Abstract
Background
Light is most effective at changing the timing of the circadian clock when applied close to the core body temperature minimum. The present study investigated, in a home setting, if individually tailored light treatment using flashing blue light delivered through closed eyelids during the early part of the sleep period delayed circadian phase and sleep in a population of healthy older adults and in those suffering from early awakening insomnia.
Methods
Twenty-eight participants (9 early awakening insomniacs) completed an 8-week, within-subjects study. Twice, participants collected data during two baseline weeks and one intervention week. During the intervention week, participants wore a flashing blue (active) or a flashing red (control) light mask during sleep. Light was expected to delay circadian phase. Saliva samples for dim light melatonin onset (DLMO) were collected at the end of each baseline and intervention week. Wrist actigraphy and Daysimeter, a calibrated light and activity meter, data were collected during the entire study.
Results
Compared to baseline, flashing blue light, but not flashing red light, significantly (p<0.05) delayed DLMO. The mean ± standard deviation phase shift (minutes) was 0:06 ± 0:30 for the flashing red light and 0:34 ± 0:30 for the flashing blue light. Compared to Day 1, sleep start times were significantly delayed (by approximately 46 minutes) at Day 7 after the flashing blue light. The light intervention did not affect sleep efficiency.
Conclusions
The present study demonstrated the feasibility of using light through closed eyelids during sleep for promoting circadian alignment and sleep health.
Keywords: Circadian phase, dim light melatonin onset, light through closed eyelids, flashing blue light, sleep, early awakening insomniacs
Background
Humans are under the influence of two mechanisms: the homeostatic (sleep drive) and the circadian (alerting force) systems (1, 2). The sleep drive and the alerting force are distinct mechanisms, independent from each other, although they normally work together to ensure that we are asleep at night and awake during the day. Sleep drive is low when we wake up and increases steadily throughout the day, and then diminishes rapidly within the first hours of sleep. The alerting force is regulated by the biological clock and follows a daily, circadian rhythm. The biological clock sends the body an alerting signal during the daytime and a sleeping signal at night. The interaction between the sleep drive and the alerting force determines when we fall asleep and how well we sleep at night. Misalignment between these two systems will lead to disturbed sleep or to sleep times that are not optimum with societal norms, such as what may be experienced by those suffering from early sleep onset or delayed sleep phase disorder.
The retinal light/dark exposure pattern is the main synchronizer of the circadian system to the local position on Earth (3). When this pattern is disrupted or misaligned with social requirements, a tailored light intervention can be used to promote sleep consolidation and efficiency by aligning the signals from the biological clock with the sleep drive mechanism, thus controlling the timing of the sleep/wake cycle (4). The relationship between the timing of a light intervention and the changes (magnitude and direction) in circadian time is described by a phase response curve (PRC) (5). The point on the PRC where the application of a light stimulus changes circadian phase from maximum delay to maximum advance is known as the crossover point. In humans, this occurs near the time of the core body temperature minimum (CBTmin), which typically occurs during the latter portion of the sleep period and corresponds to when people are most sleepy. In an individual normally entrained to the local 24-hour (h) light/dark cycle, evening light exposure (i.e., prior to CBTmin) delays circadian phase, and morning light exposure (i.e., after CBTmin) advances circadian phase (5).
Since CBTmin occurs during sleep, the first challenge for applying light when it has the most impact on circadian phase, is to determine eyelid transmittance. Robinson et al. (6) were among the first to measure light transmission through human closed eyelids. Their results suggested that the eyelids function as a red-pass filter, with transmission of 14.5% of 700-nanometer (nm) light, but only 3% or less transmission of light at 580 nm and below. Similarly, Ando and Kripke (7) showed that red light (615-635 nm) was attenuated to 5%, while blue (400-510 nm) and green (540-565 nm) lights were attenuated to less than 1%.
Recently Bierman et al. (8) modeled the spectral transmittance of the human eyelid. Using predictions from their spectral transmittance model, Figueiro and Rea (9) used 527-nm (green) light-emitting diodes (LEDs) to deliver 60 minutes (min) of continuous light to the eyelids of sleeping participants. Depending on the spectral transmittance of each participant's eyelids, it was determined that between 17,000 and 50,000 lux at the eyelid from the green LEDs was needed to reliably stimulate the circadian system as measured in terms of nocturnal melatonin suppression and of delaying circadian phase, as measured by dim light melatonin onset (DLMO) (9). Although effective, the heat generated by the continuously operated LEDs was very high and constituted a significant barrier to the development of a practical device that could be used at home to correct circadian misalignment.
Although the underlying retinal mechanisms responsible for photic stimulation of the circadian system are still under investigation, Rea et al. (10, 11) proposed a model of human circadian phototransduction. The intrinsically photosensitive retinal ganglion cell (ipRGC) (12) is the central element in the phototransduction model, but it also receives input from classical photoreceptors (13). Their proposed model was shown to be able to quantitatively predict light-induced nocturnal melatonin suppression from both narrow-band and polychromatic light sources (9, 14, 15). Based on the phototransduction model, Figueiro et al. (16) hypothesized that a train of brief flashes of short-wavelength (blue) light could provide photic information to the biological clock and phase shift DLMO. The advantage of delivering a train of brief flashes is the lack of heat buildup from the light source, which makes it possible to use this light mask at home. Figueiro et al. (16) demonstrated, in a laboratory study, that 2-second (s) pulses of 480-nm light acutely suppressed melatonin and phase shifted DLMO. More recently, Figueiro et al. (17) demonstrated, in a pilot study using 10 subjects, that 1 h exposure to 2-s pulses of flashing blue light delivered every 30 s for 7 consecutive days phase delayed DLMO by about 24 min in those living at home. The present study extends from those by Figueiro et al. (16, 17) by investigating if a flashing 480-nm (blue) light delivered through closed eyelids during the early part of the sleep period delayed circadian phase and sleep start times in a population of healthy older adults and in those suffering from early awakening insomnia.
Unlike the previous published studies (16, 17), the present study used a flashing 640-nm (red) light mask as the control condition. Another novelty of the present study was that data from a calibrated light measuring device worn by subjects was used in combination with an algorithm to make daily adjustments to the timing and duration of light exposures to more effectively achieve a target circadian phase delay. In addition, this is the first field study that tested the effectiveness and feasibility of delivering a light treatment through closed eyelids during sleep in a group of early awakening insomniacs. It was hypothesized that circadian phase, as measured by DLMO, and sleep start and end times, as measured by actigraphy, would be significantly delayed after the flashing blue light, but not after the flashing red light. The use of flashing light, rather than continuous light exposures is practical because its brief duration reduces the blue light hazard risk (18) and reduces heat generation, which is one of the major barriers for the development of a commercial light mask product.
