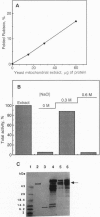
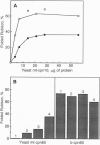
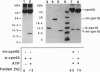
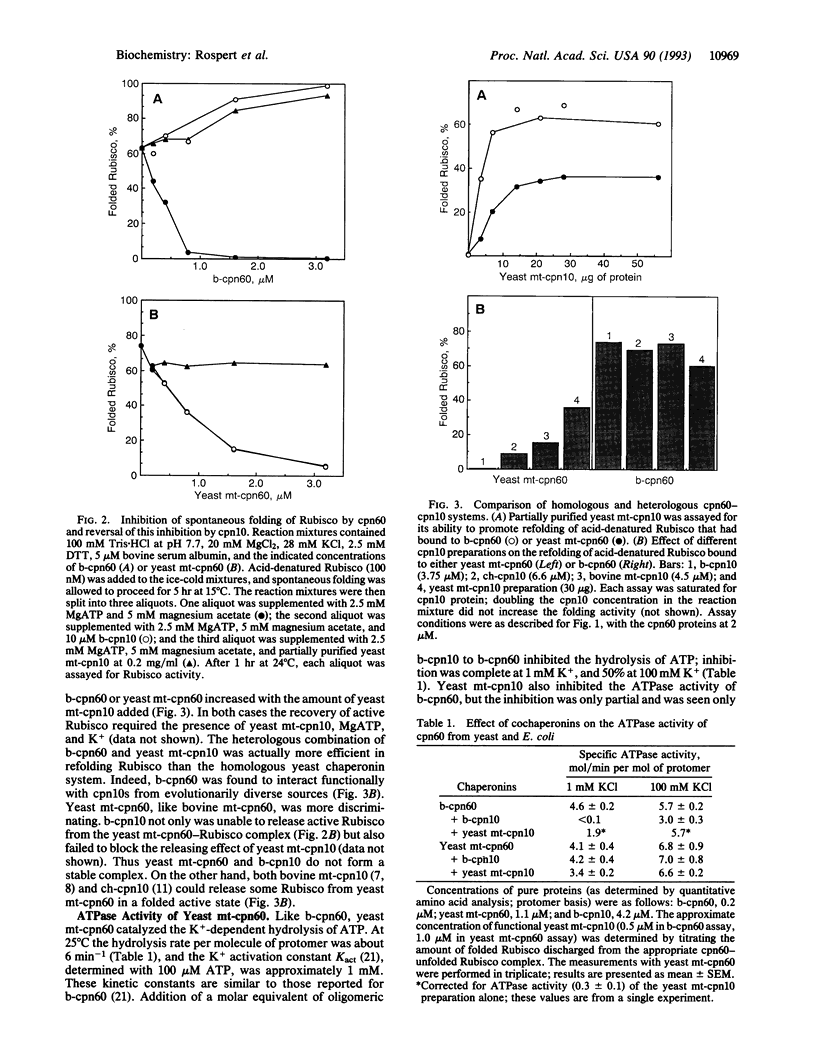
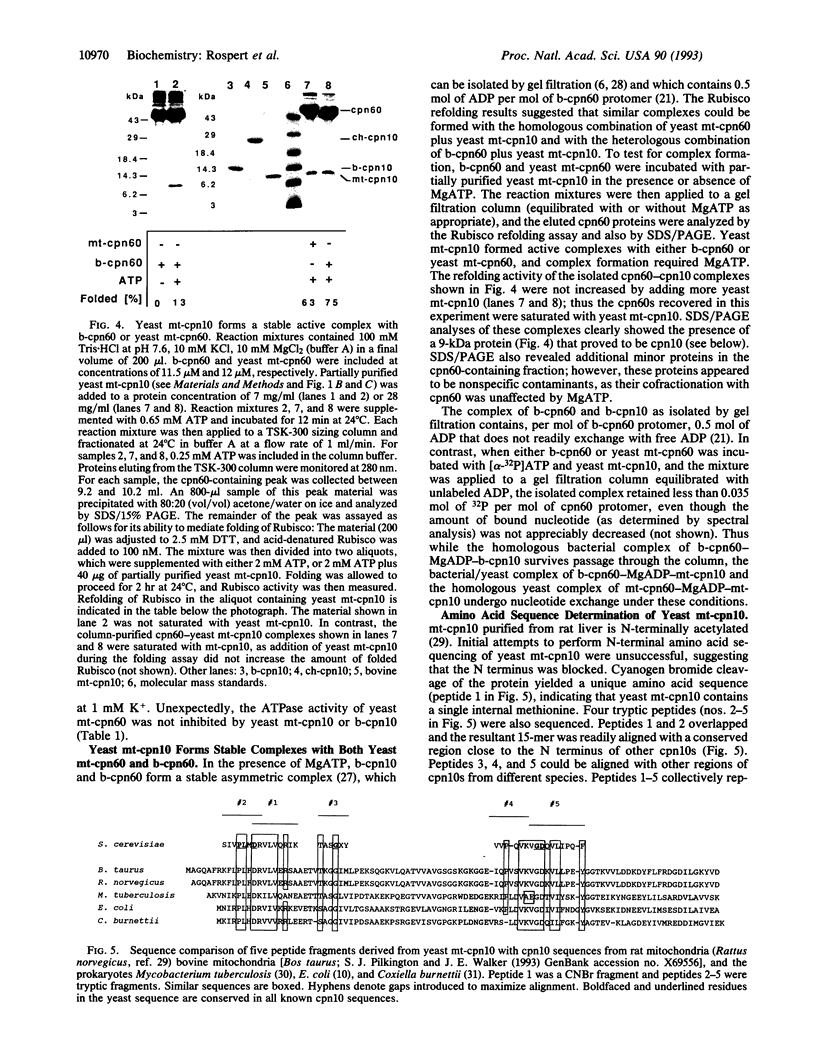
Abstract
Chaperonin 60 (cpn60) and chaperonin 10 (cpn10) constitute the chaperonin system in prokaryotes, mitochondria, and chloroplasts. In Escherichia coli, these two chaperonins are also termed groEL and groES. We have used a functional assay to identify the groES homolog cpn10 in yeast mitochondria. When dimeric ribulose-1,5-bisphosphate carboxylase (Rubisco) is denatured and allowed to bind to yeast cpn60, subsequent refolding of Rubisco is strictly dependent upon yeast cpn10. The heterologous combination of cpn60 from E. coli plus yeast cpn10 is also functional. In contrast, yeast cpn60 plus E. coli cpn10 do not support refolding of Rubisco. In the presence of MgATP, yeast cpn60 and yeast cpn10 form a stable complex that can be isolated by gel filtration and that facilitates refolding of denatured Rubisco. Although the potassium-dependent ATPase activity of E. coli cpn60 can be inhibited by cpn10 from either E. coli or yeast, neither of these cpn10s inhibits the ATPase activity of yeast cpn60. Amino acid sequencing of yeast cpn10 reveals substantial similarity to the corresponding cpn10 proteins from rat mitochondria and prokaryotes.
