Abstract
Background: The introduction of fundus photography has impacted retinal imaging and retinal screening programs significantly. Literature Review: Fundus cameras play a vital role in addressing the cause of preventive blindness. More attention is being turned to developing countries, where infrastructure and access to healthcare are limited. One of the major limitations for tele-ophthalmology is restricted access to the office-based fundus camera. Results: Recent advances in access to telecommunications coupled with introduction of portable cameras and smartphone-based fundus imaging systems have resulted in an exponential surge in available technologies for portable fundus photography. Retinal cameras in the near future would have to cater to these needs by featuring a low-cost, portable design with automated controls and digitalized images with Web-based transfer. Conclusions: In this review, we aim to highlight the advances of fundus photography for retinal screening as well as discuss the advantages, disadvantages, and implications of the various technologies that are currently available.
Key words: : fundus camera, retinal photography, ophthalmic screening, tele-ophthalmology, portable camera system, smartphone-based camera system, portable eye examination kit, slit-lamp adaptors
Introduction
Jackman and Webster1 first published photographs of the retina in 1886. The next breakthrough was the first commercially available fundus camera produced by Carl Zeiss in 1926, following which considerable improvements to the field of view (FoV) were made.2 In 1927, Metzger published stereoscopic fundus photographs taken with the method of side-to-side shifting, as reported by Donaldson.3 The invention of the electronic flash tube enabled light to be directed through the pupil, and Hansell and Beeson4 successfully attached it to the camera in 1953. A ground-breaking 148° FoV camera emerged in 1960 (the Pomerantzeff Equator-Plus fundus camera), and nerve fiber layer photography using the traditional camera by Carl Zeiss was first reported by Behrendt and Wilson5 in 1965. As reported by Dobbins,6 Steven Sasson invented the first digital camera at Eastman Kodak in 1975, and the subsequent shift from analog to digital revolutionized medical record keeping. In recent years, confocal scanning laser ophthalmoscopy has emerged as a solution to minimize aberrations through poor dilation and to produce high-contrast, detailed images.7 Through the years, camera systems have evolved to boast sharper images, nonmydriatic wide-field options, pupil tracking, and, most recently, portability. Popular manufacturers in the market today are Topcon, Zeiss, Canon, Nidek, Kowa, CSO, and CenterVue.
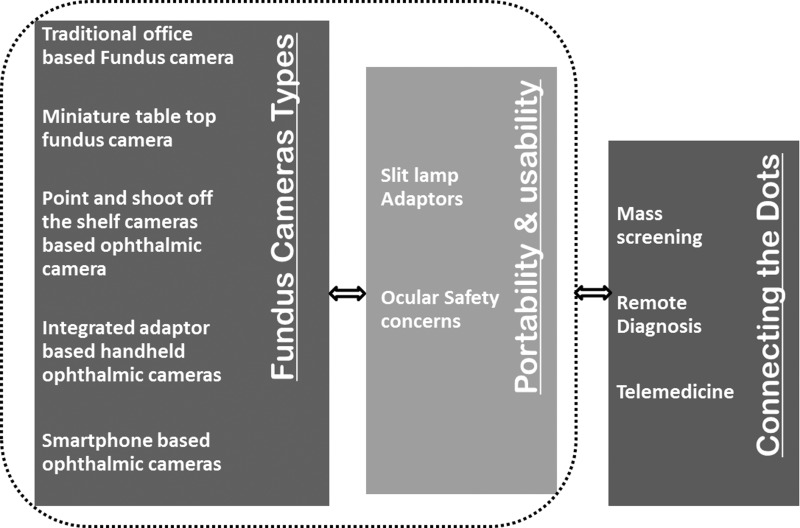
Traditional fundus cameras offer good-quality images but are bulky, office based, technician dependent, and costly. Besides access to the retinal imaging device, affordability is of paramount importance in screening programs, especially in the remotest of places. Recently, there have been significant technological advances that have radicalized retinal photography. Improvements in telecommunications and smartphones are two remarkable breakthroughs that have made ophthalmic screening in remote areas a realizable possibility (Fig. 1).
Fig. 1.
Flowchart depicting evolution and scope of retinal screening and fundus photography.
According to World Health Organization statistics, the number of people with visual morbidity worldwide, as of 2010, was in excess of 285 million, of which 39 million were blind.8 The advances in fundus imaging will hopefully decrease preventable visual morbidity by allowing easy and timely fundus screening. This review attempts to summarize the evolutionary journey from traditional fundus cameras to the newest models in retinal photography. Most of these models, each with their image-forming parameters, are covered in the following sections, with technical specifications listed in Table 1.
Table 1.
