Abstract
Background: Surgical site infection (SSI) after spine surgery is classified as a “never event” by the Centers for Medicare and Medicaid. Intra-wound antibiotics (IWA) have been proposed to reduce the incidence of SSI, but robust evidence to support its use is lacking.
Methods: Prospective cohort undergoing spine fusion at 20 Washington State hospitals (July 2011 to March 2014) participating in the Spine Surgical Care and Outcomes Assessment Program (Spine SCOAP) linked to a discharge tracking system. Patient, hospital, and operative factors associated with SSI and IWA use during index hospitalizations through 600 days were analyzed using a random effects logistic model (index), and a time-to-event analysis (follow-up) using Cox proportional hazards.
Results: A total of 9,823 patients underwent cervical (47%) or lumbar (53%) procedures (mean age, 58; 54% female) with an SSI rate of 1.1% during index hospitalization. Those with SSI were older, more often had diabetes mellitus, and more frequently underwent lumbar (versus cervical) fusion compared with those without SSI (all p < 0.01). Unadjusted rates of SSI during index hospitalization were lower in patients who received IWA (0.8% versus 1.5%). After adjustment for patient, hospital, and operative factors, no benefit was observed in those receiving IWA (odds ratio [OR] 0.65, 95% confidence interval [CI]: 0.42–1.03). At 12 mo, unadjusted rates of SSI were 2.4% and 3.0% for those who did and did not receive antibiotics; after adjustment there was no significant difference (hazard ratio [HR] 0.94, 95% CI: 0.62–1.42).
Conclusions: Whereas unadjusted analyses indicate a nearly 50% reduction in index SSI using IWA, we did not observe a statistically significant difference after adjustment. Despite its size, this study is underpowered to detect small but potentially relevant improvements in rates of SSI. It remains to be determined if IWA should be promoted as a quality improvement intervention. Concerns related to bias in the use of IWA suggest the benefit of a randomized trial.
More than 800,000 spinal operations are performed annually in the United States [1,2]. Surgical site infection (SSI) after spine surgery is a rare but devastating event leading to increases in patient morbidity with an estimated cost of $33,000–$100,000 per infection [3,4]. The Center for Medicare and Medicaid Services (CMS) now considers SSI after spine surgery to be a “never event” because it is believed to be largely preventable [5,6]. Large studies reviewing hospital discharge data show that SSI after spine surgery occurs in approximately 2.5% of patients [7], but rates as high as 14% have been reported in the literature [8]. In 2009, The National Healthcare Safety Network reported nationally that SSI occurs in 1.0%, 1.5%, and 3.1% of cases after laminectomy, fusion, and revision fusion, respectively [9].
Whereas certain SSI prevention techniques have high levels of evidence and have achieved broad uptake (e.g., antibiotics on time, maintenance of euthermia and euglycemia), other approaches, such as the use of intra-wound antibiotics (IWA), are supported by less compelling data. Intra-wound antibiotics may alter rates of SSI and are often used in spine surgery patients. The use of IWA involves placing antibiotics, most commonly powdered vancomycin, directly in the surgical incision prior to closure [10] although there does not appear to be a standardized procedure. It has been shown to be a safe intervention, with few adverse events attributable to IWA use [3,10]. The first published use of IWA in spine surgery was in 1996, but it likely has been used for several decades prior to this [11]. Only one randomized controlled trial (RCT) has been performed to date, showing no difference in infection rates between patients who did and did not receive IWA (1.61% versus 1.68%; not significant), although the study was underpowered to detect an effect [12]. Meta-analyses of observational studies combined with data from the one RCT suggest that IWA reduces SSI rates, with pooled ORs of 0.19–0.43 (all p < 0.05) [13–15]. The observation that the effect of IWA on SSI is not observed in the setting of an RCT highlights the possibility of confounding and bias in the way IWA is applied. Other issues related to the existing studies of this issue include a lack of information about long-term SSI. Surgical site infection can occur as late as 12 mo or more after skeletal surgery with implants [16–17] and biofilms that form on implants may contribute to SSI in ways that have not been well assessed [18]. Despite these gaps and the uncertain evidence, clinical guidelines from the North American Spine Society include a recommendation to add IWA “in patients with comorbidities or for those undergoing complicated spine surgery” [19].
We sought to perform an effectiveness evaluation comparing spine surgery patients who did and did not receive IWA in Washington State, monitoring the risk of SSI to see if the effect of IWA changes over time. We hypothesized that the rate of SSI would be lower in patients who received IWA, both during the index hospitalization as well as during the follow-up period
Patients and Methods
Many Washington State hospitals participate in a novel data surveillance and benchmarking network called the Spine Surgical Care and Outcomes Assessment Program (Spine SCOAP), a quality improvement and benchmarking collaborative [20]. Participation in Spine SCOAP is voluntary, but approximately 75% of eligible spinal procedures in Washington State take place within the Spine SCOAP network. The Comparative Effectiveness Research Translation Network (CERTAIN) partners with Spine SCOAP to provide research and analytic support once the data have been collected [21].
