesis by recruiting BMDCs [74, 168].
In addition to escape from treatment, distinct subsets of patients fail to achieve any response and may be intrinsically resistant to anti-VEGF therapy [83]. Evidence suggests nonresponding late-stage breast cancer patients may have pre-existing activation of parallel proangiogenic pathways [169]. A recent study in newly diagnosed GBM patient tissue reported that expression of neuronal pentraxin-2 and aquaporin-3 were increased in edematous tumors in the absence of increased VEGF expression, suggesting the existence of VEGF-independent mechanisms of tumor edema [170]. Increasingly, clinical studies are simultaneously targeting multiple angiogenic pathways in an attempt to preempt intrinsic resistance (Table 1). Finally, stem-like brain tumor cells were shown to grow in a highly infiltrative, angiogenesis-independent manner in a murine model [171].
Biomarkers of Response and Resistance
Predictive biomarkers are essential in order to realize the goal of “personalized” therapy for cancer patients. However, there are currently no validated biomarkers predictive of antiangiogenic efficacy or failure. Recent data indicate that imaging techniques (see above) and the serial evaluation of circulating cytokines and cell populations are promising biomarker candidates [172].
Candidate blood biomarkers include the plasma proteins collagen IV, VEGF, bFGF, PlGF, soluble VEGFR-2, Tie-2, SDF-1α, and soluble intercellular adhesion molecule 1, as well as circulating endothelial cells. In a study of recurrent GBM patients, radiographic tumor progression on cediranib was associated with elevated bFGF, SDF-1α, Tie-2, and circulating endothelial cells. Tissue biomarkers are also being evaluated, and one study suggested that VEGF protein expression levels predict radiographic response in patients treated with bevacizumab [173]. In that study, the authors also reported that tumor expression of hypoxia-induced carbonic anhydrase 9 was associated with shorter survival. Many correlative studies are ongoing, and validation of these preliminary findings in large prospective cohorts is required.
Monotherapy or Combination Therapy
An open question remains whether antiangiogenic agents are effective as single agents or only in combination with cytotoxic therapies. In non-CNS cancers, bevacizumab is approved for use only in combination with chemotherapy, whereas the TKIs sorafenib and sunitinib are active as single agents in renal cell carcinoma and hepatocellular carcinoma. One hypothesis is that bevacizumab may principally be serving to transiently “normalize” tumor vasculature and improve chemotherapy delivery and efficacy [82], whereas the observed clinical efficacy of broad-spectrum TKIs as single agents may result from their ability to inhibit multiple pathogenic signaling pathways concurrently. Recent evidence suggests that bevacizumab may have efficacy as a single agent in recurrent GBM patients. Two prospective studies have reported PFS6 rates of 35% [110] and 29% [111] with bevacizumab monotherapy. Cumulative evidence suggests, however, that combination therapy will likely be necessary to treat this refractory malignancy.
Toxicity
The risks for thrombosis and hemorrhage with antiangiogenic therapy in GBM patients have been continuing concerns. Although an increased risk for thromboembolic events in patients treated with bevacizumab has been reported in some studies [105], thrombotic complications in the absence of anti-VEGF therapy are common in the GBM patient population [174]. The CNS hemorrhage risk appears to be low, and observed events are often asymptomatic. The risk for epistaxis is increased; however, major systemic bleeding is rare. Notably, among 11 patients treated concurrently with bevacizumab and anticoagulation in one study, only one episode of mild epistaxis was seen [102].
Anti-VEGF antibodies and VEGFR TKIs appear to have shared as well as distinct toxicities. Shared toxicities include fatigue, which is nearly universal, and hypertension. Less frequently, hemorrhage and GI perforation are observed. Impaired wound healing is a concern with these agents, and may be problematic after surgery in newly diagnosed GBM patients [112]. Proteinuria is more frequently observed in patients treated with bevacizumab, whereas hypertension, diarrhea, mucositis, and skin toxicity are relatively more common in patients who receive TKIs. Other rare but serious complications include thrombotic thrombocytopenic purpura [106], myocardial infarction, arterial stroke, and RPLS.
Recurrent AG and Newly Diagnosed MG Patients
Although several of the trials discussed above included both recurrent AG (WHO grade III) and recurrent GBM (WHO grade IV) patients, antiangiogenic drugs have mainly been studied in the recurrent GBM population. The efficacy of antiangiogenic drugs in patients with recurrent AG, newly diagnosed AG, or newly diagnosed GBM has not been rigorously assessed. One phase II study of 33 AG patients treated with bevacizumab and irinotecan reported a PFS6 rate of 55% [175], which compares favorably with the PFS6 rate achieved with temozolomide in this population (46%) [108]. Antiangiogenic therapy has not been reported in newly diagnosed AG patients. In newly diagnosed GBM patients, several ongoing trials are evaluating the addition of bevacizumab, aflibercept, cediranib, cilengitide, or XL184 to standard temozolomide and radiation therapy. The role of antiangiogenic therapy in these patient subsets remains to be determined.
Conclusion
Current evidence suggests that antiangiogenic agents, either alone or when combined with cytotoxic therapies, may have efficacy in patients with recurrent MG. Antiangiogenic drugs also appear to have potent antiedema and steroid-sparing effects in the MG patient population. However, definite survival benefits have yet to be demonstrated, and any observed improvements may be modest. Validation in larger prospective clinical trials is required to determine the efficacy of these agents. In addition, the contribution of the antiedema effect to the overall clinical benefit of these drugs is currently unknown. Further studies are necessary to address this question as well as the remaining uncertainties regarding the mechanisms of action of antiangiogenic therapy, the different mechanisms of antiangiogenic drug resistance, and the role of antiangiogenic agents in newly diagnosed MG patients. Directed preclinical investigations and clinical trials with well-integrated imaging and molecular studies may lead to answers as well as to new insights that may be exploited to improve upon the clinical benefits realized thus far. Despite these obstacles, the progress made in antiangiogenic therapy has provided a novel therapeutic framework that, when built upon, may ultimately improve the outcomes for patients with MG.
Acknowledgments
We thank David N. Louis for providing the histopathological images.
Footnotes
Disclosures: Andrew S. Chi: None; A. Gregory Sorenson: Consultant/advisory role: ACRIN Image Metrix, Genentech, Epix Pharmaceuticals, Millennium, AstraZeneca, Mitsubishi Pharma; Research funding/contracted research: NIH, Siemens Medical Solutions, GE Healthcare, GlaxoSmithKline, Novartis Pharmaceuticals, Exelixis, Schering Plough, Amgen, AstraZeneca; Rakesh K. Jain: Consultant/advisory role: AstraZeneca, Dyax, Millennium; Honoraria: AstraZeneca, Dyax; Ownership interest: SynDevRx; Tracy T. Batchelor: Consultant/advisory role: Millennium, AstraZeneca, ImClone, Exelixis, Vertex, McCleon; Honoraria: Genentech, Schering-Plough, Enzon.
The article discusses bevacizumab (Genentech) and cediranib (AstraZeneca).