Technical Specifications of Fundus Cameras Under Different Categories
| CATEGORY, NAME | DESIGN PRINCIPLE | PUPIL | FIELD OF VIEW | FOCUSING RANGE | FIXATION TARGETS | IMAGE SENSOR/DISPLAY | ADDITIONAL FEATURES |
|---|---|---|---|---|---|---|---|
| Miniature table-top design | |||||||
| iCam13 | Reflective imaging using white light | Nonmydriatic | 45° | Manual, −35D to 30D | 7 | 12-bit CCD, 5.2 MP, computer interface | Multiple image views, image sharpening, color, postprocessing for red-free images |
| 3nethra (Classic and Royal)15 | Reflective imaging using white light | Nonmydriatic | 45° | Adjustable, −18D to +18D Sph and −8D to +8D Cyl (Royal) | Not specified | 3 MP, computer interface | Corneal imaging, color, red-free images |
| dRS16 | Reflective imaging using white light | Nonmydriatic | 45° H 40° V |
Autofocus, −15D to +15D | 7 | 5 MP, 10.4-inch touchscreen color display; WiFi and Ethernet connected | Multiple field acquisition, color, red-free, stereo pair images |
| EasyScan17 | Confocal SLO, with green, NIR | Nonmydriatic | 60° H 45° V |
Autofocus | Not specified | Photodetector-based computer interface; network connectivity | Enhanced view of periphery, better resolution, automated iris detection, pseudo-color |
| Topcon TRC-NW8Fplus19 | Reflective imaging using white light | Nonmydriatic | 45° | Autofocus, −13D to 12D (without lens correction) | Internal/external fixation target (can be selected) | 8 MP digital SRL camera | Stereo photography, color, red-free images, fluorescein angiography |
| Zeiss VISUCAM 20020 | Reflective imaging using white light | Nonmydriatic | 45° and 30° | Autofocus −35D to +35D | Internal/external fixation target (can be selected) | CCD 5.0 MP, 19-inch TFT | Color, red-free images, blue and red autoflorescence, anterior segment imaging, stereo image mode |
| Kowa Nonmyd721 | Reflective imaging using white light | Nonmydriatic | 45° | Not specified | Not specified | 12 MP digital camera | Optic nerve head color, red-free images, pseudo-3D display |
| Canon CR-222 | Reflective imaging using white light | Nonmydriatic | 45° | Autofocus | Internal and external | 18 MP EOS digital camera | Cobalt- and red-free imaging |
| OCULUS ImageCam 2 digital slit lamp camera23 | Slit lamp-based | Not specified | Not specified | Not specified | Not specified | 2 MP resolution | Viewer software for anterior segment, fundus, sclera, etc., with USB interface |
| California ultra-widefield retinal imaging24 | Reflective imaging using multiple wavelengths | Nonmydriatic | 200° | Not specified | Not specified | Not specified | Composite color, red-free, autofluorescence, fluorescein angiography, indocyanine green angiography |
| Point-and-shoot off-the-shelf digital camera-based11 | Conventional optics + camera lens | Mydriatic | 50° | Manual | No | Camera CMOS sensor | — |
| Integrated adaptor-detector-based (hand-held) | |||||||
| iExaminer35 + PanOptic ophthalmoscope25 | iPhone + PanOptic ophthalmoscope | Nonmydriatic | 25° | –20D to +20D | No | iPhone 4S camera | Color, corneal imaging, cobalt blue filter |
| Volk Pictor26 | Reflective imaging using white light | Nonmydriatic | 40° | –20D to +20D | 9 | 5 MP, TFT LCD detector, WiFi/USB connectivity | Color, corneal imaging |
| VersaCam27 | Reflective imaging using white light | Nonmydriatic | 40° | –20D to +20D | Not specified | 2 MP camera, 3.5-inch color LCD | Color imaging |
| JedMed Horus Scope28 | Reflective imaging | Nonmydriatic | Not specified | 5–30 mm | Not specified | 2 MP HD camera, 3.5-inch color LCD, PC connectivity through USB | Color imaging, general examinations for ear, nose, and throat and women's health |
| Optomed Smartscope29 | Conventional optics | Nonmydriatic | 40° | –20D to+ 20D | Not specified | 5 MP CMOS image sensor, 2.4-inch TFT LCD detector, PC connectivity through USB | Anterior eye module, otoscope, dermatoscope |
| Kowa Genesis-D30 | Conventional optics | Mydriatic | Not specified | Not specified | Not specified | 2 MP digital camera, 2.5-inch TFT LCD display | — |
| Riester ri-screen multifunctional digital camera system31 | Slit lamp-based | Nonmydriatic | 25° or 40° | 25° or 40° | Not specified | 3.5-inch full HD full color TFT-LCD display | Additional modules for otoscopy and dermatoscopy |
| Smartphone-based (hand-held) | |||||||
| Ocular Cellscope33 | iPhone + conventional optics | Not specified | 55° | Autofocus | No | iPhone | — |
| PEEK34 | iPhone + external lens | Not specified | Not specified | Filmic Pro application | No | iPhone | — |
| Harvard Medical School prototype36 | iPhone + external lens | Mydriatic | Not specified | Filmic Pro application | No | iPhone | — |
3D, three-dimensional; CCD, charge-coupled device; Cyl, cylindrical; D, diopters; H, horizontal; HD, high-definition; LCD, liquid crystal display; MP, megapixels; NIR, near-infrared; PC, personal computer; PEEK, Portable Eye Examination Kit; SLO, scanning laser ophthalmoscopy; Sph, spherical; V, vertical; TFT, thin-film transistor.
Method of Literature Search
A comprehensive Medline and Scopus search was conducted initially using the following key words: “retinal imaging,” “fundus camera,” “ophthalmoscope,” “miniature,” “handheld,” “telemedicine,” and “smartphone fundus camera.” The original relevant articles were retrieved and evaluated. Also, because the review focuses on latest technological advancements in fundus imaging, search engines like Google and Bing were prompted for these key words. The information about commercial products was obtained from the online articles and Web pages of the respective companies, and major statistics reports were sought from the official database available on the World Health Organization Web site.8 The review relied primarily on articles written in English.
Traditional Fundus Camera
The design of the traditional fundus camera system is based on monocular indirect ophthalmoscopy.9 A common design by Knoll9 and others10 can be considered as a reference layout for a traditional table-top fundus camera. It consists of a sequence of optic components including objective and condensing lenses, beam splitters, mirrors, masks, diffusers, and polarizers, which altogether direct the illuminating light through the pupil of the eye, collecting light reflected from the retinal surface and relaying it to imaging optics forming an image of the retina on the detector screen.11 Advanced versions of these systems are equipped with additional features like automated analysis and algorithms. Filters can be applied to camera systems for autofluorescence, fundus fluorescein angiography, and indocyanine green angiography.12
Limitations of Traditional Fundus Cameras
The need for a miniature fundus camera device has emerged from specific limitations that accompany the use of traditional table-top fundus cameras. First, they form a bulky system, incorporating a host of optical and mechanical components, and the alignment of every part with respect to another is a critical parameter for good-quality images. Second, the operation of such a sophisticated system requires skilled personnel. Third, the bulkiness and complexity of the instrument restrict its use only in high-end clinical settings, such that it is difficult to be accessible in remote rural settings. Fourth, the number of optical components and add-on features in more recent devices renders the cost of the cameras exorbitantly high for them to be installed in rural locales where much of the population is subjected to ailments amounting to visual morbidity.
