Abstract
Despite the rise in popularity of both acupuncture and manual therapy in veterinary medicine, and the increasing number of Canadian veterinarians practising these techniques, there is little research demonstrating their effectiveness. In this repeated measures, therapeutic trial, 47 client-owned dogs with naturally occurring lameness were assessed for clinical response to treatment. Owners were blinded to the treatment schedule and completed questionnaires to assess their dogs’ comfort and mobility. Comparison between pre- and post-treatment results demonstrated that combined acupuncture and manual therapy provides immediate short-term improvement in comfort and mobility, as demonstrated by owner observed changes in play behavior (P = 0.015), walking (P < 0.001), trotting (P = 0.002), jumping (P < 0.001), descending stairs (P = 0.003), rising from a lying position (P < 0.001), and reduced stiffness after rest (P < 0.001) or following exercise (P < 0.001). Mood and attitude also improved, but did not attain statistical significance.
Résumé
Efficacité de l’acupuncture et de la thérapie manuelle combinées par rapport à l’absence de traitement pour la douleur musculo-squelettique canine. Malgré la croissance de la popularité de l’acupuncture et de la thérapie manuelle en médecine vétérinaire et le nombre grandissant de vétérinaires canadiens qui pratiquent ces techniques, il existe peu de recherche démontrant leur efficacité. Dans cet essai thérapeutique à mesures répétées, 47 chiens appartenant à des clients atteints de boiterie naturelle ont été évalués pour leur réponse clinique au traitement. Les propriétaires n’ont pas été informés du calendrier de traitement et ils ont rempli des questionnaires afin d’évaluer le confort et la mobilité de leurs chiens. La comparaison entre les résultats avant et après le traitement ont démontré que l’acupuncture et la thérapie manuelle combinées offraient une amélioration immédiate à court terme pour le confort et la mobilité, comme le démontrent les observations faites par les propriétaires pour le comportement de jeu (P = 0,015), la marche (P < 0,001), le galop (P = 0,002), le saut (P < 0,001), la descente d’escaliers (P= 0,003), le lever d’une position couchée (P < 0,001) et une raideur réduite après le repos (P < 0,001) ou après de l’exercice (P < 0,001). L’humeur et l’attitude se sont aussi améliorées, mais sans atteindre une importance statistique.
(Traduit par Isabelle Vallières)
Introduction
The demand for manual therapy and acupuncture medicine is increasing among dog owners, as is the number of Canadian veterinarians graduating from programs that teach these skills. The recently established specialty of veterinary sports medicine and rehabilitation routinely employs both these techniques to address musculoskeletal disorders. There are veterinarians who perform manual therapy concurrently with acupuncture as part of their regular practice when treating musculoskeletal pain. Many, including the primary author of this paper, do so because they believe that the combination of these 2 modalities yields better results than they see with either therapy alone.
Despite this rise in popularity and common acceptance of these techniques, the effectiveness of acupuncture in addressing musculoskeletal pain in dogs has only been superficially examined (1–6). The authors could find no research publications regarding the effectiveness of manual therapy in dogs, and only few papers examining the effectiveness of combined acupuncture and manual therapy (CAMT), all of which were from human medicine (7–10).
Manual therapy is an umbrella term for therapeutic techniques involving the “hands on” movement of joints and stretching of muscles. It can be divided into the broad categories of manipulations, mobilizations, and stretching or massage. The International Federation of Orthopaedic Manipulative Physical Therapists defines manipulations as “a passive, high velocity, low amplitude thrust applied to a joint complex within its anatomical limit with the intent to restore optimal motion, function, and/or to reduce pain.” Similarly, they define mobilizations as a “therapy technique comprising a continuum of skilled passive movements to the joint complex that are applied at varying speeds and amplitudes.” Although physiotherapists and chiropractors can each perform manipulations and mobilizations, manipulation techniques are predominantly performed by chiropractors (11).
Traditional Chinese Medicine acupuncture involves the stimulation of specific anatomic locations known as acupuncture points with an irritant, often a thin needle. Alternatively, needles can be placed in palpable regions of hyperirritable skeletal muscle. This latter technique is known as myofascial trigger point dry needling or intramuscular stimulation (12).