All consecutive adult patients undergoing elective cervical or lumbar spinal fusion in Spine SCOAP hospitals between July 2011 and March 2014 were included in this study. Spine SCOAP relies on trained abstractors at each hospital who review clinical records individually to collect data. The data are collected primarily for quality improvement purposes but the protocol used for abstraction is developed prospectively. The clinical records from SCOAP were linked to records in the Washington State Comprehensive Hospital Abstract Reporting System (CHARS) to obtain follow-up rates of SSI during subsequent hospitalizations. The Comprehensive Hospital Abstract Reporting System is an administrative database derived from discharge records from all public and private hospitals in Washington State, excluding Veterans Affairs and U.S. military hospitals. The University of Washington Human Subjects Division and the Washington State Institutional Review Board approved this study.
The exposure of interest was IWA receipt during the time of operation. Use of IWA was abstracted from the medical record and was recorded as a yes/no parameter. The dose, timing, and type of antibiotic were not recorded. Patients were excluded from this analysis if they were missing IWA status (1% of cohort); no patient was missing SSI data. The primary outcome measured was SSI during the index hospitalization, defined by documentation of one of the following: Antibiotics ordered for presumed infection, antibiotics ordered for confirmed infection, or incision re-opening/debridement.
Hospital-specific IWA usage and SSI rates were compared to assess overall trends and association between the two. Descriptive statistics were used to analyze baseline characteristics between individuals who did and did not receive IWA, as well as those who did and did not develop SSI. Included in this analysis were parameters describing patient characteristics (age, gender, race, body mass index [BMI], insurance status); comorbidities (coronary artery disease, hypertension, asthma, diabetes mellitus, sleep apnea, osteoporosis, prior spine surgery, history of infection after prior spine surgery); medications (beta blockers, statin, angiotensin-converting enzyme inhibitors/angiotensin receptor blockers, therapeutic anti-coagulation, steroids, narcotics); operative characteristics (surgical site, surgeon type, length of operation, surgical approach, invasiveness index); SSI prevention metrics (on-time antibiotics, euthermia, glucose monitoring, appropriate use of insulin); and hospital characteristics (hospital type, presence of surgical residency). Surgical site was defined as cervical versus lumbar. The invasiveness index was calculated based on six possible interventions on each operated vertebra: Anterior/posterior decompression, anterior/posterior fusion, and anterior/posterior instrumentation. Each intervention at each level was scored one point and summed. Previous work has shown that increasing levels of invasiveness are associated with risk of SSI in spine surgery patients [22,23]. Surgeon type was coded as neurosurgeon, orthopedic surgeon, or other/missing. Surgical method was defined as “open” versus “minimally invasive.”
Patient characteristics were stratified by both SSI outcomes and receipt of IWA and summarized using frequency distributions for categorical parameters; continuous parameters were summarized using means, medians and standard deviations. Categorical parameters were compared using Pearson χ2 statistic. Continuous parameters were compared using the two-tailed Student t test.
We used multivariable logistic regression with random effects, clustering by hospital site, to estimate the odds of incident SSI for patients who received IWA compared with those who did not. Given the low rate of SSI in the population, we used a stepwise regression method with a priori selection of parameters to select the most important parameters to include in the random effects model. Any parameter with a univariable association probability above 0.2 was not included in our model unless the parameter was known to be clinically significant in the development of SSI. Random effects regression models were used to account for variance because of unmeasured factors within a hospital that may affect patient outcomes systematically. To the extent that this is true, models using random effects are expected to provide more precise outcome estimates for correlated data [24].
A propensity score match was performed as a sensitivity analysis. Patients were assigned a predictive score from zero to one based on their likelihood of receiving IWA. Patients who did and did not receive IWA were then matched 1:1 with their nearest neighbor using calipers of 0.03 width based on their propensity score. Patients who were not matched were not included in the propensity score analysis. Standardized differences were examined for balance in the baseline characteristics between the IWA and matched non-IWA patients. A standardized difference of less than 10% was considered to represent good balance between the two groups [25]. Patients who did and did not receive IWA were then compared with one another to determine rates of SSI, analyzed using two-tailed paired Student t test.
The secondary outcome was SSI rates during the follow-up period, up to 600 days post-operatively, after which there were few patients with complete follow-up data. In addition to the definitions previously described for SSI at index hospitalization, International Statistical Classification of Diseases and Health Related Problems (ICD-9) codes were used to identify SSI occurrences (Appendix A). Kaplan-Meier curves, stratified by IWA receipt, were constructed to compare rates of SSI over time to take into account censoring because of differential follow-up. A Cox proportional hazard model clustered at the hospital level was used to adjust for differences between those who did and did not receive IWA. The model included patient characteristics (age, gender, race, insurance status, BMI, diagnosis, albumin, and diabetes mellitus, American Society of Anesthesia class [ASA] class, smoking status), operative characteristics (indication, surgical method, length of operation), and hospital characteristics (type of hospital, presence of surgical residency). All statistical analyses were performed using Stata version 11.0 (StataCorp, College Station, TX). All tests were two sided and p values <0.05 were considered statistically significant.
