Abstract
Background: Age is a critical factor in outcome for patients with well-differentiated thyroid cancer. Currently, age 45 years is used as a cutoff in staging, although there is increasing evidence to suggest this may be too low. The aim of this study was to assess the potential for changing the cut point for the American Joint Committee on Cancer/Union for International Cancer Control (AJCC/UICC) staging system from 45 years to 55 years based on a combined international patient cohort supplied by individual institutions.
Methods: A total of 9484 patients were included from 10 institutions. Tumor (T), nodes (N), and metastasis (M) data and age were provided for each patient. The group was stratified by AJCC/UICC stage using age 45 years and age 55 years as cutoffs. The Kaplan–Meier method was used to calculate outcomes for disease-specific survival (DSS). Concordance probability estimates (CPE) were calculated to compare the degree of concordance for each model.
Results: Using age 45 years as a cutoff, 10-year DSS rates for stage I–IV were 99.7%, 97.3%, 96.6%, and 76.3%, respectively. Using age 55 years as a cutoff, 10-year DSS rates for stage I–IV were 99.5%, 94.7%, 94.1%, and 67.6%, respectively. The change resulted in 12% of patients being downstaged, and the downstaged group had a 10-year DSS of 97.6%. The change resulted in an increase in CPE from 0.90 to 0.92.
Conclusions: A change in the cutoff age in the current AJCC/UICC staging system from 45 years to 55 years would lead to a downstaging of 12% of patients, and would improve the statistical validity of the model. Such a change would be clinically relevant for thousands of patients worldwide by preventing overstaging of patients with low-risk disease while providing a more realistic estimate of prognosis for those who remain high risk.
Introduction
The incidence of well-differentiated thyroid cancer (WDTC) is rapidly rising (1–4). The biology of WDTC is highly dependent on age, with young patients outperforming older patients in terms of survival (5). As such, staging of WDTC is unique among adult malignancies in that the American Joint Committee on Cancer/Union for International Cancer Control (AJCC/UICC) includes age within the staging system for this disease (6). All patients <45 years old are considered stage I, unless there is evidence of distant metastases, which is considered stage II disease. However, patients ≥45 years are staged dependent on tumor characteristics, nodal involvement, and distant metastatic disease in a fashion similar to squamous-cell carcinoma of the head and neck.
Age 45 years was chosen as a cutoff based on historical patient cohorts that gave rise to many of the early staging systems (7). The median age of most cohorts is 45 years, making this a convenient cutoff for categorical variable analysis. Recent trends, however, suggest that the average age of patients presenting with disease is increasing (8), thus placing more patients at risk of being placed at a higher-stage group. In addition, there is mounting evidence that age cutoff of 45 years is too low, and that many older patients remain at low risk of disease-specific death (9–11).
A recent analysis of survival for patients treated at Memorial Sloan Kettering Cancer Center (MSKCC) between 1986 and 2005 concluded that a change from 45 to 55 years in the current AJCC/UICC model would lead to a significant increase in the number of patients being considered at a lower stage, while maintaining the excellent outcomes for those patients considered to have early-stage disease (12). The aim of this study was to validate the proposed change in age cutoff in a large international cohort of patients.
Methods
Approaches were made to a number of international groups with an interest in thyroid cancer outcomes analysis. All centers received approval from their Institutional Review Board or equivalent. Only patients treated at the respective institutions were considered for inclusion. In order to ensure high-quality, reliable data, all groups were asked to provide clinician-collected data on tumor characteristics, nodal involvement, and distant metastatic disease (TNM), age at first treatment, disease-specific survival (DSS) status, and time to last follow-up.
A total of 10 institutions provided a total of eight data sets. The cohort included patients treated at MSKCC between 2005 and 2010. This MSKCC cohort was separate from the cohort used to develop the age 55 years model, who were treated between 1986 and 2005. Each institution was responsible for the accuracy of data provided. Following collation of the overall cohort at the coordinating center (MSKCC), any patients who could not be staged due to missing data were excluded. The remaining cohort was assigned an AJCC/UICC stage, and then re-staged using the AJCC/UICC model with age 55 years as a cutoff rather than age 45 years. Data from the Manitoba registry were also included. These data have previously been analyzed regarding the age cutoff for thyroid cancer staging, and for that reason, a subgroup analysis was also performed without these data (13,14).
In addition to analysis of the group as a whole, the outcomes of all patients who were effectively downstaged by the change were analyzed as a separate subgroup to confirm the effect on specific patient groups within the cohort.
The Kaplan–Meier method was used to express outcomes. A concordance probability estimate (CPE) was used to estimate the degree of concordance between stage and observed outcomes for each model (15). Ties from the calculation of CPE were excluded to prevent untoward consequences of a small number of prognostic categories.