Other techniques employed by veterinary acupuncture practitioners include aquapuncture, the injection of fluid into acupuncture or myofascial trigger points (12,13), and electro-acupuncture, which involves running electric current between 2 or more needles (10,14).
Many studies on the effectiveness of manual therapy have been done in humans (15–20). The underlying mechanism of manual therapy is unclear, with many suggesting that the originally proposed mechanistic model should be replaced with a neurophysiological one (21,22). Although meta-analytic review demonstrates a positive benefit to manual therapy, a common criticism is that more randomized controlled studies are needed, and that designing a study in which the patients are blinded is difficult to achieve (15–20).
Side-by-side comparison of the effectiveness of mobilizations versus manipulations have found that overall the 2 techniques have similar efficacy (23,24).
Meta-analyses of acupuncture research also found that it is effective in treating chronic lower back pain in humans, but there is inadequate evidence to evaluate its effectiveness in acute back pain (25). There are concerns that there is a lack of evidence stemming from quality research, particularly blinded research (20,26,27).
The purpose of this experiment was to determine if the combination of manual therapy and acupuncture is an effective treatment for improving comfort or mobility in dogs. We hypothesized that the combined therapies will result in increased comfort and mobility as assessed by owner-completed questionnaires. In order to maximize clinical relevance, the experiment was designed to mimic typical patient presentation and appointment structure.
Materials and methods
Sample
Owners of dogs with “painful or restricted movement” were invited, via Facebook, television, and newspaper interviews, to visit a website providing information about the study. Specific information about the duration of the study, the nature of the treatment involved, and the costs/benefits to participants were provided on the website, and individuals were requested to complete a short questionnaire about the dog’s signalment, presenting complaints, and answer 12 validated questions about behavior and comfort (28–31). A summary of the presenting complaints taken from the online application form, and key examination findings that were later analyzed statistically, are detailed in Table 1. In order to mimic the wide variety of patient presentations, few limitations were placed on applicants: dogs needed to be ambulatory, could not have been previously diagnosed with an irreversible progressive neurologic condition, and must have difficulty in performing at least 1 of the activities identified on the application form. Patients with ongoing pain-modifying prescriptions were eligible to participate as long as they were on a regular daily schedule that did not vary during the data collection period.
Table 1.
Presenting complaints, historic and diagnostic findings for all dogs
| Presenting complaint | Incidence | Historic findings | Incidence | Diagnostic findings | Incidence |
|---|---|---|---|---|---|
| Deteriorated mood | 4/47 (9%) | Single leg lameness | 12/47 (26%) | CrCL | 6/47 (13%) |
| Deteriorated attitude | 14/47 (30%) | Urinary incontinence | 5/47 (11%) | Elbow DJD | 17/47 (36%) |
| Reduced playing | 15/47 (32%) | Prior paresis | 6/47 (13%) | Carpal DJD | 10/47 (21%) |
| Difficulty walking | 25/47 (53%) | Shoulder muscle pain | 35/47 (74%) | ||
| Painful vocalizations | 17/47 (36%) | Anticlinal pain | 37/47 (79%) | ||
| Difficulty trotting | 39/47 (83%) | Iliopsoas pain | 18/47 (38%) | ||
| Difficulty jumping | 43/47 (91%) | Shifted/Fixed Pelvis | 34/47 (72%) | ||
| Difficulty upstairs | 35/46 (76%) | HL CP deficits | 9/47 (19%) | ||
| Difficulty downstairs | 25/46 (54%) | ||||
| Difficulty rising | 29/47 (62%) | ||||
| Stiffness after rest | 37/47 (79%) | ||||
| Stiffness after exercise | 37/47 (79%) |
Prior paresis — a clinical history of hind-limb paresis that left the patient recumbent for 3 or more consecutive days; CrCL — cranial cruciate ligament insufficiency (either unilateral or bilateral); DJD — degenerative joint disease diagnosed by the presence of palpable exostoses, reduced range of motion, and pain at end range of motion; Shifted/Fixed Pelvis — asymetric position of ilial wings coupled with reduced sacro-iliac or lumbosacral joint mobility on palpation; HL CP — hind limb conscious proprioceptive deficits.