Note Added in Proof
While this manuscript was in press, the U.S. Food and Drug Administration (FDA) granted accelerated approval of Avastin® (bevacizumab; Genentech, Inc., South San Francisco, CA) monotherapy for patients with glioblastoma (GBM) with progressive disease following prior therapy. The new indication for Avastin® was granted under the FDA’s accelerated approval program that permits the use of certain surrogate endpoints or an effect on a clinical endpoint other than survival or irreversible morbidity as bases for approvals of products intended for serious or life-threatening illnesses or conditions. The approval was based on demonstration of improved objective response rates observed in two historically-controlled, single-arm or noncomparative phase II trials [110, 111].
The FDA independently reviewed an open-label, multicenter, noncomparative phase II study that randomized 167 recurrent GBM patients to receive bevacizumab alone or bevacizumab in combination with irinotecan [110], although only efficacy data from the bevacizumab monotherapy arm (n = 85) were used to support drug approval. Response was assessed by magnetic resonance imaging (MRI) and measured using World Health Organization radiographic criteria along with decreased or stable corticosteroid use. According to the FDA analysis of this study, tumor responses were observed in 26% of patients treated with bevacizumab alone, and the median duration of response in these patients was 4.2 months. In this study, the incidence of adverse events known to be associated with bevacizumab did not appear to be significantly increased in GBM patients based on this externally controlled trial.
The FDA used the same response assessment criteria to independently assess another single-arm, single-institution trial in which 56 recurrent GBM patients were treated with bevacizumab alone [111]. Responses were observed in 20% of patients, and the median duration of response was 3.9 months. This approval will significantly impact the general treatment approach for patients with recurrent GBM. Currently, however, no data are available from prospective, randomized controlled trials demonstrating improvement in disease-related symptoms or increased survival with bevacizumab in GBM. These data will be necessary to measure the actual clinical benefit of bevacizumab in this population.
Author Contributions
Conception/Design: Andrew S. Chi, Tracy T. Batchelor
Collection/assembly of data: Andrew S. Chi
Data analysis: Andrew S. Chi, A. Gregory Sorensen, Rakesh K. Jain, Tracy T. Batchelor
Manuscript writing: Andrew S. Chi, A. Gregory Sorensen, Rakesh K. Jain, Tracy T. Batchelor
Final approval of manuscript: Tracy T. Batchelor
References
- 1.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 2.Wong ET, Hess KR, Gleason MJ, et al. Outcomes and prognostic factors in recurrent glioma patients enrolled onto phase II clinical trials. J Clin Oncol. 1999;17:2572–2578. doi: 10.1200/JCO.1999.17.8.2572. [DOI] [PubMed] [Google Scholar]
- 3.Folkman J. Tumor angiogenesis: Therapeutic implications. N Engl J Med. 1971;285:1182–1186. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 4.Folkman J. Angiogenesis. Annu Rev Med. 2006;57:1–18. doi: 10.1146/annurev.med.57.121304.131306. [DOI] [PubMed] [Google Scholar]
- 5.Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407:249–257. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- 6.Kerbel RS. Tumor angiogenesis. N Engl J Med. 2008;358:2039–2049. doi: 10.1056/NEJMra0706596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Folkman J. Angiogenesis: An organizing principle for drug discovery? Nat Rev Drug Discov. 2007;6:273–286. doi: 10.1038/nrd2115. [DOI] [PubMed] [Google Scholar]
- 8.Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 9.Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355:2542–2550. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 10.Miller K, Wang M, Gralow J, et al. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med. 2007;357:2666–2676. doi: 10.1056/NEJMoa072113. [DOI] [PubMed] [Google Scholar]
- 11.Escudier B, Eisen T, Stadler WM, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007;356:125–134. doi: 10.1056/NEJMoa060655. [DOI] [PubMed] [Google Scholar]
- 12.Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 13.Motzer RJ, Hutson TE, Tomczak P, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356:115–124. doi: 10.1056/NEJMoa065044. [DOI] [PubMed] [Google Scholar]
- 14.Demetri GD, van Oosterom AT, Garrett CR, et al. Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib:Arandomised controlled trial. Lancet. 2006;368:1329–1338. doi: 10.1016/S0140-6736(06)69446-4. [DOI] [PubMed] [Google Scholar]
- 15.Fischer I, Gagner JP, Law M, et al. Angiogenesis in gliomas: Biology and molecular pathophysiology. Brain Pathol. 2005;15:297–310. doi: 10.1111/j.1750-3639.2005.tb00115.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kargiotis O, Rao JS, Kyritsis AP. Mechanisms of angiogenesis in gliomas. J Neurooncol. 2006;78:281–293. doi: 10.1007/s11060-005-9097-6. [DOI] [PubMed] [Google Scholar]
- 17.Jain RK, di Tomaso E, Duda DG, et al. Angiogenesis in brain tumours. Nat Rev Neurosci. 2007;8:610–622. doi: 10.1038/nrn2175. [DOI] [PubMed] [Google Scholar]
- 18.Winkler F, Kozin SV, Tong RT, et al. Kinetics of vascular normalization by VEGFR2 blockade governs brain tumor response to radiation: Role of oxygenation, angiopoietin-1, and matrix metalloproteinases. Cancer Cell. 2004;6:553–563. doi: 10.1016/j.ccr.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 19.Gagner JP, Law M, Fischer I, et al. Angiogenesis in gliomas: Imaging and experimental therapeutics. Brain Pathol. 2005;15:342–363. doi: 10.1111/j.1750-3639.2005.tb00119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jain RK, Duda DG, Clark JW, et al. Lessons from phase III clinical trials on anti-VEGF therapy for cancer. Nat Clin Pract Oncol. 2006;3:24–40. doi: 10.1038/ncponc0403. [DOI] [PubMed] [Google Scholar]
- 21.Kleihues P, Cavenee WK, editors. World Health Organization Classification of Tumours: Pathology and Genetics: Tumours of the Nervous System. Lyon, France: IARC Press; 2000. pp. 1–314. [Google Scholar]
