Abstract
Background: Multiple organ dysfunction (MOD) has plagued intensive care units (ICUs) for more than four decades, and its epidemiology has evolved because more patients are surviving previously lethal insults. Over the years, different predominant phenotypes of MOD have been described, all of which have consumed tremendous healthcare resources and have been associated with prolonged ICU stays and prohibitive mortality rates.
Methods: Review of the English-language literature.
Results: By the 1990s, it became widely accepted that MOD could ensue after both infectious and non-infectious insults by what appeared to be a similar auto-destructive systemic inflammatory response. A 1996 analysis recognized that MOD was a bimodal phenomenon. As a result of years of implementation efforts, fewer patients died of early fulminant sepsis, and those who developed MOD survived hospitalization. Unfortunately, a substantial portion of these patients enter a state of persistent inflammation, immunosuppression, and catabolism (PICS) marked by persistent loss of lean body mass with failure to rehabilitate, sepsis recidivism necessitating re-hospitalization, increasing functional dependence, and an indolent path to death.
Conclusion: Unfortunately, as our population ages and peri-operative care improves, PICS will become an insurmountable epidemic. We believe PICS is the next horizon in surgical critical care and have developed a program to study the pathogenesis and novel therapies for this vexing problem.
William A. Altemeier, MD, was a godfather of academic surgery and one of the founders of the Surgical Infection Society. He pioneered the understanding of the epidemiology and pathophysiology of surgical infections with a special interest in intra-abdominal infection (IAI) and its microbiology. Having received countless honors, accolades, and awards for research and service, Dr. Altemeier understood the importance of translational research in improving medical care. With this in mind, a multidisciplinary research team was developed at the University of Florida Sepsis and Critical Illness Research Center (UF SCIRC) to pursue a recently funded NIGMS P-50 program project grant entitled “PICS: A New Horizon for Surgical Critical Care.”
The purpose of this lecture is to discuss the evolving epidemiology of multiple organ dysfunction (MOD) into the persistent inflammation immunosuppression and catabolism syndrome (PICS), discuss PICS as the new paradigm for MOD, and provide an overview of the research focus of UF SCIRC. We believe these concepts are noteworthy because PICS has become a predominant MOD phenotype and represents the next challenging horizon in surgical critical care.
The Evolving Epidemiology of MOD to PICS
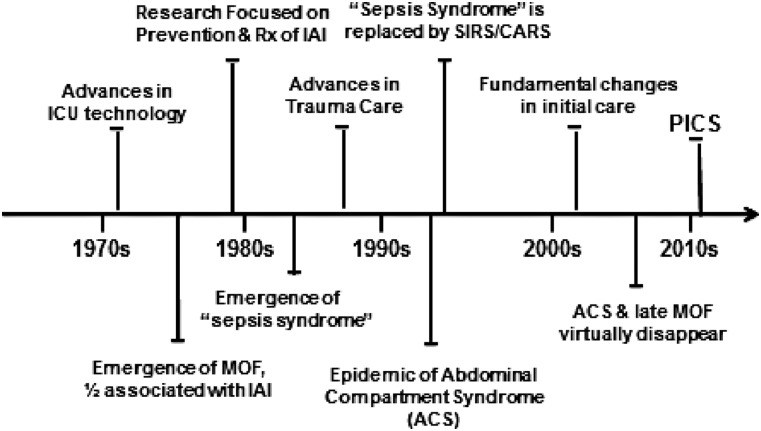
Multiple organ dysfunction has plagued intensive care units (ICUs) for more than four decades, and its epidemiology has evolved because of research and advances in care that have allowed patients to survive previously lethal insults. Over the years, different predominant phenotypes of MOD have been described. Some have come and gone, but all have consumed tremendous healthcare resources and have been associated with prolonged ICU stays and prohibitive mortality rates (Fig. 1) [1].
FIG. 1.
Evolution of multiple organ dysfunction (MOD) resulting in persistent inflammation, immunosuppression, catabolism syndrome (PICS) as a new phenotype. Abbreviations: CARS = compensatory anti-inflammatory response syndrome; IAI = intra-abdominal infection; SIRS = systemic inflammatory response syndrome.