Modern Table-Top Fundus Cameras
Advancements in the field of optical sources and detectors have led to miniaturization of optical assemblies at a lower cost. In line with these developments, miniature table-top fundus camera system designs have emerged that provide retinal images comparable to those of traditional fundus cameras.
Icam
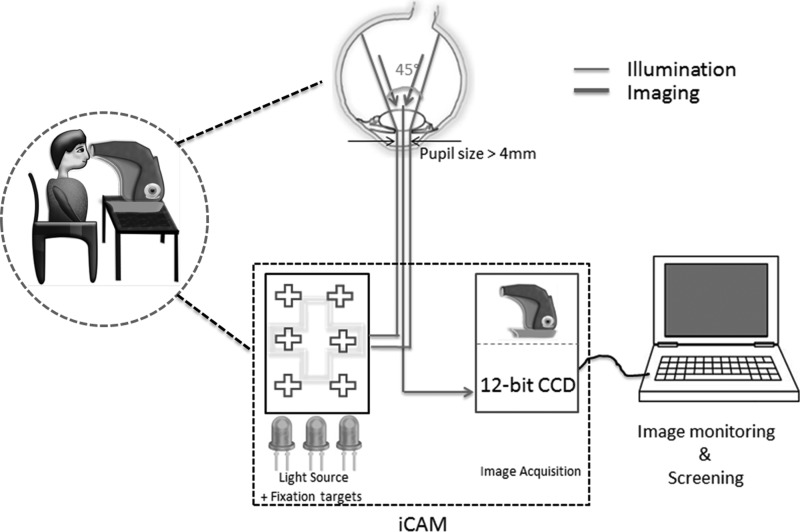
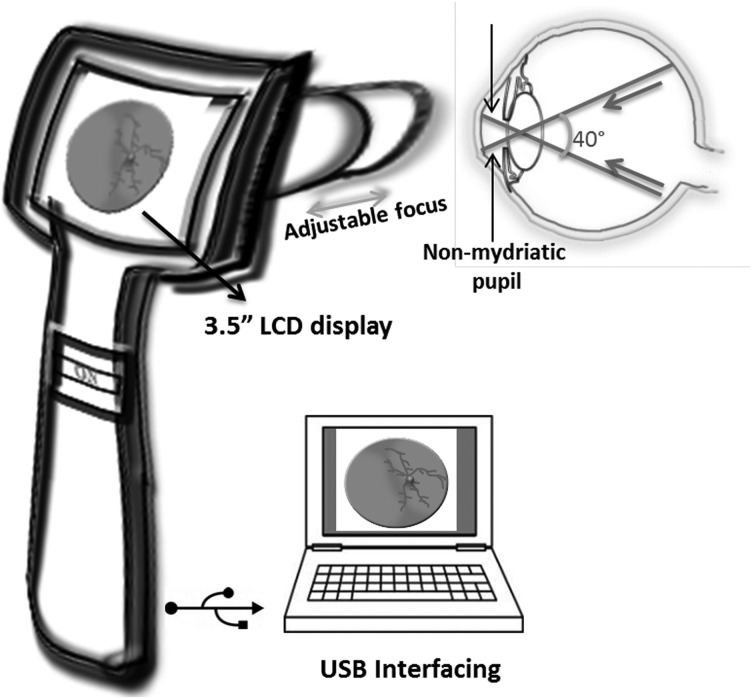
The iCam is a 4.5-kg table-top fundus camera designed by Optovue, Inc. (Fremont, CA).13 A near-infrared light-emitting diode (LED) provides illumination during alignment of the patient's eye, and the white LED is used for image capture. This allows for a smaller machine design, longer life expectancy, and less heat emission compared with the typical xenon flash lamp. LEDs maintain light characteristics such as color temperature, lumens of output, and distribution of light, which allows a controlled and reproducible illumination unit. The pupil is manually aligned using the split-image technique in the direction of each of the targets such that images of different regions of the retina are acquired. Computer interfacing is used for viewing images. Figure 2 is an illustrative representation of the basic design methodology behind the iCam. iFusion is a combination of a nonmydriatic fundus camera (iCam) and spectral-domain optical coherence tomography (iVue).14
Fig. 2.
Illustrative representation of the miniature table-top fundus camera iCam. The table-top device images the retina by light-emitting diode illumination covering different parts of the retina through fixation targets. CCD, charge-coupled device.
3nethra
3nethra is a portable, integrated table-top fundus camera designed and developed by Forus Inc. (Bengaluru, India).15 3nethra Classic is the basic version, and 3nethra Royal incorporates an additional automated refractometer. Low-power LED light is used for image capture and can image both posterior and anterior segments. It has focus guidance, and images are viewed with a laptop computer. 3nethra intelligently uses a digital Web-based telemedicine feature to transmit images over to mobile phones and via e-mails, and the device is targeted at primary healthcare population eye screening.