Due to scheduling constraints, a maximum of 59 participants could be accepted into the study. Because this research was occurring concurrently with a project on the relationship between urinary incontinence and lower back pain, 14 dogs with a history of urinary incontinence were preferentially included in this study. Statistical comparisons were conducted between the incontinent dogs and the rest of our sample, and there was no discernible difference between the groups on any relevant variables. The incontinent dogs were therefore included in the main sample, and will not be discussed further. The remaining 45 of 68 eligible applicants were randomly chosen by having a young child select from a shuffled array of face down application forms.
At the beginning of the first appointment, owners signed a consent form for participation in the research, as is required by the Canadian Council on Animal Care in science.
Study design
A repeated measures design was selected to minimize the number of required participants, and to evaluate improvements as a function of treatment relative to each dog’s presenting baseline. Starting the day of the orientation appointment, enrolled dogs were placed on a restricted exercise regimen to mimic the instructions that are routinely given following a manual therapy and acupuncture treatment. By instructing owners to initiate exercise restriction a minimum of 2 wk prior to the first treatment rather than immediately following treatment, we were able to control for this variable by standardizing the experience of all participating dogs. Exercise restriction instructions consisted of not allowing any galloping, jumping a distance greater than 1 body length, exercising more than 75% of the amount that would normally result in fatigue (e.g., if a 1-hour walk would result in noticeable fatigue by the end of the walk, or flare in lameness the following day, then walks were to be restricted to 45 min or less), and no aggressive playing. Exercise restriction remained constant throughout the experiment for all dogs.
To enhance the “blinding” of dog owners, the experiment was designed with a double crossover format, meaning that during the first 2 appointments used in the analysis, group A dogs rested in a cage for 30 min with no treatment before being returned to the owner, and group B dogs received a 30-minute examination and treatment. For the third and fourth appointments, group A dogs received a 30-minute examination and treatment (identical in format to that received by group B) and group B dogs rested in a cage. Treatment consisted of CAMT personalized for each dog’s examination findings.
All dogs, regardless of their group allocation, were seen 14 d prior to the first treatment, for the first treatment, 6 d later at the time of the second treatment, and 8 d following the second treatment (Table 2). The appointment schedule was filled to accommodate participant availability, and then appointments that started at the top of the hour were assigned to group A, and appointments that started on the half hour were assigned group B. Each participant’s allocation to group A or B remained the same for the remainder of the experiment.
Table 2.
Appointment schedule for each group
| Date | Group A | Group B |
|---|---|---|
| Orientation appointment Day 0 (28 days before Treatment 1 for Group A, 14 days before Treatment 1 for Group B) |
Orientation appointment Initiation of exercise restriction program Visit: Pre-treatment 1 |
Orientation appointment Initiation of exercise restriction program Visit: Pre-treatment 1 |
| Appointment #1 Day 14 (14 days before Treatment 1 for Group A, 0 days before Treatment 1 for Group B) |
No treatment received Visit: Pre- treatment 2 (Sham) |
1st treatment administered after survey completed Visit: Treatment 1 |
| Appointment #2 Day 20 (8 days before Treatment 1 for Group A, 6 days after Treatment 1 and 0 days before Treatment 2 for Group B) |
No treatment received Visit: Pre-treatment 3 (Sham) |
2nd treatment administered after survey completed Visit: Treatment 2 |
| Appointment #3 Day 28 (0 days before Treatment 1 for Group A, 8 days after Treatment 2 for Group B) |
1st treatment administered after survey completed Visit: Treatment 1 |
No treatment received Visit: Post-treatment 1 (Sham) |
| Appointment #4 Day 34 (6 days after Treatment 1 and 0 days before Treatment 2 for Group A, 14 days after Treatment 2 for Group B) |
2nd treatment administered after survey completed Visit: Treatment 2 |
No treatment received Visit: Post- treatment 2 (Sham) |
| Appointment #5 Day 42 (8 days after Treatment 2 for Group A, 22 days after Treatment 2 for Group B) |
No treatment received Visit: Post-treatment 1 |
No treatment received Visit: Post-treatment 3 |
Prior to the commencement of each appointment, to confirm that there had been no deviation from the instructions given during the orientation appointment, owners were specifically asked if their dogs had changed their medication schedule in any way, and what activities their dogs had engaged in the previous week. Owners were separated from their dogs and blinded to the treatment schedule. To reduce any placebo effect, owners were informed of the existence of sham appointments but knew nothing further about the treatment schedule, except that their dogs would receive at least 1 treatment. Questionnaires were completed prior to the start of each appointment.
