Abstract
Rhabdomyosarcoma (RMS) is a common soft-tissue neoplasm in the pediatric age group. Common locations are head and neck, genitourinary areas, trunk, and extremities. Two pathologic variants of this malignancy are embryonal and alveolar. The involvement of breast is rare. Herein, we report two cases of alveolar RMS of the breast of which one is an isolated breast metastasis from an orbital primary, whereas the other is a primary RMS of the breast. Both the patients were treated with surgery followed by adjuvant chemotherapy and radiotherapy and are currently disease free at six and three year respectively, following completion of treatment.
KEY WORDS: Pediatric soft tissue sarcoma, pediatric tumors, rhabdomyosarcoma (RMS), rhabdomyosarcoma breast
INTRODUCTION
Rhabdomyosarcoma (RMS) is the commonest soft-tissue neoplasm in children. It is the third most common extracranial childhood solid tumor with an annual incidence of 4-7/million.[1] Common sites of involvement are head and neck areas, genitourinary tract, extremities, trunk, retroperitoneum, and biliary tract. RMS of the breast is rare. Most of the reported cases are metastatic RMS involving the breast. Less than 15 cases of primary RMS of the breast have been reported to the best of our knowledge. Here, we report two cases of which one is a primary RMS of the breast, whereas the other is metastatic RMS from an orbital primary.
CASE REPORTS
Case 1
A 16-year-old postmenarchal girl at posttreatment follow-up for RMS presented with complaint of lump in the right breast. The patient had nonmetastatic alveolar RMS of the orbit diagnosed 15 months before. She was treated with chemotherapy based on International Rhabdomyosarcoma Study 4 (IRS 4) protocol and radiotherapy. After a disease-free interval of 4 months, the patient presented with relapse. On clinical examination a well-defined firm nodule of 2 × 1 cm was palpable in the upper outer quadrant of right breast with no palpable axillary or supraclavicular lymphadenopathy. Fine-needle aspiration cytology (FNAC) was consistent with round cell tumor. Ultrasonogram (USG) showed an ill-defined hypoechoic lesion [Figure 1]. Complete metastatic workup including bone marrow study, bone scan, and computerized tomogram (CT) scan of the thorax was negative for metastasis. Wide excision of lump with axillary sampling was performed. Histopathology revealed an alveolar RMS. All axillary nodes sampled were free of malignancy.
Figure 1.
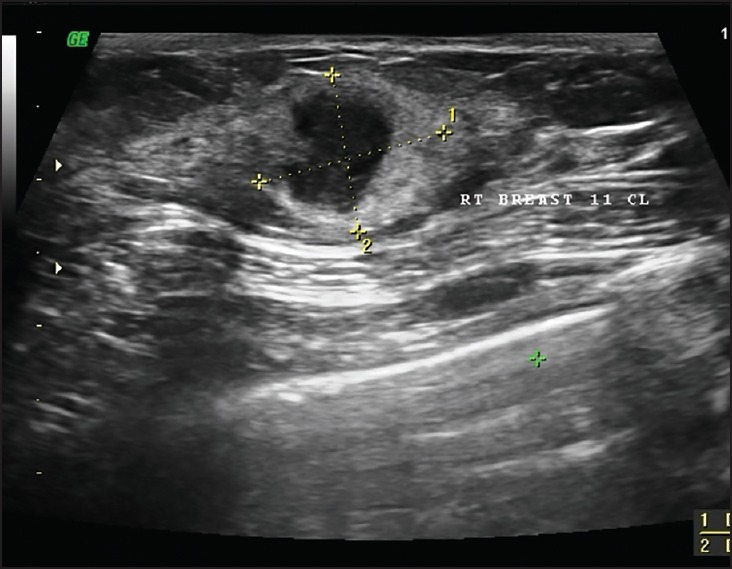
USG reveals a heterogeneous solid lesion at 12 o’clock position of the right breast. It has postacoustic enhancement with edge shadowing
The patient was given six cycles of second-line chemotherapy with vincristine, topotecan, and cyclophosphamide followed by RT (41.4 Gy/23#) to the breast. The patient continues to be disease free at the follow-up of 72 months from completion of the treatment.