Results
In total, 10 institutions provided data in eight separate data sets, which were combined for two units. In total, the combined data set described 9484 patients with a median follow-up of 5.3 years (range 0–44 years). The 10-year DSS for the entire cohort was 96.9%. A total of 224 patients died of disease. The details of the patients from each data set and the combined cohort, including patient number, follow-up, and DSS, are shown in Table 1.
Table 1.
Data Relating to Each Contributing Data Set and Combined Cohort, Including Patient Number, Follow-Up, and Disease-Specific Survival
| Institution | Total contribution | Inclusion dates | Median follow-up of censored patients (years) | Range follow-up of censored patients (years) | DSS—5 years | DSS—10 years |
|---|---|---|---|---|---|---|
| Combined | 9484 | 1963–2012 | 5.3 | 0–44.0 | 98.3% | 96.9% |
| Memorial Sloan Kettering Cancer Center | 2173 | 2005–2010 | 3.3 | 0–9.5 | 99.10% | Not reached |
| University of Manitoba | 1998 | 1970–2010 | 11.4 | 0–43.9 | 97.20% | 95.70% |
| University of California, San Francisco | 1851 | 1994–2004 | 4.6 | 0–25.4 | 98.20% | 97.40% |
| University of Sydney Endocrine Surgical Unit | 1129 | 1963–2012 | 3.0 | 0–44.0 | 98.20% | 96.20% |
| Mount Sinai Hospital, Toronto | 925 | 1963–2011 | 6.0 | 0–43.9 | 99.10% | 97.50% |
| Endocrine Service, Instituto Nacional do Cancer/Universidade Federal do Rio de Janeiro | 646 | 1986–2014 | 8.3 | 2.7–28 | 99.40% | 97.30% |
| Barretos Cancer Hospital/ACCamargo Cancer Center | 519 | 1980–2001 | 7.5 | 0–25.1 | 96.90% | 96.10% |
| MD Anderson | 243 | 2005–2012 | 3.8 | 0.5–8.3 | 100% | Not reached |
DSS, disease-specific survival.
The median age of the cohort was 45 years (range 4–96 years). Almost half of the patients (49.1%) had T1 tumors, and most (69.7%) were N0/NX. Nearly all patients had no metastatic disease, and so they were recorded as M0/MX (97.4%). Table 2 shows the breakdown of all elements required for AJCC/UICC staging.
Table 2.
Entire Cohort Stratified by Variables Required for AJCC/UICC Staging Model
| Variable | N (%) |
|---|---|
| Age | |
| <45 years | 4546 (47.9) |
| ≥45 years | 4938 (52.1) |
| <55 years | 6648 (70.1) |
| ≥55 years | 2836 (29.9) |
| T Stage | |
| T1 | 4655 (49.1) |
| T2 | 1847 (19.5) |
| T3 | 2307 (24.3) |
| T4 | 510 (5.4) |
| T0/X | 165 (1.7) |
| N Stage | |
| N0/Nx | 6611 (69.7) |
| N1a | 1424 (15) |
| N1b | 1391 (14.7) |
| N1 NOS | 58 (0.6) |
| M Stage | |
| M0/MX | 9234 (97.4) |
| M1 | 250 (2.6) |
AJCC/UICC, American Joint Committee on Cancer/Union for International Cancer Control.
Initially, patients were staged with the AJCC/UICC staging system using 45 years as the cutoff for age. A total of 6600 patients (69.6%) were reported as stage I, 741 patients (7.8%) as stage II, 1230 (13%) as stage III, and 913 (9.6%) as stage IV. The 10-year survival rates for stages I–IV were 99.7%, 97.3%, 96.6%, and 76.3%, respectively. Figure 1A shows the Kaplan–Meier plot for the AJCC/UICC staging with an age cutoff of 45 years.
FIG. 1.