Outcome measures
The 12 questions selected as dependent variables for this research project had all been assessed previously and found to be repeatable and reliable (28–31). However, in their original form, many of these questions employed a Likert-type response scale. Critics of Likert scales have suggested that the data provided by them is ordinal and not interval, which can limit the statistical analysis that can be applied (32). Visual analogue scales (VAS), which consist of a 10-cm line with the same anchors as a Likert scale, and onto which the participant is instructed to mark an “X” over the location that bests corresponds with their answer, allow for more nuanced responses, as well as providing interval data for subsequent analysis (32). For this study, a VAS format was employed. Scores were obtained by measuring along the line (in mm) to the point indicated by owners (range: 0 to 100). Items from the survey are listed in Table 3. Owners completed this questionnaire during the orientation appointment, and before the start of each subsequent appointment.
Table 3.
Survey questions completed by owners (with the VAS line removed)
| Lower limit | Question | Upper limit |
|---|---|---|
| Very indifferent | Your dog’s mood over the last few days has been: | Very alert |
| Very grumpy or worried | Your dog’s attitude over the last few days has been: | Very happy or good natured |
| Never plays | Your dog plays: | Very willingly |
| Very often | Rate how often your dog vocalized pain (e.g., whimpering, complaining, whining, crying out): | Never |
| With great difficulty | Your dog walks: | With great ease |
| Cannot trot at all | Your dog trots: | With great ease |
| Cannot jump at all | Your dog jumps (e.g., into car, onto sofa): | With great ease |
| Cannot climb at all | Your dog climbs up stairs (leave blank if your dog doesn’t encounter stairs): | With great ease |
| Cannot climb at all | Your dog climbs down stairs (leave blank if your dog doesn’t encounter stairs): | With great ease |
| With great difficulty | Your dog rises from a lying position: | With great ease |
| With great difficulty | Your dog moves after a long rest (e.g., first thing in the morning): | With great ease |
| With great difficulty | Your dog moves after major activity or exercise (e.g., at the end of an active day): | With great ease |
All statistics were calculated using SPSS (version 21; 2012 © IBM, Armonk, New York, USA).
Statistical model
Data relating to vocalization showed poor internal consistency (i.e., inclusion of this variable reduced Cronbach’s alpha values for the overall measure) with the other survey items. Follow-up questions were posed to the owners in an attempt to learn more about their responses, and it was determined that in many cases, vocalizations that were unlikely to be related to comfort level (e.g., barking to be let outside) had been factored into the owners’ survey responses. For that reason, survey results relating to vocalization were excluded from further analysis.
Prior to testing the hypothesis, responses to survey items were compared across scheduling groups at each of the 4 time intervals of interest for this study, using independent samples t-tests, and no significant differences emerged. Data were therefore collapsed across scheduling groups for all subsequent analyses.
The cases comprising complete data were examined using repeated measures analysis of variance (ANOVA) of owners’ ratings of 11 aspects of their dogs’ behavior at 4 points in time (Pre-treatment 1, Treatment 1, Treatment 2, Post-treatment 1), with paired comparison follow-up tests for clarification. Although a multivariate model was originally planned, multiple univariate models were run to avoid excluding cases on the basis of small amounts of missing data. Missing data points did not follow any systematic pattern within the sample as a whole. Although most assumptions (normality, sample size) were met for all analyses, as is commonly the case in repeated measures analyses, the assumption of sphericity was violated. These violations were dealt with by interpreting Greenhouse-Geisser corrected values for all ANOVA models.
