Abstract
Glioblastomas are among the most vascular tumors because they oversecrete vascular endothelial growth factor (VEGF), a potent stimulator of angiogenesis. Consequently, new drug regimens are being developed to target the VEGF signaling pathway in an attempt to halt tumor growth. Antibodies that bind VEGF, decoy molecules that sequester VEGF, and small molecule tyrosine kinase inhibitors that block receptor activation are being tested. Preliminary results with these agents have been promising, with prolonged progression-free survival reported. The antipermeability effects of anti-VEGF agents have important consequences for tumor imaging and for patient quality of life by decreasing corticosteroid dependence. However, because most patients eventually relapse, more work is needed to understand mechanisms of disease escape, including vascular cooption of native brain blood vessels.
Introduction
Glioblastoma (GBM) is the most common and most aggressive type of primary brain tumor, with a 5-year survival rate below 5%. Standard treatment for patients with GBM includes maximal safe resection; focal, fractionated radiation with concurrent temozolomide, an oral alkylating chemotherapeutic agent; and 6 to 12 monthly cycles of temozolomide. Despite this aggressive and prolonged treatment, the median survival is only 15 months [1]. In light of this poor prognosis, researchers are actively seeking new therapeutic options, and current efforts have begun to exploit the fact that GBMs are highly vascularized tumors characterized by activation of multiple proangiogenic signaling pathways. Angiogenesis-targeting agents, particularly drugs that target the vascular endothelial growth factor (VEGF) pathway, increasingly are being incorporated into drug regimens.
Angiogenesis in Gliomas
Angiogenesis in GBM involves complex interactions among glioma cells, stromal cells, and endothelial cells. Tumor growth eventually reaches a point at which the existing blood supply is no longer adequate and areas within the tumor become hypoxic, leading to cell death and necrosis. In response to this hypoxia, GBMs undergo an “angiogenic switch” and increase secretion of various growth factors to promote new blood vessel formation. Although VEGF is one such critical growth factor and is the focus of this review, other molecules and proangiogenic signaling pathways clearly are important for tumor angiogenesis [2•]. Low oxygen levels increase VEGF mRNA transcription in glioma cells by increasing the stability of hypoxia-inducible factor-1 (HIF-1), which binds to the VEGF gene promoter to induce transcription [3,4]. Elevated HIF-1 and VEGF correlate with advanced tumor grade, and GBMs have a 50-fold greater expression of VEGF than lower-grade astrocytomas, which are not characterized by robust angiogenesis [3,5].
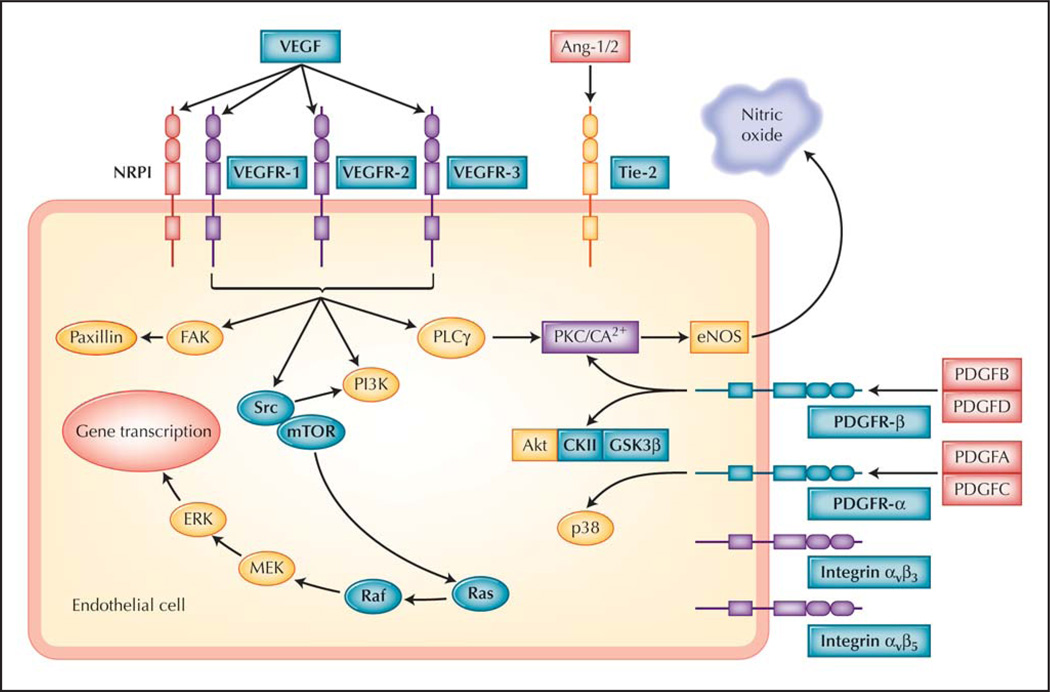
VEGF interacts with three tyrosine kinase receptors (VEGFR-1, VEGFR-2, and VEGFR-3) on endothelial cells to stimulate angiogenesis. VEGFR-2 is considered the critical receptor associated with cancer-related angiogenesis and activates a variety of intracellular pathways, including phosphatase and tensin homologue/phosphoinositide 3-kinase/Akt [6], mitogen-activated protein kinase/extracellular signal-regulated kinase (MAPK/ERK) [7], and nitric oxide [8]. In addition, VEGF upregulates Notch–Deltalike ligand 4 (Dll4) expression in tumor vasculature [9]. The Dll4 pathway has been recognized as an important mediator of tumor-related angiogenesis, although the precise role of Dll4 in angiogenesis is unclear [10]. VEGFR-1, VEGFR-2, and the downstream molecules in the VEGF signaling pathway represent possible points of therapeutic intervention (Table 1 and Fig. 1). In principle, inhibiting VEGF-induced angiogenesis should selectively target actively dividing tumor endothelial cells because normal brain endothelial cells rarely participate in active angiogenesis, making this pathway an attractive target.