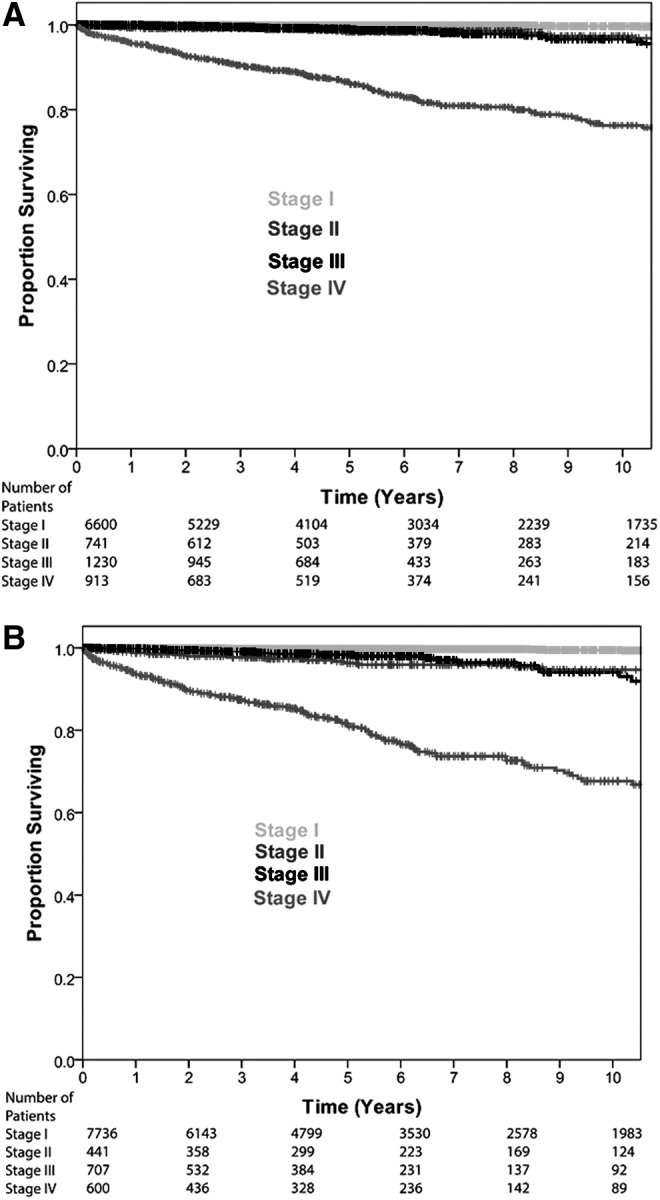
Comparison of disease-specific survival by American Joint Committee on Cancer/Union for International Cancer Control (AJCC/UICC) staging system using an age cutoff of (A) 45 years and (B) 55 years.
The same cohort of patients was then staged with the AJCC/UICC staging system using 55 years as the cutoff for age. Similarly, stage I had the most patients with 7736 patients (81.5%). Stages II–IV made up less than a quarter of the total number of patients. Stage II had the fewest number of patients (441), stage III had 707 patients (7.5%), and stage IV had 600 patients (6.3%). The 10-year survival rates for stages I–IV were 99.5%, 94.7%, 94.1%, and 67.6%, respectively. Figure 1B shows the Kaplan–Meier curve for the AJCC/UICC staging with an age cutoff of 55 years.
The stage distributions of the entire cohort using age 45 years and age 55 years as a cutoff are shown in Table 3.
Table 3.
Entire Cohort Described by AJCC/UICC Staging Model Using Cutoffs Age of 45 Years and 55 Years
| AJCC/UICC staging | N (%) | 10-year DSS |
|---|---|---|
| Cutoff of 45 years | ||
| I | 6600 (69.6) | 99.7% |
| II | 741 (7.8) | 97.3% |
| III | 1230 (13.0) | 96.6% |
| IV | 913 (9.6) | 76.3% |
| Cutoff of 55 years | ||
| I | 7736 (81.5) | 99.5% |
| II | 441 (4.6) | 94.7% |
| III | 707 (7.5) | 94.1% |
| IV | 600 (6.3) | 67.6% |
By changing the age cutoff in the AJCC/UICC staging system from 45 to 55 years, 1165 patients were downstaged (12.3%). A total of 329 patients (3.5%) went from stage II with the cutoff of 45 years to stage I with the cutoff of 55 years. A total of 523 patients (5.5%) were downstaged from stage III to stage I; 284 patients (3.0%) were downstaged from stage IV to stage I. Additionally, 29 patients (<1%) changed from stage IV to stage II. These data are shown in Table 4.
Table 4.
Changes in AJCC/UICC Staging When Age 45 Years Is Substituted for Age 55 Years
| Age 55 I | Age 55 II | Age 55 III | Age 55 IV | |
|---|---|---|---|---|
| AJCC/UICC 45 I | 6600 | 0 | 0 | 0 |
| AJCC/UICC 45 II | 329 | 412 | 0 | 0 |
| AJCC/UICC 45 III | 523 | 0 | 707 | 0 |
| AJCC/UICC 45 IV | 284 | 29 | 0 | 600 |
The DSS rate of those 1165 patients directly affected by the change in staging model was 97.6% at 10 years. When stratified by new stage, those patients downstaged to stage I (1136; 98%) have a 10-year DSS rate of 98.2%, whereas those downstaged to stage II (29, 2%) have a 10-year DSS rate of 75.5% (Fig. 2).
FIG. 2.