Full text
PDF
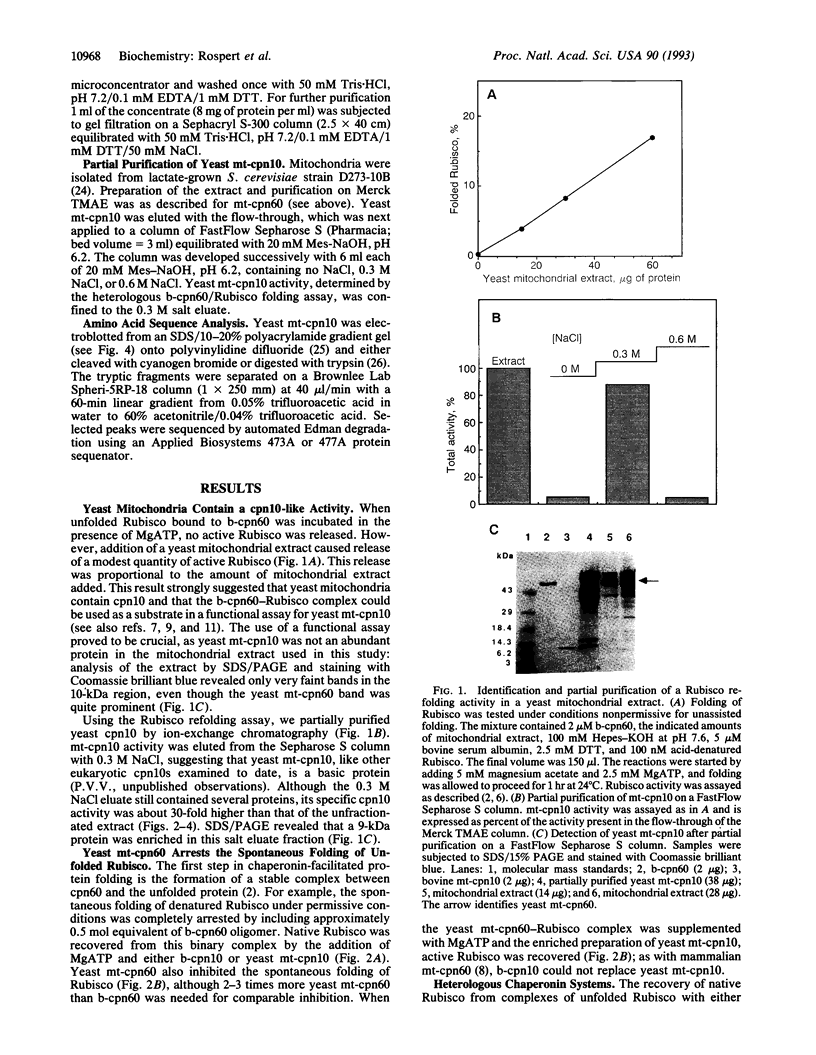

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baird P. N., Hall L. M., Coates A. R. A major antigen from Mycobacterium tuberculosis which is homologous to the heat shock proteins groES from E. coli and the htpA gene product of Coxiella burneti. Nucleic Acids Res. 1988 Sep 26;16(18):9047–9047. doi: 10.1093/nar/16.18.9047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertsch U., Soll J., Seetharam R., Viitanen P. V. Identification, characterization, and DNA sequence of a functional "double" groES-like chaperonin from chloroplasts of higher plants. Proc Natl Acad Sci U S A. 1992 Sep 15;89(18):8696–8700. doi: 10.1073/pnas.89.18.8696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Buchner J., Schmidt M., Fuchs M., Jaenicke R., Rudolph R., Schmid F. X., Kiefhaber T. GroE facilitates refolding of citrate synthase by suppressing aggregation. Biochemistry. 1991 Feb 12;30(6):1586–1591. doi: 10.1021/bi00220a020. [DOI] [PubMed] [Google Scholar]
- Chandrasekhar G. N., Tilly K., Woolford C., Hendrix R., Georgopoulos C. Purification and properties of the groES morphogenetic protein of Escherichia coli. J Biol Chem. 1986 Sep 15;261(26):12414–12419. [PubMed] [Google Scholar]
- Daum G., Böhni P. C., Schatz G. Import of proteins into mitochondria. Cytochrome b2 and cytochrome c peroxidase are located in the intermembrane space of yeast mitochondria. J Biol Chem. 1982 Nov 10;257(21):13028–13033. [PubMed] [Google Scholar]
- Goloubinoff P., Christeller J. T., Gatenby A. A., Lorimer G. H. Reconstitution of active dimeric ribulose bisphosphate carboxylase from an unfoleded state depends on two chaperonin proteins and Mg-ATP. Nature. 1989 Dec 21;342(6252):884–889. doi: 10.1038/342884a0. [DOI] [PubMed] [Google Scholar]
- Goloubinoff P., Gatenby A. A., Lorimer G. H. GroE heat-shock proteins promote assembly of foreign prokaryotic ribulose bisphosphate carboxylase oligomers in Escherichia coli. Nature. 1989 Jan 5;337(6202):44–47. doi: 10.1038/337044a0. [DOI] [PubMed] [Google Scholar]
- Gutteridge S., Reddy G. S., Lorimer G. The synthesis and purification of 2'-carboxy-D-arabinitol 1-phosphate, a natural inhibitor of ribulose 1,5-bisphosphate carboxylase, investigated by 31P n.m.r. Biochem J. 1989 Jun 15;260(3):711–716. doi: 10.1042/bj2600711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman D. J., Hoogenraad N. J., Condron R., Høj P. B. Identification of a mammalian 10-kDa heat shock protein, a mitochondrial chaperonin 10 homologue essential for assisted folding of trimeric ornithine transcarbamoylase in vitro. Proc Natl Acad Sci U S A. 1992 Apr 15;89(8):3394–3398. doi: 10.1073/pnas.89.8.3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman D. J., Hoogenraad N. J., Condron R., Høj P. B. The complete primary structure of rat chaperonin 10 reveals a putative beta alpha beta nucleotide-binding domain with homology to p21ras. Biochim Biophys Acta. 1993 Jul 10;1164(2):219–222. doi: 10.1016/0167-4838(93)90251-l. [DOI] [PubMed] [Google Scholar]