- 22.Plate KH, Mennel HD. Vascular morphology and angiogenesis in glial tumors. Exp Toxicol Pathol. 1995;47:89–94. doi: 10.1016/S0940-2993(11)80292-7. [DOI] [PubMed] [Google Scholar]
- 23.Bullitt E, Zeng D, Gerig G, et al. Vessel tortuosity and brain tumor malignancy: A blinded study. Acad Radiol. 2005;12:1232–1240. doi: 10.1016/j.acra.2005.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guo P, Hu B, Gu W, et al. Platelet-derived growth factor-B enhances glioma angiogenesis by stimulating vascular endothelial growth factor expression in tumor endothelia and by promoting pericyte recruitment. Am J Pathol. 2003;162:1083–1093. doi: 10.1016/S0002-9440(10)63905-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yuan F, Salehi HA, Boucher Y, et al. Vascular permeability and microcirculation of gliomas and mammary carcinomas transplanted in rat and mouse cranial windows. Cancer Res. 1994;54:4564–4568. [PubMed] [Google Scholar]
- 26.Deeken JF, Löscher W. The blood-brain barrier and cancer: Transporters, treatment, and Trojan horses. Clin Cancer Res. 2007;13:1663–1674. doi: 10.1158/1078-0432.CCR-06-2854. [DOI] [PubMed] [Google Scholar]
- 27.Rampling R, Cruickshank G, Lewis AD, et al. Direct measurement of pO2 distribution and bioreductive enzymes in human malignant brain tumors. Int J Radiat Oncol Biol Phys. 1994;29:427–431. doi: 10.1016/0360-3016(94)90432-4. [DOI] [PubMed] [Google Scholar]
- 28.Boucher Y, Leunig M, Jain RK. Tumor angiogenesis and interstitial hypertension. Cancer Res. 1996;56:4264–4266. [PubMed] [Google Scholar]
- 29.Helmlinger G, Yuan F, Dellian M, et al. Interstitial pH and pO2 gradients in solid tumors in vivo: High-resolution measurements reveal a lack of correlation. Nat Med. 1997;3:177–182. doi: 10.1038/nm0297-177. [DOI] [PubMed] [Google Scholar]
- 30.Jain RK, Tong RT, Munn LL. Effect of vascular normalization by antiangiogenic therapy on interstitial hypertension, peritumor edema, and lymphatic metastasis: Insights from a mathematical model. Cancer Res. 2007;67:2729–2735. doi: 10.1158/0008-5472.CAN-06-4102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yancopoulos GD, Davis S, Gale NW, et al. Vascular-specific growth factors and blood vessel formation. Nature. 2000;407:242–248. doi: 10.1038/35025215. [DOI] [PubMed] [Google Scholar]
- 32.Holash J, Maisonpierre PC, Compton D, et al. Vessel cooption, regression, and growth in tumors mediated by angiopoietins and VEGF. Science. 1999;284:1994–1998. doi: 10.1126/science.284.5422.1994. [DOI] [PubMed] [Google Scholar]
- 33.Zagzag D, Amirnovin R, Greco MA, et al. Vascular apoptosis and involution in gliomas precede neovascularization: A novel concept for glioma growth and angiogenesis. Lab Invest. 2000;80:837–849. doi: 10.1038/labinvest.3780088. [DOI] [PubMed] [Google Scholar]
- 34.Santarelli JG, Udani V, Yung YC, et al. Incorporation of bone marrowderived Flk-1-expressing CD34+ cells in the endothelium of tumor vessels in the mouse brain. Neurosurgery. 2006;59:374–382. doi: 10.1227/01.NEU.0000222658.66878.CC. discussion 374–382. [DOI] [PubMed] [Google Scholar]
- 35.Duda DG, Cohen KS, Kozin SV, et al. Evidence for incorporation of bone marrow-derived endothelial cells into perfused blood vessels in tumors. Blood. 2006;107:2774–2776. doi: 10.1182/blood-2005-08-3210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Murdoch C, Muthana M, Coffelt SB, et al. The role of myeloid cells in the promotion of tumour angiogenesis. Nat Rev Cancer. 2008;8:618–631. doi: 10.1038/nrc2444. [DOI] [PubMed] [Google Scholar]
- 37.Plate KH, Breier G, Weich HA, et al. Vascular endothelial growth factor is a potential tumour angiogenesis factor in human gliomas in vivo. Nature. 1992;359:845–848. doi: 10.1038/359845a0. [DOI] [PubMed] [Google Scholar]
- 38.Ferrara N. Vascular endothelial growth factor: Basic science and clinical progress. Endocr Rev. 2004;25:581–611. doi: 10.1210/er.2003-0027. [DOI] [PubMed] [Google Scholar]
- 39.Mizukami Y, Kohgo Y, Chung DC. Hypoxia inducible factor-1 independent pathways in tumor angiogenesis. Clin Cancer Res. 2007;13:5670–5674. doi: 10.1158/1078-0432.CCR-07-0111. [DOI] [PubMed] [Google Scholar]
- 40.Shweiki D, Itin A, Soffer D, et al. Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature. 1992;359:843–845. doi: 10.1038/359843a0. [DOI] [PubMed] [Google Scholar]
- 41.Kaur B, Khwaja FW, Severson EA, et al. Hypoxia and the hypoxia-inducible- factor pathway in glioma growth and angiogenesis. Neuro Oncol. 2005;7:134–153. doi: 10.1215/S1152851704001115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer. 2003;3:721–732. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- 43.Fukumura D, Xu L, Chen Y, et al. Hypoxia and acidosis independently up-regulate vascular endothelial growth factor transcription in brain tumors in vivo. Cancer Res. 2001;61:6020–6024. [PubMed] [Google Scholar]
- 44.Fukumura D, Kashiwagi S, Jain RK. The role of nitric oxide in tumour progression. Nat Rev Cancer. 2006;6:521–534. doi: 10.1038/nrc1910. [DOI] [PubMed] [Google Scholar]
- 45.Sun L, Hui AM, Su Q, et al. Neuronal and glioma-derived stem cell factor induces angiogenesis within the brain. Cancer Cell. 2006;9:287–300. doi: 10.1016/j.ccr.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 46.Lamszus K, Heese O, Westphal M. Angiogenesis-related growth factors in brain tumors. Cancer Treat Res. 2004;117:169–190. doi: 10.1007/978-1-4419-8871-3_12. [DOI] [PubMed] [Google Scholar]
- 47.Zagzag D, Zhong H, Scalzitti JM, et al. Expression of hypoxia-inducible factor 1α in brain tumors: Association with angiogenesis, invasion, and progression. Cancer. 2000;88:2606–2618. [PubMed] [Google Scholar]
- 48.Fukumura D, Xavier R, Sugiura T, et al. Tumor induction of VEGF promoter activity in stromal cells. Cell. 1998;94:715–725. doi: 10.1016/s0092-8674(00)81731-6. [DOI] [PubMed] [Google Scholar]
- 49.Plate KH, Breier G, Weich HA, et al. Vascular endothelial growth factor and glioma angiogenesis: Coordinate induction of VEGF receptors, distribution of VEGF protein and possible in vivo regulatory mechanisms. Int J Cancer. 1994;59:520–529. doi: 10.1002/ijc.2910590415. [DOI] [PubMed] [Google Scholar]