In the mid-1970s, widespread introduction of advanced ICU care allowed patients to survive single organ dysfunction, only to develop MOD. In the late 1970s, seminal reports from inner city U.S.A. “Knife and Gun Clubs” concluded that MOD was a “fatal expression of uncontrolled infection.” Because of a predominance of penetrating trauma in the study populations, IAIs were found in more than 50% of cases, and “blind laparotomy” became a common practice to diagnose and treat IAI before MOD became an irreversible condition.
The concern over IAI focused research efforts in the 1980s including: (1) Optimal peri-operative and therapeutic antibiotics; (2) better surgical technique carried out by dedicated emergency surgeons; (3) early enteral nutrition (EEN); (4) improved computed tomographic (CT) scanning for early diagnosis; and (5) increased use of interventional radiology methods [2–6]. By the 1990s, although IAI continued to occur after penetrating trauma, it disappeared as a common inciting event for MOD.
In the mid-1980s, studies from Europe increasingly reported that MOD was occurring after severe blunt trauma, but curiously, in many cases, there was no inciting infection. The term “sepsis syndrome” was coined to describe MOD patients who appeared to be septic but had no identifiable site of infection. It became widely accepted that MOD could ensue after both infectious and non-infectious insults by what appeared to be a similar auto-destructive systemic inflammatory response, which, if unabated, resulted in MOD and death [7–9].
Although several potential pathologic mechanisms were proposed and studied, bacterial translocation garnered the most attention and provided the mechanism (although the initial view was too simple) for a variety of ICU maladies and explained the beneficial effects of different gut-directed therapies in reducing nosocomial infections (including sucrafate stress gastritis prophylaxis, selective gut decontamination, and EEN) [10–18]. Although research efforts at reducing IAI and defining the mechanism(s) of sepsis syndrome continued throughout the 1980s, tremendous advances in the care of severe trauma were being implemented widely, including: (1) Trauma systems; (2) advanced trauma life support; (3) damage control surgery; and (4) surgical critical care. As a result, by the early 1990s, fewer patients exsanguinated in the operating room, but disturbingly, larger numbers developed abdominal compartment syndrome (ACS) soon after ICU admission.
By the mid-1990s, this new MOD phenotype emerged as an epidemic in trauma centers worldwide [19–21]. As clinicians were trying to understand and decrease the risk of ACS, on the research side, the concept “sepsis syndrome” was replaced by the systemic inflammatory response syndrome/compensatory anti-inflammatory response syndrome (SIRS/CARS) paradigm. In part, this was secondary to translational research at the University of Colorado. A 1996 analysis of the prospective Denver MOD database recognized that MOD was a bimodal phenomenon [22]. An initial severe insult could cause early MOD (∼one-third of cases). This was followed by a relatively quiescent period until late infections began to occur, which appeared to precipitate a second peak in MOD (∼two-thirds of cases). On the basis of contemporary immunologic insights, it was proposed that the initial insult induced a pro-inflammatory systemic inflammatory response syndrome (SIRS) mediated by increased innate immunity activity (principally by neutrophils). If this reaction was severe enough, early MOD ensued [23,24]. In an attempt to protect against auto-destructive SIRS pro-inflammation, the body induced CARS, mediated by decreased adaptive immunity (principally through lymphocytes), which set the stage for nosocomial infections and late MOD.
For the next decade, SIRS/CARS remained the dominant paradigm to explain MOD pathogenesis. In the 1990s, researchers performed numerous clinical trials testing a host of early anti-inflammatory interventions to counter SIRS. However, out of frustration over repeated negative results, they shifted their focus to interventions to modulate CARS to prevent late nosocomial infections. Unfortunately, these efforts proved ineffective. Into the mid-2000s, CARS remained a nebulous concept, and basic immunologists focused their research efforts on characterizing the multiple defects in adaptive immunity that defined it.