- Hemmingsen S. M., Woolford C., van der Vies S. M., Tilly K., Dennis D. T., Georgopoulos C. P., Hendrix R. W., Ellis R. J. Homologous plant and bacterial proteins chaperone oligomeric protein assembly. Nature. 1988 May 26;333(6171):330–334. doi: 10.1038/333330a0. [DOI] [PubMed] [Google Scholar]
- Johnson R. B., Fearon K., Mason T., Jindal S. Cloning and characterization of the yeast chaperonin HSP60 gene. Gene. 1989 Dec 14;84(2):295–302. doi: 10.1016/0378-1119(89)90503-9. [DOI] [PubMed] [Google Scholar]
- Langer T., Pfeifer G., Martin J., Baumeister W., Hartl F. U. Chaperonin-mediated protein folding: GroES binds to one end of the GroEL cylinder, which accommodates the protein substrate within its central cavity. EMBO J. 1992 Dec;11(13):4757–4765. doi: 10.1002/j.1460-2075.1992.tb05581.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubben T. H., Gatenby A. A., Donaldson G. K., Lorimer G. H., Viitanen P. V. Identification of a groES-like chaperonin in mitochondria that facilitates protein folding. Proc Natl Acad Sci U S A. 1990 Oct;87(19):7683–7687. doi: 10.1073/pnas.87.19.7683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning-Krieg U. C., Scherer P. E., Schatz G. Sequential action of mitochondrial chaperones in protein import into the matrix. EMBO J. 1991 Nov;10(11):3273–3280. doi: 10.1002/j.1460-2075.1991.tb04891.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin J., Langer T., Boteva R., Schramel A., Horwich A. L., Hartl F. U. Chaperonin-mediated protein folding at the surface of groEL through a 'molten globule'-like intermediate. Nature. 1991 Jul 4;352(6330):36–42. doi: 10.1038/352036a0. [DOI] [PubMed] [Google Scholar]
- Matsudaira P. Sequence from picomole quantities of proteins electroblotted onto polyvinylidene difluoride membranes. J Biol Chem. 1987 Jul 25;262(21):10035–10038. [PubMed] [Google Scholar]
- Mendoza J. A., Rogers E., Lorimer G. H., Horowitz P. M. Chaperonins facilitate the in vitro folding of monomeric mitochondrial rhodanese. J Biol Chem. 1991 Jul 15;266(20):13044–13049. [PubMed] [Google Scholar]
- Ostermann J., Horwich A. L., Neupert W., Hartl F. U. Protein folding in mitochondria requires complex formation with hsp60 and ATP hydrolysis. Nature. 1989 Sep 14;341(6238):125–130. doi: 10.1038/341125a0. [DOI] [PubMed] [Google Scholar]