Digital Retinography System
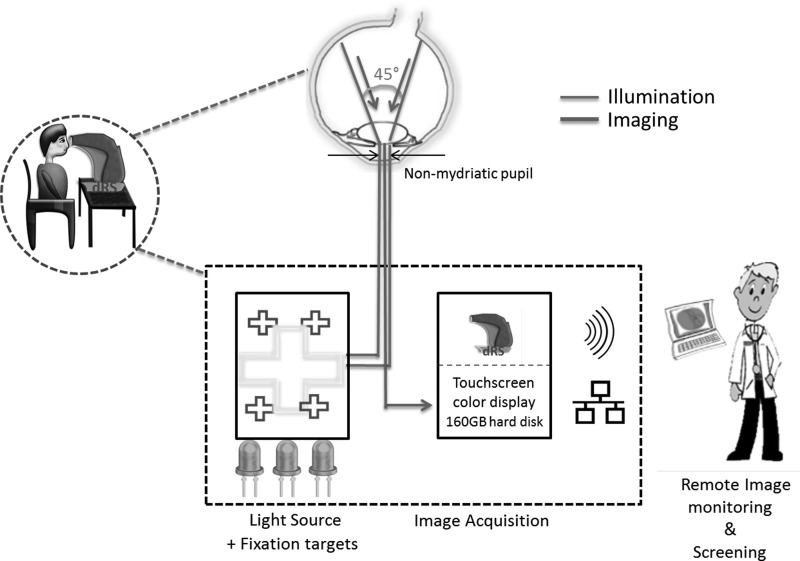
Designed by CenterVue (Padova, Italy), the digital retinography system (dRS) is also a nonmydriatic miniature table-top fundus camera16 requiring a pupil size of 4 mm. Marketed as being compact (19 kg), it has a built-in screen featuring patient autosensing, autoalignment, and autofocus, and usage requires minimal operator skill. The illumination schematic is presented in Figure 3.
Fig. 3.
Diagrammatic representation of fundus imaging by the digital retinography system (dRS). Equipped with wireless and Ethernet connectivity, this miniature table-top version also acquires fundus images through light-emitting diode illumination and fixation targets.
EasyScan
EasyScan is an 11-kg retinal imaging system developed by iOptics (Den Haag, The Netherlands),17 which works on the principle of scanning laser ophthalmoscopy18 and boasts high-resolution images up to the sixth bifurcation. There are three capture modes: green (532 nm), near-infrared (785 nm), and combined (pseudo-color). It is fully automated with iris detection, autofocus, autocapture, and auto-exposure. No external power source is required, and the images are viewed on a computer screen connected to the device for uploading.
TRC-NW8Fplus
The nonmydriatic retinal camera developed by Topcon (Tokyo, Japan)19 has the feature of autofocus, which helps in image acquisition in difficult conditions like excess accommodation, and has the option of autoshoot, which helps in quicker capture, which is beneficial in cases of eyes with poor or variable fixation. High-resolution images are acquired with the help of a true red-free filter, which is replaceable to acquire the color images with a 45° angle of coverage. Good-quality images can be taken with lower flash intensities, and stereophotography is also possible.
VISUCAM 200
This nonmydriatic fundus camera from Zeiss (Oberkochen, Germany) has the optional feature of macular pigment optical density assessments for age-related macular degneration. Weighing 30 kg, VISUCAM 200 comes with a pupil size marker and positional aid along with the autofocus and autoflash options.20 Wide-field imaging generates an automontage for panoramic retinal views. Images can be transferred via through a network for comprehensive report management by Zeiss Systems.
Nonmyd7
The fourth-generation nonmydriatic fundus camera from Kowa (Torrance, CA), Nonmyd7 is provided with a special feature that allows magnification over 20° FoV to image the optic nerve head.21 Small pupil mode can be activated, and the software montage function can create mosaics using the four fixed dots switching-type internal fixation targets. The images are uploaded to Kowa's VK-2 digital imaging software.
Canon CR-2
The CR-2 non-mydriatic digital retinal camera from Canon (Tokyo) uses low-power LEDs for illumination and photography.22 It has autofocus, autofundus, and autocapture modes and a digital filter processor. It provides 45° and 35° viewing angles in small pupil mode (pupil size, 3.3 mm). The CR-2 PLUS AF has an additional fundus autofluorescence imaging modality for undilated pupils.
Oculus Imagecam 2 Digital Slit Lamp Camera
Different segments of the eye, such as anterior segment, fundus, sclera, etc., can be conveniently imaged by setting suitable exposure time, light magnification, and white balance.23 Additional video sequences can be recorded by the high-resolution, digital video camera in the beam path of the slit lamp.
California Ultra-Widefield Retinal Imaging
This recent mini-table-top version for retinal screening from Optos (Dunfermline, Scotland, United Kingdom)24 offers an ultra-widefield that covers as wide as 200° or up to 82% of the retina capture in a single image. It includes an additional imaging modality: indocyanine green can be simultaneously captured with other imaging modalities through interweaved angiography.
Limitations of Modern Table-Top Fundus Cameras
Most of the models have incorporated add-on features that contribute to additional size and weight of the camera. Patients have to be seated upright for photographs to be taken. It is essentially an office-based procedure, hence warranting a visit to the clinic. The overall cost of most table-top models is high, and application in primary healthcare may be limited due to financial constraints. These factors limit their use to high-end speciality healthcare units.