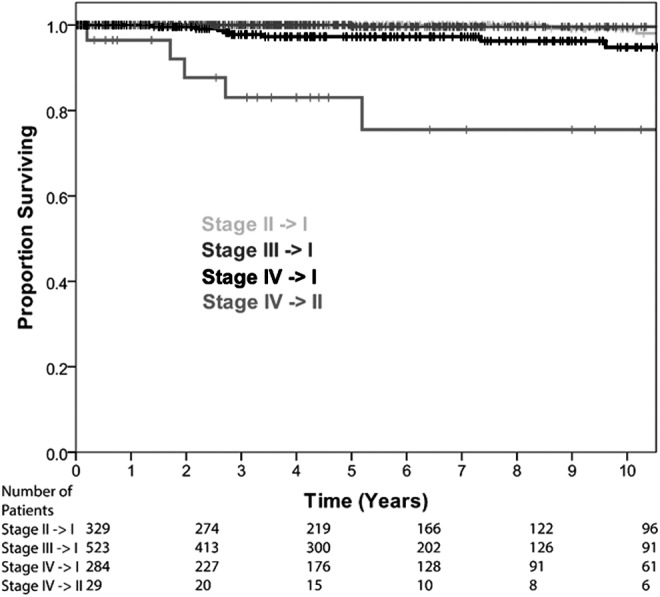
Disease-specific survival of patients directly affected by the proposed change stratified by AJCC/UICC stage change.
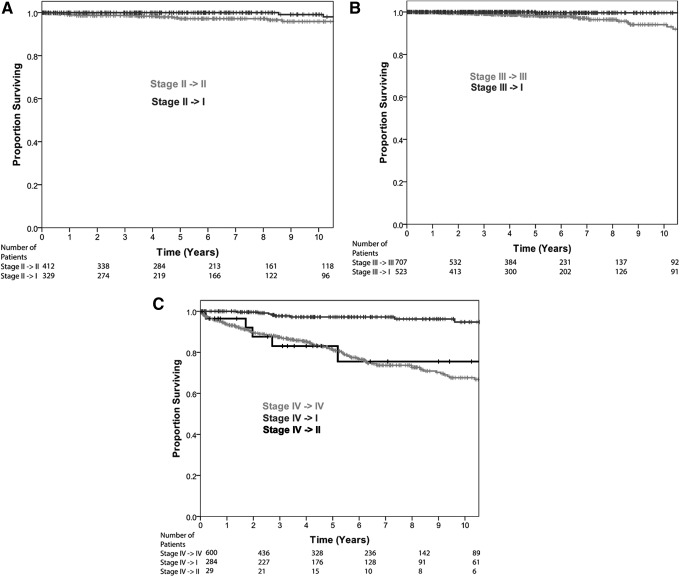
Detailed analysis of the effect of the proposed staging changes on each current stage group is shown in Figure 3A–C. Patients currently considered AJCC/UICC stage II (age ≥45 years T2N0M0, or young patients who are M1) continue to have excellent outcomes. As expected, those older patients with T2N0M0 disease who are considered as stage I with the proposed change have slightly superior outcomes to those who remain stage II (10-year DSS 99.1% vs. 95.9%; p = 0.027; Fig. 3A). Those patients currently considered to have stage III disease (≥45 years with T3N0M0 or T1-3N1aM0 disease) may either remain in stage III if ≥55 years or transition to stage I disease (those between 45 and 54 years). Those younger patients who are considered stage I with the proposed change have excellent outcomes compared with those who remain in stage III (10-year DSS 99.6% vs. 94.1%; p < 0.001; Fig. 3B). Patients currently considered stage IV are aged >45 years with advanced local (T4) regional (N1b), or distant (M1) disease. Those patients who are M0 (T4/N1b) and are aged between 45 and 54 years transition to stage I and have excellent outcomes (10-year DSS 94.8%). In contrast, those who transition to stage II disease (age 45–54 years M1) have poor outcomes compared with the current stage IV group (10-year DSS 75.5% vs. 67.6%; p = 0.7; Fig. 3C). Only 29 (0.3%) patients transition into stage II disease.
FIG. 3.
Detailed analysis of disease-specific survival by initial and re-stage groupings (A) initially stage II on AJCC/UICC, (B) initially stage III on AJCC/UICC, and (C) initially stage IV on AJCC/UICC.
The CPE calculated for the AJCC model using age 45 years as a cutoff is 0.90 (standard error 0.02) compared with 0.92 (standard error 0.01) for the same model using age 55 years as a cutoff, suggesting an improvement.
Subgroup analysis with exclusion of the Manitoba data set was performed in an identical manner. Again, an improvement in CPE was seen when the cutoff was moved from 45 to 55 years (CPE 45-year cutoff 0.87 [SE = 0.03]; CPE 55-year cutoff 0.89 [SE = 0.03]).
Discussion
WDTC is being diagnosed with increased frequency worldwide. Few patients die of the disease, and therefore the vast majority of patients should not be considered to be at an advanced stage or high risk at the time of diagnosis.
Following the recognition in the 1960s and 1970s that histology was critical in predicting outcome for those patients with thyroid cancer, refinements in risk prediction were based on the experience of major centers across the world (16–19).
In 1976, Cady et al. published work that showed the association of age with outcome. They stratified their cohort as age <40 years, 40–50 years, and >50 years.