- 50.Shibuya M, Claesson-Welsh L. Signal transduction by VEGF receptors in regulation of angiogenesis and lymphangiogenesis. Exp Cell Res. 2006;312:549–560. doi: 10.1016/j.yexcr.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 51.Roy H, Bhardwaj S, Ylä-Herttuala S. Biology of vascular endothelial growth factors. FEBS Lett. 2006;580:2879–2887. doi: 10.1016/j.febslet.2006.03.087. [DOI] [PubMed] [Google Scholar]
- 52.Grau SJ, Trillsch F, Herms J, et al. Expression of VEGFR3 in glioma endothelium correlates with tumor grade. J Neurooncol. 2007;82:141–150. doi: 10.1007/s11060-006-9272-4. [DOI] [PubMed] [Google Scholar]
- 53.Hu B, Guo P, Bar-Joseph I, et al. Neuropilin-1 promotes human glioma progression through potentiating the activity of the HGF/SF autocrine pathway. Oncogene. 2007;26:5577–5586. doi: 10.1038/sj.onc.1210348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kowanetz M, Ferrara N. Vascular endothelial growth factor signaling pathways: Therapeutic perspective. Clin Cancer Res. 2006;12:5018–5022. doi: 10.1158/1078-0432.CCR-06-1520. [DOI] [PubMed] [Google Scholar]
- 55.Neufeld G, Kessler O. The semaphorins: Versatile regulators of tumour progression and tumour angiogenesis. Nat Rev Cancer. 2008;8:632–645. doi: 10.1038/nrc2404. [DOI] [PubMed] [Google Scholar]
- 56.Lee TH, Seng S, Sekine M, et al. Vascular endothelial growth factor mediates intracrine survival in human breast carcinoma cells through internally expressed VEGFR1/FLT1. PLoS Med. 2007;4:e186. doi: 10.1371/journal.pmed.0040186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gerber HP, Malik AK, Solar GP, et al. VEGF regulates haematopoietic stem cell survival by an internal autocrine loop mechanism. Nature. 2002;417:954–958. doi: 10.1038/nature00821. [DOI] [PubMed] [Google Scholar]
- 58.Lee S, Chen TT, Barber CL, et al. Autocrine VEGF signaling is required for vascular homeostasis. Cell. 2007;130:691–703. doi: 10.1016/j.cell.2007.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kendall RL, Thomas KA. Inhibition of vascular endothelial cell growth factor activity by an endogenously encoded soluble receptor. Proc Natl Acad Sci U S A. 1993;90:10705–10709. doi: 10.1073/pnas.90.22.10705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gagnon ML, Bielenberg DR, Gechtman Z, et al. Identification of a natural soluble neuropilin-1 that binds vascular endothelial growth factor: In vivo expression and antitumor activity. Proc Natl Acad Sci U S A. 2000;97:2573–2578. doi: 10.1073/pnas.040337597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lyden D, Hattori K, Dias S, et al. Impaired recruitment of bone-marrow-derived endothelial and hematopoietic precursor cells blocks tumor angiogenesis and growth. Nat Med. 2001;7:1194–1201. doi: 10.1038/nm1101-1194. [DOI] [PubMed] [Google Scholar]
- 62.Ohm JE, Gabrilovich DI, Sempowski GD, et al. VEGF inhibits T-cell development and may contribute to tumor-induced immune suppression. Blood. 2003;101:4878–4886. doi: 10.1182/blood-2002-07-1956. [DOI] [PubMed] [Google Scholar]
- 63.Reiss Y, Machein MR, Plate KH. The role of angiopoietins during angiogenesis in gliomas. Brain Pathol. 2005;15:311–317. doi: 10.1111/j.1750-3639.2005.tb00116.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lakka SS, Gondi CS, Rao JS. Proteases and glioma angiogenesis. Brain Pathol. 2005;15:327–341. doi: 10.1111/j.1750-3639.2005.tb00118.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang D, Anderson JC, Gladson CL. The role of the extracellular matrix in angiogenesis in malignant glioma tumors. Brain Pathol. 2005;15:318–326. doi: 10.1111/j.1750-3639.2005.tb00117.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rege TA, Fears CY, Gladson CL. Endogenous inhibitors of angiogenesis in malignant gliomas: Nature’s antiangiogenic therapy. Neuro Oncol. 2005;7:106–121. doi: 10.1215/S115285170400119X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gridley T. Vascular biology: Vessel guidance. Nature. 2007;445:722–723. doi: 10.1038/445722a. [DOI] [PubMed] [Google Scholar]
- 68.Benedito R, Duarte A. Expression of Dll4 during mouse embryogenesis suggests multiple developmental roles. Gene Expr Patterns. 2005;5:750–755. doi: 10.1016/j.modgep.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 69.Williams CK, Li JL, Murga M, et al. Up-regulation of the Notch ligand Delta-like 4 inhibits VEGF-induced endothelial cell function. Blood. 2006;107:931–939. doi: 10.1182/blood-2005-03-1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Thurston G, Noguera-Troise I, Yancopoulos GD. The Delta paradox: DLL4 blockade leads to more tumour vessels but less tumour growth. Nat Rev Cancer. 2007;7:327–331. doi: 10.1038/nrc2130. [DOI] [PubMed] [Google Scholar]
- 71.Ridgway J, Zhang G, Wu Y, et al. Inhibition of Dll4 signalling inhibits tumour growth by deregulating angiogenesis. Nature. 2006;444:1083–1087. doi: 10.1038/nature05313. [DOI] [PubMed] [Google Scholar]
- 72.Noguera-Troise I, Daly C, Papadopoulos NJ, et al. Blockade of Dll4 inhibits tumour growth by promoting non-productive angiogenesis. Nature. 2006;444:1032–1037. doi: 10.1038/nature05355. [DOI] [PubMed] [Google Scholar]
- 73.Bertolini F, Shaked Y, Mancuso P, et al. The multifaceted circulating endothelial cell in cancer: Towards marker and target identification. Nat Rev Cancer. 2006;6:835–845. doi: 10.1038/nrc1971. [DOI] [PubMed] [Google Scholar]
- 74.Aghi M, Cohen KS, Klein RJ, et al. Tumor stromal-derived factor-1 recruits vascular progenitors to mitotic neovasculature, where microenvironment influences their differentiated phenotypes. Cancer Res. 2006;66:9054–9064. doi: 10.1158/0008-5472.CAN-05-3759. [DOI] [PubMed] [Google Scholar]
- 75.Natori T, Sata M, Washida M, et al. G-CSF stimulates angiogenesis and promotes tumor growth: Potential contribution of bone marrow-derived endothelial progenitor cells. Biochem Biophys Res Commun. 2002;297:1058–1061. doi: 10.1016/s0006-291x(02)02335-5. [DOI] [PubMed] [Google Scholar]