Results
Between July 2011 and March 2014, 9,823 patients underwent cervical or lumbar fusion across 20 hospitals. The mean age of subjects was 58 y (standard deviation [SD] 12.8), 54% were female. Overall, 55% of patients received IWA, but this varied widely across hospitals (range, 10%–98%). Surgical volume varied across hospitals (range, 5–1,512 cases).
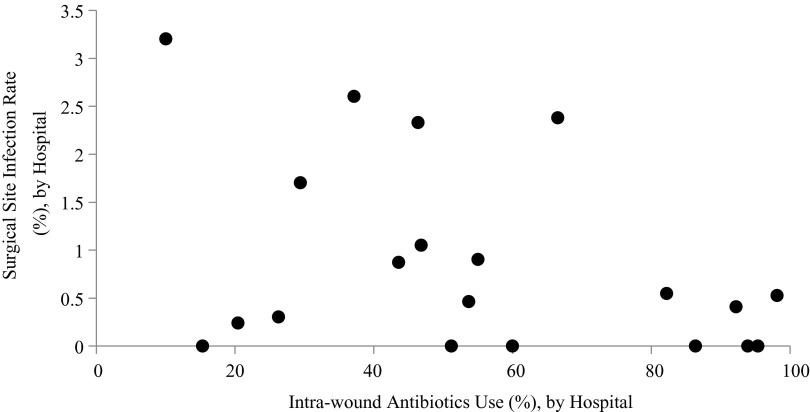
Overall, 111 (1.1%) patients developed SSI. The rate of SSI in patients who did and did not receive IWA was 0.8% and 1.5%, respectively (p < 0.01). Rates of SSI varied by hospital (range 0 to 3.2%) but there was no association between IWA use and SSI at the hospital level (p = 0.08) or number of procedures performed and SSI rate (p = 0.10) (Fig. 1).
FIG. 1.
Rates of intra-wound antibiotics (IWA) use and surgical site infection across 20 Spine Surgical Care and Outcomes Assessment Program (SCOAP) hospitals. No significant association was found between rates of IWA use at the hospital level and rates of SSI (p = 0.08).
Patients with SSI were older (64 versus 58); had greater BMI (32 versus 30); were less often white (78% versus 87%); more frequently had albumin ≤3.5 mg/dL (11% versus 3%); more often had diabetes mellitus (32% versus 17%) and hypertension (69% versus 52%); more frequently used beta blockers (32% versus 21%); more frequently underwent a lumbar procedure (76% versus 52%) versus a cervical procedure and had operative duration longer than four hours (42% versus 20%); and more frequently were treated at an academic hospital (90% versus 66%) (all p < 0.01). There were no differences with regard to gender, insurance status, smoking status, rates of degenerative disk disease, prior surgery, asthma, sleep apnea, coronary artery disease, previous SSI, use of home statins, angiotensin-converting enzyme inhibitors/angiotensin receptor blockers use, therapeutic anticoagulation, steroid use, or baseline narcotic use. Infection prophylaxis measures such as maintenance of euglycemia, euthermia, and timely administration of prophylactic antibiotics were similar between the two groups, as were operative characteristics such as approach (open versus laparoscopic), invasiveness index, and surgeon type (Table 1).
Table 1.
Patient, Operative, and Hospital Characteristics Associated with Surgical Site Infection during Index Hospitalization
| No surgical site infection | Surgical site infection | ||||
|---|---|---|---|---|---|
| n = 9,712 | n = 111 | ||||
| Count | % | Count | % | p | |
| Patient characteristics | |||||
| Mean age (years) | 58 | 64 | <0.01a | ||
| Male | 4,497 | 46% | 44 | 40% | 0.16b |
| Mean body mass index (kg/m2) | 30 | 32 | 0.01 | ||
| Race: White | 8,497 | 87% | 87 | 78% | <0.01 |
| Private Insurance | 6,677 | 69% | 72 | 65% | 0.39 |
| Current smoker | 8,730 | 20% | 98 | 15% | 0.35 |
| Low albumin (≤3.5 mg/dL) | 301 | 3% | 12 | 11% | <0.01 |
| Comorbid conditions | |||||
| Degenerative disk disease | 2,213 | 23% | 21 | 19% | 0.34 |
| Prior surgery | 1,643 | 17% | 21 | 19% | 0.58 |
| Hypertension | 5,080 | 52% | 77 | 69% | <0.01 |
| Diabetes mellitus | 1,651 | 17% | 36 | 32% | <0.01 |
| Asthma | 1,392 | 14% | 20 | 18% | 0.26 |
| Sleep apnea | 1,516 | 16% | 21 | 19% | 0.32 |
| Coronary artery disease | 828 | 9% | 14 | 13% | 0.12 |
| SSIc after previous spine surgery | 53 | 1% | 2 | 2% | 0.08 |
| Osteoporosis | 201 | 2% | 8 | 8% | <0.01 |
| Home medications | |||||
| Statin | 3,085 | 32% | 43 | 39% | 0.12 |
| Beta blocker | 2,005 | 21% | 36 | 32% | <0.01 |
| ACE-Ic or ARBd | 3,252 | 33% | 46 | 41% | 0.08 |
| Therapeutic anticoagulation | 253 | 3% | 6 | 5% | 0.07 |
| Steroids | 367 | 4% | 2 | 2% | 0.28 |
| Narcotics | 5,245 | 54% | 57 | 51% | 0.58 |
| Infection prophylaxis measures | |||||
| Maintenance of euglycemia | |||||
| Insulin started for hyperglycemiae | 211 | 45% | 4 | 31% | 0.31 |
| Glucose tested among patients with diabetes mellitus | 1,169 | 71% | 29 | 81% | 0.21 |
