Abstract
Background
To explore the efficacy of tranexamic acid (TXA) on reducing hidden blood loss (HBL) in total knee arthroplasty (TKA) by conducting a comparative study and meta-analysis.
Material/Methods
A total of 108 patients underwent TKA was equally distributed to experimental and control groups. The only difference between two groups was the administrations of 15 mg of TXA mixed in 100 mL normal saline for experimental group and 100 mL of normal saline for control group. The volumes of blood loss, red blood loss (RBL) were recorded, calculated and analyzed. Stata 12.0 software was applied for data analysis.
Results
The intraoperative and postoperative blood loss volume in experimental group were remarkably reduced compared with those in control group (intraoperative: 105.1±12.1 mL vs. 185.5±20.3 mL, P<0.001; postoperative: 220.7±16.8 mL vs. 290.5±22.4 mL, P<0.001). Accordingly, the control group had significantly higher transfusion rate than experimental group (3.7% vs.25.9%, P=0.001). Our results also found that both the measured and hidden RBL were obviously reduced in experimental group compared with control group (measured RBL: 96.9±11.8 mL vs. 135.2±13.5 mL, P<0.001; hidden RBL: 170.8±37.2 mL vs. 364.2±41.5 mL, P<0.001). Furthermore, meta-analysis confirmed that TXA can notably decrease HBL (SMD=2.68, 95%CI=1.55~3.80, P<0.001).
Conclusions
TXA can significantly reduce the intraoperative and postoperative blood loss and HBL, therefore decreasing the transfusion need in TKA.
MeSH Keywords: Blood Loss, Surgical; Meta-Analysis; Tranexamic Acid
Background
Total knee arthroplasty (TKA) remains one of the most effective treatments for advanced knee arthritis to correct deformity, relieve the repeatable pain and maintain stability of the knee [1]. The requirement of hip and knee arthroplasties in United States has been steadily increasing [2]. However, there can be considerable blood loss during the TKA which may be due to the extensive dissections and increased fibrinolysis [3]. Moreover, TKA usually complicated with higher volume of hidden blood loss (HBL) [4]. HBL refers to the intraoperative hemorrhagic volume, postoperative drainage and volume of blood spread in to tissue space and articular cavity [5,6]. And it has been reported that the early postoperative HBL can significantly contribute to the postoperative lower limb swelling [7]. Currently, remarkable blood loss after TKA as well as postoperative blood transfusion requirement remains a major concern for patients undergone TKA [8]. However, given its significant impact on TKA patients, HBL has not received enough attention [9].
Nowadays, plenty of blood-conserving techniques have been introduced in TKA procedure to decrease blood loss and transfusion requirements, including controlled hypotension, cell salvage, use of drain, the use of tourniquet and antifibrinolytic agents [10]. Tourniquet is commonly used in TKA to obtain a clear surgical field and to reduce intraoperative blood loss [11]. However, the main advantages of tourniquet remain controversial and evidence has been demonstrated that the application of tourniquet in TKA has not been shown to improve the performance and may increase the risk of complications [11]. In recent years, antifibrinolytic agents such as tranexamic acid (TXA) have gained widely popularity in TKA surgery to decrease blood loss and transfusion [12]. Among the available antifibrinolytic agents, TXA is believed to have the maximum of antifibrinolytic potency and minimum of side effects [13]. Therefore, it has been widely applied to stop bleeding in dental surgery, cardiac surgery, heavy menstrual bleeding [14,15]. TXA is a reversible lysine analogue that competes with the lysine residues on fibrin for the binding of plasminogen thus inhibiting the fibrinolysis and stabilizing clot [16]. It has been suggested that TXA is safe and effective on reducing postoperative bleeding in orthopedic procedures [17]. The role of TXA in decreasing death risk within the first three hours in patients who suffered from heavy blood due to trauma was well documented [18]. Moreover, a variety of studies confirmed that TXA can serve a potent inhibitor in reducing blood loss during TKA [8,19,20]. However, limited information has been presented regarding the efficiency of TXA in reducing HBL. Therefore, to further clarify the role of TXA regarding HBL in TKA surgery, a comparative study and a meta-analysis was also performed.
Material and Methods
Ethics statement
The study was performed with the approval of the Ethics committee of West China Center of Medical Sciences, Sichuan University. All patients provided written informed consents before study. All procedures in this study were in accordance with the Helsinki Declaration [21].