- 76.Ellis LM, Hicklin DJ. VEGF-targeted therapy: Mechanisms of antitumour activity. Nat Rev Cancer. 2008;8:579–591. doi: 10.1038/nrc2403. [DOI] [PubMed] [Google Scholar]
- 77.Willett CG, Boucher Y, di Tomaso E, et al. Direct evidence that the VEGF-specific antibody bevacizumab has antivascular effects in human rectal cancer. Nat Med. 2004;10:145–147. doi: 10.1038/nm988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Duda DG, Jain RK, Willett CG. Antiangiogenics: The potential role of integrating this novel treatment modality with chemoradiation for solid cancers. J Clin Oncol. 2007;25:4033–4042. doi: 10.1200/JCO.2007.11.3985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kerbel RS. Antiangiogenic therapy: A universal chemosensitization strategy for cancer? Science. 2006;312:1171–1175. doi: 10.1126/science.1125950. [DOI] [PubMed] [Google Scholar]
- 80.Shaked Y, Kerbel RS. Antiangiogenic strategies on defense: On the possibility of blocking rebounds by the tumor vasculature after chemotherapy. Cancer Res. 2007;67:7055–7058. doi: 10.1158/0008-5472.CAN-07-0905. [DOI] [PubMed] [Google Scholar]
- 81.Gorski DH, Beckett MA, Jaskowiak NT, et al. Blockage of the vascular endothelial growth factor stress response increases the antitumor effects of ionizing radiation. Cancer Res. 1999;59:3374–3378. [PubMed] [Google Scholar]
- 82.Jain RK. Normalization of tumor vasculature: An emerging concept in antiangiogenic therapy. Science. 2005;307:58–62. doi: 10.1126/science.1104819. [DOI] [PubMed] [Google Scholar]
- 83.Batchelor TT, Sorensen AG, di Tomaso E, et al. AZD2171, a pan-VEGF receptor tyrosine kinase inhibitor, normalizes tumor vasculature and alleviates edema in glioblastoma patients. Cancer Cell. 2007;11:83–95. doi: 10.1016/j.ccr.2006.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fischer I, Cunliffe CH, Bollo RJ, et al. High-grade glioma before and after treatment with radiation and Avastin: Initial observations. Neuro Oncol. 2008;10:700–708. doi: 10.1215/15228517-2008-042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Karaman MW, Herrgard S, Treiber DK, et al. A quantitative analysis of kinase inhibitor selectivity. Nat Biotechnol. 2008;26:127–132. doi: 10.1038/nbt1358. [DOI] [PubMed] [Google Scholar]
- 86.Chi A, Norden AD, Wen PY. Inhibition of angiogenesis and invasion in malignant gliomas. Expert Rev Anticancer Ther. 2007;7:1537–1560. doi: 10.1586/14737140.7.11.1537. [DOI] [PubMed] [Google Scholar]
- 87.Kerbel RS, Kamen BA. The anti-angiogenic basis of metronomic chemotherapy. Nat Rev Cancer. 2004;4:423–436. doi: 10.1038/nrc1369. [DOI] [PubMed] [Google Scholar]
- 88.Browder T, Butterfield CE, Kräling BM, et al. Antiangiogenic scheduling of chemotherapy improves efficacy against experimental drug-resistant cancer. Cancer Res. 2000;60:1878–1886. [PubMed] [Google Scholar]
- 89.Kim JT, Kim JS, Ko KW, et al. Metronomic treatment of temozolomide inhibits tumor cell growth through reduction of angiogenesis and augmentation of apoptosis in orthotopic models of gliomas. Oncol Rep. 2006;16:33–39. [PubMed] [Google Scholar]
- 90.Shaked Y, Emmenegger U, Man S, et al. Optimal biologic dose of metronomic chemotherapy regimens is associated with maximum antiangiogenic activity. Blood. 2005;106:3058–3061. doi: 10.1182/blood-2005-04-1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cham K, Baker J, Takhar K, et al. Metronomic gemcitabine exerts antitumor effects in primary orthotopic human pancreatic cancer xenografts by altering the tumor microenvironment. Presented at the 2008 American Association for Cancer Research Annual Meeting; April 12–16, 2008; San Diego, CA. [Google Scholar]
- 92.Singh SK, Hawkins C, Clarke ID, et al. Identification of human brain tumour initiating cells. Nature. 2004;432:396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- 93.Bao S, Wu Q, Sathornsumetee S, et al. Stem cell-like glioma cells promote tumor angiogenesis through vascular endothelial growth factor. Cancer Res. 2006;66:7843–7848. doi: 10.1158/0008-5472.CAN-06-1010. [DOI] [PubMed] [Google Scholar]
- 94.Gilbertson RJ, Rich JN. Making a tumour’s bed: Glioblastoma stem cells and the vascular niche. Nat Rev Cancer. 2007;7:733–736. doi: 10.1038/nrc2246. [DOI] [PubMed] [Google Scholar]
- 95.Calabrese C, Poppleton H, Kocak M, et al. A perivascular niche for brain tumor stem cells. Cancer Cell. 2007;11:69–82. doi: 10.1016/j.ccr.2006.11.020. [DOI] [PubMed] [Google Scholar]
- 96.Folkins C, Man S, Xu P, et al. Anticancer therapies combining antiangiogenic and tumor cell cytotoxic effects reduce the tumor stem-like cell fraction in glioma xenograft tumors. Cancer Res. 2007;67:3560–3564. doi: 10.1158/0008-5472.CAN-06-4238. [DOI] [PubMed] [Google Scholar]
- 97.Lee CG, Heijn M, di Tomaso E, et al. Anti-vascular endothelial growth factor treatment augments tumor radiation response under normoxic or hypoxic conditions. Cancer Res. 2000;60:5565–5570. [PubMed] [Google Scholar]
- 98.Macdonald DR, Cascino TL, Schold SC, Jr, et al. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol. 1990;8:1277–1280. doi: 10.1200/JCO.1990.8.7.1277. [DOI] [PubMed] [Google Scholar]
- 99.Sorensen AG, Batchelor TT, Wen PY, et al. Response criteria for glioma. Nat Clin Pract Oncol. 2008;5:634–644. doi: 10.1038/ncponc1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Stark-Vance V. Bevacizumab and CPT-11 in the treatment of relapsed malignant glioma [abstract] Neuro Oncol. 2005;7:369. [Google Scholar]
- 101.Pope WB, Lai A, Nghiemphu P, et al. MRI in patients with high-grade gliomas treated with bevacizumab and chemotherapy. Neurology. 2006;66:1258–1260. doi: 10.1212/01.wnl.0000208958.29600.87. [DOI] [PubMed] [Google Scholar]
- 102.Norden AD, Young GS, Setayesh K, et al. Bevacizumab for recurrent malignant gliomas: Efficacy, toxicity, and patterns of recurrence. Neurology. 2008;70:779–787. doi: 10.1212/01.wnl.0000304121.57857.38. [DOI] [PubMed] [Google Scholar]
- 103.Guiu S, Taillibert S, Chinot O, et al. Bevacizumab/irinotecan. An active treatment for recurrent high grade gliomas: Preliminary results of an ANOCEF multicenter study. Rev Neurol (Paris) 2008;164:588–594. doi: 10.1016/j.neurol.2008.04.003. In French. [DOI] [PubMed] [Google Scholar]