Case 2
A 12-year-old premenarchal girl presented with a lump in the right breast of 5 months duration. On examination, the breast was enlarged and had a 9 cm × 9 cm mass with the involvement of the nipple areolar complex [Figure 2]. Multiple enlarged ipsilateral axillary nodes, the largest measuring 3 cm × 3 cm, were present. Core biopsy revealed alveolar RMS. Metastatic workup including bone marrow study and Positron emission tomography (PET) scan were negative. The patient received chemotherapy based on IRS 4 protocol. Modified radical mastectomy (MRM) was performed after the induction chemotherapy. Histopathology showed a viable residual tumor of size 3.5 cm × 3.5 cm × 2 cm. Three out of the total 17 axillary nodes showed metastasis with perinodal extension. The patient received RT, 41.4 Gy/23# to the chest wall followed by boost of 10 Gy/5# to the tumor site. The patient is disease free at the follow-up of 35 months from the completion of treatment.
Figure 2.
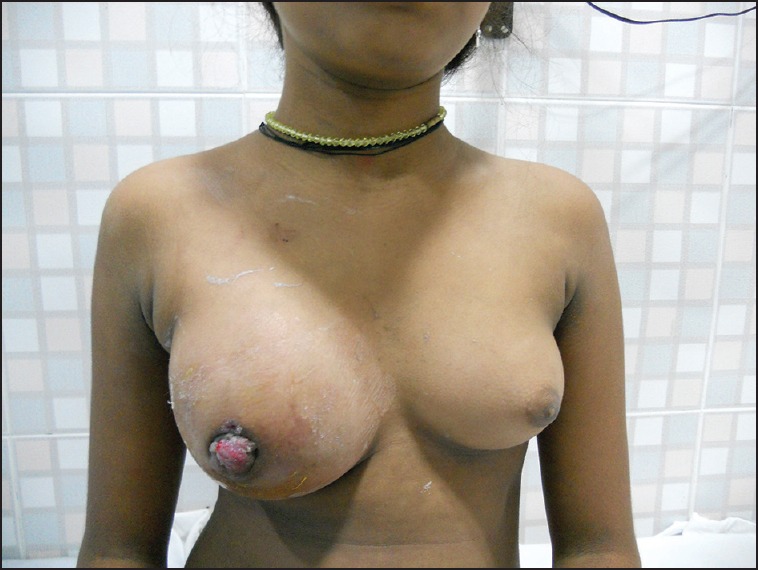
Enlarged right breast with the destruction of nipple areolar complex in a case of primary RMS of the breast
DISCUSSION
RMS is a common pediatric soft tissue neoplasm. Common locations are head and neck, genitourinary areas, trunk, and extremities. Common pathologic variants are embryonal and alveolar. The involvement of breast is rare, the primary involvement being extremely rare.[2,3,4,5,6,7,8] In a series reported from an intergroup RMS study, of the total 26 patients described, 19 had metastatic disease and the remaining 7 had primary disease in the breast. Alveolar histology was common and was seen in 24 patients and embryonal variant was seen in 1 patient. All the patients were in the adolescent age group.[2] Rarely, it is reported in adults with the oldest patient known being 60 years old.[3]
Among patients with metastatic RMS, synchronous metastasis to the breast is seen in 3.7% of cases.[4] The incidence of breast metastasis throughout the disease history is 6.7%.[5] The commonest primary site for metastatic RMS to the breast are extremities and head and neck areas.[2,4] The tendency of RMS to metastasize to breast in adolescent girls is thought to be related to the expression of insulin-like growth factor (IGF) receptors in the tumor cells. The breast epithelium and stroma are known to express growth factors IGF-I and IGF-II.[9] Increased vascularity is another reason for the preferential metastasis to the growing breast.