Table 1.
Select anti–vascular endothelial growth factor agents in trials for glioblastoma*
| Drug | Mechanism | Most advanced phase | Results |
|---|---|---|---|
| Antibodies | |||
| Bevacizumab | VEGF antibody | Phase 2: recurrent GBM | Bevacizumab alone: 29%–35% APF6 |
| Phase 3: newly diagnosed GBM† | Bevacizumab + irinotecan: 50.2% APF6 | ||
| Aflibercept | VEGF-α/β, placental growth factor “receptor decoy” | Phase 2: recurrent GBM | 30% response rate |
| Small-molecule inhibitors | |||
| Cediranib | VEGFR-1/2/3, c-kit, PDGFR-α/β inhibitor; weak FGFR-1, EGFR inhibitor | Phase 3: recurrent GBM | 25.8% APF6 |
| Phase 2: newly diagnosed GBM | Ongoing | ||
| Vatalanib | VEGFR-1/2/3, PDGFR-β, c-kit inhibitor | Phase 2: recurrent GBM | 33/47 patients with PR/SD |
| Phase 2: newly diagnosed GBM | Results pending | ||
| Sunitinib | VEGFR-2, PDGFR, c-kit inhibitor | Phase 2: recurrent GBM | Ongoing |
| XL184 | VEGFR-2, c-met, Tie-2, c-kit inhibitor | Phase 2: recurrent GBM | Ongoing |
| Pazopanib | VEGFR-1/2/3, PDGFR-α/β, c-kit inhibitor | Phase 1/2: recurrent GBM | Ongoing |
| AEE788 | VEGFR-1/2, EGFR inhibitor | Phase 1/2: recurrent GBM | Results pending |
| Sorafenib | VEGFR-2/3, PDGFR-β, Flt-3, Raf inhibitor | Phase 1/2: recurrent and newly diagnosed GBM | Ongoing |
| Vandetanib | VEGFR-1/2, EGFR, Ret kinase inhibitor | Phase 1/2: recurrent and newly diagnosed GBM | Ongoing |
Please see the National Institutes of Health’s ClinicalTrials.gov website for up-to-date information on ongoing trials (http://www.clinicaltrials.gov).
Phase 3 to open in 2009.
APF6—alive and progression free at 6 months; EGFR—epidermal growth factor receptor; FGFR-1—fibroblast growth factor receptor-1; GBM—glioblastoma; PDGFR—platelet-derived growth factor receptor; PR/SD—partial response/stable disease; VEGF—vascular endothelial growth factor; VEGFR—VEGF receptor.
Figure 1.
Simplified brain tumor angiogenesis pathway and potential points of intervention. Bold lettering highlights molecules known to be targeted by drugs that are currently in clinical trials. Vascular endothelial growth factor (VEGF) is targeted by bevacizumab, whereas tyrosine kinase inhibitors such as cediranib target mainly VEGF receptors 1–3 (VEGFR-1, VEGFR-2, VEGFR-3), Tie-2 (as well as Tie-1, which interacts with Tie-2), and platelet-derived growth factor (PDGF) receptors α and β (PDGFR-α and PDGFR-β). Agents targeting other pathways, such as inhibitors of mammalian target of rapamycin (mTOR) (eg, temsirolimus), Src, or integrins (eg, αvβ3 and αvβ5), are also in clinical development for brain tumors. Ang-1/2—angiopoietin 1/2; CKII—casein kinase II; eNOS—endothelial nitric oxide synthase; ERK—extracellular signal-regulated kinase; FAK—focal adhesion kinase-1; GSK3 β —glycogen synthase kinase-3 β ; MEK—mitogen-activated protein kinase ERK kinase; NRP1—neuropilin-1; PI3K—phosphatidylinositol-3 kinase; PKC—protein kinase C; PLC γ—phospholipase C γ. (From Jain et al. [2•], with permission.)
Several other growth factors can also increase activation of the VEGF pathway through stimulation of alternate tyrosine kinase receptors. Platelet-derived growth factor-β (PDGF-β) [11], epidermal growth factor [12,13], tumor necrosis factor-α, and basic fibroblast growth factor (bFGF) [11,14] can all upregulate expression of VEGF in gliomas. The angiopoietins, Ang-1 and Ang-2, have a complex interaction with VEGF through their tyrosine kinase receptors, Tie-1 and Tie-2. In the presence of VEGF, Ang-2 promotes vessel sprouting, but in the absence of VEGF, Ang-2 causes vessel regression [15]. Consequently, selective inhibition of the VEGF signaling axis may ultimately prove insufficient for a sustained antiangiogenic effect.