- 104.Vredenburgh JJ, Desjardins A, Herndon JE, 2nd, et al. Phase II trial of bevacizumab and irinotecan in recurrent malignant glioma. Clin Cancer Res. 2007;13:1253–1259. doi: 10.1158/1078-0432.CCR-06-2309. [DOI] [PubMed] [Google Scholar]
- 105.Vredenburgh JJ, Desjardins A, Herndon JE, 2nd, et al. Bevacizumab plus irinotecan in recurrent glioblastoma multiforme. J Clin Oncol. 2007;25:4722–4729. doi: 10.1200/JCO.2007.12.2440. [DOI] [PubMed] [Google Scholar]
- 106.Wagner SA, Desjardins A, Reardon DA, et al. Update on survival from the original phase II trial of bevacizumab and irinotecan in recurrent malignant gliomas [abstract] J Clin Oncol. 2008;26(suppl 15):2021. [Google Scholar]
- 107.Yung WK, Albright RE, Olson J, et al. A phase II study of temozolomide vs. procarbazine in patients with glioblastoma multiforme at first relapse. Br J Cancer. 2000;83:588–593. doi: 10.1054/bjoc.2000.1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yung WK, Prados MD, Yaya-Tur R, et al. Multicenter phase II trial of temozolomide in patients with anaplastic astrocytoma or anaplastic oligoastrocytoma at first relapse. Temodal Brain Tumor Group. J Clin Oncol. 1999;17:2762–2771. doi: 10.1200/JCO.1999.17.9.2762. [DOI] [PubMed] [Google Scholar]
- 109.Chen W, Delaloye S, Silverman DH, et al. Predicting treatment response of malignant gliomas to bevacizumab and irinotecan by imaging proliferation with [18F] fluorothymidine positron emission tomography: A pilot study. J Clin Oncol. 2007;25:4714–4721. doi: 10.1200/JCO.2006.10.5825. [DOI] [PubMed] [Google Scholar]
- 110.Cloughesy TF, Prados MD, Wen PY, et al. A phase II, randomized, noncomparative clinical trial of the effect of bevacizumab (BV) alone or in combination with irinotecan (CPT) on 6-month progression free survival (PFS6) in recurrent, treatment-refractory glioblastoma (GBM) [abstract] J Clin Oncol. 2008;26(suppl 15):2010b. [Google Scholar]
- 111.Kreisl TN, Kim L, Moore K, et al. Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J Clin Oncol. 2009;27:740–745. doi: 10.1200/JCO.2008.16.3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lai A, Filka E, McGibbon B, et al. Phase II pilot study of bevacizumab in combination with temozolomide and regional radiation therapy for upfront treatment of patients with newly diagnosed glioblastoma multiforme: Interim analysis of safety and tolerability. Int J Radiat Oncol Biol Phys. 2008;71:1372–1380. doi: 10.1016/j.ijrobp.2007.11.068. [DOI] [PubMed] [Google Scholar]
- 113.Narayana A, Golfinos JG, Fischer I, et al. Feasibility of using bevacizumab with radiation therapy and temozolomide in newly diagnosed high-grade glioma. Int J Radiat Oncol Biol Phys. 2008;72:383–389. doi: 10.1016/j.ijrobp.2008.05.062. [DOI] [PubMed] [Google Scholar]
- 114.Holash J, Davis S, Papadopoulos N, et al. VEGF-Trap: A VEGF blocker with potent antitumor effects. Proc Natl Acad Sci U S A. 2002;99:11393–11398. doi: 10.1073/pnas.172398299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.De Groot JF, Wen PY, Lamborn K, et al. Phase II single arm trial of aflibercept in patients with recurrent temozolomide-resistant glioblastoma: NABTC 0601 [abstract] J Clin Oncol. 2008;26(suppl 15):2020. [Google Scholar]
- 116.Batchelor T, Sorensen G, di Tomaso E, et al. A multidisciplinary phase II study of AZD2171 (cediranib), an oral pan-VEGF receptor tyrosine kinase inhibitor, in patients with recurrent glioblastoma. Presented at the 2008 American Association for Cancer Research Annual Meeting; April 12–16, 2008; San Diego, CA. [Google Scholar]
- 117.Thomas AL, Morgan B, Horsfield MA, et al. Phase I study of the safety, tolerability, pharmacokinetics, and pharmacodynamics of PTK787/ZK 222584 administered twice daily in patients with advanced cancer. J Clin Oncol. 2005;23:4162–4171. doi: 10.1200/JCO.2005.09.034. [DOI] [PubMed] [Google Scholar]
- 118.Conrad C, Friedman H, Reardon D, et al. A phase I/II trial of single-agent PTK 787/ZK 222584 (PTK/ZK), a novel, oral angiogenesis inhibitor, in patients with recurrent glioblastoma multiforme (GBM) [abstract] J Clin Oncol. 2004;22(14 suppl):1512. [Google Scholar]
- 119.Reardon D, Friedman H, Brada M, et al. A phase I/II trial of PTK 787/ZK 222584 (PTK/ZK), a multi-VEGF receptor tyrosine kinase inhibitor, in combination with either temozolomide or lomustine for patients with recurrent glioblastoma multiforme (GBM) [abstract] Neuro Oncol. 2004;6:307–400. [Google Scholar]
- 120.Kirkpatrick JP, Rich JN, Vredenburgh JJ, et al. Final report: Phase I trial of imatinib mesylate, hydroxyurea, and vatalanib for patients with recurrent malignant glioma (MG) [abstract] J Clin Oncol. 2008;26(suppl 15):2057. [Google Scholar]
- 121.Hess C, Vuong V, Hegyi I, et al. Effect of VEGF receptor inhibitor PTK787/ZK222584 [correction of ZK222548] combined with ionizing radiation on endothelial cells and tumour growth. Br J Cancer. 2001;85:2010–2016. doi: 10.1054/bjoc.2001.2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Morabito A, De Maio E, Di Maio M, et al. Tyrosine kinase inhibitors of vascular endothelial growth factor receptors in clinical trials: Current status and future directions. The Oncologist. 2006;11:753–764. doi: 10.1634/theoncologist.11-7-753. [DOI] [PubMed] [Google Scholar]
- 123.Bergers G, Hanahan D. Modes of resistance to anti-angiogenic therapy. Nat Rev Cancer. 2008;8:592–603. doi: 10.1038/nrc2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.D’Amato RJ, Loughnan MS, Flynn E, et al. Thalidomide is an inhibitor of angiogenesis. Proc Natl Acad Sci U S A. 1994;91:4082–4085. doi: 10.1073/pnas.91.9.4082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Marx GM, Pavlakis N, McCowatt S, et al. Phase II study of thalidomide in the treatment of recurrent glioblastoma multiforme. J Neurooncol. 2001;54:31–38. doi: 10.1023/a:1012554328801. [DOI] [PubMed] [Google Scholar]
- 126.Short SC, Traish D, Dowe A, et al. Thalidomide as an anti-angiogenic agent in relapsed gliomas. J Neurooncol. 2001;51:41–45. doi: 10.1023/a:1006414804835. [DOI] [PubMed] [Google Scholar]