Modified Hand-Held Fundus Cameras Using Off-the-Shelf Point-and-Shoot Cameras
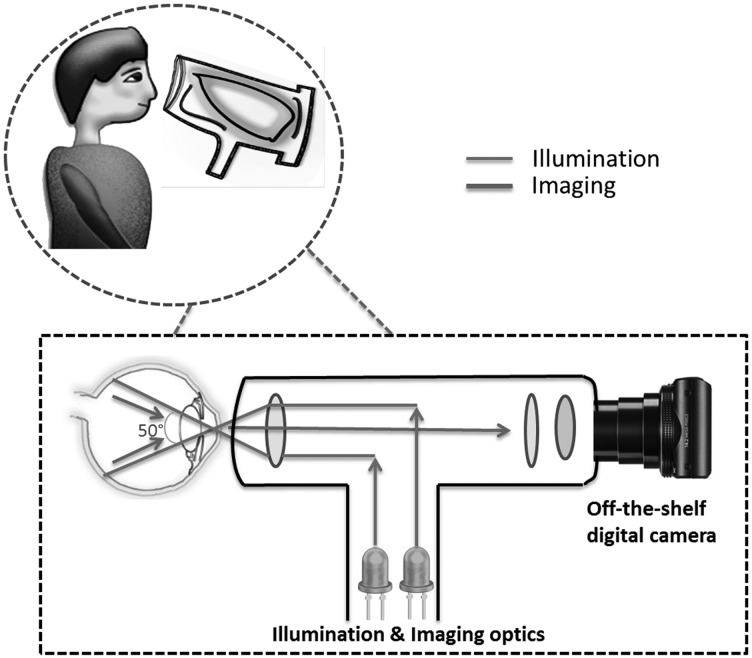
Research groups have incorporated low-cost commercially available optics into digital cameras, depicted in Figure 4, for acquiring retinal images.11 These prototype designs are light and capable of acquiring quality fundus images. The light sources used for illumination are LEDs and the xenon flash lamp. The camera-based CMOS sensor with its automatic focus and exposure capabilities provides a 50° retinal FoV. The images produced by the prototype camera in the study were comparable to standard fundus cameras.11
Fig. 4.
Modified hand-held fundus cameras using off-the-shelf point-and-shoot cameras. Additional optical components are assembled to transform a commercial digital camera into a fundus camera.
Limitations of Modified Hand-Held Fundus Cameras Using Digital Point-And-Shoot Cameras
Although commercial point-and-shoot cameras offer an easy, lightweight, and portable alternative, there are no fixation targets on the camera for proper focusing. In addition, reflections from cornea and common optics cause artifacts in images, limiting the use of such prototypes in clinical settings.
Integrated Adaptor-Detector-Based Hand-Held Ophthalmic Cameras
To overcome the technical issues highlighted in previous sections, illuminating optics were integrated with high-resolution detectors to ensure a consistent high-definition image capture. The basic architecture of the integrated adaptor-detector-based hand-held camera design is shown in Figure 5. The following section elaborates on commercially available designs.
Fig. 5.
Integrated adaptor-detector-based hand-held ophthalmic cameras. The optical assembly is integrated with a display console, making it a user-friendly hand-held instrument. LCD, liquid crystal display.
Panoptic Ophthalmoscope
The PanOptic™ ophthalmoscope, developed by WelchAllyn (Skaneateles Falls, NY), uses a halogen lamp for illumination providing a 5× zoom for images acquired through undilated pupils.25 The device can be accessorized with a smartphone (Apple [Cupertino, CA] iPhone®) for acquiring, storing, and transferring fundus images.
Volk Pictor
Volk Pictor enables nonmydriatic fundus examination with an improved 40° FoV.26 It has modifications allowing still images and videos of the optic disc, macula, and retinal vasculature. The illumination levels and focusing can be adjusted to produce high-resolution reflection-free images. The attached detector increases the overall cost, and the acquired images require a wired interface for transfer.
VersaCam
VersaCam from Nidek Co., Ltd. (Gamagori, Japan) enables one-handed operation by an ophthalmoscopic adaptor to image the retina.27 It is a lightweight, portable device, weighing 400 g. The infrared and white LEDs provide illumination for nonmydriatic capture. Good-quality images of normal healthy fundus have been demonstrated. Acquired images are transferred via a mini-USB to a computer.
Horus Scope
The Horus Scope from JedMed (St. Louis, MO) is a hand-held ophthalmoscopic adaptor for viewing the retina and capturing video and still images that can be easily transferred to the personal computer.28 The built-in infrared technology allows nonmydriatic imaging capturing. Although the focusing range is adjustable, there is no information about the FoV of retina covered.
Smartscope
The Smartscope® from Optomed (Oulu, Finland) offers a FoV of 40° while imaging through an undilated pupil (3.5 mm).29 IR and white LEDs are used for image targeting and capturing. The compact optical module offers adjustable focus. The acquired images have been demonstrated to distinguish diabetic retinopathy, age-related macular degeneration, retinopathy of prematurity, and glaucoma.29
Genesis-D
A hand-held digital retinal camera from Kowa Optimed consists of a compact flash memory and an liquid crystal display monitor.30 It has an indirect lens holder accessory with the VK-2 digital imaging system and electronic medical record connectivity and also facilitates telemedicine. Mydriasis is required for color photography and fundus fluorescein angiography.
Riester Ri-Screen Multifunctional Digital Camera System
This slit lamp-based system, along with attachable ophthalmoscopic lens, enables retinal ophthalmic imaging and nonmydriatic eye fundus examination.31 The Riester (Jungingen, Germany) ri-screen provides digital images and video to support screening and documentation of ocular lesions and anomalies.
Limitations of Hand-Held Ophthalmic Cameras
There are no qualitative reports showing the comparison of images acquired by these cameras with the standard fundus cameras in clinical trials. The manual alignment of the illuminating beam with the optical axis is a crucial requirement for good-quality images and is time consuming. Most important is that technical descriptions about the illumination and imaging light energies and their adherence to ocular safety limits are not stated.
Smartphone-Based Ophthalmic Cameras
Smartphones equipped with faster processors, larger storage memory, smaller batteries, and advanced operating systems have paved the way for numerous applications (apps). Research on the use of smartphones for medicine is growing, but there are limited data about their utility, feasibility, and their impact as medical tools.