Preventing angiogenesis is hypothesized to arrest tumor growth through several mechanisms. The initial hypothesis was that antiangiogenic agents prevent new blood vessel formation and prune the existing tumor vessels, leading to tumor deprivation of oxygen and nutrients [16]. Vascular normalization is a second potential mechanism by which antiangiogenic agents may achieve an antitumor effect when combined with cytotoxic therapies. GBM vessels are highly abnormal and are characterized by enlarged vessel diameter, increased permeability, lack of adequate pericyte coverage, and abnormally thickened basement membranes. The result is a heterogeneous, disorganized tumor vascular network with areas of increased perfusion and areas of decreased perfusion leading to hypoxia and, potentially, inefficient or inhomogeneous delivery of chemotherapy drugs or the oxygen that is needed for radiation to be effective. A number of anti-VEGF agents have been shown to revert this abnormal vascular network to a more normalized state [17]. Normalization improves tumor perfusion and delivery of cytotoxic therapies, thus improving the efficacy of concomitantly administered drugs [18]. As opposed to pruning or destroying blood vessels, vascular normalization induces structural and functional changes in the abnormal tumor vasculature that transforms these vessels to a more “normal” morphologic state. Finally, another possible mechanism of action of anti-VEGF drugs is disruption of the perivascular cancer–stem cell niche. GBM stem cells, the self-renewable cells that potentially give rise to gliomas, exist in a highly specialized stem cell–vascular niche that allows these cells to remain in a self-renewing state [19]. Anti-VEGF agents may disrupt the critical interaction between these GBM stem cells and endothelial cells, thus contributing to their death [20].
Many drugs that block the VEGF pathway are in clinical development. They differ primarily in their route of administration, specificity and potency of VEGF blockade, and additional targets blocked. In this review, we focus on the drugs in latest stages of clinical development.
Antibodies to VEGF
Antibodies have the advantage of being highly specific for VEGF, with limited cross-reactivity and fewer “off-target” side effects. The half-life of an antibody is generally long, which allows less frequent dosing. Conversely, antibodies are costly to produce and must be administered by intravenous infusion. They are large molecular weight protein molecules with limited penetration of the normal blood–brain barrier (BBB). However, when targeting endothelial cells that line tumor blood vessels or in the setting of a disrupted BBB, this may be less of an impediment.
Bevacizumab
Bevacizumab, a recombinant, humanized monoclonal antibody, inhibits VEGFR-mediated cell signaling by sequestration of its ligand, VEGF-A. VEGF-A is the best-characterized VEGF isoform and likely the most important for brain tumor angiogenesis. Because the half-life of bevacizumab is about 20 days, it is administered every 2 weeks and sometimes every 3 weeks. A phase 2 study of 35 patients with recurrent high-grade gliomas treated with bevacizumab and irinotecan, a cytotoxic chemotherapeutic that inhibits topoisomerase I, demonstrated promising results [21•]. Forty-six percent of patients were alive and progression free at 6 months (APF6), a commonly used metric in studies of recurrent malignant glioma, and median progression-free survival (PFS) was 24 weeks. Median overall survival was 42 weeks. This finding compares favorably with historical databases of studies in recurrent GBM in which the median APF6 was 15% [22]. High VEGF expression in the original tumor tissue correlated with radiographic response, whereas high expression of carbonic anhydrase 9, a marker of hypoxia and acidosis, was correlated with reduced survival [23].
A subsequent randomized, noncomparative phase 2 trial of patients with recurrent GBM confirmed the impact on APF6; 167 patients were randomized to bevacizumab alone (85 patients) or bevacizumab plus irinotecan (82 patients) [24••]. Because of the possible normalizing effects on tumor vasculature, bevacizumab in combination with irinotecan (a cytotoxic agent) was postulated to be more effective than bevacizumab alone. The APF6 was 35.1% in the bevacizumab-alone arm and 50.2% in the combination therapy arm. Median overall survival was 9.7 months in the bevacizumab arm and 8.9 months in the combination arm, with more frequent grade 3 toxicities in the combination arm (67% vs 48%). Consequently, it is unclear whether there is a significant advantage to combining bevacizumab with irinotecan over bevacizumab alone, considering the increased rate of toxicity observed in the combination arm and the similar median overall survival between the two arms of the study. A recent trial of single-agent bevacizumab followed by combination therapy for patients who progressed supported these findings, with an APF6 of 29% with single-agent bevacizumab [25]. Adding irinotecan after failure of single-agent bevacizumab did not lead to any objective responses. Alternate drug combinations with bevacizumab such as erlotinib, an endothelial growth factor receptor (EGFR) tyrosine kinase inhibitor (TKI), and etoposide are being explored in patients with recurrent GBM [26,27]. In addition, bevacizumab is being studied in combination with temozolomide and radiation in newly diagnosed GBM patients; this regimen was determined to be relatively safe in a small pilot study [28]. A large randomized phase 3 trial of bevacizumab in combination with temozolomide and radiation is in the planning stages.
Aflibercept
Aflibercept, also called VEGF Trap, is a recombinant human chimeric fusion protein that blocks two VEGF subtypes, VEGF-A and VEGF-B, as well as placental growth factor (PlGF), another ligand for VEGFR-1. It has a higher affinity for VEGF than bevacizumab, a half-life of about 25 days, and works as a decoy receptor to sequester extracellular VEGF ligand and prevent its interaction with VEGFRs [29]. Preliminary data from a phase 2 trial of aflibercept in patients with recurrent malignant glioma demonstrated a 30% radiographic response rate in the subset of patients with GBM (14 patients with stable disease, eight patients with a partial response) [30]. Treatment was terminated in 12 patients (25%) due to toxicity; one patient had central nervous system ischemia, and one patient had a systemic hemorrhage. In a mouse xenograft model, aflibercept combined with radiation was more effective than radiation alone, suggesting that it might be beneficial in combination with radiation and temozolomide in newly diagnosed GBM patients. A trial is ongoing in this patient population [31].
Tyrosine Kinase Inhibitors
Small-molecule TKIs interfere with tyrosine or serine/threonine kinase growth factor receptor signaling by attaching to the intracellular adenosine triphosphate–binding pocket of the receptor. These agents have the advantage of being orally active. Most of the agents in this class lack specificity for one tyrosine kinase receptor, thus allowing broad inhibition of a variety of growth factor pathways, albeit at the risk of increasing potential side effects. A number of TKIs are in clinical development. The primary factors that distinguish these drugs are their selectivity and potency at different receptors.