Results
Sample description
Forty-seven of 59 dogs remained eligible at the end of the data collection period. Of those, 21 were spayed females, 24 neutered males, and 2 were intact males. The average age was 9.65 y [standard deviation (SD) = 3.30 y; range: 2.5 to 14 y]. The average female age was 7.95 y (SD = 3.13 y; range: 2.5 to 14 y), which is significantly younger than the average male age of 11.02 y (SD = 2.79 y; range: 5.5 to 14 y), t (45) = 3.54, P = 0.001 (2-tailed).
There were 21 breeds: 13 of the subjects were mixed breed, 10 were Labrador retrievers, 3 German shepherds, and the remaining purebreds were represented by only 1 or 2 individuals. The average patient weighed 26.23 kg (SD = 12.31 kg; range: 5.5 to 44.3 kg). There were no significant differences in dog weight by gender: females averaged 22.62 kg (SD = 12.09 kg; range: 5.5 to 41.9 kg) and males averaged 29.14 kg (SD = 11.92 kg; range: 6.3 to 44.3 kg).
A total of 11 dogs which failed to attend any of the scheduled appointments were excluded from the experiment. Data from a single patient whose owner abruptly stopped a daily anti-inflammatory prescription were also excluded.
Hypothesis tests
Repeated measures ANOVAs for each survey item revealed significant differences for 9 of the 11 outcome measures (all but mood and attitude) (Table 4). Post-hoc paired comparisons were then conducted to determine which schedule intervals were significantly different from one another (Table 5). Except for difficulty with moving upstairs, differences were observed between responses provided immediately prior to the first treatment (Treatment 1) and responses provided 8 d after the second treatment (Post-treatment 1) for variables that produced a significant ANOVA result. The difference associated with scores at these time intervals for the upstairs variable failed to achieve significance once corrections for multiple comparisons were applied, but the trend was similar (i.e., comparison of pre-treatment and post-treatment scores was in the same direction and approached conventional levels of significance). Further, for trotting, jumping, rising, stiffness after rest, and stiffness after exercise items, significant differences between baseline (Pre-treatment 1) and scores taken immediately before the second treatment (Treatment 2) were significantly different. For trotting, jumping, moving downstairs, rising, stiffness after rest, and stiffness after exercise, significant differences were observed between baseline (Pre-treatment 1) and 8 d after the second treatment (Post-treatment 1). Walking, trotting, jumping, rising, stiffness after rest and stiffness after exercise showed significant change between the first and second treatment. Finally, no significant differences were observed between Pre-treatment 1 and the first treatment (the period over which exercise restriction alone had been imposed), or between Treatment 2 and Post-treatment 1.
Table 4.
Repeated measures ANOVA results for complete set of owners’ ratings of dog behavior
| Measure | n | F(df)a | Significance | Partial eta squared |
|---|---|---|---|---|
| Mood | 47 | F(2,108) = 0.665 | P = 0.539 | 0.014 |
| Attitude | 47 | F(2,104) = 2.522 | P = 0.078 | 0.052 |
| Play | 47 | F(2,104) = 4.152 | P = 0.015 | 0.083 |
| Walk | 47 | F(2,109) = 8.582 | P < 0.001 | 0.157 |
| Trot | 45 | F(2,94) = 6.568 | P = 0.002 | 0.130 |
| Jump | 46 | F(2.84) = 13.027 | P < 0.001 | 0.224 |
| Upstairs | 41 | F(2,91) = 4.195 | P = 0.014 | 0.095 |
| Downstairs | 42 | F(2,88) = 5.891 | P = 0.003 | 0.126 |
| Rising | 47 | F(2,103) = 11.964 | P < 0.001 | 0.206 |
| After rest | 47 | F(2,116) = 13.331 | P < 0.001 | 0.225 |
| After exercise | 47 | F(2,99) = 16.448 | P < 0.001 | 0.263 |
Note that Greenhouse-Geisser corrections have been applied to all DVs to correct for violations of the assumption of sphericity.
Table 5.