Subject
From March 2012 to December 2014, 108 patients in our hospital prepared for unilateral TKA or one-stage bilateral TKA were included in current study. Patients were excluded if they presented with any blood disease, or diabetes, or any coagulation disorders or any history of thromboembolism. Finally, the 108 patients underwent TKA (49 males and 59 females) with mean age of 65.8±11.2 years (range 45~78 years) were randomly assigned to experimental group and control group (1:1) using a simple randomization test.
Intervention methods
All procedures were performed by surgeons in the same team and all implants were cement artificial prostheses. The only treatment difference between groups was the administrations of TXA and normal saline (intravenous drip). The surgery duration was controlled under 90 min. Patients were under intravenous combined anesthesia before surgery. An incision was made by knee centric notch inside knee cap through medial patella for patella repair. Limb tourniquet and exsanguination were applied preoperatively and the pressures for tourniquet were 100 mmHg higher than patients’ systolic pressure. Drains were left in situ for postoperative bleeding, followed by incision closure and pressure bandaging. Blood pressure were monitored and measured each day in the morning using an automated device (Omron model 705 IT; Omron Corporation – Healthcare, Kyoto, Japan). Coded ampoules containing either TXA (100 mg/mL Cyklokapron; Pharmacia) or saline as placebo were prepared by Apoteksbolaget, Umeå, Sweden. Immediately after the operation, the patients in experimental group received an administration of TXA (15 mg/kg) mixed in 100 mL normal saline, while control group were given for the same volume of normal saline [22]. In the morning, 5 mL venous blood was extracted from each patient with empty stomach. Then blood samples were added with ethylenediaminetetraacetic acid disodium salt (EDTA-Na2) for anticoagulation and maintained at room temperature for 30 min. After centrifugation at 3000r/min for 10 min, the samples were under supernatant separation and saved at Eppendorf (EP) tubes in a refrigerator at −80°C for further usage. Enzyme linked immunosorbent assay (ELISA) was applied for blood test using an automatic microplate reader (Finland, Thermo muhiscan ascent).
Outcome assessment
The volumes of blood loss, drainage and transfusion in each group were recorded to calculate the measured/hidden red blood loss (RBL). Hematocrit (Hct) was recorded preoperatively and 72 h postoperatively. Coagulation indexes including contractinogen (Fib), prothrombin time (PT) and activated partial thromboplastin time (APPT) were observed intraoperatively and postoperatively. Transfusion volume and transfusion requirements during the whole procedure were recorded for further analysis. Measured blood loss refers to the intraoperative and postoperative blood loss. In this case, with the application of tourniquet and exsanguination, the blood loss during the surgery was negligible. Postoperative blood loss refers to the sum of drainage and net increase of bandages. Routine blood tests were done in the morning on the preoperative day and on the first and third postoperative day to record hemoglobin and Hct.
Patient’s blood volume (PBV) was estimated according to the formula of Nadler et al. [23]. PBV= k1 × height (m) + k2 × weight (kg) + k3; where k1 = 0.3669, k2 = 0.03219, k3 = 0.6041, for men; and k1 = 03561, k2 = 0.03308, k3 = 0.1833 for women. HBL was calculated based on Gross equation [24]: Total blood loss = PBV × (preoperative Hct – postoperative Hct); Measured blood loss = (intraoperative blood loss + postoperative drainage) × (preoperative Hct + postoperative Hct) ÷ 2; HBL = total blood loss − measured blood loss + RBL transfusion.
Statistical analysis
All data analyses were performed by use of Stata 12.0 (Stata Corporation, College Station, TX, USA). Categorical data are presented with percentages and frequency counts. The χ2 test was used to compare the percentages and frequencies, and continuous data are presented with mean ± standard deviation (mean ±SD). PubMed, WANFANG, China National Knowledge Infrastructure (CNKI), and VIP databases were thoroughly searched for articles published prior to 2013, utilizing the search terms (“tranexamic acid” or “AMCHA” or “TXA”) and (“arthroplasty replacement knee” or “total knee replacement” or “TKA”) and (“Hidden blood loss” or “HBL”). More than 2 investigators were employed for data extraction, with disagreement solved by discussion or consultation. Standard mean difference (SMD) with 95% confidence intervals (95%CI) was calculated by either random-effects model or fixed-effects model to evaluate the correlation between TXA and HBL in TKA. The Z test was utilized to evaluate the effect size [25]. Cochran Q and I2 were applied to measure the heterogeneity [26,27]. Significant heterogeneity was confirmed with P<0.05 or I2 ≥50%. The fixed-effects model was used with the absence of heterogeneity; otherwise, the random-effects model was employed [28].