Methods
Participant Selection
Twenty-nine participants over the age of 65 were recruited through email and posted notices. Nineteen participants [mean age ± standard deviation (SD) = 69 ± 5 years] (9 males) were healthy older adults who did not report having early awakening insomnia and ten participants (mean age ± SD = 70 ± 4.5 years) (2 males) reported a history of early awakening insomnia. No a priori power calculations were performed. One early awakening participant withdrew and his data are not reported here. Participants were not included in the study if they had sleep apnea, restless legs syndrome (RLS), cognitive impairment or reported using beta blockers. Sleep apnea was screened for using the Sleep Apnea Scale of the Sleep Disorders Questionnaire, a 12-item scale, yielding scores between 0 and 60. A score of 29 was used as a cutoff for men (sensitivity 75%, specificity 65%), and a cutoff of 26 was used for women (sensitivity 80%, specificity 67%) (19). RLS was screened for using the RLS Rating Scale, a 10-item scale that yields scores between 0 and 40. A cutoff of ≥11 (indicating the presence of symptoms that are at least moderate) was used as a positive screen for RLS (20). The cognitive status exclusion criterion consisted of a score of 24 or less on the Mini-Mental State Examination (21).
Normal sleepers were healthy older adults who reported going to bed after 21:00 and waking up after 05:30. Early awakening insomnia was characterized using self-reports. Potential participants who complained that they could not stay awake past 19:00 (even though their actual bedtimes during the study weeks may have been later) and could not stay asleep past 04:00 (even though their wakeup times during the study weeks may have been later) were accepted into the study. All of the early awakening insomniacs had PSQI scores above 6. Five normal sleepers had PSQI scores between 6 and 9. All other normal sleepers had PSQI scores below 6. The mean ± SD Pittsburgh Sleep Quality Index (PSQI) (22) scores for the normal sleepers were 4.6 ± 2.8 and for the early awakening insomniacs were 10.5 ± 3.2. The mean ± SD self-reported bedtimes and wakeup times (h:min) for the normal sleepers were 22:18 ± 1:11 and 06:24 ± 1:01. The mean ± SD self-reported bedtimes and wakeup times for the early awakening insomniacs were 20:12 ± 0:56 and 04:22 ± 0:54. All participants provided written informed consent approved by Rensselaer Polytechnic Institute's Institutional Review Board and were paid for their participation. The study was conducted in accordance with the Declaration of Helsinki (23) and conformed to international ethical standards.
Light Conditions
Active light was delivered to both retinae through closed eyelids using the flashing blue light mask previously described by Figueiro et al. (16) and shown in Figure 1. In brief, the active light mask contained two blue LED arrays (wavelength of peak intensity [λmax] = 480 nm, full-width-half-maximum [FWHM] = 24 nm), one for each eyelid. Using the elastic strap around the back of the head, the light mask held the LED arrays in front of the eyelids. A control light mask containing two red LED arrays (λmax = 640 nm, FWHM = 25 nm) was used. The light-stimulus condition was a train of blue or red light pulses: 2-s duration light pulses spaced apart 30 s for no more than 3 h. The light mask was programmed to be turned on no earlier than 1 h after bedtimes and to be turned off no later than 1 h prior to CBTmin, which was estimated to occur 7 h after baseline DLMO. Therefore, the train of light pulses always occurred during the expected delay portion of the PRC. A target phase shift of 2 h was established at the start of the study. Every evening, participants were asked to report their expected bedtimes and to download the light data from the Daysimeter to a laptop that was provided to each participant. Once the download was complete, a modified algorithm based on the model of human circadian entrainment by Kronauer et al. (24) was used to program the most effective timing to apply circadian light, each night, using the active light mask. The Kronauer model consists of a phototransduction process (L) driving a limit cycle oscillator process (P). The modified Kronauer model (CS-oscillator model) proposed by Rea and colleagues (25), is based on the understanding of the spectral and absolute sensitivities of the human circadian system to retinal light exposures. Circadian stimulus (CS) (25, 26), a metric reflecting the spectral and absolute sensitivities of the human circadian system, was used as scaled input to process P; process L was eliminated under the assumption that CS accurately characterized the conversion of optical radiation on the retina to neural signals to the biological clock. Rea et al. (25) also proposed to adjust two process P parameters (q and k) to achieve a 1:1 proportional relationship between the CS-oscillator model predictions and the delta DLMO observed in two independent field studies designed to deliver an advancing or a delaying light intervention to participants living at home. The correlation between delta DLMO measured in these two field studies and the predicted phase changes calculated using the CS-oscillator model were statistically significant (R2 = 0.66, p<0.0001) with a prediction uncertainty of 1.75 h (95% confidence) (25). Input to the CS-oscillator model was continuously measured (24 h per day) light-dark exposures from the Daysimeter and assumed a fixed circadian tau of 24.2 h. The same CS-oscillator model was used in the present study.
Figure 1.
Active light was delivered through closed eyelids during sleep using a light mask. The light mask contained two blue LED arrays (λmax = 480 nm, FWHM = 24 nm), one for each eyelid.
Participants were also asked to follow the same experimental procedure (i.e., download the Daysimeter data daily) when experiencing the flashing red light mask, but because this light mask was not expected to shift the timing of the biological clock, the program automatically turned the mask on starting 1 h after bedtimes and kept it on for 3 h. A Daysimeter, which also records actigraphy and temperature, was incorporated into the light mask to verify compliance and to allow the experimenters to verify that the light mask was being worn and that the lights were flashing during the expected time. Post hoc analyses of the Daysimeter data revealed that over the course of the week, the mean ± SD duration that the flashing blue light mask was turned on was 2.76 ± 0.6 h. In 4 of the 28 subjects, the flashing blue light intervention was turned on for only 3 of the 7 days of the intervention period and it was turned off slightly before the 3 h period. The mean ± SD duration that the flashing blue light mask was turned on was 2.9 ± 0.1 h on night 1, 2.9 ± 0.1 h on night 2, 2.8 ± 0.5 on night 3, 2.8 ± 0.6 h on night 4, 2.7 ± 0.8 h on night 5, 2.6 ± 0.9 h on night 6 and 2.6 ± 0.7 h on night 7.