Cell means (standard deviations) and results of post-hoc pairwise comparisons for survey variables
| Measure | Pre-treatment | Treatment 1 | Treatment 2 | Post-treatment | n |
|---|---|---|---|---|---|
| Mood | 68.96 (21.30) | 67.85 (22.75) | 71.66 (20.88) | 70.21 (21.73) | 47 |
| Attitude | 69.77 (21.90) | 69.55 (21.82) | 74.00 (17.63) | 75.49 (18.70) | 47 |
| Play | 59.06 (29.47) | 58.70 (28.89)a | 62.94 (27.50) | 67.21 (27.33)a | 47 |
| Walk | 50.53 (23.24) | 44.57 (23.57)a,b | 54.85 (23.78)a | 58.21 (24.48)b | 47 |
| Trot | 46.84 (29.13)a | 44.80 (27.47)b,c | 50.83 (28.09)b | 56.27 (27.99)a,c | 45 |
| Jump | 31.92 (26.77)a,b | 35.72 (27.55)c,d | 42.67 (30.43)a,c | 45.24 (31.58)b,d | 46 |
| Upstairs | 45.73 (27.08) | 48.90 (23.73) | 51.98 (26.26) | 54.61 (27.18) | 41 |
| Downstairs | 42.95 (23.43)a | 45.57 (22.64)b | 48.93 (24.94) | 53.21 (25.33)a,b | 42 |
| Rising | 38.06 (24.82)a,b | 41.66 (21.22)c,d | 48.13 (23.99)a,c | 50.66 (24.91)b,d | 47 |
| After rest | 32.96 (21.09)a,b | 38.17 (22.47)c,d | 46.66 (25.32)a,c | 48.11 (26.33)b,d | 47 |
| After exercise | 30.55 (23.40)a,b | 34.68 (22.65)c,d | 45.74 (23.49)a,c | 47.77 (24.61)b,d | 47 |
Means within rows with matching subscripts are significantly different (after Bonferroni correction for multiple comparisons, family-wise alpha = 0.05). Subscripts separated by a comma denote separate comparisons.
Additional analyses
Although the main hypothesis did not suggest that treatment would be differentially effective for dogs with different presenting complaints, we explored the possibility by means of re-rerunning the repeated measures ANOVA model using each dog’s history and diagnostic variables (Table 1) as between group factors. Dogs were grouped as either having or not having specific problems, which created substantial variation in the sizes of groups to be compared. To avoid spurious results, only diagnostic variables that produced sub-samples of 12 or more (25% or more of the total sample) were examined. Specifically, history of single leg lameness, a diagnosis of elbow degenerative joint disease, shoulder muscle pain, iliopsoas pain, and an assymetrically positioned/hypomobile pelvis met our criterion for evaluation of comparison of groups with and without the presenting problem (Table 1). Of these, only a history of single leg lameness produced a significant result when included in the model [i.e., a 2 (presence/absence of presenting problem) × 4 (appointment) mixed-model ANOVA for each of the survey items]. In all cases, owner responses to the survey items were of equivalent or greater magnitude when the dogs had a history of single leg lameness than responses from owners of dogs without such a history. The general pattern of improvement over the course of treatment was the same as for the repeated measures ANOVAs reported, though the pattern of significant paired comparison analyses within the groups with and without a history of lameness was not as consistent. The pattern of significant differences in the paired comparisons should be interpreted with some caution, however, given the smaller group sizes. No history × appointment interaction was observed in any of the mixed model ANOVAs. These results are summarized in Tables 6 and 7.
Table 6.