- Pierce J., Gutteridge S. Large-scale preparation of ribulosebisphosphate carboxylase from a recombinant system in Escherichia coli characterized by extreme plasmid instability. Appl Environ Microbiol. 1985 May;49(5):1094–1100. doi: 10.1128/aem.49.5.1094-1100.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce J., Reddy G. S. The sites for catalysis and activation of ribulosebisphosphate carboxylase share a common domain. Arch Biochem Biophys. 1986 Mar;245(2):483–493. doi: 10.1016/0003-9861(86)90241-9. [DOI] [PubMed] [Google Scholar]
- Reading D. S., Hallberg R. L., Myers A. M. Characterization of the yeast HSP60 gene coding for a mitochondrial assembly factor. Nature. 1989 Feb 16;337(6208):655–659. doi: 10.1038/337655a0. [DOI] [PubMed] [Google Scholar]
- Schloss J. V., Phares E. F., Long M. V., Norton I. L., Stringer C. D., Hartman F. C. Ribulosebisphosphate carboxylase/oxygenase from Rhodospirillum rubrum. Methods Enzymol. 1982;90(Pt E):522–528. doi: 10.1016/s0076-6879(82)90179-3. [DOI] [PubMed] [Google Scholar]
- Todd M. J., Viitanen P. V., Lorimer G. H. Hydrolysis of adenosine 5'-triphosphate by Escherichia coli GroEL: effects of GroES and potassium ion. Biochemistry. 1993 Aug 24;32(33):8560–8567. doi: 10.1021/bi00084a024. [DOI] [PubMed] [Google Scholar]
- Viitanen P. V., Lorimer G. H., Seetharam R., Gupta R. S., Oppenheim J., Thomas J. O., Cowan N. J. Mammalian mitochondrial chaperonin 60 functions as a single toroidal ring. J Biol Chem. 1992 Jan 15;267(2):695–698. [PubMed] [Google Scholar]
- Viitanen P. V., Lubben T. H., Reed J., Goloubinoff P., O'Keefe D. P., Lorimer G. H. Chaperonin-facilitated refolding of ribulosebisphosphate carboxylase and ATP hydrolysis by chaperonin 60 (groEL) are K+ dependent. Biochemistry. 1990 Jun 19;29(24):5665–5671. doi: 10.1021/bi00476a003. [DOI] [PubMed] [Google Scholar]
- Vodkin M. H., Williams J. C. A heat shock operon in Coxiella burnetti produces a major antigen homologous to a protein in both mycobacteria and Escherichia coli. J Bacteriol. 1988 Mar;170(3):1227–1234. doi: 10.1128/jb.170.3.1227-1234.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeilstra-Ryalls J., Fayet O., Georgopoulos C. The universally conserved GroE (Hsp60) chaperonins. Annu Rev Microbiol. 1991;45:301–325. doi: 10.1146/annurev.mi.45.100191.001505. [DOI] [PubMed] [Google Scholar]
- van der Vies S. M., Viitanen P. V., Gatenby A. A., Lorimer G. H., Jaenicke R. Conformational states of ribulosebisphosphate carboxylase and their interaction with chaperonin 60. Biochemistry. 1992 Apr 14;31(14):3635–3644. doi: 10.1021/bi00129a012. [DOI] [PubMed] [Google Scholar]