| On-time antibioticsf | 5,125 | 99% | 66 | 100% | 0.52 |
| Euthermia (36+) | 8,902 | 97% | 104 | 98% | 0.56 |
| Operative/hospital characteristics | |||||
| Open surgical approach | 9,139 | 94% | 101 | 91% | 0.16 |
| Invasiveness indexg | 9.13 | 9.88 | 0.06 | ||
| Anatomic location | |||||
| Cervical | 4,619 | 48% | 27 | 24% | <0.01 |
| Lumbar | 5,093 | 52% | 84 | 76% | |
| Surgeon type | |||||
| Neurosurgeon | 5,254 | 54% | 51 | 46% | 0.15 |
| Orthopedic surgeon | 4,377 | 45% | 58 | 52% | |
| Unknown | 81 | 1% | 2 | 2% | |
| Operation duration (hours) % | |||||
| ≤ 2 | 3,403 | 35% | 15 | 14% | <0.01 |
| 2–4 | 4,325 | 45% | 49 | 44% | |
| >4 | 1,944 | 20% | 47 | 42% | |
| Academic hospital | 6,369 | 66% | 100 | 90% | <0.01 |
Comparison using Student t test for continuous variables.
Comparison of patients who did and did not receive intra-wound antibiotics using χ2 tests for heterogeneity unless otherwise indicated.
Hyperglycemia defined as >180 mg/dL.
This number describes the proportion of patients who received on-time antibiotics, among those who had this recorded. This was not included as a metric in 2013.
Based on number of instrumentation levels, decompressive levels and fusion levels.
SSI = surgical site infection; ACE- I = angiotensin converting enzyme inhibitor; ARB = antiogensin II receptor blockers.
Patients who received IWA more frequently had private insurance (71% versus 67%); more often were treated by a neurosurgeon (63% versus 44%); had lower rates of degenerative disk disease (21% versus 24%), prior surgery (16% versus 18%), and smoking (19% versus 22%); had lower rates of sleep apnea (14% versus 18%); and had greater rates of baseline narcotic use (57% versus 50%) (all p < 0.01). Although statistically significant, BMI was similar between the two groups (30.2 versus 29.8, p < 0.01). There were no significant differences with regards to age, gender, race, albumin status, hypertension, diabetes mellitus, asthma, previous SSI, and use of non-narcotic home medications between the two groups. Patients who received IWA less frequently had glucose tested among patients with diabetes mellitus (67% versus 76%, p < 0.01) but were otherwise similar with regards to maintenance of euthermia and on-time antibiotics. There were no differences with regards to invasiveness index or anatomic location (Table 2).
Table 2.
Patient, Operative, and Hospital Characteristics Associated with Receipt of Intra-Wound Antibiotics during their Initial Operation
| No intra-wound antibiotics | Intra-wound antibiotics | ||||
|---|---|---|---|---|---|
| n = 4,464 | n = 5,359 | ||||
| Count | % | Count | % | p | |
| Patient characteristics | |||||
| Mean age | 58 | 58 | 0.80a | ||
| Male | 2,084 | 47% | 2457 | 46% | 0.41b |
| Mean body mass index (kg/m2) | 29.8 | 30.2 | <0.01 | ||
| Race: White | 3,870 | 87% | 4,714 | 88% | 0.06 |
| Private insurance | 2,970 | 67% | 3,779 | 71% | <0.01 |
| Current smoker | 3,995 | 22% | 4,833 | 19% | <0.01 |
| Low albumin (≤3.5 mg/dL) | 133 | 3% | 180 | 3% | 0.29 |
| Comorbid conditions | |||||
| Degenerative disk disease | 1,093 | 24% | 1,141 | 21% | <0.01 |
| Prior surgery | 807 | 18% | 857 | 16% | <0.01 |
| Hypertension | 2,365 | 53% | 2,792 | 52% | 0.38 |
| Diabetes | 778 | 17% | 909 | 17% | 0.55 |
| Asthma | 653 | 15% | 759 | 14% | 0.51 |
| Sleep apnea | 789 | 18% | 748 | 14% | <0.01 |
| Coronary artery disease | 412 | 9% | 430 | 8% | 0.04 |
| SSI after previous spine surgery | 29 | 1% | 26 | 0% | 0.28 |
| Osteoporosis | 111 | 3% | 98 | 2% | <0.01 |
| Home medications | |||||
| Statin | 1,412 | 32% | 1,716 | 32% | 0.68 |
| Beta blocker | 942 | 21% | 1,099 | 21% | 0.46 |
| ACE-I or ARB | 1,479 | 33% | 1,819 | 34% | 0.41 |
| Therapeutic anticoagulation | 119 | 3% | 140 | 3% | 0.87 |
| Steroids | 178 | 4% | 191 | 4% | 0.28 |
| Narcotics | 2,246 | 50% | 3,056 | 57% | <0.01 |
| Infection prophylaxis measures | |||||