Cediranib
Cediranib is a TKI that blocks all VEGFRs (VEGFR-1/2/3), c-kit, and PDGF receptors α and β (PDGFR-α/β). It is administered orally and, because its half-life is 12.5 to 35.4 hours, can be administered once daily. In a phase 2 study of 30 patients with recurrent GBM treated with cediranib alone, the radiographic response was 56%, median PFS was 117 days, and overall survival was 227 days [32••]. The APF6 was 25.8%, which compares favorably with historical controls. Patients underwent specialized MRI scans to allow examination of the tumor vasculature. Cediranib treatment led to a transient decrease in vessel size and permeability, suggesting normalization of the abnormal GBM vascular network. This was the first study to identify a “normalization window” secondary to anti-VEGF therapy in cancer patients. A positive consequence of reduced vascular permeability was reduction of vasogenic cerebral edema, a cause of major morbidity in this patient population. The antiedema effect of cediranib also resulted in a steroid-sparing effect, with most subjects able to reduce or discontinue corticosteroids as the cerebral edema resolved. A randomized phase 3 trial of cediranib versus cediranib with lomustine, an oral alkylating agent that has been a standard therapeutic in patients with recurrent GBM, is ongoing. In addition, a phase 1b/2 trial of cediranib is under way in combination with radiation and temozolomide for patients with newly diagnosed GBM.
Vatalanib
Vatalanib (PTK787) is a pan-VEGFR, PDGFR, and c-kit inhibitor with a short half-life [33]. Preclinical studies using C6 glioma cell lines that expressed VEGF demonstrated that vatalanib treatment leads to smaller tumor volumes and increased necrosis [34]. In a phase 1/2 trial of 47 patients with recurrent GBM treated with vatalanib alone, two patients had a partial response and 31 patients had stable disease as measured by MRI [35]. The drug was well tolerated; dose-limiting toxicities were observed only in patients taking at least 1000 mg daily. Vatalanib has also been studied in combination with other drugs. In a phase 1/2 trial of 51 patients with recurrent GBM treated with vatalanib with lomustine or temozolomide, four subjects had a partial response (three with vatalanib and temozolomide; one with vatalanib and lomustine), and 27 had stable disease (19 with vatalanib and temozolomide; eight with vatalanib and lomustine) [36]. The median time to progression was 15.7 weeks for the 51 patients. In another study, eight of 37 patients with recurrent GBM had a partial response when treated with imatinib, hydroxyurea, and vatalanib [37]. Because imatinib is a PDGFR inhibitor and hydroxyurea induces double-strand DNA breaks, the combination was thought to affect multiple tumor targets. Vatalanib is currently being studied in combination with radiation and temozolomide for newly diagnosed GBM patients.
Other TKIs
Several broader-spectrum TKIs that target VEGFRs are under study in the GBM patient population (Table 1). Sorafenib has a wide spectrum of targets in tumor cells, including VEGFR-1, VEGFR-2, PDGFR-β, c-kit, B-Raf, and Raf-1 kinases. It was determined to be safe in a phase 1 trial of patients with recurrent malignant glioma [38]. A phase 1/2 study exploring sorafenib in combination with temsirolimus (a mammalian target of rapamycin [mTOR] inhibitor) or erlotinib (an EGFR inhibitor) is ongoing.
Vandetanib (ZD6474) inhibits VEGFR-1, VEGFR-2, EGFR, and Ret kinases. In preclinical glioma mouse models, vandetanib prolonged survival, decreased tumor growth, decreased VEGF secretion, and disrupted tumor vascularity [39,40]. Combination therapy of vandetanib with radiation or temozolomide was also more effective than vandetanib alone [40]. Clinical studies of vandetanib in the GBM patient population are ongoing.
AEE788 is a combined inhibitor of EGFR, VEGFR-1, and VEGFR-2 that inhibited VEGFR-driven angiogenesis in preclinical models [41]. In one such mouse model of glioma, AEE788 decreased tumor growth and increased median survival when used in combination with an mTOR inhibitor (RAD001) [42]. A phase 1/2 trial in patients with recurrent GBM has been completed with acceptable toxicity, but the final results have not been published [43].
Resistance to Anti-VEGF Therapy
Although anti-VEGF therapies have been associated with prolonged PFS and overall survival in several uncontrolled clinical trials, most glioma patients treated with these drugs eventually relapse. It appears that some patients do not respond at all to anti-VEGF therapy. These nonresponders may have a tumor that is not dependent on VEGF for angiogenesis or growth. The patients who initially respond but then relapse have likely developed escape mechanisms to bypass VEGF inhibition. Hypothesized escape mechanisms include upregulation of alternate proangiogenic pathways, improved protection of tumor neovasculature by increased pericyte coverage, and increased invasiveness of tumor cells that co-opt native brain blood vessels [44•]. Support for these mechanisms has predominantly come from preclinical models, and there are increasing efforts to identify tumor escape mechanisms in humans using blood or MRI biomarkers. In one clinical study, patients with recurrent GBM who relapsed after treatment with cediranib had elevated stromal-derived growth factor-1α, bFGF, and Tie-2, all of which have been implicated in alternate proangiogenic signaling pathways [32••]. Genetic alteration in the VEGF molecule itself or its receptor, a commonly cited mechanism for acquired drug resistance in traditional chemotherapeutics, has not been clearly demonstrated.