Results
Baseline characteristics
The basic details of the patients in both experimental and control groups are presented in Table 1. No significant differences in sex, body mass index (BMI), or age were detected between the 2 groups (all P>0.05). Preoperative hemoglobin and Hct in the control group were not significantly different from those in the experimental group (both P>0.05).
Table 1.
The basic characteristics of sex, age, body mass index (BMI), preoperative hemoglobin, and preoperative hematocrit (Hct) in experimental and control groups.
| Groups | Control group (n=54) | Experimental group (n=54) | χ2/t | P |
|---|---|---|---|---|
| Gender (M/F) | 18/36 | 12/42 | 1.66 | 0.197 |
| Age (year) | 60.5±5.2 | 71.1±5.8 | 1.70 | 0.092 |
| BMI (kg/cm2) | 26.2±1.9 | 25.8±2.1 | 1.04 | 0.302 |
| Hemoglobin (g/L) | 121.5±12.2 | 122.1±11.5 | 0.26 | 0.792 |
| Preoperative Hct (%) | 37.8±2.8 | 38.5±3.5 | 1.15 | 0.254 |
M – male; F – female; BMI – Body Mass Index; Hct – hematocrit.
Blood loss and transfusion
Our results demonstrated that intraoperative and postoperative blood loss in the experimental group were both significantly decreased compared with those in the control group (intraoperative blood loss: 105.1±12.1 mL vs. 185.5±20.3 mL, t=25.01, P<0.001; postoperative blood loss: 220.7±16.8 mL vs. 290.5±22.4 mL, t=18.34, P<0.001). There were 2 cases (3.70%) of transfusion in the experimental group, which is in contrast to the 14 cases (25.93%) in the control group (χ2=10.57, P=0.001).
RBL and Hct
Postoperative Hct in the control group was notably reduced compared to that in the experimental group (P<0.001), but no significant difference was found in preoperative Hct (P=0.086) (Table 2). Increased PBV and total RBL were observed in the control group but not in the experimental group (both P<0.001). Furthermore, measured RBL was reduced to 38 mL and hidden RBL was 193 mL in the experimental group in comparison with the control group. Clinical assessment did not find any deep vein thrombosis (DVT) in either group.
Table 2.
The comparison on preoperative hematocrit (Hct), postoperative Hct, total red blood loss (RBL), measured RBL and hidden RBC loss in experimental and control groups.
| Groups | Control group (n=54) | Experimental group (n=54) | t | P |
|---|---|---|---|---|
| PBV (mL) | 2480.0± 260.0 | 3080.0±310.0 | 10.90 | <0.001 |
| Total RBL(mL) | 265.4±28.5 | 437.4±33.9 | 28.54 | <0.001 |
| Measured RBL (mL) | 96.9±11.8 | 135.2±13.5 | 15.70 | <0.001 |
| Hidden RBL (mL) | 170.8±37.2 | 364.2±41.5 | 25.50 | <0.001 |
| Preoperative Hct (%) | 37.2±3.7 | 36.0±3.5 | 1.73 | 0.086 |
| Postoperative Hct (%) | 26.5±3.2 | 21.8±3.6 | 7.17 | <0.001 |
PBV – patient blood volume; RBL – red blood cell loss; Hct – hematocrit.
Coagulation indexes
The comparisons of intraoperative Fib between the 2 groups was not statistically significant (P=0.427), and similar results was found in postoperative Fib at 3 h and 6 h (postoperative 3 h: P=0.254; postoperative 6h: P=0.349). The PT and APPT were also not significantly different between the groups, both intraoperatively and postoperatively (3 h and 6 h) (all P>0.05) (Table 3).
Table 3.