While these research efforts were being directed at the SIRS/CARS paradigm, tremendous advances in the clinical care of trauma patients were occurring on two fronts. First, there were fundamental changes in the initial care of patients arriving with severe bleeding that were directed at minimizing the risk of ACS, including (1) Massive transfusion protocols; (2) more rapid hemorrhage control; (3) limiting early crystalloid resuscitation in the emergency department; and (4) less aggressive goal-oriented ICU resuscitation [25]. Second, in high-performing trauma centers, there was a concerted effort to implement evidence-based standard operating procedures (SOPs) to avoid harmful iatrogenic care, including: (1) High tidal-volume mechanical ventilation; (2) liberal blood transfusions; (3) early TPN; and (4) intermittent dialysis, and to decrease the risk of late nosocomial infections, including: (1) Hand washing; (2) full sterile precautions; (3) ventilator bundle; and (4) antibiotic protocols. As a result, ACS and the second peak in late MOD deaths after severe trauma virtually disappeared.
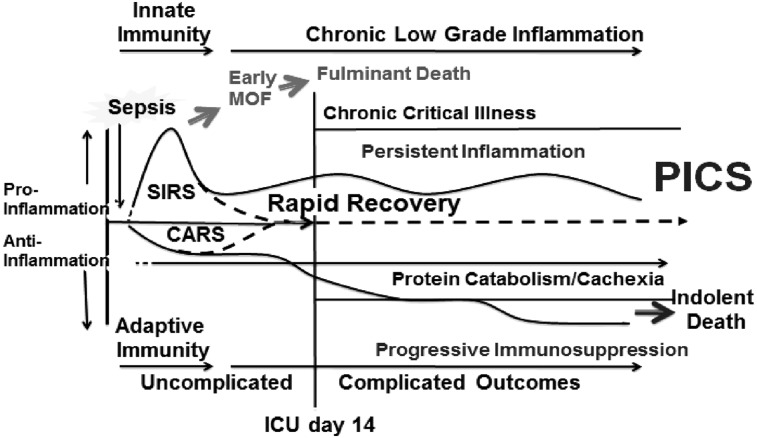
Over this same time period, however, there was a tremendous increase in the occurrence of severe sepsis/septic shock (SS/SS) in non-trauma hospitalized patients. It was recognized that SS/SS was a disease of the elderly, the diagnosis of sepsis was frequently delayed, and sepsis-directed SOPs were delivered haphazardly. Thus, SS/SS became the leading cause of prolonged ICU stays and had a persistent prohibitive in-hospital mortality rate exceeding 40%. This provided the rationale for routine sepsis screening and more consistent implementation of evidence-based sepsis SOPs (e.g., the Surviving Sepsis campaign). As a result of years of concerted implementation efforts, fewer patients died of early fulminant sepsis, and those who developed MOD survived hospitalization with supportive ICU care. Unfortunately, a substantial portion of these patients entered a state of chronic critical illness (CCI), defined as an ICU stay of >14 days with continued low-grade organ dysfunction). In the surgical ICU, many of these CCI patients exhibited a new predominant MOD phenotype called the “persistent inflammation, immunosuppression, catabolism syndrome” (PICS) (Fig. 2).
FIG. 2.
Clinical pathway of persistent inflammation, immunosuppression, catabolism syndrome (PICS) manifestation. Abbreviations: CARS = compensatory anti-inflammatory response syndrome; MOD = multiple organ dysfunction; SIRS =systemic inflammatory response syndrome.
PICS: The New Paradigm for MOD
In recent years, the SIRS/CARS paradigm has been challenged on several fronts. The compensatory nature of CARS has been questioned. In the commonly used animal model of cecal ligation and puncture (CLP), blocking the early pro-inflammation activity has no effect on either anti-inflammation or suppression of adaptive immunity. In addition, in CLP, CARS is not delayed relative to SIRS. The production of pro- and anti-inflammatory cytokines occurs simultaneously. Of note, the Glue Grant has provided consistent human evidence for this conclusion. Looking at genome-wide expression analysis of blood leukocytes after severe blunt trauma, those investigators observed downregulation of genes involved in T-cell responses and antigen presentation and upregulation of anti-inflammatory genes, which paralleled increased expression of pro-inflammatory genes.