Toxicities of Antiangiogenic Agents
Agents that target the VEGF pathway have several uncommon but serious complications (Table 2) [45]. The toxicity profile also varies depending on the agent. Hypertension has been a notable side effect because VEGF blocks nitric oxide and prostacyclin synthesis, impairs baroreceptor response, and perturbs endothelial cell function. As many as 60% of patients, particularly those with borderline hypertension, may require treatment with antihypertensive agents, and early intervention is important to preempt development of severe hypertension. Arterial and venous thrombosis as well as hemorrhage have been reported, which are a concern for patients with GBM because they are at risk for intratumoral hemorrhage and deep vein thrombosis in the absence of VEGF inhibition. Reassuringly, though, intracerebral hemorrhage has been reported in less than 5% of GBM patients treated with anti-VEGF therapy in studies to date. Poor wound healing because of angiogenic blockade is another potential complication, particularly in patients who have recently undergone craniotomy for resection of their tumor. Bowel perforation and reversible posterior leukoencephalopathy have also been rarely reported.
Table 2.
Toxicities of anti–vascular endothelial growth factor agents
| Toxicity | Possible mechanism |
|---|---|
| Bleeding; impaired wound healing | Platelet dysfunction |
| Thrombotic events | EC apoptosis Platelet activation |
| Hypertension | Blockade of nitric oxide and prostacyclins Decreased capillary density Impaired baroreceptor response |
| Proteinuria | Podocyte dysfunction in kidney glomeruli |
| Rash; hand-foot syndrome | Epidermal cell apoptosis EC dysfunction |
| Gastrointestinal perforation | Mucosal cell apoptosis EC dysfunction |
| Hypothyroidism | Decreased thyroid vascularity |
| Fatigue | Hypothyroidism |
| Posterior reversible encephalopathy syndrome | Blood–brain barrier dysregulation Hypertension |
EC—endothelial cell.
VEGF Inhibitors as Antiedema Therapies
Malignant gliomas are often associated with a significant amount of vasogenic edema obligating patients to chronic corticosteroid use. Unfortunately, this often leads to steroid-related toxicities, such as osteoporosis, weight gain, insomnia, and psychiatric effects—all of which impair the patient’s quality of life. Vasogenic edema is the result of oversecretion of VEGF leading to disruption of the BBB and increased vascular permeability. Vascular permeability permits the influx of macromolecules and fluid into the brain parenchyma, resulting in vasogenic edema and elevated interstitial fluid pressure. By blocking VEGF, anti-VEGF agents restore the integrity of the BBB and decrease vasogenic edema. Vatalanib, cediranib, and bevacizumab have all been observed to decrease vasogenic edema as measured by serial MRI scans [32••,35,46–48].
The antipermeability effect of anti-VEGF therapies may be beneficial in another cancer-related condition associated with elevated vascular permeability: cerebral radiation necrosis. Radiation necrosis occurs several months to years after therapeutic doses of radiation and is thought to be caused by endothelial cell damage and the release of VEGF [49]. Patients with cerebral radiation necrosis develop progressive vasogenic cerebral edema and typically require long-term corticosteroid use to control swelling. In a study of eight patients, bevacizumab was shown to ameliorate radiation necrosis based on improvement in MRI scans (decreased T2 signal and contrast enhancement) and decreased requirement for corticosteroids [48].
Assessing Glioblastoma Response and Progression With Anti-VEGF Therapies
By convention, glioma response to therapy is determined by a reduction in contrast enhancement on post-contrast T1-weighted MRI images. GBMs enhance after the intravenous administration of contrast agents because the tumor vasculature has a dysfunctional, permeable BBB that enables leakage of contrast from the intravascular space into the brain parenchyma. A decrease in enhancement after therapy with standard cytotoxic chemotherapy agents is typically interpreted as a decrease in tumor burden. However, in the setting of anti-VEGF agents, the association between reduction in contrast enhancement and decreased tumor burden is less clear. Anti-VEGF agents restore the integrity of the BBB, which results in a reduction in permeability and contrast leakage on post-contrast T1-weighted MRI scans (Fig. 2). It is unclear to what extent, if any, that anti-VEGF agents cause direct tumor cytotoxicity independent of their vascular effects. With reduced vessel permeability, tumor growth may not be visible on standard post-contrast MRI sequences. This suggests that alternative methods to measure tumor response to anti-VEGF therapies need to be developed.
Figure 2.
MRI of a patient with a recurrent left temporal glioblastoma. Contrast-enhanced MRI prior to therapy with bevacizumab and irinotecan (A) and 6 months later (B). Fluid-attenuated inversion recovery (FLAIR) prior to therapy with bevacizumab and irinotecan (C) and 6 months later (D). Note the remarkable decrease in contrast enhancement and reduction in FLAIR hyperintensity with decreased mass effect on the lateral ventricle.
In fact, preclinical models have suggested that blocking VEGF and thus angiogenesis may lead to the tumor co-opting native brain blood vessels as an alternate way of maintaining an adequate blood supply [50–52]. Histologic sections from gliomas in rats treated with a VEGF murine antibody have shown tumor cells infiltrating into normal surrounding brain and tracking along native brain blood vessels. Because these blood vessels maintain an intact BBB, the perivascular tumor is not visible on contrast-enhanced MRI studies. Although not yet histologically confirmed, there is a concern that observed changes on fluid-attenuated inversion recovery (FLAIR) sequences or diffusion imaging represent tumor growth without an associated increase in contrast enhancement. Therefore, new neuroimaging criteria or biomarkers may be needed to assess glioma response in the setting of anti-VEGF therapy.