| Maintenance of euglycemia | |||||
| Insulin started for hyperglycemiac | 110 | 45% | 105 | 44% | 0.93 |
| Glucose tested among diabetics | 588 | 76% | 610 | 67% | <0.01 |
| On-time antibioticsd | 2,289 | 99% | 2,902 | 99% | 0.38 |
| Euthermia (36+) | 4,188 | 97% | 4,818 | 97% | 0.73 |
| Operative/hospital characteristics | |||||
| Open surgical approach | 4,171 | 94% | 5,069 | 95% | 0.03 |
| Invasiveness indexe | 9.11 | 9.16 | 0.54 | ||
| Anatomic location | |||||
| Cervical | 2,146 | 48% | 2,500 | 47% | 0.16 |
| Lumbar | 2,318 | 52% | 2,859 | 53% | |
| Surgeon type | |||||
| Neurosurgeon | 1,940 | 44% | 3,365 | 63% | <0.01 |
| Orthopedic surgeon | 2,496 | 56% | 1,939 | 36% | |
| Unknown | 28 | 1% | 55 | 1% | |
| Operation duration (hours) | |||||
| ≤2 | 1,449 | 33% | 1,969 | 37% | <0.01 |
| 2–4 | 1,929 | 43% | 2,445 | 46% | |
| >4 | 1,070 | 24% | 921 | 17% | |
| Academic hospital | 3,224 | 72% | 3,245 | 61% | <0.01 |
Comparison using Student t test for continuous variables.
Comparison of patients who did and did not receive intra-wound antibiotics using χ2 tests for heterogeneity unless otherwise indicated.
Hyperglycemia defined as >180 mg/dL.
This number describes the proportion of patients who received on-time antibiotics, among those who had this recorded. This was not included as a metric in 2013.
Based on number of instrumentation levels, decompressive levels and fusion levels.
SSI = surgical site infection; ACE- I = angiotensin converting enzyme inhibitor; ARB = antiogensin II receptor blockers.
After adjustment for patient, operative, and hospital characteristics in the random effects model, IWA was associated with a non-significant 35% reduction in SSI (OR 0.65, 95% CI: 0.42, 1.03). Risk factors for SSI included increasing age (OR 1.03, 95% CI 1.01, 1.05) albumin ≤3.5 mg/dL (OR 2.08, 95% CI: 1.04, 4.17), diabetes mellitus (OR 1.68, 95% CI: 1.06, 2.67), operation time longer than four hours (OR 2.65, 95% CI 1.23, 5.74), and treatment at an academic hospital (OR 2.63, 95% CI: 1.14, 6.08). Gender, race, insurance status, smoking status, BMI, surgical location, surgical approach, ASA class, presence of a surgical residency program, and invasiveness index score were not associated with increased SSI risk (Table 3).
Table 3.
Odds Ratios (Index Hospitalization) and Hazard Ratio (Follow-Up Period) for Incidence of Surgical Site Infection
| Variable | Unadjusted odds ratio | 95% CI | Adjusted odds ratio | 95% CI | Hazard ratio | 95% CI |
|---|---|---|---|---|---|---|
| Intra-wound antibiotics | 0.67 | 0.44–1.03 | 0.65 | 0.42–1.03 | 0.94 | 0.62–1.42 |
| Age | 1.04 | 1.02–1.05 | 1.03 | 1.01–1.05 | 1.02 | 1.01–1.04 |
| Male | 0.74 | 0.51–1.08 | 0.68 | 0.44–1.04 | 0.78 | 0.59–1.04 |
| Race: White | 0.68 | 0.42–1.08 | 0.65 | 0.39–1.10 | 0.82 | 0.52–1.04 |
| Current smoker | 0.77 | 0.45–1.30 | 1.08 | 0.60–1.94 | 1.42 | 1.13–1.80 |
| Private insurance | 0.85 | 0.57–1.27 | 0.87 | 0.56–1.36 | 0.70 | 0.52–0.93 |
| BMI >30 | 1.45 | 0.99–2.11 | 1.35 | 0.87–2.10 | 1.31 | 1.02–1.69 |
| Albumin <3.0 | 3.62 | 1.98–6.60 | 2.08 | 1.04–4.17 | 2.39 | 1.23–4.64 |
| Diabetes mellitus | 2.32 | 1.56–3.45 | 1.68 | 1.06–2.67 | 1.15 | 0.80–1.65 |
| Prior surgery | 1.08 | 0.67–1.75 | 0.82 | 0.48–1.40 | 0.81 | 0.55–1.19 |
| Cervical sitea | 0.41 | 0.26–0.63 | 0.68 | 0.41–1.13 | 0.54 | 0.39–0.75 |
| Operating time (hours) | ||||||
| <2 | Ref | Ref | Ref | Ref | Ref | Ref |
| 2–4 | 1.96 | 1.13–3.40 | 1.94 | 0.98–3.83 | 1.67 | 1.07–2.59 |
| >4 | 3.83 | 2.14–6.86 | 2.65 | 1.23–5.74 | 2.12 | 1.51–2.97 |
| ASA3 class | ||||||
| 1 | Ref | Ref | Ref | Ref | Ref | Ref |
| 2 | 1.99 | 0.48–8.27 | 1.91 | 0.26–14.28 | 4.37 | 0.82–23.20 |
| 3 | 4.84 | 1.17–19.93 | 3.05 | 0.40–23.19 | 6.39 | 0.97–42.01 |
| 4 | 15.96 | 3.11–81.78 | 7.67 | 0.80–73.78 | 10.59 | 1.47–76.52 |
| Academic hospital | 5.11 | 1.84–14.20 | 2.63 | 1.14–6.08 | 2.25 | 1.45–3.49 |
Versus lumbar site.