Thus, following an inflammatory insult (either severe trauma or sepsis), SIRS and CARS occur simultaneously (Fig. 2). In some cases, SIRS can become overwhelming, leading to early MOD and a fulminant trajectory leading to death. Fortunately, modern ICU care is directed at early detection and prevention of this trajectory's fatal expression. If the severely insulted patient does not die of early MOD, there are two alternatives. Either the aberrant immunology response reverses rapidly (i.e., achieves homeostasis) or its dysfunction persists. In the traditional SIRS/CARS paradigm, pro-inflammation occurs early and is terminated by the development of CARS. This is contrary to experimental data from critically ill patients with immunosuppression, where inflammation is persistent but attenuated compared with that after the initial inflammatory insult. In these CCI patients, both immunosuppression and inflammation continue. This state is characterized by lymphopenia with hyporesponsive lymphocytes, a greater plasma interleukin (IL)-6 concentration, neutrophilia with increased immature granulocytes, and anemia.
Importantly, there is an associated acute-phase response with high CRP and low pre-albumin concentration with persistent catabolism. Despite aggressive nutritional intervention, there is a continued loss of lean body mass and a proportional decrease in functional status with poor wound healing. An estimated 30%–50% of these patients with CCI deteriorate into PICS. Clinically, patients with PICS suffer from recurrent nosocomial infections, poor healing, and discharge to long-term acute-care facilities (LTACs), where they experience sepsis recidivism necessitating re-hospitalization, failure to rehabilitate, and an indolent death. At one year after ICU discharge, as many as half of patients with PICS are dead and another quarter are bedridden and fully functionally dependent. Thus, it appears that through our best efforts at advances in care, the epidemiology of MOD has again changed, and PICS is a new predominant MOD phenotype. As the population ages and peri-operative care continues to improve, PICS will become the next challenging horizon in surgical critical care.
Overview of Research Focus at the UF SCIRC
The original descriptions of CCI were of patients admitted to LTACs requiring prolonged mechanical ventilation. They suffered from a variety of chronic disease states, and multiple pathogenic mechanisms undoubtedly were involved. The UF SCIRC is interested in CCI that occurs in surgical ICUs and has coined the term “PICS” to describe its pathophysiology. This condition occurs after insults that involve persistent or recurrent inflammation. Examples include major burns (>30% body surface area), torso trauma leading to damage control surgery, necrotizing pancreatitis, and SS/SS. This latter condition can occur either as a primary inciting event (e.g., patients presenting with an IAI) or as a complication of major surgery or trauma. The fact that numerous novel interventions for SS/SS have been proved to be clinically ineffective indicates that our underlying understanding of SS/SS pathophysiology is incomplete. Much of this understanding comes from acute animal models of sepsis, which fail to recapitulate the human condition.