In 1986, the Mayo Clinic reported outcomes for 859 patients with papillary thyroid cancer (PTC) treated between the 1940s and 1970s. Their results suggested that older age as well as extrathyroidal extension and distant metastases were predictors of disease-specific death. A number of groups, including Mazzaferri et al., reported similar findings (20,21).
The Mayo Clinic combined the risk factors of age, tumor grade, extent of disease, and size of primary lesion in to the AGES system for risk prediction, which was an early method of disease staging (17). Staging patients allowed low- and high-risk groups to be identified. This would later be refined to include completeness of surgical resection and reported as a MACIS score (22), which applied a greater weight to age from the cutoff of 40 years.
The experience of >800 patients managed at the Lahey Clinic over four decades was reported by Cady et al. in 1988 (18). They found similar results, introducing the AMES system, which included age, distant metastases, extrathyroidal extension, and size, again to stratify patients into high- and low-risk groups. This system used a different age cutoff dependent on sex (41 years for men and 51 years for women).
A similar group of risk factors was reported by Shah et al. from MSKCC (19), which gave rise to the GAMES system of stratification (including tumor grade on histology) (23). Age 45 years was chosen as the cutoff for this system. Sherman et al. reported the findings of the National Thyroid Cancer Treatment Cooperative Study in 1998, again using age 45 years as a cutoff for PTC (24).
The AJCC published the second edition of its staging manual in 1977 (25), and first used an age cutoff of 45 years in 1983 (26). This has remained in use since that time, and has gained international acceptance (6). Age 45 years is used as a cutoff, with younger patients being limited to stage II disease in the presence of distant metastasis and stage I without. Patients who are ≥45 years are stage I or stage II with T1N0M0 or T2N0M0 disease, respectively. If older patients have pT3 primary disease or metastases to the central neck nodes, they are considered stage III. Those with pT4 primary disease, superior mediastinal, lateral neck, or distant metastases are considered stage IV.
Although younger patients outperform older patients in terms of survival, irrespective of the age cutoff selected, clinical experience led to the observation that many older patients remained at low risk of disease-specific death, despite the stage grouping assigned by the AJCC/UICC model.
This observation led to an analysis of the MSKCC institutional database in order to determine a statistically robust age cutoff using the current AJCC/UICC model. A process of recursive partitioning was used to determine that although no cutoff was perfect, age 55 years was more suitable than age 45 years (12). Such a change resulted in a wider spread of DSS between stage groups (stage I–IV for AJCC/UICC using 45 years 99.6–81% vs. 99.2–74%) while resulting in 12% of patients being downstaged. The vast majority of the downstaged patients (97%) were then considered as stage I disease, and the DSS rate of this specific group of patients was 98% at 10 years. This analysis suggested that using an age cutoff of 45 years leads to a significant number of patients who are at very low risk of death being inappropriately assigned an advanced disease stage. Not only does this approach lead to heightened patient anxiety, but it also encourages clinicians to recommend more aggressive primary therapy in a group of patients with little potential for gain. In addition, those patients who remain in stage groups III and IV have outcomes more in keeping with the label of advanced disease (67.6% 10-year DSS in stage IV patients) (12).
International reports of the analysis of multiple institutional data sets have also suggested that age 45 years may not be the ideal cutoff for WDTC. Analysis of the National Thyroid Cancer Treatment Cooperative Study Group by Jonklaas et al. questioned whether a cutoff >45 years would improve current staging systems in a U.S. population (10). Study of a Japanese cohort of around 1000 patients by Ito et al. revealed that a change in survival was observed at 60 years (27).
Analysis of the Surveillance, Epidemiology, and End Results (SEER) database has also scrutinized the relationship between age and outcome. Oyer et al. demonstrated that prognosis remains unaffected by age until 35 years (28). Although Bischoff et al. found that survival deteriorated with age, no specific inflection point was seen at 45 years, and survival for all patients <65 years exceeded 90%, again raising the question of whether a cutoff of 45 years is appropriate (9). More recently, Kim et al. included >35,000 patients treated between 1988 and 2010, and found age 57 years to be the optimal age cutoff for determining DSS (29).
However, investigation into outcomes from WDTC is limited by the number of events. With such excellent overall outcomes, large cohorts and long follow-up are required. Although national registries such as SEER and the National Cancer Data Base provide such a resource, they lack critical detail, including that relating to lymph node characteristics, which may have a significant impact on the overall results (30). In contrast, single institutional data sets provide high-quality data, usually collected by clinicians and subjected to rigorous quality control. However, such resources are limited by patient numbers.
The aim of this study was to attempt to address the weaknesses of both approaches by combining a group of clinician-collected data sets into one multi-institutional cohort, which would provide large patient numbers and reliable clinical data.