The prescribed corneal irradiance levels for the active, flashing blue light mask were based upon CS calculations (26). CS is defined in terms of equivalence to the estimated percentage, from 0% to 70%, of light-induced nocturnal melatonin suppression following 1 h exposure to the retina (eyes open) from a non-pulsing light through a fixed pupil of 2.3 mm diameter. Because individual eyelid transmittance measurements were not made for the participants in the study, and to ensure that all subjects received a significant corneal light stimulus, the irradiance for the 480-nm light stimulus was set to provide a CS of 0.30 for the thickest eyelids measured in a previous eyelid transmittance study (8). A CS of 0.3 is equivalent to 30% melatonin suppression after 1 h exposure to the light stimulus. The mean ± SD eyelid irradiances from the flashing blue light mask needed to achieve this CS value were 225 W/m2 ± 7.6 W/m2 or 27,493 lux ± 966 lux. The corresponding CS for a person having mean eyelid transmittance is 0.43. The red light eyelid irradiances were matched to the blue light eyelid irradiances. After taking into account eyelid transmissions at these two wavelengths, the mean ± SD red light eyelid irradiances were set to 7 W/m2 ± 0.3 W/m2 or 1507 lux ± 4.6 lux. The calculated CS for a person having mean eyelid transmittance for the flashing red light mask was <0.001. It is also important to note that all of the calculations were made based on a continuous light exposure and that the retinal light exposure (irradiance × time) in the present experiment is only 7% of that from a continuous 1 h exposure. The uncertainties associated with quantifying retinal light exposures are described in Figueiro et al. (16)
Procedures
Participants were required to complete an 8-week protocol (Figure 2). During all 8 weeks of the protocol, participants wore a Daysimeter (27) as a pendant while awake and a wrist actigraph (Philips Respironics, Pittsburgh, PA, USA) at all times, except for exercising and showering. They were also asked to keep sleep logs for the duration of the study. Figueiro et al. (27) previously documented the physical and calibration characteristics of the Daysimeter. The Daysimeter has three orthogonally oriented, solid-state accelerometers that are calibrated in terms of gravitational forces on the device, which allow for continuous measures of rest/activity patterns, and can be used to verify compliance.
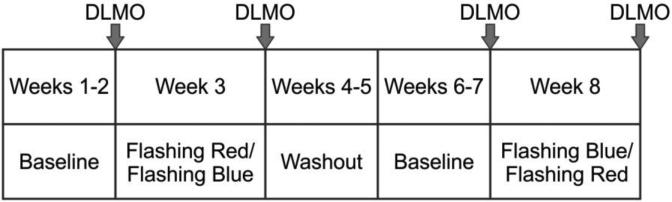
Figure 2.
Study protocol. The 8-week study consisted of 2 baseline weeks, an intervention week, a washout period, another 2 baseline weeks, and a second intervention week. Saliva samples were collected four times during the protocol for circadian phase assessment by DLMO.
The 8-week study consisted of 2 baseline weeks (baseline 1) followed by an intervention week during which participants wore a flashing red (control) or a flashing blue (active) light mask for 7 nights at home (intervention 1). After a 10-to-14-day washout period, participants were asked to collect another 2 baseline weeks (baseline 2), followed by a second intervention week (intervention 2). Those who received the flashing red light during the intervention 1 week received the flashing blue light during the intervention 2 week and vice-versa. The order in which subjects started experiencing the flashing blue and flashing red lights was randomly assigned to each individual. The first subject who was recruited for the study experienced the flashing blue light first, then the second subject started the study with the flashing red light. Given that we had some equipment availability issues, at the end of the study, 16 participants had experienced the flashing blue light first and 12 participants had experienced the flashing red light first.
At the end of the first baseline week, participants collected saliva samples for circadian phase assessment by DLMO. Participants collected saliva samples at home (timing was based upon their sleep schedule), every 30 min during a 4.5-h window that terminated approximately 1.5 h after the predicted DLMO calculated using their habitual sleep schedules (28). Participants were instructed to remain in dim light and refrain from eating and drinking during the saliva collection period. Saliva samples were refrigerated until the next morning, when the research nurse collected the samples and brought them to the laboratory, where they were immediately frozen and assayed within the next 2 days.
Participants began using the mask at the end of the second week of the baseline period. The second baseline week allowed us to assay the saliva samples for DLMO times, which were then used to estimate CBTmin and therefore, the start and end times of the light exposure, as detailed above. At the end of the intervention week, participants again collected and refrigerated saliva samples for circadian phase assessment. After the washout period, participants started two additional baseline weeks. Again, at the end of the first week of baseline 2, participants collected saliva samples at home for circadian phase assessment. Following the completion of the baseline weeks, the light mask was again worn for 7 days using the opposite light condition as the first intervention week. At the end of the second intervention week period, participants collected saliva samples for their last circadian phase assessment.
Data Analyses
Two normal sleepers and two early awakening participants did not provide enough saliva samples and their melatonin data are not included in the analyses. Melatonin concentrations in the saliva samples were determined by radioimmunoassay using a commercially available kit from Labor Diagnostika Nord (Nordhorn, Germany). The limit of detection was 1.4 pg/mL and the intra-assay and inter-assay coefficients of variability were determined to be 11.4% and 14.6%, respectively.
DLMO times were calculated using one of two techniques published in the literature, either by taking the average of the three lowest points plus twice the standard deviation of these points (“3L”)(29) or by taking the average of the five continuous lowest points plus 15% of the five continuous highest points (“5H/5L”)(30). The different techniques were employed to account for individual differences in the observed melatonin profiles and to account for the fact that four subjects did not provide enough saliva for melatonin assay at the latter part of the night (31). The 3L method was used for those subjects with steep melatonin profiles whereas the 5H/5L method was used for those with shallow melatonin profiles. The 5H/5L method better characterized DLMO than the 3L method for shallow profiles because the latter method is based upon the first three, lowest melatonin concentrations, which were very often the same value with no standard deviation (16, 17, 32). The 5H/5L method was used to calculate DLMO for 13 subjects and the 3L method was used to calculate DLMO for 12 subjects. For every subject, the same threshold method was used in baseline and interventions weeks. Changes in circadian phase were calculated as the difference between DLMO at baseline and DLMO after the light intervention week. Data for 17 normal sleepers and 8 early awakening participants were included in the analyses and are reported below.