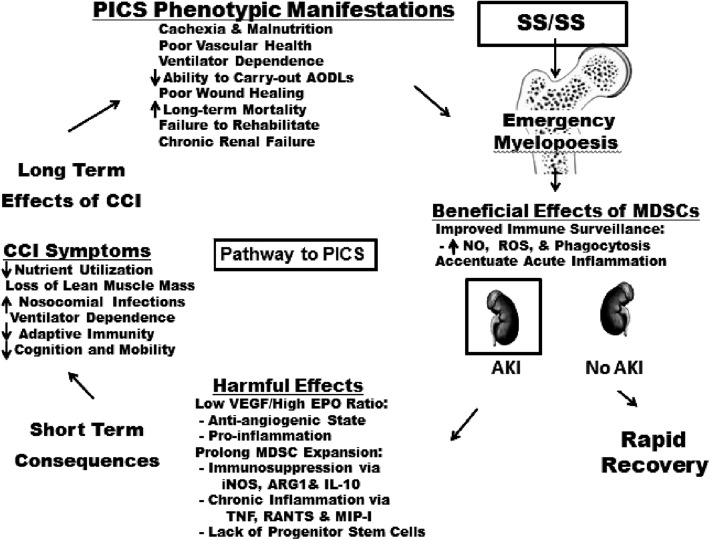
Recently, SCIRC colleagues have developed a model of chronic CLP-induced sepsis and have identified emergency myelopoiesis as an explanation for the initiation and persistence of the aberrant immunology response that characterizes PICS (Fig. 3) [26–28]. Emergency myelopoiesis is a well-conserved bone marrow response to a variety of chronic and acute inflammatory diseases, including malignant disease, autoimmune disease, infection, trauma, and burns. It involves the expansion of immature myeloid-derived suppressor cells (MDSCs). Hemopoietic stems cells are directed down the common myeloid progenitor line of innate immunity to produce MDSCs, whereas lymphocyte production is shut down, sacrificing adaptive immunity. These MDSCs are not allowed to mature into granulocytes, macrophages, and dendritic cells but are released prematurely from the bone marrow to establish residence in non-bone marrow hematopoietic sites (e.g., lymph nodes, spleen, and liver). The MDSCs can fight infections by improving immune surveillance and accentuating acute inflammation, but chronic expansion suppresses lymphocyte function through multiple mechanisms, including inducible nitric oxide synthase (iNOS), arginase-1 (ARG-1), and IL-10, and induces chronic inflammation through different mediators (including tumor necrosis factor (TNF), regulated on activation, normal T-cell expressed and secreted protein (RANTES), and macrophage inflammatory protein (MIP)-1), which presumably mediate catabolism. Doctors Moldawer and Efron are leading a proof of concept project aimed at demonstrating that MDSC expansion persists in surgical patients after SS/SS and that it is associated with the clinical phenotype of PICS. A Glue Grant retrospective database analysis and our UF SCIRC prospective preliminary data support this concept. In the laboratory, this project will pursue novel interventions to modulate favorably the number, function, and maturation of MDSCs. Of note, there is some evidence that depletion of MDSCs in the chronic CLP model is harmful.
FIG. 3.
Schematic depiction of how four projects of P50 grant research program are interwoven.
Other UF SCIRC investigators (Doctors Bihorac and Segal) have identified acute kidney injury (AKI) as a powerful harbinger of dismal outcomes after SS/SS (i.e., it is the pathway to PICS). Given that the serum creatinine concentration is a poor biomarker of AKI, they are interested in identifying early biomarkers (e.g., urine insulin-like growth factor-binding protein 7 and tissue inhibitor of metalloproteinases-2) [29–33]. Given that AKI after SS/SS is often worsened by additional insults (e.g., nephrotoxic drugs, contrast agents, hypotension, and hypovolemia), they are interested in devising treatment algorithms to minimize further injury in patients with sepsis with early AKI. These investigators also are studying how AKI contributes to the persistent pro-inflammatory organ “cross talk” involved in the progression of MOD. They also are interested in how AKI contributes to prolonged MDSC expansion leading to a lack of progenitor stem cells and an anti-angiogenic growth factor profile; e.g., low vascular endothelial growth factor (VEGF)/high erythropoietin (EPO) ratio, both of which limit renal repair processes leading to chronic renal insufficiency with progressive PICS pro-inflammation and catabolism.