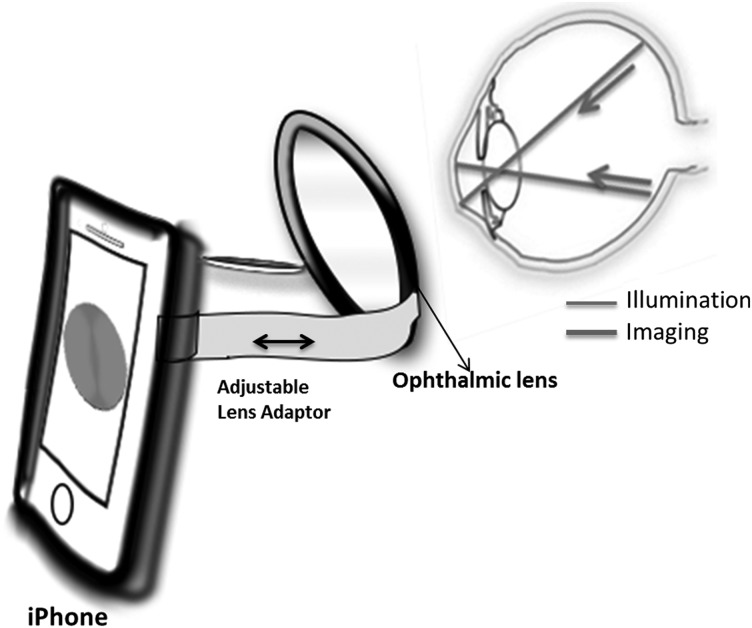
Technological advancements allow smartphone-based attachments32–35 and integrated lens adaptors to transform the smartphone into a fundus camera (Fig. 6).36 Some versions use apps designed for screening of abnormalities based on artificial intelligence–driven algorithms. The following section gives an overview of the smartphone-based fundus camera systems.
Fig. 6.
Smartphone-based fundus camera system. An external ophthalmic lens is placed in front of the smartphone's lens for acquiring retinal images using the phone's built-in flash for illumination.
Iexaminer
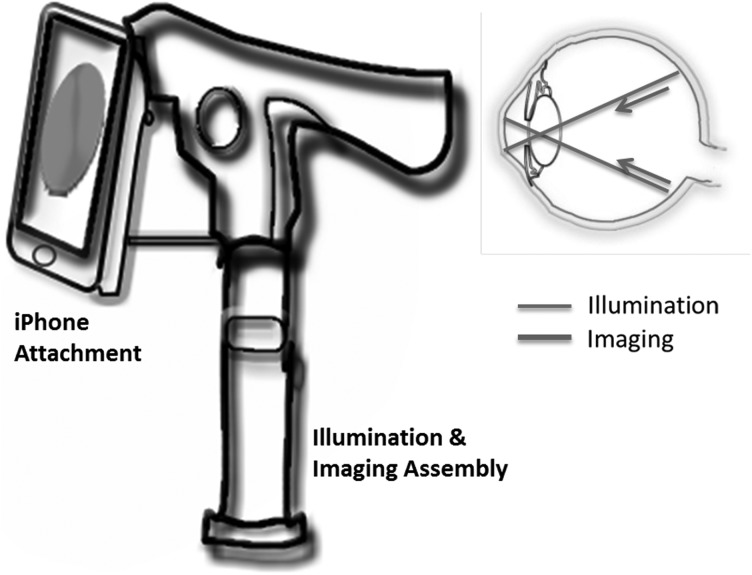
The Welch Allyn iExaminer™ turns the PanOptic ophthalmoscope into a mobile digital imaging device.35 Figure 7 is an illustration of the system. It aligns the optical axis of the PanOptic ophthalmoscope to the visual axis of the iPhone 4 or 4S camera to capture high-resolution pictures of the fundus and retinal nerve. There is an iExaminer app that enables storing and printing of images.
Fig. 7.
Adaptor-based fundus camera system. A smartphone is used as a display console for retinal images, and illumination and imaging optics are integrated to it externally.
Ocular CellScope
A mobile phone–based retinal camera has been described by researchers at the Francis I. Proctor Foundation and the University of California, San Francisco, named the Ocular CellScope.33 It comprises a mobile phone, a housing that contains the illuminating and collecting optics, and an integrated phone holder that ensures alignment with the phone's camera. Both the optical components and the iPhone are integrated within a polymer casing. It uses a single 54D ophthalmic lens for focusing and capturing reflected light, in turn using the autofocus mechanism of the iPhone's camera to correct for variability in axial length and refractive error in the subject's eye. The illumination is provided by three white LEDs powered by the iPhone itself. However, artifacts are seen in final images, even though the cross-polarization technique is used for reducing back reflections.
Portable Eye Examination Kit
Andrew Bastawrous and collaborators have developed a clip-on hardware and an app for less than $100 US that can be used for imaging the retina, as reported by Navitsky.34 The Portable Eye Examination Kit (PEEK) facilitates automatic grading of acquired images, with an additional option of sending the images to experts around the world. The team conducted extensive field trials on thousands of patients over the past 3 years over 100 locations. The system also has the capability to store GPS data for each patient screened for ease of referencing.
D-Eye System
Similar to the PEEK system, the D-eye System uses an add-on adaptor with an built-in lens attachment to the smartphone.37 It started as a prospective clinic-based comparative study conducted in the ophthalmic diabetic center of the Spedali Civili di Brescia, Brescia, Italy. With the D-eye adaptor attached to the smartphone, it is able to capture a FoV of approximately 20°.
Other Similar Prototypes
A similar approach has been demonstrated in human and rabbit eyes by a team of ophthalmologists from Harvard Medical School, Boston, MA.32 They used the iPhone camera's built-in flash for acquiring images and an external 20D lens for focusing. They used the Filmic Pro app (£5.99) for control of light intensity, exposure, and focus. A Koeppe lens was used for imaging patients under anesthesia. Still images were then retrieved from the recorded video. When imaging the fundus of rabbits, 28D or 30D lenses have shown to give better results.