Actigraph data were analyzed using the Actiware-Sleep Version 3.4 from Mini Mitter Co., Inc., now Philips Respironics (Pittsburg, PA, USA). Visual inspection of the data was performed prior to analyses. Actigraph data recorded while the monitor was not worn were removed from the analyses. This included periods identified as such in the subjects’ sleep logs, in addition to actigraphy data that indicated no activity for more than 30 consecutive min. Days that contained more than 10% of missing data were excluded from the analyses and data sets with fewer than 2 days of valid data were excluded entirely; 3 subjects were excluded for non-compliance and 4 subjects were excluded due to equipment failure. Using the average of the 7 days of the last week of the baseline and the 7 days of the intervention weeks, sleep efficiency was calculated and compared between baseline and intervention weeks. Data from 14 normal sleepers and 7 early awakening participants were included in the average data and are reported below. In order to determine whether the flashing blue light delayed sleep compared to the flashing red light, the sleep start times and sleep end times at Day 1 and Day 7 of baseline and intervention weeks were calculated for the flashing blue and flashing red light conditions. Data from 14 normal sleepers and 8 early awakening participants were included in the Day 1 and Day 7 data analyses.
Statistical Analyses
A 2 (light conditions: blue and red flashing lights) × 2 (weeks: baseline and intervention) within and 1 between (group: normal sleepers and early awakening insomniacs) mixed analyses of variance (ANOVA) was performed on DLMO times, sleep efficiency and midsleep times (sleep start times minus sleep end times).
In order to compare the change in sleep start and end times during the intervention weeks, a 2 (light conditions: blue and red flashing lights) × 2 (days: Day 1 and Day 7 of the intervention week) within and 1 between (group: normal sleepers and early awakening insomniacs) was also performed. Two-tailed paired Student's t-tests were performed to further compare significant main effects and interactions. For the group comparisons, two-tailed independent samples t-tests (equal variances assumed) were performed.
A criterion probability of a Type I error (p) less than 0.05 was considered significant for all inferential statistical analyses unless Bonferroni corrections were required. All statistical analyses were performed using PASW Statistics 18.0 Software (SPSS, Chicago, IL, USA).
Results
Circadian Phase
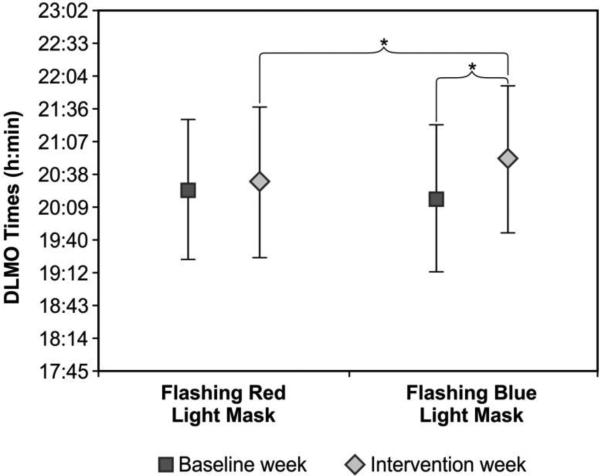
The ANOVA revealed a significant main effect of weeks (F1,23 = 16.8; p<0.0001; partial eta squared = 0.42) and a significant light conditions × weeks interaction (F1,23 = 15.3; p = 0.001; partial eta squared = 0.40). The light conditions × weeks × group interaction was close to significant (F1,23 = 3.2; p<0.09; partial eta squared = 0.12). DLMO times were significantly later after the intervention week than after the baseline week. Post hoc t-tests revealed that compared to the respective baseline weeks, DLMO time was significantly later after the flashing blue light exposure (t(24) = 5.6; p < 0.0001) but not after the flashing red light exposure (t(24) = 1.3; p = 0.22). Post hoc t-tests also revealed that DLMO after the flashing blue light exposure was significantly later than DLMO after the flashing red light exposure (t(24) = 2.4; p = 0.025). Figure 3 shows DLMO times for the baseline and intervention weeks for both light conditions. The mean ± SD DLMO times (h:min) after the baseline weeks was 20:25 ± 1:02 for the flashing red light and 20:17 ± 1:05 for the flashing blue light. The mean ± SD DLMO times after the intervention weeks was 20:32 ± 1:06 for the flashing red light and 20:52 ± 1:06 for the flashing blue light.
Figure 3.
Mean ± SD DLMO times for baseline and intervention weeks for both light conditions (flashing red and flashing blue lights). DLMO times were not significantly different between the two baseline weeks (p> 0.05). DLMO time after flashing blue light was significantly later than DLMO time after flashing red light (t(24) = 2.4; p = 0.025). DLMO time after flashing blue light was also significantly later than after baseline (t(24) = 5.6; p < 0.0001). * = statistically significant
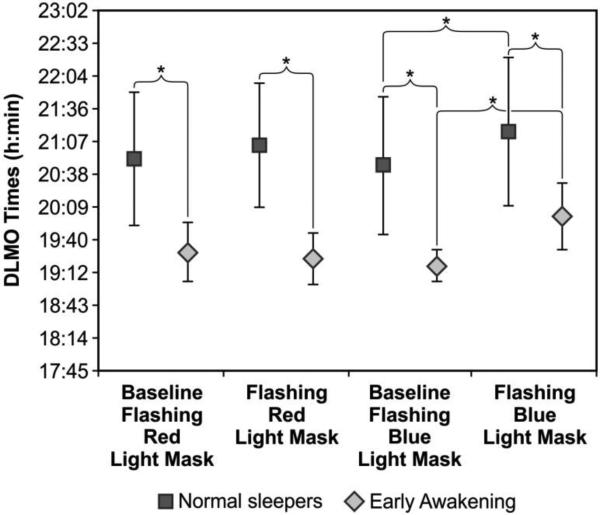
As shown in Figure 4, there was also a significant main effect of group (F1,23 = 18.5; p<0.0001; partial eta squared = 0.45). As expected, DLMO times were significantly earlier for the early awakening insomniacs than for the normal sleepers. The mean ± SD DLMO times after the flashing red light baseline week was 19:30 ± 0:25 for the early awakening insomniacs and 20:51 ± 0:57 for the normal sleepers. The mean ± SD DLMO times after the flashing red light intervention week was 19:24 ± 0:22 for the early awakening insomniacs and 21:04 ± 0:54 for the normal sleepers. The mean ± SD DLMO times after the flashing blue light baseline week was 19:17 ± 0:13 for the early awakening insomniacs and 20:46 ± 1:01 for the normal sleepers. The mean ± SD DLMO times after the flashing blue light intervention week was 20:01 ± 0:29 for the early awakening insomniacs and 21:16 ± 01:05 for the normal sleepers.
Figure 4.