CI = confidence interval; BMI = body mass index, kg/m2; ASA = American Society of Anesthesiologists class.
Propensity score matching found 6,910 patients who did and did not receive IWA with similar characteristics (3,455 in each cohort). After matching, the only significant differences between the two groups were surgeon and hospital type: Patients who received IWA were more likely to be treated by an orthopedic surgeon (50% versus 47%, p = 0.02) or at a hospital with a surgical residency program (30% versus 28%, p = 0.04). We did not observe a statistically significant difference in rates of SSI between propensity score matched patients who did and did not receive IWA (0.93% versus 1.30%, p = 0.14).
The secondary analysis of rates of SSI during the follow-up period included 7,179 patients because we were limited to evaluation of those patients with data linkable to CHARS. At 30 d post-operatively, patients who received IWA had non-significantly lower rates of SSI compared with those who did not (1.8% versus 2.4%, p = 0.09). These trends continued at 90 d (2.2% versus 2.8%, p = 0.06), 180 d (2.2% versus 2.9%, p = 0.06), and 600 d (2.7% versus 3.6%, p = 0.06). After adjustment for patient, operative, and hospital characteristics in the Cox proportional hazard model we did not observe a difference between the two groups. The hazard of SSI was 0.94 (95% CI 0.62–1.42) for patients who received IWA compared with those who did not.
Discussion
Surgical site infection after spine fusion is a challenging but avoidable complication to patients and the health care system. Recent payment policy changes have increased pressure on hospitals to utilize risk-reducing interventions for SSI, but the evidence base for SSI prevention in spine surgery is limited. In this study of patients in Spine SCOAP, those undergoing cervical or lumbar fusion in Washington State had an overall SSI rate of 1.1%. Whereas unadjusted rates of SSI were lower in patients who received IWA, those who received IWA were also at lower risk for SSI development prior to surgery. After adjustment for patient, hospital, and operative factors, the odds of SSI and rates of SSI in patients with IWA were not statistically lower than in patients who did not receive IWA. Of particular note, especially given the interest in hospital-level quality improvement interventions, we did not find that the increased use of IWA within hospitals was associated with lower rates of SSI. Both groups of patients also demonstrated increasing rates of SSI outside of the 30-d time frame, with approximately 80% of infections occurring by 90 d and the residual by 12 mo. This suggests that the 30-d window included in many SSI assessment schemes may be inadequate in assessing SSI after spine surgery.
Although SSI after spine surgery is an infrequent event, it is recognized as a substantial problem. Surgical site infection can predispose susceptible patients to failure of fusion, sometimes requiring revision instrumentation procedures many years after the index operation [26]. Furthermore, calculating precise rates of SSI in the population at large can be challenging. Using billing claims that are of questionable validity for certain diagnoses [27], investigators using the Healthcare Cost and Utilization Project found rates of SSI in spine surgery patients to be 2.5% (as defined by a single ICD-9 code of 998.59), leading to substantially greater costs and length of stay [7]. Although limited data exist, the cost of SSI after traumatic or elective spine surgery costs between $33,000 and $100,000, respectively [3,4]. If there are 800,000 spine procedures each year [2], the impact on the health care system could be as high as $660 million to $2 billion annually.