Comparison of coagulation indexes on intraoperative tourniquet relief at 3 and 6 h after surgery in experimental and control group patients.
| Groups | Control group (n=54) | Experimental group (n=54) | t | P |
|---|---|---|---|---|
| Intraoperative Fib (g/L) | 4.1±0.7 | 4.2±0.6 | 0.8 | 0.427 |
| Postoperative 3 h Fib (g/L) | 4.1±0.4 | 4.2±0.5 | 1.15 | 0.254 |
| Postoperative 6 h Fib (g/L) | 4.1±0.5 | 4.2±0.6 | 0.94 | 0.349 |
| Intraoperative PT (s) | 13.6±1.7 | 13.5±1.5 | 0.32 | 0.747 |
| Postoperative 3 h PT (s) | 13.8±1.2 | 13.6±1.5 | 0.77 | 0.446 |
| Postoperative 6 h PT (s) | 13.9±1.3 | 13.6±1.4 | 1.15 | 0.251 |
| Intraoperative APPT (s) | 34.6±5.8 | 35.7±6.1 | 0.96 | 0.339 |
| Postoperative 3 h APPT (s) | 35.2±6.2 | 36.1±6.5 | 0.74 | 0.463 |
| Postoperative 6 h APPT (s) | 35.4±6.3 | 36.2±6.6 | 0.64 | 0.521 |
Fib – contractinogen; PT – prothrombin time; APPT – activated partial thromboplastin time.
Meta-analysis outcomes
The predefined searching strategy resulted in a total of 480 articles. After removing the duplications (n=12), letters or reviews (n=20), unrelated to human (n=68), and unrelated to research topic (n=240), 140 studies were left for further evaluation. Then a total of 133 studies were excluded, resulting in the inclusion of 7 comparative studies in the present meta-analysis. The 7 studies enrolled 530 patients for TKA, with 250 patients in the experimental group receiving TXA, in contrast to 280 patients in the control group subject to sterile placebo (natrium muriaticum). Among the 7 included studies, 4 studies were conducted in Asia, 2 in Sweden, and 1 in Spain [29–35]. The sample size was from 51 to 100. The publication year of the included study was from 2003 to 2014. Cochran’s Q-statistic and I2 test revealed the presence of heterogeneity; therefore, the random-fixed model was applied for data analysis (I2=96.5%, Ph<0.001). The meta-analysis results found that the application of TXA can significantly decreased the HBL, with statistical significance (SMD=2.68, 95%CI=1.55~3.80, P<0.001) (Figure 1).
Figure 1.
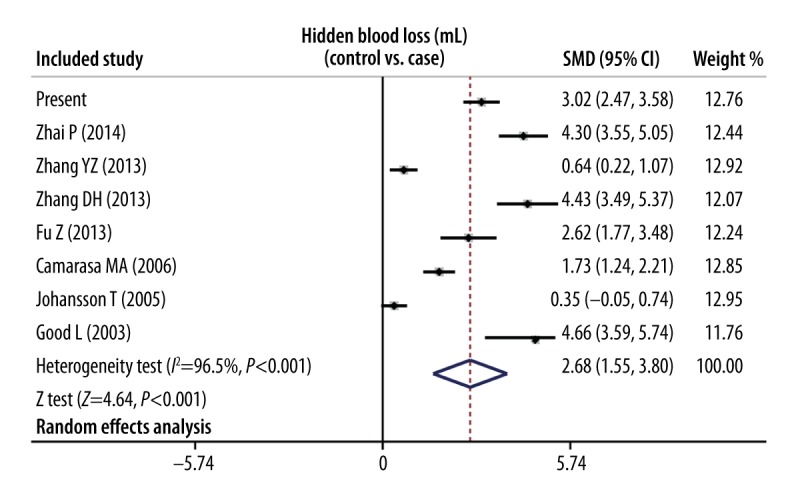
Forest plot comparing the efficacy of tranexamic acid (TXA) and sterile placebo (natrium muriaticum) in reducing hidden blood loss in total knee arthroplasty (TKA).
Discussion
Blood loss and blood transfusion requirements are significant risk factors in TKA. Blood transfusion often has several complications and several methods have been introduced to reduce blood loss volume and transfusion need, with conflicting results [10,36,37]. The major results in this comparative study revealed that the group administered TXA had a remarkable decreased volume of intraoperative and postoperative blood loss, as well as notably decreased transfusion need, which was in agreement with the summarized results from our meta-analysis, suggesting that the administration of TXA can remarkably reduce total blood loss and postoperative blood loss in TKA and reduce need for transfusion. TXA plays an important role in plasminogen activation by acting as a competitive inhibitor and it interferes with fibrinolysis, resulting in inhibition of fibrinolysis; therefore, potentially decreasing blood loss and transfusion need [4,12]. TXA exerts an antifibrinolytic effect by blocking lysine binding sites, thereby inhibiting the interaction of plasmin fibrin [38,39]. This observation is consistent with a study in 124 patients by Wong et al. that reported TXA can significantly reduce postoperative bleeding after TKA [17]. Haoran Zhang reported that TXA significantly decreased postoperative blood loss after TKA, resulting in a significant reduction of blood transfusion requirement [37].