Mean ± SD DLMO times for baseline and intervention weeks for each group (normal sleepers and early awakening insomniacs) and for both light conditions (flashing red and flashing blue). DLMO times were significantly later in normal sleepers than in early awakening insomniacs both at baseline (t(23) = 3.8; p = 0.001 for baseline flashing red light and t(23) = 4.0; p = 0.001 for baseline flashing blue light) and after the two light interventions (t(23) = 4.9; p < 0.0001 for flashing red light and t(23) = 3.1; p = 0.005 for flashing blue light). There was also a statistically significant difference between DLMO times at baseline and after the flashing blue light condition for normal sleepers (t(16) = 3.9; p = 0.001) and for early awakening insomniacs (t(7) = 4.3; p = 0.004).
* = statistically significant
DLMO phase shift for each light condition was calculated by subtracting DLMO times after the intervention week from DLMO times at baseline. Post hoc t-tests revealed a significant difference between phase shift obtained after flashing blue and flashing red lights (t(24) = 3.3; p = 0.003). The mean ± SD phase shift (min) was 0:06 ± 0:30 for the flashing red light and 0:34 ± 0:30 for the flashing blue light. Table 1 shows the individual DLMO times for baseline and intervention weeks for each group together with the DLMO phase shifts for both light conditions.
Table 1.
DLMO times and DLMO phase shift (Night 2 – Night 1) for baseline and intervention weeks for each group (normal sleepers and early awakening insomniacs)
| Normal Sleepers | ||||||
|---|---|---|---|---|---|---|
| Subject # | DLMO Baseline Flashing Red (h:min) | DLMO Flashing Red (h:min) | Shift N2 – N1 (h:min)* | DLMO Baseline Flashing Blue (h:min) | DLMO Flashing Blue (h:min) | Shift N2 – N1 (h:min)* |
| 1 | 21:30 | 21:41 | –0:11 | 20:50 | 20:45 | 0:05 |
| 2 | 20:20 | 21:06 | –0:46 | 20:20 | 21:00 | –0:40 |
| 3 | 22:40 | 22:15 | 0:25 | 22:40 | 22:50 | –0:10 |
| 4 | 20:45 | 22:00 | –1:15 | 20:45 | 21:00 | –0:15 |
| 5 | 21:40 | 21:00 | 0:40 | 21:40 | 22:30 | –0:50 |
| 6 | 21:10 | 21:22 | –0:12 | 21:00 | 21:30 | –0:30 |
| 7 | 21:30 | 21:30 | 0:00 | 20:10 | 21:20 | –1:10 |
| 8 | 20:14 | 21:10 | –0:56 | 20:08 | 20:55 | –0:47 |
| 9 | 21:00 | 21:52 | –0:52 | 21:15 | 22:30 | –1:15 |
| 10 | 21:04 | 20:30 | 0:34 | 20:54 | 22:15 | –1:21 |
| 11 | 21:24 | 20:57 | 0:27 | 22:30 | 21:49 | 0:41 |
| 12 | 21:11 | 21:51 | –0:40 | 21:46 | 22:42 | –0:56 |
| 13 | 19:30 | 20:00 | –0:30 | 19:48 | 20:03 | –0:15 |
| 14 | 19:05 | 19:31 | –0:26 | 19:05 | 19:35 | –0:30 |
| 15 | 21:30 | 21:35 | –0:05 | 20:30 | 21:01 | –0:31 |
| 16 | 19:00 | 18:52 | 0:08 | 19:02 | 19:03 | –0:01 |
| 17 | 21:07 | 21:00 | 0:07 | 20:40 | 20:48 | –0:08 |
| Early Awakening Insomniacs | ||||||
| 1 | 19:37 | 19:40 | –0:03 | 19:18 | 21:04 | –1:46 |
| 2 | 19:38 | 19:05 | 0:33 | 19:03 | 19:51 | –0:48 |
| 3 | 19:00 | 18:48 | 0:12 | 18:55 | 19:30 | –0:35 |
| 4 | 19:48 | 19:42 | 0:06 | 19:30 | 19:45 | –0:15 |
| 5 | 18:45 | 19:03 | –0:18 | 19:36 | 20:07 | –0:31 |
| 6 | 19:35 | 19:42 | –0:07 | 19:18 | 19:37 | –0:19 |
| 7 | 20:05 | 19:47 | 0:18 | 19:27 | 20:15 | –0:48 |
| 8 | 19:32 | 19:30 | 0:02 | 19:15 | 20:00 | –0:45 |
negative values = phase delay
Actigraphy data
The ANOVA using sleep efficiency revealed only a significant main effect of light conditions (F1,19 = 16.2; p = 0.001; partial eta squared = 0.46). Sleep efficiency was not significantly different (p > 0.05) between groups. The average sleep efficiency for both groups during baseline and intervention weeks after the flashing red light condition was significantly lower (mean ± SD = 81.5% ± 5 %) than after the baseline and intervention weeks after the flashing blue light condition (mean ± SD = 85% ± 4%). In order to investigate whether sleep efficiency was reduced in the intervention weeks compared to the baseline weeks, post hoc t-tests were performed comparing sleep efficiency during the baseline and the intervention weeks for each light conditions. Post hoc t-tests revealed that sleep efficiency after both the flashing blue and the flashing red light was not significantly different from baseline weeks (t(20) = 0.48; p = 0.6 and t(20) = 1.9; p = 0.07 respectively).
Using midsleep times as the outcome measure, the ANOVA revealed a significant main effect of light conditions (F1,19 = 6.6; p = 0.02; partial eta squared = 0.26). Mean ± SD midsleep times (h:min) was 2:15 ± 0:52 after the flashing blue light condition and 2:05 ± 0:55 after the flashing red light condition. The main effects of group was also significant (F1,19 = 5.5; p = 0.03; partial eta squared = 0.22). Mean ± SD midsleep times (h:min) was 02:27 ± 0:50 in normal sleepers and 01:35 ± 0:44 in early awakening insomniacs. No other main effects or interactions were statistically significant (p> 0.05).