Patients with sepsis who develop AKI frequently have concurrent acute lung injury (ALI) necessitating mechanical ventilation, which is pro-inflammatory and causes nosocomial infections that set the stage for CCI progression to PICS. Recent studies have shown that mechanical ventilation is associated with surprisingly rapid atrophy of diaphragmatic and skeletal muscles. Our bedside ultrasound studies reveal a 50% reduction in the cross-sectional area (CSA) of the diaphragm and vastus lateralis within 2–3 wks of mechanical ventilation after SS/SS. The UF SCIRC investigators (Doctors Martin and Gabrielli) have had a career-long interest in weaning patients from mechanical ventilation and have developed an inspiratory muscle strength training (IMST) regimen. In a recent randomized clinical trial (RCT) they demonstrated that IMST patients could be weaned faster from mechanical ventilation and showed better inspiratory muscle strength than control patients. In the current grant, the RCT is being repeated with the endpoints of diaphragm strength, measured by standard deep breathing and phrenic nerve stimulation, CSA by ultrasound imaging, and a panel of biomarkers reflecting inflammation, immunosuppression, and catabolism. Additionally, this project is in collaboration with Doctor Leeuwenburgh from the UF Institute on Aging (IOA), who studies aging sarcopenia [34–37]. Given that sepsis is a disease of the elderly and that elderly patients are especially prone to PICS, studies related to sarcopenia are likely to be relevant to sepsis catabolism. The IOA has extensive experience studying the success of resistance strength training in reversing aging sarcopenia. Therefore, this project will perform a second round of CRT early quadriceps strength training after SS/SS. In addition to the endpoint of muscle strength testing, muscle biopsies will be obtained to assess: (1) Mitochondrial respiration and reactive oxygen species production using high-resolution respirometry; (2) histochemistry studies to document changes in the CSA and types of muscle fibers; and (3) genomic and proteomic markers of anabolism/catabolism cross-talk, apoptosis, and mitochondrial biogenesis.
Although we recognize the phenotypic manifestations of full-blown PICS (Fig. 3), it is unclear what events in the progression of SS/SS through CCI to PICS need to be targeted to prevent to this vexing problem. To address this issue, the UF SCIRC is characterizing the clinical epidemiology of PICS in combination with serial novel biomarkers. There are three goals. The first is to identify key pathogenic mechanisms that can be linked to specific phenotypic manifestations of PICS to be targeted in future interventions. The second is to develop precise early prediction models (within 48 h) for high-risk CCI patients who are likely to benefit from a novel intervention. The third is to identify a composite endpoint such as a CCI score on a specific day (e.g., day 14 or 21 after the onset of SS/SS) that reflects a dismal long-term outcome (e.g., death or full functional dependence at one y). As we have improved our ability to decrease the short-term mortality rate, the gold standard Food and Drug Association endpoint of 30-d mortality rate has become obsolete. Additionally, the traditional phase II CRTs leading to definitive Phase III CRT testing of a specific intervention is not logical. As with cancer and cardiovascular disease, future successful sepsis interventions undoubtedly will be multimodality. Clinical testing will need to be done in an iterative fashion, where a promising intervention will be tested in a relatively small RCT, and if it favorably modulates the composite outcome (e.g., CCI score), it becomes standard of care in the next RCT, which will test a second intervention that targets another important pathogenic mechanism linked to a key clinical manifestation of PICS. Over time, a cocktail will be devised that can be tested in a large definitive RCT. Based on our Glue Grant experience, genomic biomarkers will be helpful in identifying appropriate interventions for specific patients.
Conclusions
Multiple organ dysfunction has challenged ICU clinicians for the past four decades. Through tremendous research efforts and advances in care, MOD outcomes have improved, but the epidemiology of the disorder has evolved through a series of clinical phenotypes. Some have come and gone; all have been associated with prolonged ICU stays and prohibitive mortality rates. Recent epidemiology studies have identified a substantial decrease in early fulminant MOD, and the second peak—late full-blown MOD—has disappeared. Unfortunately, many high-risk patients are surviving prolonged ICU stays only to enter CCI and be discharged to LTACs. Exactly what happens to these CCI patients once discharged is not clear, but a substantial portion or these patients are deteriorating into a new MOD phenotype of persistent inflammation, immunosuppression, and catabolism (i.e., PICS). Their clinical course is characterized by persistent loss of lean body mass with failure to obtain rehabilitation, sepsis recidivism necessitating re-hospitalization, increasing functional dependence, and an indolent path to death. Unfortunately, as our population ages and peri-operative care improves, PICS will increase into an insurmountable epidemic. Given the ineffectiveness of our current treatment paradigms, we believe PICS is the next horizon in surgical critical care and have developed the UF SCIRC to study the pathogenesis of and novel therapies for this vexing problem.