The metastatic RMS patient in our study had the primary in the orbit and presented with a very short disease-free interval of 4 months. Orbital involvement for RMS is generally considered to be a favorable site. There has been no previously reported case of metastasis to the breast from an orbital primary. After excluding metastatic disease elsewhere breast conserving surgery was performed.
Primary RMS is mostly reported in adolescent girls. Since benign breast diseases are more common in this age group the diagnosis of primary RMS is often delayed. Generally they are locally advanced or metastatic at diagnosis.[2] Our patient also experienced a delay in referral because the initial diagnosis by the primary physician was fibroadenoma. The patient had a large tumor with the involvement of nipple areola complex. Hence, MRM was performed after induction chemotherapy with the option of delayed breast reconstruction. RMS of the breast is an aggressive malignancy, and long-term survival is approximately 50% in primary cases and around 20% among metastatic cases.[2]
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Kramer S, Meadows AT, Jarrett P, Evans AE. Incidence of childhood cancer: Experience of a decade in a population-based registry. J Natl Cancer Inst. 1983;70:49–55. [PubMed] [Google Scholar]
- 2.Hays DM, Donaldson SS, Shimada H, Crist WM, Newton WA, Jr, An-drassy RJ, et al. Primary and metastatic rhabdomyosarcoma in the breast: Neoplasms of adolescent females, a report from the Intergroup Rhabdomyosarcoma Study. Med Pediatr Oncol. 1997;29:181–9. doi: 10.1002/(sici)1096-911x(199709)29:3<181::aid-mpo4>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 3.Bhosale SJ, Kshirsagar AY, Sulhyan SR, Sulhyan SR. Rhabdomyosarcoma of the breast: A rare malignancy. Am J Case Rep. 2013;14:250–2. doi: 10.12659/AJCR.883976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Howarth CB, Caces JN, Pratt CB. Breast metastases in children with rhabdomyosarcoma. Cancer. 1980;46:2520–4. doi: 10.1002/1097-0142(19801201)46:11<2520::aid-cncr2820461134>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 5.D’Angelo P, Carli M, Ferrari A, Manzitti C, Mura R, Miglionico L, et al. ; AIEOP Soft Tissue Sarcoma Committee. Breast metastases in children and adolescents with rhabdomyosarcoma: Experience of the Italian Soft Tissue Sarcoma Committee. Pediatr Blood Cancer. 2010;55:1306–9. doi: 10.1002/pbc.22729. [DOI] [PubMed] [Google Scholar]
- 6.Persic M, Roberts JT. Alveolar rhabdomyosarcoma metastatic to the breast: Long-term survivor. Clin Oncol (R Coll Radiol) 1999;11:417–8. doi: 10.1053/clon.1999.9096. [DOI] [PubMed] [Google Scholar]
- 7.Binokay F, Soyupak SK, Inal M, Celiktas M, Akgül E, Aksungur E. Primary and metastatic rhabdomyosarcoma in the breast: Report of two pediatric cases. Eur J Radiol. 2003;48:282–4. doi: 10.1016/s0720-048x(03)00041-x. [DOI] [PubMed] [Google Scholar]
- 8.Jung SP, Lee Y, Han KM, Lee SK, Kim S, Bae SY, et al. Breast metastasis from rhabdomyosarcoma of the anus in an adolescent female. J Breast Cancer. 2013;16:345–8. doi: 10.4048/jbc.2013.16.3.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Minniti CP, Tsokos M, Newton WA, Jr, Helman LJ. Specific expression of insulin-like growth factor-II in rhabdomyosarcoma tumor cells. Am J Clin Pathol. 1994;101:198–203. doi: 10.1093/ajcp/101.2.198. [DOI] [PubMed] [Google Scholar]