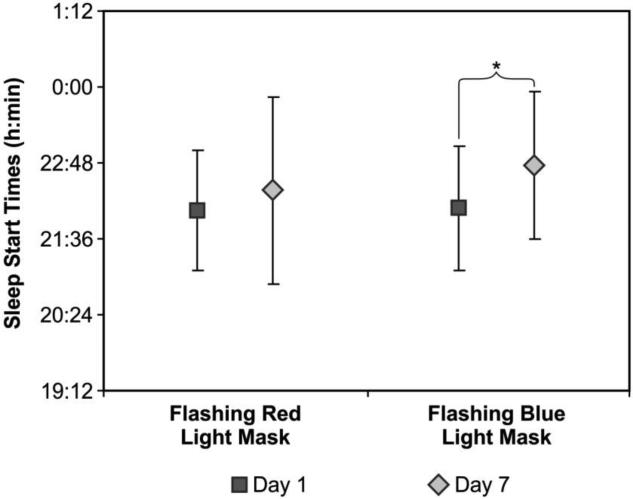
For the comparison between sleep start and end times at Day 1 and Day 7, the ANOVA using sleep start times revealed a significant main effect of days (F1,20 = 16.1; p = 0.001; partial eta squared = 0.44), a significant light conditions × days interaction (F1,20 = 4.6; p = 0.045; partial eta squared = 0.12) and a significant groups × light conditions × days interaction (F1,20 = 6.8; p = 0.17; partial eta squared = 0.25). The main effect of light conditions was close to significance (F1,20 = 3.6; p = 0.07; partial eta squared = 0.15). Sleep start times after flashing blue light at Day 7 were significantly later than at Day 1 (t(21) = 3.2; p = 0.004). During the flashing blue light intervention week, the mean ± SD sleep start times at Day 1 and Day 7 were 22:05 ± 0:59 and 22:46 ± 01:10, respectively. During the flashing red light week, the mean ± SD sleep start times at Day 1 and Day 7 were 22:03 ± 0:57 and 22:22 ± 1:29, respectively (Figure 5).
Figure 5.
Mean ± SD sleep start times at Day 1 and Day 7 during the intervention weeks for both light conditions (flashing red and flashing blue lights). Sleep start times after flashing blue light at Day 7 were significantly later than at Day 1 (t(21) = 3.2; p = 0.004) but there was no significant difference in sleep start time from Day 1 to Day 7 during the week that participants were exposed to the flashing red light mask. * = statistically significant
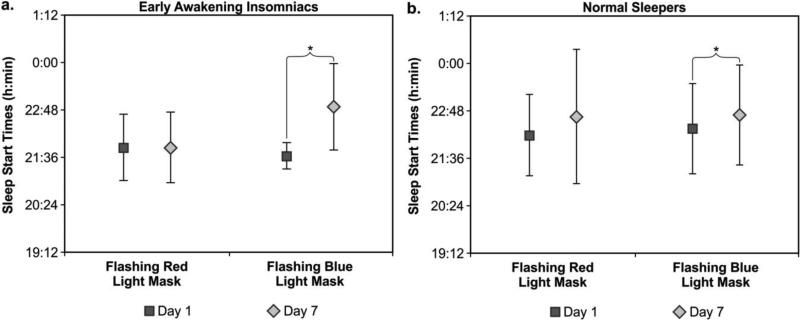
Sleep start times after flashing blue light at Day 7 was later than at Day 1 in both, normal sleepers (t(13) = 2.1; p = 0.05) and early awakening insomniacs (t(7) = 2.8; p = 0.027). There were no significant differences (p > 0.05) between sleep start times at Day 7 and Day 1 after the flashing red light week for either group. During the flashing blue light intervention week, the early awakening insomniacs exhibited mean ± SD sleep start times at Day 1 and Day 7 of 21:38 ± 0:20 and 22:53 ± 01:05, respectively. The normal sleepers had mean ± SD sleep times at Day 1 and Day 7 of 22:21 ± 01:09 and 22:42 ± 01:16. During the flashing red light week, the early awakening insomniacs exhibited mean ± SD sleep start times at Day 1 and Day 7 of 21:51 ± 0:51 and 21:51 ± 0:53, respectively. The normal sleepers had mean ± SD sleep times at Day 1 and Day 7 of 22:11 ± 1:02 and 22:39 ± 1:42 (Figure 6a and 6b).
Figures 6a (left) and 6b (right).
Mean ± SD sleep start times at Day 1 and Day 7 during the intervention weeks for each group (normal sleepers and early awakening insomniacs) and for both light conditions (flashing red and flashing blue). Sleep start times after flashing blue light at Day 7 were significantly later than at Day 1 in both, normal sleepers (t(13) = 2.1; p = 0.05) and early awakening insomniacs (t(7) = 2.8; p = 0.027). There were no significant differences (p > 0.05) between sleep start times at Day 7 and Day 1 after the flashing red light week for either group. * = statistically significant
The ANOVA using the sleep end times revealed an almost significant main effect of days (F1,20 = 3.8; p = 0.07; partial eta squared = 0.16). Sleep end times at Day 1 were earlier than at Day 7 during the flashing blue light intervention week (05:44 ± 01:48 and 06:14 ± 01:22, respectively), albeit the difference did not reach statistical significance (t(21) = 1.5; p = 0.15).
Discussion
The present within-subjects, placebo-controlled field study demonstrated that flashing blue light delivered through closed eyelids prior to predicted CBTmin delays circadian phase and sleep start times in normal sleepers and in early awakening insomniacs living at home. Compared to the control, flashing red light, participants who received the active, flashing blue light for one week delayed DLMO by an average of 34 min. When taking into account the phase shift obtained with the flashing red light (6 min), the net phase delay after one week of the active light intervention was about 28 min, similar to results obtained by Figueiro et al. (17), who showed that the same flashing blue light mask delayed DLMO by about 24 min in healthy adults living at home. One other laboratory study also demonstrated that much shorter flashes of light (i.e., milliseconds) delivered to awake participants, with eyes open, phase shifted DLMO by about 45 min (33). The larger phase shift obtained in the Zeitzer et al. (33) study may have been due to the more controlled protocol employed by these authors (i.e., constant routine, dim light conditions during the study, which may have increased sensitivity to light, and controlled meal times) and due to the fact that the subjects’ eyes were open during the light exposure. Recently, the same group published a laboratory study involving 13 subjects, where 6 subjects received a flashing light pulse via closed eyelids. Their results showed that, compared to a dark night, those who received the flashing light for one night phase delayed DLMO by about 30 min (34).
While a net circadian phase change of 28 min in one week may not seem large, it is likely representative of what one would be able to achieve while living a normal life, without controlling the entire daily light exposure. A few factors may have played a role in this reduced effect: 1) the uncertainty of the participants’ eyelid transmittances, 2) flashing light may be less effective at phase shifting the clock than continuous light, and 3) exposure to light in the morning may have counteracted the delaying effect of the evening light. The magnitude of the phase shift obtained in this study, however, is in line with phase shifts obtained in other field studies where participants received continuous light while awake. Appleman et al. (35) found that young adults who were placed on an advance sleep/wake schedule and asked to experience a delaying light intervention (i.e., 2 h of orange-tinted glasses that remove short-wavelength light in the morning and 3 h of 470-nm light in the evening prior to going to bed) showed a phase delay of about 59 min after one week of the light intervention. Figueiro et al. (36) showed, in another field study, that early chronotypes that were given a similar light intervention as the one employed by Appleman et al. (35) (orange-tinted glasses in the morning and short-wavelength light in the evening) delayed by 37 min after one week of the light intervention. It is likely that a greater delay would have been observed in the present study if participants were asked to wear orange-tinted glasses for 2 h in the morning. Nevertheless, this novel method of delivering light through closed eyelids during sleep is consistent with the phototransduction model proposed by Rea et al. (10, 11) and can reliably change circadian phase in the home setting.