Previous research on the effectiveness of IWA has shown a mixed effect with overall benefit among pooled results from observational, uncontrolled trials and one underpowered RCT [12–15]. The additional morbidity associated with SSI, along with the high cost associated with SSI, may explain why the use of IWA is so common (55% within Spine SCOAP). It is also unclear whether surgeons who use IWA incorporate it as standard practice, or whether they select patients at greater risk for IWA use. We found substantial differences between IWA and non-IWA patients that would suggest differential use, but the patients receiving IWA were surprisingly more likely to have attributes that placed them at lower risk for SSI, such as greater rates of private insurance, lower rates of smoking, and lower rates of prior surgery. This is contrary to the recommendations from the North American Spine Society [19] and may reflect surgeon or site preference, rather than patient risk stratification. These risk factors need to be accounted for when comparing the rates of SSI, and ideally controlled for in a large randomized trial. The size of such a trial makes it a challenge to fund and organize, but it is necessary because SSI occurs so infrequently. Another complicating issue of a trial of IWA for SSI is that the time window for evaluation of SSI needs to extend beyond the convenient window of 30 d post-operatively that is typically tracked. This study found that the effects of IWA do not appear to vary over time but that complete capture of SSI rates is dependent on having an adequate follow-up period.
This study has several limitations. One consideration is that we did not record the specific antibiotic used, timing of the use during the operation, dose, volume, and location of IWA use. A more complete analysis would discuss the variability in dose, volume, and location between surgeons and centers. While 1 g of vancomycin powder is the antibiotic and dose reported most commonly in the literature [12–15] it is possible that other antibiotics are used within the Spine SCOAP network that may affect our study outcomes. A second limitation is that the definition of SSI used for this analysis may not reflect accurately the true rates of SSI. In-hospital assessments of SSI in Spine SCOAP follow closely, but are not identical, to those used by the U.S. Centers for Disease Control. The definition of SSI that occurred after the index hospitalization may be even more problematic because the sensitivity of ICD-9 diagnostic code schema for SSI in spine patients has not been validated. Some SSIs may not be treated as in-patients and will have been missed by this approach. Some of the hospitalizations with the ICD-9 diagnostic codes used in this schema may reflect other SSIs unrelated to the index surgery. To account for this we did perform a sensitivity analysis using a restricted set of diagnostic codes and found similar rates of SSI. This study is also underpowered to detect the 30% reduction in SSI that we observed, which may be significant clinically. To detect accurately a 30% decrease in baseline SSI rate (1.1% to 0.77%), we would need a study with more than 36,000 patients. Last, the time-to-event analysis was restricted to a subset of patients with data linkable to CHARS and may introduce bias that is difficult to assess.
In conclusion, this study did not observe differences in adjusted rates of SSI between patients who did and did not receive IWA. However, this study is underpowered to detect small but potentially important differences in SSI rates. There were substantial differences in the types of patients receiving IWA, the clinicians using IWA, and the centers where procedures in which IWA was used are performed. Future studies should be randomized and should characterize the dose, timing, and type of antibiotic use, as well as patient characteristics related to effectiveness of IWA. This is especially the case given the importance of this problem, the perception of benefit of IWA, and the increasing use of this practice for the growing numbers of patients undergoing spine surgery around the world.
Appendix A:
ICD-9 Codes Used for Surgical Site Infection Definition
| Code | Definition |
|---|---|
| 996.60 | Infection and inflammatory reaction due to internal prosthetic device, implant, and graft. |
| 996.67 | Infection and inflammatory reaction due to other internal orthopedic device, implant, and graft. |
| 998.30 | Disruption of wound, unspecified. |
| 998.31 | Disruption of internal surgical wound (fascia, muscle). |
| 998.32 | Disruption of external surgical wound (subcutaneous/skin layer). |
| 998.5 | Postoperative infection not elsewhere classified. |
| 998.51 | Infected postoperative seroma. |
| 998.59 | Other postoperative infection. |
| 998.6 | Persistent postoperative fistula. |
| 998.83 | Non-healing surgical wound. |
Acknowledgments
Dr. Ehlers was supported by a training grant from the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health under Award Number T32DK070555. Research reported in this publication was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Number R21AR068632. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Weiss AJ, Elixhauser A. Trends in Operating Room Procedures in U.S. Hospitals, 2001–2011: Statistical Brief #171. Rockville, MD: Healthcare Cost and Utilization Project (HCUP) Statistical Briefs, 2006 [Google Scholar]