The results show that a change in the age cutoff for the AJCC/UICC model would impact a large number of patients. The change leads to a widening of DSS outcomes at 10 years: 99.7–76.3% for age 45 years to 99.5–67.6% for 55 years. More importantly, however, 12% of patients are downstaged by the change, with 9% moving from “advanced” stage III or IV disease to stage I or II disease. It is critical to note that despite this downstaging, outcomes in the stage I and II disease categories remain excellent (99.5% and 94.7% 10-year DSS, respectively).
Patients who would be affected by this change are those between the ages of 45 and 54 years. A change in age cutoff would render all patients in this age category as stage I, unless they were M1 at presentation, which would result in assignment to stage II. The overwhelming majority of this group (98%) would be restaged to AJCC/UICC stage I. These patients have a 10-year DSS rate of 98.2%, suggesting that they are currently incorrectly assigned to a more advanced stage category. For almost all patients who are currently considered stage II–IV, the change in staging is appropriate, with 10-year DSS rates in re-staged patients of ≥95% (Fig. 3A–C).
Those patients who are between 45 and 54 years of age and have M1 disease would be restaged as stage II. The patients in this group have less favorable outcomes (10-year DSS 75.5%; Fig. 3C). This change has a number of detrimental effects on the overall staging system. The impact on stage II disease overall is to lower the DSS rate from 97.3% with age 45 years as a cutoff to 94.7% at 10 years with age 55 years as a cutoff. This results in the DSS of stage II being slightly lower than stage III (96.3% stage II vs. 98.3% stage III) due to the outcome of patients aged 45–54 years with distant metastases. In addition, these specific patients would be assigned to a stage group with significantly better outcomes than they would have if analyzed as a separate subgroup (96.3% vs. 83%). However, the number of patients who fall in this group is small (2% of those re-staged and 0.3% of the entire cohort in this study). In addition, those patients who present with distant metastases do not present a management dilemma, as all will be treated with total thyroidectomy, appropriate neck dissection, if indicated, and postoperative radioactive iodine (RAI).
The patients with limited disease represent the group in whom treatment remains most controversial. The need for aggressive treatment to primary and potential metastatic disease in terms of total thyroidectomy, elective central neck dissection, and postoperative RAI is unproven in the majority of these cases. Indeed, the approach to therapy will be tailored to the perceived nature of the disease on an individual basis. This highlights the importance of avoiding unnecessary overstaging of patients with biologically non-aggressive disease.
In particular, the presence of limited regional disease (particularly small-volume N1a disease) and moderately locally advanced (T3) disease, which can be managed surgically, currently upstages older patients to stage III, and very few will go on to die of their disease. The current results show that this group have excellent outcomes, irrespective of whether they are 45–54 years (99.6% 10-year DSS) or >55 years (94.1 10-year DSS), demonstrating the fact that such disease features do not seem to reflect aggressive disease, irrespective of age.
Assessment of the discriminatory power of the two staging models shows that both age cutoffs perform well. Using the current system, the CPE is 0.90. However, this rises to 0.92 when the cutoff of age 55 is applied. This suggests that such a change would be both clinically relevant and statistically robust.
This work is not without limitations. All groups collected data retrospectively, which limits the conclusions that can be drawn from any such study. In addition, diagnostic, treatment, and follow-up regimens vary between groups and have evolved over the decades described. Although this study includes a high number of patients with data from reliable sources, the follow-up time was limited. The vast majority of surgically treated recurrences occur within two years of initial therapy (31). However, it is likely that events will continue to occur beyond the five-year median reported here. It is also clear that no single age cutoff will be perfect. Analysis of outcomes for patients managed at MSKCC between 1986 and 2010 (including both the cohort of patients used to generate the initial model and patients used in this study) suggest that no specific cutoff is ideal (32). Indeed, it is likely that modular systems based upon continuous variables will outperform any such system. However, the AJCC/UICC model is simple to apply and internationally accepted. For this reason, it has been widely adopted, and the AJCC/UICC committee is not likely to abandon the system in the next edition of the staging manual. Patients who transition into stage II have poor outcomes using this system (75.5% 10-year DSS). This has a limited impact on the overall performance of patients with stage II disease, and affects only 29 patients, which constitutes <0.5% of the overall cohort.
This work represents the largest multi-institutional clinician-collected cohort of patients treated for WDTC reported to date. Prospective studies of this size on this subject are not feasible, and cohorts described in national registries lack the accurate data required to draw robust conclusions in relation to disease-specific outcomes.
In conclusion, the present results suggest that the AJCC/UICC should consider a change in the staging model for WDTC to incorporate an age cutoff of 55 years rather than 45 years. Such a change would improve the distribution of outcomes between stage I and IV disease and lead to a downstaging of 12% of patients. Almost 10% of low-risk patients would move out of the advanced disease stage category, which would maintain excellent outcomes in those with stage I–III disease while providing more accurate prognostic information for those considered to have stage IV disease. This improved recognition of disease biology would enable clinicians to counsel their patients more accurately and select appropriate therapy accordingly.