The uncertainty of the algorithm that was used to predict optimum timing and duration of light exposure may have also affected the magnitude of the observed phase delay. For example, in 4 of the 28 subjects, the flashing blue light intervention was turned on for only 3 of the 7 days of the intervention period and it was turned off slightly before the 3 h period. The algorithm interpreted that the participants had achieved the 2-h target phase delay during the first 3-4 days of the intervention and, therefore, did not program the masks to be turned on during the last 3-4 nights of the intervention period. This does not completely explain the present results, however, because removing the 4 subjects that did not receive 7 days of light intervention from the DLMO calculations resulted in a DLMO phase delay of 29 min rather than 28 min. Nevertheless, preliminary calculations suggest that the model is predicting a much larger phase change than the actual measured DLMO phase delay (2.3 h and 0.5 h, respectively). Given that the model was not developed to predict the effect of flashing blue light on circadian phase shift, future work should utilize the present data set to refine the CS-oscillator model so that light treatment from flashing blue light can be more precisely applied.
Even though the sample size difference between the two groups (normal sleepers and early awakening insomniacs) did not allow us to determine whether there is a significant difference in the response by these two groups, based on the magnitude of the phase shifting and sleep start time delay exhibited by the two groups, the present results suggest that the early awakening insomniacs may be more sensitive to the phase delaying light stimulus. Albeit not statistically significant, most likely due to the smaller sample size in this group and the large individual differences, early awakening insomniacs had a later DLMO as well as a later sleep start time as a result of the flashing blue light. Given that the Daysimeter data revealed that there were no significant differences in total light exposures during waking hours between the two groups, it would be reasonable to hypothesize that this observed difference was due to different sensitivity to the light intervention. It should be noted that in the early awakening insomniacs, but not in the normal sleepers, the magnitude of the delay in sleep start times from Day 1 to Day 7 of the intervention was much greater than in DLMO times. Sleep start times were delayed by 75 min and DLMO times were delayed by 34 min in the early awakening insomniacs. These response differences are in line with those suggested by Cole et al. (37), who showed the effectiveness of a light mask for advancing circadian phase in those suffering from delayed sleep phase syndrome. Another limitation of the present study was that 5 out of the 19 normal sleepers had PSQI scores between 7 and 9, suggesting that they had some sleep disturbances. Future studies should further investigate the relationship between DLMO and sleep times and whether early awakening insomniacs are indeed more sensitive to delaying light than normal sleepers who have PSQI scores below 6 (i.e., no sleep disturbances).
Not unlike the studies by Cole et al. (37) and Grandner et al. (38), the light mask was well tolerated by the participants, especially after a couple of days into the intervention. Sleep efficiency, as measured by the actigraph data, did not differ between baseline and intervention weeks, suggesting that participants were able to sleep through the light mask. Out of the 29 participants accepted into the study, only one withdrew from the study because he was not able to sleep while wearing the mask. All others reported being able to sleep while the light mask was energized.
The results of the present study have some practical implications for sleep health in populations other than older adults. The use of a light mask delivering light while people are asleep may help increase compliance with light interventions in populations suffering from circadian sleep disorders. For example, teenagers who want to advance their sleep during the school week may prefer to receive light treatment 1 h prior to waking rather than getting up 1 h earlier to receive the light. Travelers who will cross many time zones may want to start light treatment prior to initiating their trip and the use of a light mask delivering light during sleep may be a practical way to accomplish this.
In conclusion, the present study demonstrates that flashing blue light delivered through closed eyelids during sleep can change circadian phase and delay sleep start times in those living at home. This novel device can potentially increase compliance to light treatment and promote circadian alignment with social obligations, and therefore, sleep health in those suffering from circadian sleep disorders.
Acknowledgements
The study was funded by the National Institute on Aging (# R01AG042602). Mark S. Rea, Barbara Plitnick, Rebekah Mullaney, Greg Ward, Andrew Bierman, Sharon Lesage, Erin Ryan, Martin Overington, Dennis Hull and Dennis Guyon of the Lighting Research Center are acknowledged for their technical and editorial support.
References
- 1.Achermann P, Borbély AA. Mathematical models of sleep regulation. Front Biosci. 2003;8:s683–93. doi: 10.2741/1064. [DOI] [PubMed] [Google Scholar]
- 2.Borbély AA. A two process model of sleep regulation. Hum Neurobiol. 1982;1(3):195–204. [PubMed] [Google Scholar]
- 3.Wright KP, McHill AW, Birks BR, Griffin BR, Rusterholz T, Chinoy ED. Entrainment of the human circadian clock to the natural light-dark cycle. Curr Biol. 2013;23:1554–8. doi: 10.1016/j.cub.2013.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith MR, Eastman CI. Shift work: health, performance and safety problems, traditional countermeasures, and innovative management strategies to reduce circadian misalignment. Nat Sci Sleep. 2012;4:111. doi: 10.2147/NSS.S10372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khalsa SBS, Jewett ME, Cajochen C, Czeisler CA. A phase response curve to single bright light pulses in human subjects. J Physiol. 2003;549(3):945–52. doi: 10.1113/jphysiol.2003.040477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Robinson J, Bayliss S, Fielder A. Transmission of light across the adult and neonatal eyelid in vivo. Vision Res. 1991;31(10):1837–40. doi: 10.1016/0042-6989(91)90031-y. [DOI] [PubMed] [Google Scholar]
- 7.Ando K, Kripke DF. Light attenuation by the human eyelid. Biol Psychiatry. 1996;39(1):22–5. doi: 10.1016/0006-3223(95)00109-3. [DOI] [PubMed] [Google Scholar]