Limitations of Smartphone-Based Ophthalmic Cameras
The light sources of the iPhone 5 or higher versions need to be verified against safety standards.38 Beam alignment is problematic with the use of smartphone-based imaging systems. The iPhone's built-in flash has a fairly high intensity, which constricts the pupil. Thus, image capture is difficult and requires pupillary dilation or the use of special apps to tailor the intensity and exposure time of flash for optimal illumination. At the moment, such lens adaptors use the built-in flash of the iPhone itself as a coaxial light source, but efforts are being put to design lens adaptors with provision of including coaxial light sources like external LEDs with varying intensity levels.11.32,33
The Future of Ophthalmic Photography
There has been a recent surge of telecommunications in the developing world. For example, teledensity in India has increased from a mere 12.1% to 74.02%, registering a growth of 600% during the past 5 years.39 There are many portable systems used today, and most of the gadgets can be used by semiskilled personnel. The advent of teletechnology (Fig. 8) could create a greater community outreach by decentralizing the delivery of healthcare services. In third-world settings where residents in rural areas have limited access to proper healthcare, a cost-effective, practical tool for accurate screening of the retina is of significant relevance in facilitating appropriate referral to an eye specialist in a timely manner.
Fig. 8.
The proposed complete cycle of tele-ophthalmology.
Smartphones have become an integral part of the medical field lately to provide fast and clear access to electronically mailed digital images,40 instant messaging and virtual private network,41 user–interface services,42 and mobile healthcare computing devices.43 Statistical analysis for comparison of the quality of nonmydriatic fundus photographs displayed on an iPhone 3G against a desktop computer has shown the iPhone's image quality to be superior to that same image viewed on a computer display.44 This unexpected phenomenon has been attributed to the advanced features of the smartphone's display (i.e., higher dot pitch and brightness). Nevertheless, the smartphone cannot, in its present form, replace the current value of in-person ophthalmic examination, but it remains a very promising device for ophthalmic telemedicine.
Conclusions
New inexpensive, portable, easy-to-operate fundus cameras have revolutionized retinal screening programs, which have grown exponentially over the last decade.11,45,46 Tele-ophthalmology is already making waves in linking remote villages to ophthalmologists. Ocular fundoscopic examination in young children is being made easier with nonmydriatic fundus cameras, especially to diagnose conditions such as retinopathy of prematurity and retinoblastoma.
In this overview we have outlined the technical advantages and disadvantages of all the different systems. However, the assessment of these camera systems needs to be validated in clinical studies.
Acknowledgments
This work was supported by the National Institute for Health Research Biomedical Research Centre based at Moorfields Eye Hospital, the NHS Foundation Trust, the University College London Institute of Ophthalmology, and Nanyang Technological University.
Disclosure Statement
No competing financial interests exist.
N.P. wrote the first draft of the manuscript, and P.H. and J.L. have further edited the manuscript. P.A.K. has critically reviewed the manuscript and provided valuable input. T.S.C. is the supervisor for N.P. and provided critical technical input. R.A., S.T., and T.H.L. provided the overall conceptualization of the project and the manuscript and critically reviewed and edited the manuscript. A.R. has organized the technical specifications and technology for each camera.
References
- 1.Jackman WT, Webster JD. On photographing the retina of the living human eye. Philadel Photogr 1886;23:340–341 [Google Scholar]
- 2.Yannuzzi LA. The Retinal Atlas. New York: Elsevier, 2010 [Google Scholar]
- 3.Donaldson DD. A new camera for stereoscopic fundus photography. Trans Am Ophthalmol Soc 1964;62:429–458 [PMC free article] [PubMed] [Google Scholar]
- 4.Hansell P, Beeson EJG. Retinal photography in color. Br J Ophthalmol 1953;37:65–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Behrendt T, Wilson LA. Spectral reflectance photography of the retina. Am J Ophthalmol 1965;59:1079–1088 [PubMed] [Google Scholar]
- 6.Dobbin B. Kodak engineer had revolutionary idea: The first digital camera. The Associated Press; 2005. Available at www.seattlepi.com/business/article/Kodak-engineer-had-revolutionary-idea-the-first-1182624.php (last accessed February25, 2015) [Google Scholar]
- 7.LaRocca F, Nankivil D, Farsiu S, Izatt JA. Handheld simultaneous scanning laser ophthalmoscopy and optical coherence tomography system. Biomed Opt Express 2013;4:2307–2321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mariotti SP. Global data on visual impairments 2010. World Health Organization. Publication WHO/NMH/PBD/12.01. Available at www.who.int/blindness/publications/globaldata/en/ (last accessed May8, 2014)
- 9.Knoll HA. Ophthalmic instruments. In: Kingslake R, ed. Applied optics and optical engineering, Vol. 5: Optical instruments, Part 2. New York: Academic Press, 1969:281–304 [Google Scholar]
- 10.Shibata N, Torii M. Fundus camera. U.S. Patent Application 20020067919. June 6, 2002 [Google Scholar]
- 11.Tran K, Mendel TA, Holbrook KL, Yates PA. Construction of an inexpensive, hand-held fundus camera through modification of a consumer “point-and-shoot” camera. Invest Ophthalmol Vis Sci 2012;53:7600–7607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Young CW. Interpretation of fundus fluorescein angiography. Arch Ophthalmol 1979;97:564–565 [Google Scholar]