Although complications due to thrombosis caused by fibrinolytic inhibition have been a concern in TKA [40], no DVT was found in either group in this study, implying that TXA does not increase the risk of DVT, which was in agreement in a previous meta-analysis [4]. In the current study, serial changes in Hct levels were also obtained to more accurately estimate the total blood loss. The results in our comparative study also demonstrated that the experimental group using TXA had notably reduced postoperative Hct, PBV, and total RBL, suggesting that TXA can notably decrease both measured and hidden RBL in TKA. Furthermore, this observation was in agreement with the results in our meta-analysis, revealing the potency of TXA in HBL reduction in TKA.
The originality of this study needs to be mentioned. Firstly, different from previous studies investigating the efficiency of TXA in blood loss in TKA, our study was performed from a totally different perspective, focusing on the potency of TXA in HBL reduction in TKA, since TKA can have considerable HBL. Secondly, to better confirm the results in our meta-analysis, we also added a comparative study to analyze and validate the efficiency of TXA in HBL. Some limitations should be noted. First, this study had a rather small sample size with only 54 cases distributed to each group. Additionally, the differences in ethnicity, sex, and surgical technologies among studies may have contributed to the presence of heterogeneity. We failed to further explore the sources of heterogeneity. Furthermore, this experimental and control study and meta-analysis focused only on the efficacy of TXA in reducing HBL, and no evaluation of functional scoring or patient satisfaction was conducted.
Conclusions
The present comparative study and meta-analysis proved that TXA can result in the reduction of HBL in TKA and no DVTs were found as complications. Because of the small sample size in the present comparative study regarding the use of TXA, more studies with large sample sizes are needed to validate these findings.
Acknowledgments
The authors like to thank the reviewers for their helpful comments on this paper.
Footnotes
Conflict of interest
The authors state that they have no conflict of interest.
Source of support: Departmental sources
References
- 1.Noble JW, Jr, Moore CA, Liu N. The value of patient-matched instrumentation in total knee arthroplasty. J Arthroplasty. 2012;27:153–55. doi: 10.1016/j.arth.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 2.Memtsoudis SG, Chiu YL, Nurok M, et al. Utilization of critical care services among patients undergoing total hip and knee arthroplasty: epidemiology and risk factors. Anesthesiology. 2012;117:107–16. doi: 10.1097/ALN.0b013e31825afd36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mannucci PM, Levi M. Prevention and treatment of major blood loss. N Engl J Med. 2007;356:2301–11. doi: 10.1056/NEJMra067742. [DOI] [PubMed] [Google Scholar]
- 4.Kagoma YK, Douketis J, Bhandari M, et al. Use of antifibrinolytic therapy to reduce transfusion in patients undergoing orthopedic surgery: a systematic review of randomized trials. Thromb Res. 2009;123:687–96. doi: 10.1016/j.thromres.2008.09.015. [DOI] [PubMed] [Google Scholar]
- 5.Matsuda K, Nozawa M, Katsube S, et al. Reinfusion of unwashed salvaged blood after total knee arthroplasty in patients with rheumatoid arthritis. Int Orthop. 2009;33:1615–18. doi: 10.1007/s00264-008-0661-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang FJ, Xiao Y, Liu YB, et al. Clinical effects of applying a tourniquet in total knee arthroplasty on blood loss. Chin Med J (Engl) 2010;123:3030–33. [PubMed] [Google Scholar]
- 7.Gao FQ, Li ZJ, Zhang K, et al. Risk factors for lower limb swelling after primary total knee arthroplasty. Chin Med J (Engl) 2011;124:3896–99. [PubMed] [Google Scholar]