Conclusions
Results of anti-VEGF therapies for GBM have been modestly encouraging, and there might be some positive impact on PFS in patients with GBM. These results are preliminary and await validation in prospective phase 3 trials that are under way. Unfortunately, treatment eventually fails in most, if not all, patients, and therefore salvage therapies will be required. Tumor angiogenesis is a pathophysiologic process characterized by redundant pathways and multiple escape mechanisms. Agents that target other components of the angiogenesis pathway (eg, bFGF, PDGF, angiopoietins, interleukin-8) may be necessary for a sustained antiangiogenic effect. The long-term effects of prolonged inhibition of angiogenesis on normal organs have not been adequately studied and may ultimately limit application of this type of therapy. Improved identification of the normalization window in GBM by noninvasive serial imaging may allow exploitation of this anti-VEGF effect by identification of the optimal time interval in which to combine anti-VEGF and cytotoxic therapies. In addition, anti-VEGF therapies may prove effective in the treatment of conditions associated with increased vascular permeability, including vasogenic edema in the brain or spinal cord, malignant effusions, and noncancerous conditions such as macular degeneration.
Acknowledgments
Dr. Sorensen has served as a paid consultant for ACRIN Image Metrix, Genentech, Epix Pharmaceuticals, Millennium Pharmaceuticals, AstraZeneca, and Mitsubishi Pharma. He has received research support from Novartis Pharmaceuticals.
Dr. Jain has served as a consultant for Millennium Pharmaceuticals, AstraZeneca, Dyax, and SynDevRx and has received research support from AstraZeneca and Dyax.
Dr. Batchelor has served on the speakers bureaus of Schering-Plough and Enzon and has served as a consultant for Genentech, AstraZeneca, Millennium Pharmaceuticals, ImClone, Vertex, Enzon, Acceleron, and Exelixis.
Footnotes
Disclosure
No other potential conflicts of interest relevant to this article were reported.
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 2. Jain RK, di Tomaso E, Duda DG, et al. Angiogenesis in brain tumours. Nat Rev Neurosci. 2007;8:610–622. doi: 10.1038/nrn2175. The authors present a comprehensive review about glioma angiogenesis.
- 3.Jensen RL, Ragel BT, Whang K, Gillespie D. Inhibition of hypoxia inducible factor-1alpha (HIF-1alpha) decreases vascular endothelial growth factor (VEGF) secretion and tumor growth in malignant gliomas. J Neurooncol. 2006;78:233–247. doi: 10.1007/s11060-005-9103-z. [DOI] [PubMed] [Google Scholar]
- 4.Forsythe JA, Jiang BH, Iyer NV, et al. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol Cell Biol. 1996;16:4604–4613. doi: 10.1128/mcb.16.9.4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Plate KH, Breier G, Weich HA, Risau W. Vascular endothelial growth factor is a potential tumour angiogenesis factor in human gliomas in vivo. Nature. 1992;359:845–848. doi: 10.1038/359845a0. [DOI] [PubMed] [Google Scholar]
- 6.Gomez-Manzano C, Fueyo J, Jiang H, et al. Mechanisms underlying PTEN regulation of vascular endothelial growth factor and angiogenesis. Ann Neurol. 2003;53:109–117. doi: 10.1002/ana.10396. [DOI] [PubMed] [Google Scholar]
- 7.Yoshino Y, Aoyagi M, Tamaki M, et al. Activation of p38 MAPK and/or JNK contributes to increased levels of VEGF secretion in human malignant glioma cells. Int J Oncol. 2006;29:981–987. [PubMed] [Google Scholar]
- 8.Saino M, Maruyama T, Sekiya T, et al. Inhibition of angiogenesis in human glioma cell lines by antisense RNA from the soluble guanylate cyclase genes, GUCY1A3 and GUCY1B3. Oncol Rep. 2004;12:47–52. [PubMed] [Google Scholar]
- 9.Kerbel RS. Tumor angiogenesis. N Engl J Med. 2008;358:2039–2049. doi: 10.1056/NEJMra0706596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li JL, Sainson RC, Shi W, et al. Delta-like 4 Notch ligand regulates tumor angiogenesis, improves tumor vascular function, and promotes tumor growth in vivo. Cancer Res. 2007;67:11244–11253. doi: 10.1158/0008-5472.CAN-07-0969. [DOI] [PubMed] [Google Scholar]
- 11.Tsai JC, Goldman CK, Gillespie GY. Vascular endothelial growth factor in human glioma cell lines: induced secretion by EGF, PDGF-BB, and bFGF. J Neurosurg. 1995;82:864–873. doi: 10.3171/jns.1995.82.5.0864. [DOI] [PubMed] [Google Scholar]
- 12.Goldman CK, Kim J, Wong WL, et al. Epidermal growth factor stimulates vascular endothelial growth factor production by human malignant glioma cells: a model of glioblastoma multiforme pathophysiology. Mol Biol Cell. 1993;4:121–133. doi: 10.1091/mbc.4.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ryuto M, Ono M, Izumi H, et al. Induction of vascular endothelial growth factor by tumor necrosis factor alpha in human glioma cells. Possible roles of SP-1. J Biol Chem. 1996;271:28220–28228. doi: 10.1074/jbc.271.45.28220. [DOI] [PubMed] [Google Scholar]
- 14.Morrison RS, Gross JL, Herblin WF, et al. Basic fibroblast growth factor-like activity and receptors are expressed in a human glioma cell line. Cancer Res. 1990;50:2524–2529. [PubMed] [Google Scholar]
- 15.Reiss Y, Machein MR, Plate KH. The role of angiopoietins during angiogenesis in gliomas. Brain Pathol. 2005;15:311–317. doi: 10.1111/j.1750-3639.2005.tb00116.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Folkman J. Angiogenesis: an organizing principle for drug discovery? Nat Rev Drug Discov. 2007;6:273–286. doi: 10.1038/nrd2115. [DOI] [PubMed] [Google Scholar]
- 17.Jain RK, Duda DG, Clark JW, Loeffler JS. Lessons from phase III clinical trials on anti-VEGF therapy for cancer. Nat Clin Pract Oncol. 2006;3:24–40. doi: 10.1038/ncponc0403. [DOI] [PubMed] [Google Scholar]
- 18.Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005;307:58–62. doi: 10.1126/science.1104819. [DOI] [PubMed] [Google Scholar]
- 19.Calabrese C, Poppleton H, Kocak M, et al. A perivascular niche for brain tumor stem cells. Cancer Cell. 2007;11:69–82. doi: 10.1016/j.ccr.2006.11.020. [DOI] [PubMed] [Google Scholar]
- 20.Bao S, Wu Q, Sathornsumetee S, et al. Stem cell-like glioma cells promote tumor angiogenesis through vascular endothelial growth factor. Cancer Res. 2006;66:7843–7848. doi: 10.1158/0008-5472.CAN-06-1010. [DOI] [PubMed] [Google Scholar]
- 21. Vredenburgh JJ, Desjardins A, Herndon JE, 2nd, et al. Bevacizumab plus irinotecan in recurrent glioblastoma multiforme. J Clin Oncol. 2007;25:4722–4729. doi: 10.1200/JCO.2007.12.2440. This article reports on a phase 2 trial demonstrating the promising results of bevacizumab in patients with recurrent GBM.