- 2.Lee J. Rethinking spine care: Some health systems are moving beyond surgery in serving back pain patients. March 22, 2014. www.modernhealthcare.com/article/20140322/MAGAZINE/303229985 (Last accessed April8, 2015)
- 3.Godil SS, Parker SL, O'Neill KR, et al. . Comparative effectiveness and cost-benefit analysis of local application of vancomycin powder in posterior spinal fusion for spine trauma: Clinical article. J Neurosurg Spine 2013;19:550–555 [DOI] [PubMed] [Google Scholar]
- 4.Calderone RR, Garland DE, Capen DA, Oster H. Cost of medical care for postoperative spinal infections. Orthop Clin North Am 1996;27:171–182 [PubMed] [Google Scholar]
- 5.Miller A. Hospital reporting and “never events.” Medicare Patient Management 2009(May-Jun);4:20 www.medicarepatientmanagement.com/issues/04-03/mpmMJ09-NeverEvents.pdf (Last accessed April8, 2015) [Google Scholar]
- 6.Milstein A. Ending extra payments for “never events”—Stronger incentives for patients' safety. N Engl J Med 2009;360:2388. [DOI] [PubMed] [Google Scholar]
- 7.de Lissovoy G, Fraeman K, Hutchins V, et al. . Surgical site infection: Incidence and impact on hospital utilization and treatment costs. Am J Infect Control 2009;37:387–397 [DOI] [PubMed] [Google Scholar]
- 8.Radcliff KE, Neusner AD, Millhouse PW, et al. . What is new in the diagnosis and prevention of spine surgical site infections. Spine J 2015;15:336–347 [DOI] [PubMed] [Google Scholar]
- 9.Edwards JR, Peterson KD, Mu Y, et al. . National Healthcare Safety Network (NHSN) report: Data summary for 2006 through 2008, issued December 2009. Am J Infect Control 2009;37:783–805 [DOI] [PubMed] [Google Scholar]
- 10.Molinari RW, Khera OA, Molinari WJ, 3rd., Prophylactic intraoperative powdered vancomycin and postoperative deep spinal wound infection: 1,512 consecutive surgical cases over a 6-year period. Eur Spine J 2012;21(Suppl 4):S476–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rimoldi RL, Haye W. The use of antibiotics for wound prophylaxis in spinal surgery. Orthop Clin North Am 1996;27:47–52 [PubMed] [Google Scholar]
- 12.Tubaki VR, Rajasekaran S, Shetty AP. Effects of using intravenous antibiotic only versus local intrawound vancomycin antibiotic powder application in addition to intravenous antibiotics on postoperative infection in spine surgery in 907 patients. Spine 2013;38:2149–2155 [DOI] [PubMed] [Google Scholar]
- 13.Bakhsheshian J, Dahdaleh NS, Lam SK, et al. . The use of vancomycin powder in modern spine surgery: Systematic review and meta-analysis of the clinical evidence. World Neurosurg 2015;83:816–823 [DOI] [PubMed] [Google Scholar]
- 14.Evaniew N, Khan M, Drew B, et al. . Intrawoundvancomycin to prevent infections after spine surgery: A systematic review and meta-analysis. Eur Spine J 2015;24:533–542 [DOI] [PubMed] [Google Scholar]
- 15.Khan NR, Thompson CJ, DeCuypere M, et al. . A meta-analysis of spinal surgical site infection and vancomycin powder. J Neurosurg Spine 2014;21:974–983 [DOI] [PubMed] [Google Scholar]
- 16.Viola RW, King HA, Adler SM, Wilson CB. Delayed infection after elective spinal instrumentation and fusion. A retrospective analysis of eight cases. Spine 1997;22:2444–2450 [DOI] [PubMed] [Google Scholar]
- 17.Bas T, Bas P, Blasco A, Bas JL. Chronic infections of the spine. Eur J Orthop Surg Traumatol 2013;23(Suppl 1):S35–40 [DOI] [PubMed] [Google Scholar]
- 18.Goodman SB, Yao Z, Keeney M, Yang F. The future of biologic coatings for orthopaedic implants. Biomaterials 2013;34:3174–3183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shaffer WO, Baisden JL, Fernand R, et al. . An evidence-based clinical guideline for antibiotic prophylaxis in spine surgery. Spine J 2013;13:1387–1392 [DOI] [PubMed] [Google Scholar]
- 20.Lee MJ, Shonnard N, Farrokhi F, et al. . The Spine Surgical Care and Outcomes Assessment Program (Spine SCOAP): A surgeon-led approach to quality and safety. Spine 2015;40:332–341 [DOI] [PubMed] [Google Scholar]
- 21.Devine EB, Alfonso-Cristancho R, Devlin A, et al. . A model for incorporating patient and stakeholder voices in a learning health care network: Washington State's Comparative Effectiveness Research Translation Network. J ClinEpidemiol 2013;66(8 Suppl):S122–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cizik AM, Lee MJ, Martin BI, et al. . Using the spine surgical invasiveness index to identify risk of surgical site infection: A multivariate analysis. J Bone Joint Surg Am 2012;94:335–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mirza SK, Deyo RA, Heagerty PJ, et al. . Towards standardized measurement of adverse events in spine surgery: Conceptual model and pilot evaluation. BMC Musculoskelet Disord 2006;7:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hu FB, Goldberg J, Hedeker D, et al. . Comparison of population-averaged and subject-specific approaches for analyzing repeated binary outcomes. Am J Epidemiol 1998;147:694–703 [DOI] [PubMed] [Google Scholar]
- 25.Austin PC, Mamdani MM, Stukel TA, et al. . The use of the propensity score for estimating treatment effects: Administrative versus clinical data. Stat Med 2005;24:1563–1578 [DOI] [PubMed] [Google Scholar]
- 26.Weiss LE, Vaccaro AR, Scuderi G, et al. . Pseudarthrosisafter postoperative wound infection in the lumbar spine. J Spinal Disord 1997;10:482–487 [PubMed] [Google Scholar]
- 27.Schoenman J, Sutton J, Kintala S, Love D, Maw R. The Value of Hospital Discharge Databases. 2005. www.hcup-us.ahrq.gov/reports/final_report.pdf (Last accessed April9, 2015)