- 13.Optovue. iCam. Available at http://optovue.com/icam/ (last accessed July10, 2014)
- 14.iFusion. Available at http://optovue.com/ifusion/ (last accessed July18, 2014)
- 15.Forus. 3nethra. Available at forushealth.com/forus/media/3nethra-Classic4e.pdf (last accessed March7, 2014)
- 16.CenterVue. Digital retinography system. Available at www.centervue.com/product.php?id=637 (last accessed May20, 2014)
- 17.i-Optics. EasyScan. Available at http://i-optics.com/products/easyscan/ (last accessed May20, 2014)
- 18.Webb RH, Hughes GW. Scanning laser ophthalmoscope. IEEE Trans Biomed Eng 1981;BME-28:488–492 [DOI] [PubMed] [Google Scholar]
- 19.Non-mydriatic retinal camera TRC-NW8F plus. Available at www.topcon.co.jp/en/eyecare/product/diag/trc/nw8fplus.html (last accessed July10, 2014)
- 20.VISUCAM 200. All that is needed for screening. Available at www.meditec.zeiss.com/88256DE3007B916B/0/…/visucam_200_en.pdf (last accessed July10, 2014)
- 21.Kowa Nonmyd7. Available at http://kowa-europe.com/medicals/en/nonmyd7.php#nonmyd7 (last accessed July10, 2014)
- 22.Canon CR-2 retinal camera. Available at www.canon-europe.com/Medical/Eye_Care/CR-2_Digital_Retinal_Camera/ (last accessed July18, 2014)
- 23.OCULUS. ImageCam® 2 digital slit lamp camera. Available at www.oculus.de/en/products/slit-lamp-documentation/imagecam-2/highlights/ (last accessed April10, 2015)
- 24.California ultra-widefield retinal imaging. Available at http://optos.com/en/Products/Retinal-imaging-products/Ultra-widefield-imaging/California/ (last accessed May21, 2015)
- 25.Welch Allyn. PanOptic™ ophthalmoscope. Available at http://intl.welchallyn.com/apps/products/product.jsp?id=11-ac-100-0000000001138 (last accessed May12, 2014)
- 26.Volk Optical Inc. Volk Pictor. Available at www.volk.com/index.php/volk-products/volk-pictor-digital-ophthalmic-imager.html (last accessed May12, 2014)
- 27.Digital Medical Scope VersaCam. Available at www.nidek-intl.com/products/diagnosis/ds-10.html (last accessed May20, 2014)
- 28.Horus Scope portable fundus camera. Available at www.jedmed.com/products/portable-fundus-camera (last accessed May20, 2014)
- 29.Smartscope M5. Available at www.optomed.com/smartscope+m5+/ (last accessed May20, 2014)
- 30.Kowa. Genesis-D. Available at http://kowa-europe.com/medicals/en/genesisD.php (last accessed July10, 2014)
- 31.Riester ri-screen multifunctional digital camera system. Available at www.ophthalmologyweb.com/5740-Digital-Retinal-Camera/5339259-Riester-ri-screen-Multifunctional-Digital-Camera-System/ (last accessed April10, 2015)
- 32.Haddock LJ, Kim DY, Mukai S. Simple, inexpensive technique for high-quality smartphone fundus photography in human and animal eyes. J Ophthalmol 2013;518479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maamari R, Keenan J, Fletcher D, Margolis T. A mobile phone-based retinal camera for portable wide field imaging. Br J Ophthalmol 2014;98:438–441 [DOI] [PubMed] [Google Scholar]
- 34.Navitsky C. The portable eye examination kit. Retina Today. 2013. Available at http://retinatoday.com/2013/12/the-portable-eye-examination-kit (last accessed May12, 2014)
- 35.Welch Allyn. iExaminer. Available at www.welchallyn.com/en/microsites/iexaminer.html (last accessed May21, 2014)
- 36.Myung D, Jais A, He L, Blumenkranz MS, Chang RT. 3D printed smartphone indirect lens adapter for rapid, high quality retinal imaging. J Mob Technol Med 2014;3:9–15 [Google Scholar]
- 37.Russo A, Morescalchi F, Costagliola C, Delcassi L, Semeraro F. Comparison of smartphone ophthalmoscopy with slit-lamp biomicroscopy for grading diabetic retinopathy. Am J Ophthalmol 2015;159:360–364.e1. [DOI] [PubMed] [Google Scholar]
- 38.Delori FC, Webb RH, Sliney DH. Maximum permissible exposures for ocular safety (ANSI 2000), with emphasis on ophthalmic devices. J Opt Soc Am A 2007;24:1250–1265 [DOI] [PubMed] [Google Scholar]
- 39.Telecom Regulatory Authority of India. Telecom subscription data as on 31st December, 2013. Available at www.trai.gov.in/WriteReadData/PressRealease/Document/PR-TSD-Dec,%2013-17022014.pdf (last accessed May21, 2014)
- 40.Aziz SR, Ziccardi VB. Telemedicine using smartphones for oral and maxillofacial surgery consultation, communication, and treatment planning. J Oral Maxillofac Surg 2009;67:2505–2509 [DOI] [PubMed] [Google Scholar]
- 41.Sachpazidis I, Ohl R, Kontaxakis G, Sakas G. TeleHealth networks: Instant messaging and point-to-point communication over the internet. Nucl Instrum Methods Phys Res A 2006;569:631–634 [Google Scholar]
- 42.Andrade R, Wangenheim AV, Bortoluzzi MK. Wireless and PDA: A novel strategy to access DICOM-compliant medical data on mobile devices. Int J Med Inform 2003;71:157–163 [DOI] [PubMed] [Google Scholar]
- 43.Lin B, Vassar JA. Mobile healthcare computing devices for enterprise-wide patient data delivery. Int J Mob Commun 2004;2:343–353 [Google Scholar]
- 44.Lamirel C, Bruce BB, Wright DW, Newman NJ, Biousse V. Nonmydriatic digital ocular fundus photography on the iPhone 3G: The Foto-Ed Study. Arch Ophthalmol 2012;130:939–940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chalam KV, Brar VS, Keshavamurthy R. Evaluation of modified portable digital camera for screening of diabetic retinopathy. Ophthalmic Res 2009;42:60–62 [DOI] [PubMed] [Google Scholar]
- 46.Fleming AD, Philip S, Goatman KA, Olson JA, Sharp PF. Automated assessment of diabetic retinal image quality based on clarity and field definition. Invest Ophthalmol Vis Sci 2006;47:1120–1125 [DOI] [PubMed] [Google Scholar]