- 22.Wong ET, Hess KR, Gleason MJ, et al. Outcomes and prognostic factors in recurrent glioma patients enrolled onto phase II clinical trials. J Clin Oncol. 1999;17:2572–2578. doi: 10.1200/JCO.1999.17.8.2572. [DOI] [PubMed] [Google Scholar]
- 23.Sathornsumetee S, Cao Y, Marcello JE, et al. Tumor angiogenic and hypoxic profiles predict radiographic response and survival in malignant astrocytoma patients treated with bevacizumab and irinotecan. J Clin Oncol. 2008;26:271–278. doi: 10.1200/JCO.2007.13.3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cloughesy TF, Prados MD, Wen PY, et al. A phase II, randomized, non-comparative clinical trial of the effect of bevacizumab (BV) alone or in combination with irinotecan (CPT) on 6-month progression free survival (PFS6) in recurrent, treatment-refractory glioblastoma (GBM) [abstract 2010b] J Clin Oncol. 2008;26(Suppl) This article presents preliminary results from a randomized phase 2 trial of bevacizumab alone versus bevacizumab with irinotecan showing prolonged PFS but similar overall survival in the two cohorts of patients. Final results are pending.
- 25.Kreisl TN, Kim L, Moore K, et al. Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J Clin Oncol. 2009;27:740–745. doi: 10.1200/JCO.2008.16.3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sathornsumetee S, Vredenburgh J, Rich J, et al. Phase II study of bevacizumab and erlotinib in patients with recurrent glioblastoma multiforme [abstract 13008] J Clin Oncol. 2008;26(Suppl) [Google Scholar]
- 27.Rich J, Desjardins A, Sathornsumetee S, et al. Phase II study of bevacizumab and etoposide in patients with recurrent malignant glioma [abstract 2022] J Clin Oncol. 2008;26(Suppl) [Google Scholar]
- 28.Lai A, Filka E, McGibbon B, et al. Phase II pilot study of bevacizumab in combination with temozolomide and regional radiation therapy for up-front treatment of patients with newly diagnosed glioblastoma multiforme: interim analysis of safety and tolerability. Int J Radiat Oncol Biol Phys. 2008;71:1372–1380. doi: 10.1016/j.ijrobp.2007.11.068. [DOI] [PubMed] [Google Scholar]
- 29.Holash J, Davis S, Papadopoulos N, et al. VEGF-Trap: a VEGF blocker with potent antitumor effects. Proc Natl Acad Sci U S A. 2002;99:11393–11398. doi: 10.1073/pnas.172398299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.De Groot J, Wen P, Lamborn K, et al. Phase II single arm trial of aflibercept in patients with recurrent temozolomide-resistant glioblastoma: NABTC 0601 [abstract 2020] J Clin Oncol. 2008;26(Suppl) [Google Scholar]
- 31.Wachsberger PR, Burd R, Cardi C, et al. VEGF trap in combination with radiotherapy improves tumor control in u87 glioblastoma. Int J Radiat Oncol Biol Phys. 2007;67:1526–1537. doi: 10.1016/j.ijrobp.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 32. Batchelor TT, Sorensen AG, di Tomaso E, et al. AZD2171, a pan-VEGF receptor tyrosine kinase inhibitor, normalizes tumor vasculature and alleviates edema in glioblastoma patients. Cancer Cell. 2007;11:83–95. doi: 10.1016/j.ccr.2006.11.021. The authors present the first trial showing vascular normalization in patients treated with an anti-VEGF agent.