Acknowledgment
This research was funded in part through NIH/NCI Cancer Center Support Grants P30 CA008748.
Author Disclosure Statement
The authors have nothing to disclose.
References
- 1.Cramer JD, Fu P, Harth KC, Margevicius S, Wilhelm SM. 2010. Analysis of the rising incidence of thyroid cancer using the Surveillance, Epidemiology and End Results national cancer data registry. Surgery 148:1147–1152; discussion 1152–1143 [DOI] [PubMed] [Google Scholar]
- 2.Davies L, Welch HG. 2010. Thyroid cancer survival in the United States: observational data from 1973 to 2005. Arch Otolaryngol Head Neck Surg 136:440–444 [DOI] [PubMed] [Google Scholar]
- 3.Enewold L, Zhu K, Ron E, Marrogi AJ, Stojadinovic A, Peoples GE, Devesa SS. 2009. Rising thyroid cancer incidence in the United States by demographic and tumor characteristics, 1980–2005. Cancer Epidemiol Biomarkers Prev 18:784–791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen AY, Jemal A, Ward EM. 2009. Increasing incidence of differentiated thyroid cancer in the United States, 1988–2005. Cancer 115:3801–3807 [DOI] [PubMed] [Google Scholar]
- 5.Haymart MR. 2009. Understanding the relationship between age and thyroid cancer. Oncologist 14:216–221 [DOI] [PubMed] [Google Scholar]
- 6.Edge SB; American Joint Committee on Cancer 2010. AJCC Cancer Staging Manual. Seventh edition. Springer, New York: [DOI] [PubMed] [Google Scholar]
- 7.Cady B, Sedgwick CE, Meissner WA, Wool MS, Salzman FA, Werber J. 1979. Risk factor analysis in differentiated thyroid cancer. Cancer 43:810–820 [DOI] [PubMed] [Google Scholar]
- 8.Hughes DT, Haymart MR, Miller BS, Gauger PG, Doherty GM. 2011. The most commonly occurring papillary thyroid cancer in the United States is now a microcarcinoma in a patient older than 45 years. Thyroid 21:231–236 [DOI] [PubMed] [Google Scholar]
- 9.Bischoff LA, Curry J, Ahmed I, Pribitkin E, Miller JL. 2013. Is above age 45 appropriate for upstaging well-differentiated papillary thyroid cancer? Endocr Pract 19:995–997 [DOI] [PubMed] [Google Scholar]
- 10.Jonklaas J, Nogueras-Gonzalez G, Munsell M, Litofsky D, Ain KB, Bigos ST, Brierley JD, Cooper DS, Haugen BR, Ladenson PW, Magner J, Robbins J, Ross DS, Skarulis MC, Steward DL, Maxon HR, Sherman SI, National Thyroid Cancer Treatment Cooperative Study G 2012. The impact of age and gender on papillary thyroid cancer survival. J Clin Endocrinol Metab 97:E878–887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McLeod DS, Jonklaas J, Brierley JD, Ain KB, Cooper DS, Fein HG, Haugen BR, Ladenson PW, Magner J, Ross DS, Skarulis MC, Steward DL, Xing M, Litofsky DR, Maxon HR, Sherman SI. 2015. Reassessing the NTCTCS staging systems for differentiated thyroid cancer, including age at diagnosis. Thyroid 25:1097–1105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nixon IJ, Kuk D, Wreesmann V, Morris L, Palmer FL, Ganly I, Patel SG, Singh B, Tuttle RM, Shaha AR, Gonen M, Shah JP. 2016. Defining a valid age cutoff in staging of well-differentiated thyroid cancer. Ann Surg Oncol 23:410–415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hendrickson-Rebizant J, Sigvaldason H, Nason RW, Pathak KA. 2015. Identifying the most appropriate age threshold for TNM stage grouping of well-differentiated thyroid cancer. Eur J Surg Oncol 41:1028–1032 [DOI] [PubMed] [Google Scholar]
- 14.Mazurat A, Torroni A, Hendrickson-Rebizant J, Benning H, Nason RW, Pathak KA. 2013. The age factor in survival of a population cohort of well-differentiated thyroid cancer. Endocr Connect 2:154–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heller G, Mo Q. 2015. Estimating the concordance probability in a survival analysis with a discrete number of risk groups. Lifetime Data Anal 2015. May 29 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Byar DP, Green SB, Dor P, Williams ED, Colon J, van Gilse HA, Mayer M, Sylvester RJ, van Glabbeke M. 1979. A prognostic index for thyroid carcinoma. A study of the E.O.R.T.C. Thyroid Cancer Cooperative Group. Eur J Cancer 15:1033–1041 [DOI] [PubMed] [Google Scholar]