Acknowledgment
The investigators acknowledge the contribution of the Center for Sepsis and Critical Illness-supported P50 GM-111152 and T-32 GM008721 grants from the National Institute of General Medical Sciences
Author Disclosure Statement
No conflict of or competing interests have been declared, and there were no funds provided for the writing of this manuscript.
References
- 1.Gentile LF, Cuenca AG, Efron PA, et al. Persistent inflammation and immunosuppression: A common syndrome and new horizon for surgical intensive care. J Trauma Acute Care Surg 2012;72:1491–1501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moore EE, Jones TN. Benefits of immediate jejunostomy feeding after major abdominal trauma: A prospective, randomized study. J Trauma 1986;26:874–881 [DOI] [PubMed] [Google Scholar]
- 3.Moore FA, Feliciano DV, Andrassy RJ, et al. Early enteral feeding, compared with parenteral, reduces postoperative septic complications: The results of a meta-analysis. Ann Surg 1992;216:172–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moore FA, Moore EE. The evolving rationale for early enteral nutrition based on paradigms of multiple organ failure: A personal journey. Nutr Clin Pract 2009;24:297–304 [DOI] [PubMed] [Google Scholar]
- 5.Moore FA, Moore EE, Jones TN, et al. TEN versus TPN following major abdominal trauma: Reduced septic morbidity. J Trauma 1989;29:916–922 [DOI] [PubMed] [Google Scholar]
- 6.Fabian TC, Croce MA, Payne LW, et al. Duration of antibiotic therapy for penetrating abdominal trauma: A prospective trial. Surgery 1992;112:788–794 [PubMed] [Google Scholar]
- 7.Balk RA, Bone RC. The septic syndrome: Definition and clinical implications. Crit Care Clin 1989;5:1–8 [PubMed] [Google Scholar]
- 8.American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med 1992;20:864–874 [PubMed] [Google Scholar]
- 9.Levy MM, Fink MP, Marshall JC, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Intens Care Med 2003;29:530–538 [DOI] [PubMed] [Google Scholar]
- 10.Kudsk KA. Beneficial effect of enteral feeding. Gastrointest Endosc Clin North Am 2007;17:647–662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kudsk KA. Current aspects of mucosal immunology and its influence by nutrition. Am J Surg 2002;183:390–398 [DOI] [PubMed] [Google Scholar]
- 12.McClave SA, Heyland DK. The physiologic response and associated clinical benefits from provision of early enteral nutrition. Nutr Clin Pract 2009;24:305–315 [DOI] [PubMed] [Google Scholar]
- 13.Deitch EA, Winterton J, Li M, Berg R. The gut as a portal of entry for bacteremia: Role of protein malnutrition. Ann Surg 1987;205:681–692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alverdy J, Zaborina O, Wu L. The impact of stress and nutrition on bacterial–host interactions at the intestinal epithelial surface. Curr Opin Clin Nutr Metab Care 2005;8:205–209 [DOI] [PubMed] [Google Scholar]
- 15.Peng X, Yan H, You Z, et al. Effects of enteral supplementation with glutamine granules on intestinal mucosal barrier function in severe burned patients. Burns 2004;30:135–139 [DOI] [PubMed] [Google Scholar]
- 16.Kudsk K. Importance of enteral feeding in maintaining gut integrity. Techn Gastrointest Endosc 2001;3:2–8 [Google Scholar]
- 17.Dobbins WO., 3rd Gut immunophysiology: A gastroenterologist's view with emphasis on pathophysiology. Am J Physiol 1982;242:G1–G8 [DOI] [PubMed] [Google Scholar]
- 18.Moore FA, Moore EE, Poggetti R, et al. Gut bacterial translocation via the portal vein: A clinical perspective with major torso trauma. J Trauma 1991;31:629–636 [DOI] [PubMed] [Google Scholar]
- 19.Balogh Z, McKinley BA, Cocanour CS, et al. Patients with impending abdominal compartment syndrome do not respond to early volume loading. Am J Surg 2003;186:602–607 [DOI] [PubMed] [Google Scholar]