- 8.Chareancholvanich K, Siriwattanasakul P, Narkbunnam R, Pornrattanamaneewong C. Temporary clamping of drain combined with tranexamic acid reduce blood loss after total knee arthroplasty: a prospective randomized controlled trial. BMC Musculoskelet Disord. 2012;13:124. doi: 10.1186/1471-2474-13-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shen HL, Li Z, Feng ML, Cao GL. Analysis on hidden blood loss of total knee arthroplasty in treating knee osteoarthritis. Chin Med J (Engl) 2011;124:1653–56. [PubMed] [Google Scholar]
- 10.Rajesparan K, Biant LC, Ahmad M, Field RE. The effect of an intravenous bolus of tranexamic acid on blood loss in total hip replacement. J Bone Joint Surg Br. 2009;91:776–83. doi: 10.1302/0301-620X.91B6.22393. [DOI] [PubMed] [Google Scholar]
- 11.Alcelik I PR, Sukeik M, Bettany-Saltikov J, et al. A comparison of outcomes with and without a tourniquet in total knee arthroplasty: a systematic review and meta-analysis of randomized controlled trials. J Arthroplasty. 2012;27:331–40. doi: 10.1016/j.arth.2011.04.046. [DOI] [PubMed] [Google Scholar]
- 12.Eubanks JD. Antifibrinolytics in major orthopaedic surgery. J Am Acad Orthop Surg. 2010;18:132–38. [PubMed] [Google Scholar]
- 13.Roy SP, Tanki UF, Dutta A, et al. Efficacy of intra-articular tranexamic acid in blood loss reduction following primary unilateral total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2012;20:2494–501. doi: 10.1007/s00167-012-1942-5. [DOI] [PubMed] [Google Scholar]
- 14.MJ C. The use of topical crushed tranexamic acid tablets to control bleeding after dental surgery and from skin ulcers in haemophilia. Haemophilia. 2007;13:443–44. doi: 10.1111/j.1365-2516.2007.01479.x. [DOI] [PubMed] [Google Scholar]
- 15.Dhillon MS, Bali K, Prabhakar S. Tranexamic acid for control of blood loss in bilateral total knee replacement in a single stage. Indian J Orthop. 2011;45:148–52. doi: 10.4103/0019-5413.77135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.MacGillivray RG, Tarabichi SB, Hawari MF, Raoof NT. Tranexamic acid to reduce blood loss after bilateral total knee arthroplasty: a prospective, randomized double blind study. J Arthroplasty. 2011;26:24–28. doi: 10.1016/j.arth.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 17.Wong J, Abrishami A, El Beheiry H, et al. Topical application of tranexamic acid reduces postoperative blood loss in total knee arthroplasty: a randomized, controlled trial. J Bone Joint Surg Am. 2010;92:2503–13. doi: 10.2106/JBJS.I.01518. [DOI] [PubMed] [Google Scholar]
- 18.Napolitano LM CM, Cotton BA, Schreiber MA, Moore EE. Tranexamic acid in trauma: how should we use it. J Trauma Acute Care Surg. 2013;74:1575–86. doi: 10.1097/TA.0b013e318292cc54. [DOI] [PubMed] [Google Scholar]
- 19.Lee SH, Cho KY, Khurana S, Kim KI. Less blood loss under concomitant administration of tranexamic acid and indirect factor Xa inhibitor following total knee arthroplasty: a prospective randomized controlled trial. Knee Surg Sports Traumatol Arthrosc. 2013;21:2611–17. doi: 10.1007/s00167-012-2213-1. [DOI] [PubMed] [Google Scholar]
- 20.Shen PF, Hou WL, Chen JB, et al. Effectiveness and safety of tranexamic acid for total knee arthroplasty: a prospective randomized controlled trial. Med Sci Monit. 2015;21:576–81. doi: 10.12659/MSM.892768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.M PN. World Medical Association publishes the Revised Declaration of Helsinki. Natl Med J India. 2014;27:56. [PubMed] [Google Scholar]
- 22.Johansson T, Pettersson LG, Lisander B. Tranexamic acid in total hip arthroplasty saves blood and money: a randomized, double-blind study in 100 patients. Acta Orthop. 2005;76:314–19. [PubMed] [Google Scholar]
- 23.Nadler SB HJ, Bloch T. Prediction of blood volume in normal human adults. Surgery. 1962;58:277–80. [PubMed] [Google Scholar]