- 17.Hay ID, Grant CS, Taylor WF, McConahey WM. 1987. Ipsilateral lobectomy versus bilateral lobar resection in papillary thyroid carcinoma: a retrospective analysis of surgical outcome using a novel prognostic scoring system. Surgery 102:1088–1095 [PubMed] [Google Scholar]
- 18.Cady B, Rossi R. 1988. An expanded view of risk-group definition in differentiated thyroid carcinoma. Surgery 104:947–953 [PubMed] [Google Scholar]
- 19.Shah JP, Loree TR, Dharker D, Strong EW, Begg C, Vlamis V. 1992. Prognostic factors in differentiated carcinoma of the thyroid gland. Am J Surg 164:658–661 [DOI] [PubMed] [Google Scholar]
- 20.Mazzaferri EL, Young RL, Oertel JE, Kemmerer WT, Page CP. 1977. Papillary thyroid carcinoma: the impact of therapy in 576 patients. Medicine (Baltimore) 56:171–196 [PubMed] [Google Scholar]
- 21.Mazzaferri EL, Young RL. 1981. Papillary thyroid carcinoma: a 10 year follow-up report of the impact of therapy in 576 patients. Am J Med 70:511–518 [DOI] [PubMed] [Google Scholar]
- 22.Hay ID, Bergstralh EJ, Goellner JR, Ebersold JR, Grant CS. 1993. Predicting outcome in papillary thyroid carcinoma: development of a reliable prognostic scoring system in a cohort of 1779 patients surgically treated at one institution during 1940 through 1989. Surgery 114:1050–1057; discussion 1057–1058. [PubMed] [Google Scholar]
- 23.Shaha AR, Shah JP, Loree TR. 1996. Risk group stratification and prognostic factors in papillary carcinoma of thyroid. Ann Surg Oncol 3:534–538 [DOI] [PubMed] [Google Scholar]
- 24.Sherman SI, Brierley JD, Sperling M, Ain KB, Bigos ST, Cooper DS, Haugen BR, Ho M, Klein I, Ladenson PW, Robbins J, Ross DS, Specker B, Taylor T, Maxon HR., 3rd 1998. Prospective multicenter study of thyroid carcinoma treatment: initial analysis of staging and outcome. National Thyroid Cancer Treatment Cooperative Study Registry Group. Cancer 83:1012–1021 [DOI] [PubMed] [Google Scholar]
- 25.American Joint Committee on Cancer Staging and End Results Reporting, American Cancer Society 1977. Manual for Staging of Cancer, 1977. American Joint Committee for Cancer Staging and End-Results Reporting, Chicago, IL [Google Scholar]
- 26.American Joint Committee on Cancer 1983 Manual for Staging of Cancer. J.B. Lippincott, Philadelphia, PA [Google Scholar]
- 27.Ito Y, Miyauchi A, Kihara M, Takamura Y, Kobayashi K, Miya A. 2012. Relationship between prognosis of papillary thyroid carcinoma patient and age: a retrospective single-institution study. Endocr J 59:399–405 [DOI] [PubMed] [Google Scholar]
- 28.Oyer SL, Smith VA, Lentsch EJ. 2012. Reevaluating the prognostic significance of age in differentiated thyroid cancer. Otolaryngol Head Neck Surg 147:221–226 [DOI] [PubMed] [Google Scholar]
- 29.Kim SJ, Myong JP, Suh H, Lee KE, Youn YK. 2015. Optimal cutoff age for predicting mortality associated with differentiated thyroid cancer. PloS One 10:e0130848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mehra S, Tuttle RM, Milas M, Orloff L, Bergman D, Bernet V, Brett E, Cobin R, Doherty G, Judson BL, Klopper J, Lee S, Lupo M, Machac J, Mechanick JI, Randolph G, Ross DS, Smallridge R, Terris D, Tufano R, Alon E, Clain J, DosReis L, Scherl S, Urken ML. 2015. Database and registry research in thyroid cancer: striving for a new and improved national thyroid cancer database. Thyroid 25:157–168 [DOI] [PubMed] [Google Scholar]
- 31.Young S, Harari A, Smooke-Praw S, Ituarte PH, Yeh MW. 2013. Effect of reoperation on outcomes in papillary thyroid cancer. Surgery 154:1354–1361; discussion 1361–1352. [DOI] [PubMed] [Google Scholar]
- 32.Ganly I, Nixon IJ, Wang LY, Palmer FL, Migliacci JC, Aniss A, Sywak M, Eskander A, Freeman JL, Campbell MJ, Shen WT, Vaisman F, Momesso D, Corbo R, Vaisman M, Shaha ARM, Tuttle RMM, Shah JP, Patel SG. 2015. Survival from differentiated thyroid cancer What has age got to do with it? Thyroid 25:1106–1114 [DOI] [PMC free article] [PubMed] [Google Scholar]