- 33.Wood JM, Bold G, Buchdunger E, et al. PTK787/ZK 222584, a novel and potent inhibitor of vascular endothelial growth factor receptor tyrosine kinases, impairs vascular endothelial growth factor-induced responses and tumor growth after oral administration. Cancer Res. 2000;60:2178–2189. [PubMed] [Google Scholar]
- 34.Goldbrunner RH, Bendszus M, Sasaki M, et al. Vascular endothelial growth factor-driven glioma growth and vascularization in an orthotopic rat model monitored by magnetic resonance imaging. Neurosurgery. 2000;47:921–929. doi: 10.1097/00006123-200010000-00024. discussion 929–930. [DOI] [PubMed] [Google Scholar]
- 35.Conrad C, Friedman H, Reardon D, et al. A phase I/II trial of single-agent PTK 787/ZK 222584 (PTK/ZK), a novel, oral angiogenesis inhibitor, in patients with recurrent glioblastoma multiforme (GBM) [abstract 1512] ASCO Annu Meeting Proc (Post-Meeting Edition) 2004;22(Suppl):1512. [Google Scholar]
- 36.Reardon D, Friedman H, Yung WKA, et al. A phase I/II trial of PTK787/ZK 222584 (PTK/ZK), a novel, oral angiogenesis inhibitor, in combination with either temozolomide or lomustine for patients with recurrent glioblastoma multiforme (GBM) [abstract 1513] ASCO Annu Meeting Proc (Post-Meeting Edition) 2004;22(Suppl):1513. [Google Scholar]
- 37.Kirkpatrick J, Rich J, Vredenburgh J, et al. Final report: phase I trial of imatinib mesylate, hydroxyurea, and vatalanib for patients with recurrent malignant glioma (MG) [abstract 2057] J Clin Oncol. 2008;26(Suppl) [Google Scholar]
- 38.Nabors L, Rosenfeld M, Chamberlain M, et al. A phase I trial of sorafenib (BAY 43-9006) for patients with recurrent or progressive malignant glioma (NABTT 0401) ASCO Annu Meeting Proc. 2007;25(Suppl):2058. [Google Scholar]
- 39.Rich JN, Sathornsumetee S, Keir ST, et al. ZD6474, a novel tyrosine kinase inhibitor of vascular endothelial growth factor receptor and epidermal growth factor receptor, inhibits tumor growth of multiple nervous system tumors. Clin Cancer Res. 2005;11:8145–8157. doi: 10.1158/1078-0432.CCR-05-0319. [DOI] [PubMed] [Google Scholar]
- 40.Sandstrom M, Johansson M, Bergstrom P, et al. Effects of the VEGFR inhibitor ZD6474 in combination with radiotherapy and temozolomide in an orthotopic glioma model. J Neurooncol. 2008;88:1–9. doi: 10.1007/s11060-008-9527-3. [DOI] [PubMed] [Google Scholar]
- 41.Traxler P, Allegrini PR, Brandt R, et al. AEE788: a dual family epidermal growth factor receptor/ErbB2 and vascular endothelial growth factor receptor tyrosine kinase inhibitor with antitumor and antiangiogenic activity. Cancer Res. 2004;64:4931–4941. doi: 10.1158/0008-5472.CAN-03-3681. [DOI] [PubMed] [Google Scholar]
- 42.Goudar RK, Shi Q, Hjelmeland MD, et al. Combination therapy of inhibitors of epidermal growth factor receptor/vascular endothelial growth factor receptor 2 (AEE788) and the mammalian target of rapamycin (RAD001) offers improved glioblastoma tumor growth inhibition. Mol Cancer Ther. 2005;4:101–112. [PubMed] [Google Scholar]
- 43.Reardon D, Cloughesy T, Conrad C, et al. A phase I study of AEE788, a novel multitargeted inhibitor of ErbB and VEGF receptor family tyrosine kinases, in recurrent GBM patients [abstract 3063] ASCO Annu Meeting Proc. 2005;23(Suppl):3063. [Google Scholar]
- 44. Bergers G, Hanahan D. Modes of resistance to anti-angiogenic therapy. Nat Rev Cancer. 2008;8:592–603. doi: 10.1038/nrc2442. Timely review about resistance mechanisms of antiangiogenic therapy.
- 45.Verheul HM, Pinedo HM. Possible molecular mechanisms involved in the toxicity of angiogenesis inhibition. Nat Rev Cancer. 2007;7:475–485. doi: 10.1038/nrc2152. [DOI] [PubMed] [Google Scholar]
- 46.Drevs J, Muller-Driver R, Wittig C, et al. PTK787/ZK 222584, a specific vascular endothelial growth factor-receptor tyrosine kinase inhibitor, affects the anatomy of the tumor vascular bed and the functional vascular properties as detected by dynamic enhanced magnetic resonance imaging. Cancer Res. 2002;62:4015–4022. [PubMed] [Google Scholar]
- 47.Pope WB, Lai A, Nghiemphu P, et al. MRI in patients with high-grade gliomas treated with bevacizumab and chemotherapy. Neurology. 2006;66:1258–1260. doi: 10.1212/01.wnl.0000208958.29600.87. [DOI] [PubMed] [Google Scholar]
- 48.Gonzalez J, Kumar AJ, Conrad CA, Levin VA. Effect of bevacizumab on radiation necrosis of the brain. Int J Radiat Oncol Biol Phys. 2007;67:323–326. doi: 10.1016/j.ijrobp.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 49.Kim JH, Chung YG, Kim CY, et al. Upregulation of VEGF and FGF2 in normal rat brain after experimental intraoperative radiation therapy. J Korean Med Sci. 2004;19:879–886. doi: 10.3346/jkms.2004.19.6.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rubenstein JL, Kim J, Ozawa T, et al. Anti-VEGF antibody treatment of glioblastoma prolongs survival but results in increased vascular cooption. Neoplasia. 2000;2:306–314. doi: 10.1038/sj.neo.7900102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kunkel P, Ulbricht U, Bohlen P, et al. Inhibition of glioma angiogenesis and growth in vivo by systemic treatment with a monoclonal antibody against vascular endothelial growth factor receptor-2. Cancer Res. 2001;61:6624–6628. [PubMed] [Google Scholar]
- 52.Gomez-Manzano C, Holash J, Fueyo J, et al. VEGF Trap induces antiglioma effect at different stages of disease. Neuro Oncol. 2008;10:940–945. doi: 10.1215/15228517-2008-061. [DOI] [PMC free article] [PubMed] [Google Scholar]