- 24.Gross JB. Estimating allowable blood loss: corrected for dilution. Anesthesiology. 1983;58:277–80. doi: 10.1097/00000542-198303000-00016. [DOI] [PubMed] [Google Scholar]
- 25.Chen H, Manning AK, Dupuis J. A method of moments estimator for random effect multivariate meta-analysis. Biometrics. 2012;68:1278–84. doi: 10.1111/j.1541-0420.2012.01761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jackson D, White IR, Riley RD. Quantifying the impact of between-study heterogeneity in multivariate meta-analyses. Stat Med. 2012;31:3805–20. doi: 10.1002/sim.5453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peters JL, Sutton AJ, Jones DR, et al. Comparison of two methods to detect publication bias in meta-analysis. JAMA. 2006;295:676–80. doi: 10.1001/jama.295.6.676. [DOI] [PubMed] [Google Scholar]
- 28.Zintzaras E, Ioannidis JP. Heterogeneity testing in meta-analysis of genome searches. Genet Epidemiol. 2005;28:123–37. doi: 10.1002/gepi.20048. [DOI] [PubMed] [Google Scholar]
- 29.Camarasa MA, Olle G, Serra-Prat M, et al. Efficacy of aminocaproic, tranexamic acids in the control of bleeding during total knee replacement: a randomized clinical trial. Br J Anaesth. 2006;96:576–82. doi: 10.1093/bja/ael057. [DOI] [PubMed] [Google Scholar]
- 30.Fu Z, Zhang J, Yao H. Effect of tranexamic acid on the hidden blood loss after total hip arthroplasty. Journal of Chongqing Medical University. 2012 Apr 2012. [Google Scholar]
- 31.Good L, Peterson E, Lisander B. Tranexamic acid decreases external blood loss but not hidden blood loss in total knee replacement. Br J Anaesth. 2003;90:596–99. doi: 10.1093/bja/aeg111. [DOI] [PubMed] [Google Scholar]
- 32.Johansson T, Pettersson LG, Lisander B. Tranexamic acid in total hip arthroplasty saves blood and money: a randomized, double-blind study in 100 patients. Acta Orthop. 2005;76:314–19. [PubMed] [Google Scholar]
- 33.Zhai P, Sun Y, Sun J. Treating 46 cases of occult blood loss of primary osteoarthritis after the single knee joint replacement with tranexamic acid injection. Rheumatism and Arthritis. 2014:21–24. [Google Scholar]
- 34.Zhang D, Xu J. Preventive effect of tranexamic acid in patients with unilateral total knee arthroplasty perioperative blood loss. Shandong Medical Journal. 2013:56–58. [Google Scholar]
- 35.Zhang Y, Jin Y, Su L, Zhen J. Clinic research on blood loss control by tranexamic acid during primary unilateral total knee replacement. Orthopedic Journal of China. 2013:762–65. [Google Scholar]
- 36.Raleigh E, Hing CB, Hanusiewicz AS, et al. Drain clamping in knee arthroplasty, a randomized controlled trial. ANZ J Surg. 2007;77:333–35. doi: 10.1111/j.1445-2197.2007.04053.x. [DOI] [PubMed] [Google Scholar]
- 37.Zhang H CJ, Chen F, Que W. The effect of tranexamic acid on blood loss and use of blood products in total knee arthroplasty: a meta-analysis. Knee Surg Sports Traumatol Arthrosc. 2012;20:1742–52. doi: 10.1007/s00167-011-1754-z. [DOI] [PubMed] [Google Scholar]
- 38.Vera-Llonch M, Hagiwara M, Oster G. Clinical and economic consequences of bleeding following major orthopedic surgery. Thromb Res. 2006;117:569–77. doi: 10.1016/j.thromres.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 39.Cid J, Lozano M. Tranexamic acid reduces allogeneic red cell transfusions in patients undergoing total knee arthroplasty: results of a meta-analysis of randomized controlled trials. Transfusion. 2005;45:1302–7. doi: 10.1111/j.1537-2995.2005.00204.x. [DOI] [PubMed] [Google Scholar]
- 40.Onodera T, Majima T, Sawaguchi N, et al. Risk of deep venous thrombosis in drain clamping with tranexamic acid and carbazochrome sodium sulfonate hydrate in total knee arthroplasty. J Arthroplasty. 2012;27:105–8. doi: 10.1016/j.arth.2011.02.004. [DOI] [PubMed] [Google Scholar]