- 127.Fine HA, Figg WD, Jaeckle K, et al. Phase II trial of the antiangiogenic agent thalidomide in patients with recurrent high-grade gliomas. J Clin Oncol. 2000;18:708–715. doi: 10.1200/JCO.2000.18.4.708. [DOI] [PubMed] [Google Scholar]
- 128.Fine HA, Wen PY, Maher EA, et al. Phase II trial of thalidomide and carmustine for patients with recurrent high-grade gliomas. J Clin Oncol. 2003;21:2299–2304. doi: 10.1200/JCO.2003.08.045. [DOI] [PubMed] [Google Scholar]
- 129.Baumann F, Bjeljac M, Kollias SS, et al. Combined thalidomide and temozolomide treatment in patients with glioblastoma multiforme. J Neurooncol. 2004;67:191–200. doi: 10.1023/b:neon.0000021803.01170.03. [DOI] [PubMed] [Google Scholar]
- 130.Chang SM, Lamborn KR, Malec M, et al. Phase II study of temozolomide and thalidomide with radiation therapy for newly diagnosed glioblastoma multiforme. Int J Radiat Oncol Biol Phys. 2004;60:353–357. doi: 10.1016/j.ijrobp.2004.04.023. [DOI] [PubMed] [Google Scholar]
- 131.Drappatz J, Wong ET, Schiff D, et al. A pilot safety study of lenalidomide and radiotherapy for patients with newly diagnosed glioblastoma multiforme. Int J Radiat Oncol Biol Phys. 2009;73:222–227. doi: 10.1016/j.ijrobp.2008.03.046. [DOI] [PubMed] [Google Scholar]
- 132.Buckner JC, Schomberg PJ, McGinnis WL, et al. A phase III study of radiation therapy plus carmustine with or without recombinant interferon-alpha in the treatment of patients with newly diagnosed high-grade glioma. Cancer. 2001;92:420–433. doi: 10.1002/1097-0142(20010715)92:2<420::aid-cncr1338>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 133.Fine HA, Wen PY, Robertson M, et al. Aphase I trial of a new recombinant human beta-interferon (BG9015) for the treatment of patients with recurrent gliomas. Clin Cancer Res. 1997;3:381–387. [PubMed] [Google Scholar]
- 134.Grossman SA, Phuphanich S, Lesser G, et al. Toxicity, efficacy, and pharmacology of suramin in adults with recurrent high-grade gliomas. J Clin Oncol. 2001;19:3260–3266. doi: 10.1200/JCO.2001.19.13.3260. [DOI] [PubMed] [Google Scholar]
- 135.Laterra JJ, Grossman SA, Carson KA, et al. Suramin and radiotherapy in newly diagnosed glioblastoma: Phase 2 NABTT CNS Consortium study. Neuro Oncol. 2004;6:15–20. doi: 10.1215/S1152851703000127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Louis DN. Molecular Pathology of Malignant Gliomas. Annu Rev Pathol. 2006;1:97–117. doi: 10.1146/annurev.pathol.1.110304.100043. [DOI] [PubMed] [Google Scholar]
- 137.Desjardins A, Quinn JA, Vredenburgh JJ, et al. Phase II study of imatinib mesylate and hydroxyurea for recurrent grade III malignant gliomas. J Neurooncol. 2007;83:53–60. doi: 10.1007/s11060-006-9302-2. [DOI] [PubMed] [Google Scholar]
- 138.Wen PY, Yung WK, Lamborn KR, et al. Phase I/II study of imatinib mesylate for recurrent malignant gliomas: North American Brain Tumor Consortium Study 99–08. Clin Cancer Res. 2006;12:4899–4907. doi: 10.1158/1078-0432.CCR-06-0773. [DOI] [PubMed] [Google Scholar]
- 139.Dresemann G. Imatinib and hydroxyurea in pretreated progressive glioblastoma multiforme: A patient series. Ann Oncol. 2005;16:1702–1708. doi: 10.1093/annonc/mdi317. [DOI] [PubMed] [Google Scholar]
- 140.Reardon DA, Egorin MJ, Quinn JA, et al. Phase II study of imatinib mesylate plus hydroxyurea in adults with recurrent glioblastoma multiforme. J Clin Oncol. 2005;23:9359–9368. doi: 10.1200/JCO.2005.03.2185. [DOI] [PubMed] [Google Scholar]
- 141.Fine HA, Puduvalli VK, Chamberlain MC, et al. Enzastaurin (ENZ) versus lomustine (CCNU) in the treatment of recurrent, intracranial glioblastoma multiforme (GBM): A phase III study [abstract] J Clin Oncol. 2008;26(suppl 15):2005. [Google Scholar]
- 142.Iñiguez MA, Rodríguez A, Volpert OV, et al. Cyclooxygenase-2: A therapeutic target in angiogenesis. Trends Mol Med. 2003;9:73–78. doi: 10.1016/s1471-4914(02)00011-4. [DOI] [PubMed] [Google Scholar]
- 143.Reardon DA, Quinn JA, Vredenburgh J, et al. Phase II trial of irinotecan plus celecoxib in adults with recurrent malignant glioma. Cancer. 2005;103:329–338. doi: 10.1002/cncr.20776. [DOI] [PubMed] [Google Scholar]
- 144.Levin VA, Giglio P, Puduvalli VK, et al. Combination chemotherapy with 13-cis-retinoic acid and celecoxib in the treatment of glioblastoma multiforme. J Neurooncol. 2006;78:85–90. doi: 10.1007/s11060-005-9062-4. [DOI] [PubMed] [Google Scholar]
- 145.Avraamides CJ, Garmy-Susini B, Varner JA. Integrins in angiogenesis and lymphangiogenesis. Nat Rev Cancer. 2008;8:604–617. doi: 10.1038/nrc2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Nabors LB, Mikkelsen T, Rosenfeld SS, et al. Phase I and correlative biology study of cilengitide in patients with recurrent malignant glioma. J Clin Oncol. 2007;25:1651–1657. doi: 10.1200/JCO.2006.06.6514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Eskens FA, Dumez H, Hoekstra R, et al. Phase I and pharmacokinetic study of continuous twice weekly intravenous administration of cilengitide (EMD 121974), a novel inhibitor of the integrins αvβ3 and αvβ5 in patients with advanced solid tumours. Eur J Cancer. 2003;39:917–926. doi: 10.1016/s0959-8049(03)00057-1. [DOI] [PubMed] [Google Scholar]
- 148.Reardon D, Fink K, Nabors B, et al. Phase IIa trial of cilengitide (EMD121974) single-agent therapy in patients (pts) with recurrent glioblastoma (GBM): EMD 121974–009 [abstract] J Clin Oncol. 2007;25(18 suppl):2002. [Google Scholar]
- 149.Gilbert M, Lamborn K, Lassman A, et al. Tumor tissue delivery of cilengitide after intravenous administration to patients with recurrent glioblastoma (GBM): Preliminary data from NABTC protocol 03–02 [abstract] Neuro Oncol. 2007;9:525. [Google Scholar]
- 150.Stupp R, Goldbrunner R, Neyns B, et al. Phase I/IIa trial of cilengitide (EMD121974) and temozolomide with concomitant radiotherapy, followed by temozolomide and cilengitide maintenance therapy in patients (pts) with newly diagnosed glioblastoma (GBM) [abstract] J Clin Oncol. 2007;25(18 suppl):2000. doi: 10.1200/JCO.2009.26.6650. [DOI] [PubMed] [Google Scholar]