- 8.Bierman A, Figueiro MG, Rea MS. Measuring and predicting eyelid spectral transmittance. J Biomed Optics. 2011;16(6):067011. doi: 10.1117/1.3593151. [DOI] [PubMed] [Google Scholar]
- 9.Figueiro MG, Rea MS. Preliminary evidence that light through the eyelids can suppress melatonin and phase shift dim light melatonin onset. BMC Res Notes. 2012;5(1):221. doi: 10.1186/1756-0500-5-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rea MS, Figueiro MG, Bullough JD, Bierman A. A model of phototransduction by the human circadian system. Brain Res Rev. 2005;50(2):213–28. doi: 10.1016/j.brainresrev.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 11.Rea MS, Figueiro MG, Bierman A, Hamner R. Modelling the spectral sensitivity of the human circadian system. Light Res Technol. 2012;44(4):386–96. [Google Scholar]
- 12.Berson D, Dunn F, Takao M. Phototransduction by retinal ganglion cells that set the circadian clock. Science. 2002;295(5557):1070–3. doi: 10.1126/science.1067262. [DOI] [PubMed] [Google Scholar]
- 13.Hattar S, Lucas RJ, Mrosovsky N, Thompson SH, Douglas RH, Hankins MW, et al. Melanopsin and rod-cone photoreceptive systems account for all major accessory visual functions in mice. Nature. 2003;424:75–81. doi: 10.1038/nature01761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Figueiro MG, Rea MS, Bullough JD. Circadian effectiveness of two polychromatic lights in suppressing human nocturnal melatonin. Neurosci Lett. 2006;406:293–7. doi: 10.1016/j.neulet.2006.07.069. [DOI] [PubMed] [Google Scholar]
- 15.Figueiro MG, Lesniak NZ, Rea MS. Implications of controlled blue light exposure for sleep in older adults. BMC Res Notes. 2011;4:334. doi: 10.1186/1756-0500-4-334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Figueiro MG, Bierman A, Rea MS. A train of blue light pulses delivered through closed eyelids suppresses melatonin and phase shifts the human circadian system. Nat Sci Sleep. 2013;5:133–41. doi: 10.2147/NSS.S52203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Figueiro MG, Plitnick B, Rea MS. Pulsing blue light through closed eyelids: Effects on phase shifting of dim light melatonin onset in older adults living in a home setting. Nat Sci Sleep. 2015;7:1–8. doi: 10.2147/NSS.S73856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bullough JD. The blue-light hazard: A review. JIES. 2000;29(2):6–14. [Google Scholar]
- 19.Douglas AB, Bornstein R, Nino-Murcia G, Keenan S, Miles L, Zarcone VPJ, et al. The Sleep Disorders Questionnaire 1: Creation and multivariate structure of SDQ. Sleep. 1994;17(2):160–7. doi: 10.1093/sleep/17.2.160. [DOI] [PubMed] [Google Scholar]
- 20.Walters AS, LeBrocq C, Dhar A, Hening W, Rosen R, Allen RP, et al. Validation of the International Restless Legs Syndrome Study Group rating scale for restless legs syndrome. Sleep Med. 2003;4(2):121–32. doi: 10.1016/s1389-9457(02)00258-7. [DOI] [PubMed] [Google Scholar]
- 21.Folstein MF, Folstein SE, McHugh PR. Mini-mental state: A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 22.Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 23.World Medical Association Declaration of Helsinki. JAMA. 2000;284(23):3043–5. [PubMed] [Google Scholar]
- 24.Kronauer RE, Forger DB, Jewett ME. Quantifying human circadian pacemaker response to brief, extended, and repeated light stimuli over the phototopic range. J Biol Rhythms. 1999;14(6):500–15. doi: 10.1177/074873099129001073. [DOI] [PubMed] [Google Scholar]
- 25.Rea MS, Bierman A, Ward G, Figueiro MG. SLEEP 2014. Minneapolis, MN: 2014. Field tests of a model of the human circadian oscillator. [Google Scholar]
- 26.Rea MS, Figueiro MG, Bierman A, Bullough JD. Circadian light. J Circadian Rhythms. 2010;8(1):2. doi: 10.1186/1740-3391-8-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Figueiro MG, Hamner R, Bierman A, Rea MS. Comparisons of three practical field devices used to measure personal light exposures and activity levels. Light Res Technol. 2013;45(4):421–34. doi: 10.1177/1477153512450453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martin SK, Eastman CI. Sleep logs of young adults with self-selected sleep times predict the dim light melatonin onset. Chronobiol Int. 2002;19(4):695–707. doi: 10.1081/cbi-120006080. [DOI] [PubMed] [Google Scholar]
- 29.Voultsios A, Kennaway DJ, Dawson D. Salivary melatonin as a circadian phase marker: validation and comparison to plasma melatonin. J Biol Rhythms. 1997;12(5):457–66. doi: 10.1177/074873049701200507. [DOI] [PubMed] [Google Scholar]
- 30.Smith MR, Revell VL, Eastman CI. Phase advancing the human circadian clock with blue-enriched polychromatic light. Sleep Med. 2009;10(3):287–94. doi: 10.1016/j.sleep.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Molina TA, Burgess HJ. Calculating the dim light melatonin onset: the impact of threshold and sampling rate. Chronobiol Int. 2011;28(8):714–8. doi: 10.3109/07420528.2011.597531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Figueiro MG, Rea MS. The effects of red and blue lights on circadian variations in cortisol, alpha amylase, and melatonin. Int J Endocrinol. 2010;2010:829351. doi: 10.1155/2010/829351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zeitzer JM, Ruby NF, Fisicaro RA, Heller HC. Response of the Human Circadian System to Millisecond Flashes of Light. PLoS One. 2011;6(7):e22078. doi: 10.1371/journal.pone.0022078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zeitzer JM, Fisicaro RA, Ruby NF, Heller HC. Millisecond Flashes of Light Phase Delay the Human Circadian Clock during Sleep. J Biol Rhythms. 2014:0748730414546532. doi: 10.1177/0748730414546532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Appleman K, Figueiro MG, Rea MS. Controlling light-dark exposure patterns rather than sleep schedules determines circadian phase. Sleep Medicine. 2013;14(5):456–61. doi: 10.1016/j.sleep.2012.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Figueiro MG, Plitnick B, Rea MS. The effects of chronotype, sleep schedule and light/dark pattern exposures on circadian phase. Sleep Medicine. 2014 doi: 10.1016/j.sleep.2014.07.009. [epub ahead of print] DOI: 10.1016/j.sleep.2014.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cole RJ, Smith JS, Alcala YC, Elliott JA, Kripke DF. Bright-light mask treatment of delayed sleep phase syndrome. J Biol Rhythms. 2002;17(1):89–101. doi: 10.1177/074873002129002366. [DOI] [PubMed] [Google Scholar]
- 38.Grandner MA, Kripke DF, Elliott J, Cole R. Short wavelength light administered just prior to waking: a pilot study. Biol Rhythm Res. 2013;44(1):13–32. doi: 10.1080/09291016.2011.632578. [DOI] [PMC free article] [PubMed] [Google Scholar]