- 20.Balogh Z, McKinley BA, Cocanour CS, et al. Supranormal trauma resuscitation causes more cases of abdominal compartment syndrome. Arch Surg 2003;138:637–642 [DOI] [PubMed] [Google Scholar]
- 21.Balogh Z, McKinley BA, Cox CS Jr, et al. Abdominal compartment syndrome: The cause or effect of postinjury multiple organ failure. Shock 2003;20:483–492 [DOI] [PubMed] [Google Scholar]
- 22.Moore FA, Sauaia A, Moore EE, et al. Postinjury multiple organ failure: A bimodal phenomenon. J Trauma 1996;40:501–510 [DOI] [PubMed] [Google Scholar]
- 23.Moore EE, Moore FA, Franciose RJ, et al. The postischemic gut serves as a priming bed for circulating neutrophils that provoke multiple organ failure. J Trauma 1994;37:881–887 [DOI] [PubMed] [Google Scholar]
- 24.Alverdy JC, Laughlin RS, Wu L. Influence of the critically ill state on host–pathogen interactions within the intestine: Gut-derived sepsis redefined. Crit Care Med 2003;31:598–607 [DOI] [PubMed] [Google Scholar]
- 25.Gonzalez EA, Moore FA. Resuscitation beyond the abdominal compartment syndrome. Curr Opin Crit Care 2010;16:570–574 [DOI] [PubMed] [Google Scholar]
- 26.Cuenca AG, Delano MJ, Kelly-Scumpia KM, et al. A paradoxical role for myeloid-derived suppressor cells in sepsis and trauma. Mol Med 2011;17:281–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Delano MJ, Kelly-Scumpia KM, Thayer TC, et al. Neutrophil mobilization from the bone marrow during polymicrobial sepsis is dependent on CXCL12 signaling. J Immunol 2011;187:911–918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Delano MJ, Thayer T, Gabrilovich S, et al. Sepsis induces early alterations in innate immunity that impact mortality to secondary infection. J Immunol 2011;186:195–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bihorac A, Baslanti TO, Cuenca AG, et al. Acute kidney injury is associated with early cytokine changes after trauma. J Trauma Acute Care Surg 2013;74:1005–1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bihorac A, Delano MJ, Schold JD, et al. Incidence, clinical predictors, genomics, and outcome of acute kidney injury among trauma patients. Ann Surg 2010;252:158–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.White LE, Chaudhary R, Moore LJ, et al. Surgical sepsis and organ crosstalk: The role of the kidney. J Surg Res 2011;167:306–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.White LE, Hassoun HT, Bihorac A, et al. Acute kidney injury is surprisingly common and a powerful predictor of mortality in surgical sepsis. J Trauma Acute Care Surg 2013;75:432–438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bihorac A, Yavas S, Subbiah S, et al. Long-term risk of mortality and acute kidney injury during hospitalization after major surgery. Ann Surg 2009;249:851–858 [DOI] [PubMed] [Google Scholar]
- 34.Calvani R, Joseph AM, Adhihetty PJ, et al. Mitochondrial pathways in sarcopenia of aging and disuse muscle atrophy. Biol Chem 2013;394:393–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Joseph AM, Adhihetty PJ, Buford TW, et al. The impact of aging on mitochondrial function and biogenesis pathways in skeletal muscle of sedentary high- and low-functioning elderly individuals. Aging Cell 2012;11:801–809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Joseph AM, Adhihetty PJ, Wawrzyniak NR, et al. Dysregulation of mitochondrial quality control processes contribute to sarcopenia in a mouse model of premature aging. PloS One 2013;8:e69327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marzetti E, Lees HA, Manini TM, et al. Skeletal muscle apoptotic signaling predicts thigh muscle volume and gait speed in community-dwelling older persons: An exploratory study. PloS One 2012;7:e32829. [DOI] [PMC free article] [PubMed] [Google Scholar]