- 151.Herrlinger U, Rieger J, Steinbach JP, et al. UKT-04 trial of continuous metronomic low-dose chemotherapy with methotrexate and cyclophosphamide for recurrent glioblastoma. J Neurooncol. 2005;71:295–299. doi: 10.1007/s11060-004-1726-y. [DOI] [PubMed] [Google Scholar]
- 152.Tuettenberg J, Grobholz R, Korn T, et al. Continuous low-dose chemotherapy plus inhibition of cyclooxygenase-2 as an antiangiogenic therapy of glioblastoma multiforme. J Cancer Res Clin Oncol. 2005;131:31–40. doi: 10.1007/s00432-004-0620-5. [DOI] [PubMed] [Google Scholar]
- 153.Kesari S, Schiff D, Doherty L, et al. Phase II study of metronomic chemotherapy for recurrent malignant gliomas in adults. Neuro Oncol. 2007;9:354–363. doi: 10.1215/15228517-2007-006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Perry JR, Mason WP, Belanger K, et al. The temozolomide RESCUE study: A phase II trial of continuous (28/28) dose-intense temozolomide (TMZ) after progression on conventional 5/28 day TMZ in patients with recurrent malignant glioma [abstract] J Clin Oncol. 2008;26(suppl 15):2010. doi: 10.1200/JCO.2009.26.5520. [DOI] [PubMed] [Google Scholar]
- 155.Strik HM, Buhk JH, Bock C, et al. Tegwondo: Development of a novel near-continuous dose-dense temozolomide regimen for the treatment of recurrent brain tumors [abstract] J Clin Oncol. 2008;26(suppl 15):13016. [Google Scholar]
- 156.Bello L, Carrabba G, Giussani C, et al. Low-dose chemotherapy combined with an antiangiogenic drug reduces human glioma growth in vivo. Cancer Res. 2001;61:7501–7506. [PubMed] [Google Scholar]
- 157.Gonzalez J, Kumar AJ, Conrad CA, et al. Effect of bevacizumab on radiation necrosis of the brain. Int J Radiat Oncol Biol Phys. 2007;67:323–326. doi: 10.1016/j.ijrobp.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 158.Chamberlain MC, Glantz MJ, Chalmers L, et al. Early necrosis following concurrent Temodar and radiotherapy in patients with glioblastoma. J Neurooncol. 2007;82:81–83. doi: 10.1007/s11060-006-9241-y. [DOI] [PubMed] [Google Scholar]
- 159.Taal W, Brandsma D, de Bruin HG, et al. The incidence of pseudo-progression in a cohort of malignant glioma patients treated with chemoradiation with temozolomide [abstract] J Clin Oncol. 2007;25(18 suppl):2009. [Google Scholar]
- 160.Hylton N. Dynamic contrast-enhanced magnetic resonance imaging as an imaging biomarker. J Clin Oncol. 2006;24:3293–3298. doi: 10.1200/JCO.2006.06.8080. [DOI] [PubMed] [Google Scholar]
- 161.Barrett T, Brechbiel M, Bernardo M, et al. MRI of tumor angiogenesis. J Magn Reson Imaging. 2007;26:235–249. doi: 10.1002/jmri.20991. [DOI] [PubMed] [Google Scholar]
- 162.Pöpperl G, Kreth FW, Herms J, et al. Analysis of 18F-FET PET for grading of recurrent gliomas: Is evaluation of uptake kinetics superior to standard methods? J Nucl Med. 2006;47:393–403. [PubMed] [Google Scholar]
- 163.Provenzale JM. Imaging of angiogenesis: Clinical techniques and novel imaging methods. AJR Am J Roentgenol. 2007;188:11–23. doi: 10.2214/AJR.06.0280. [DOI] [PubMed] [Google Scholar]
- 164.Quant E, Norden AD, Drappatz J, et al. Role of a second chemotherapy in recurrent malignant glioma patients who progress on a bevacizumabcontaining regimen [abstract] J Clin Oncol. 2008;26(suppl 15):2008. [Google Scholar]
- 165.Kerbel RS. Therapeutic implications of intrinsic or induced angiogenic growth factor redundancy in tumors revealed. Cancer Cell. 2005;8:269–271. doi: 10.1016/j.ccr.2005.09.016. [DOI] [PubMed] [Google Scholar]
- 166.Rubenstein JL, Kim J, Ozawa T, et al. Anti-VEGF antibody treatment of glioblastoma prolongs survival but results in increased vascular cooption. Neoplasia. 2000;2:306–314. doi: 10.1038/sj.neo.7900102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Gerstner E, Chen P, Batchelor T, et al. Role of diffusion MRI in the detection of infiltrative glioblastoma after treatment with cediranib [abstract] Neuro Oncol. 2008;10:759–915. doi: 10.1093/neuonc/nop051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Du R, Lu KV, Petritsch C, et al. HIF1α induces the recruitment of bone marrow-derived vascular modulatory cells to regulate tumor angiogenesis and invasion. Cancer Cell. 2008;13:206–220. doi: 10.1016/j.ccr.2008.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Relf M, LeJeune S, Scott PA, et al. Expression of the angiogenic factors vascular endothelial cell growth factor, acidic and basic fibroblast growth factor, tumor growth factor beta-1, platelet-derived endothelial cell growth factor, placenta growth factor, and pleiotrophin in human primary breast cancer and its relation to angiogenesis. Cancer Res. 1997;57:963–969. [PubMed] [Google Scholar]
- 170.Carlson MR, Pope WB, Horvath S, et al. Relationship between survival and edema in malignant gliomas: Role of vascular endothelial growth factor and neuronal pentraxin 2. Clin Cancer Res. 2007;13:2592–2598. doi: 10.1158/1078-0432.CCR-06-2772. [DOI] [PubMed] [Google Scholar]
- 171.Sakariassen PØ, Prestegarden L, Wang J, et al. Angiogenesis-independent tumor growth mediated by stem-like cancer cells. Proc Natl Acad Sci U S A. 2006;103:16466–16471. doi: 10.1073/pnas.0607668103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Duda DG, Batchelor TT, Willett CG, et al. VEGF-targeted cancer therapy strategies: Current progress, hurdles and future prospects. Trends Mol Med. 2007;13:223–230. doi: 10.1016/j.molmed.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Sathornsumetee S, Cao Y, Marcello JE, et al. Tumor angiogenic and hypoxic profiles predict radiographic response and survival in malignant astrocytoma patients treated with bevacizumab and irinotecan. J Clin Oncol. 2008;26:271–278. doi: 10.1200/JCO.2007.13.3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Wen PY, Schiff D, Kesari S, et al. Medical management of patients with brain tumors. J Neurooncol. 2006;80:313–332. doi: 10.1007/s11060-006-9193-2. [DOI] [PubMed] [Google Scholar]
- 175.Desjardins A, Reardon DA, Herndon JE, 2nd, et al. Bevacizumab plus irinotecan in recurrent WHO grade 3 malignant gliomas. Clin Cancer Res. 2008;14:7068–7073. doi: 10.1158/1078-0432.CCR-08-0260. [DOI] [PMC free article] [PubMed] [Google Scholar]