Mixed model ANOVA results: 2 (history of lameness) × 4 (appointment) for owner responses to survey items
| Measure | F(df)a | Significance | Partial eta squared |
|---|---|---|---|
| Mood | |||
| History | F(1,45) = 2.319 | P = 0.135 | 0.049 |
| Appointment | F(2,105) = 0.642 | P = 0.552 | 0.014 |
| History × Appt | F(2,105) = 0.349 | P = 0.739 | 0.008 |
| Attitude | |||
| History | F(1,45) = 0.341 | P = 0.562 | 0.008 |
| Appointment | F(2,101) = 2.19 | P = 0.111 | 0.046 |
| History × Appt | F(2,101) = 0.500 | P = 0.629 | 0.011 |
| Play | |||
| History | F(1,45) = 1.490 | P = 0.229 | 0.032 |
| Appointment | F(2,100) = 3.779 | P = 0.022 | 0.077 |
| History × Appt | F(2,100) = 0.566 | P = 0.588 | 0.012 |
| Walk | |||
| History | F(1,45) = 4.889 | P = 0.032 | 0.098 |
| Appointment | F(2,106) = 7.303 | P = 0.001 | 0.140 |
| History × Appt | F(2,106) = 0.173 | P = 0.874 | 0.004 |
| Trot | |||
| History | F(1,43) = 11.113 | P = 0.002 | 0.205 |
| Appointment | F(2,91) = 4.362 | P = 0.014 | 0.092 |
| History × Appt | F(2,91) = 0.060 | P = 0.950 | 0.001 |
| Jump | |||
| History | F(1,44) = 4.952 | P = 0.031 | 0.101 |
| Appointment | F(2,81) = 10.518 | P < 0.001 | 0.193 |
| History × Appt | F(2,81) = 0.363 | P = 0.680 | 0.008 |
| Upstairs | |||
| History | F(1,39) = 8.634 | P = 0.006 | 0.181 |
| Apppointment | F(2,88) = 2.389 | P = 0.091 | 0.058 |
| History × Appt | F(2,88) = 0.660 | P = 0.538 | 0.017 |
| Downstairs | |||
| History | F(1,40) = 6.258 | P = 0.017 | 0.135 |
| Appointment | F(2,86) = 3.537 | P = 0.031 | 0.081 |
| History × Appt | F(2,86) = 0.417 | P = 0.674 | 0.010 |
| Rising | |||
| History | F(1,45) = 9.504 | P = 0.003 | 0.174 |
| Appointment | F(2,100) = 8.884 | P < 0.001 | 0.165 |
| History × Appt | F(2,100) = 0.208 | P = 0.836 | 0.005 |
| After rest | |||
| History | F(1,45) = 10.307 | P = 0.002 | 0.186 |
| Appointment | F(2,113) = 10.220 | P < 0.001 | 0.185 |
| History × Appt | F(2,113) = 0.656 | P = 0.555 | 0.014 |
| After exercise | |||
| History | F(1,45) = 5.163 | P = 0.028 | 0.103 |
| Appointment | F(2,98) = 17.030 | P < 0.001 | 0.275 |
| History × Appt | F(2,98) = 1.414 | P = 0.248 | 0.030 |
Note: Repeated measures factors (appointment, history × appt) evaluated using Greenhouse-Geisser corrected values. History (between-groups factor) required no correction.
Table 7.
Cell means (standard deviations) for 2 × 4 mixed model ANOVAs (N = 47)
| Pre-treatment | Treatment 1 | Treatment 2 | Post-treatment | |||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||
| Measure | No lameness history | History of lameness | No lameness history | History of lameness | No lameness history | History of lameness | No lameness history | History of lameness |
| Mood | 66.11 (20.56) | 77.25 (22.13) | 66.29 (23.38) | 72.42 (21.09) | 69.77 (21.82) | 77.17 (17.51) | 67.23 (22.83) | 78.92 (15.91) |
| n = 35 | n = 12 | n = 35 | n = 12 | n = 35 | n = 12 | n = 35 | n = 12 | |
| Attitude | 68.14 (21.59) | 74.50 (23.08) | 69.57 (21.87) | 69.50 (22.67) | 73.63 (18.09) | 75.08 (16.95) | 74.11 (17.94) | 79.50 (21.08) |
| n = 35 | n = 12 | n = 35 | n = 12 | n = 35 | n = 12 | n = 35 | n = 12 | |
| Play | 56.91 (31.31) | 65.33 (23.35) | 56.63 (30.00) | 64.75 (25.57) | 59.00 (28.93) | 74.42 (19.55) | 64.66 (28.32) | 74.67 (23.76) |
| n = 35 | n = 12 | n = 35 | n = 12 | n = 35 | n = 12 | n = 35 | n = 12 | |
| Walk | 47.26 (60.08) | 60.08 (20.31) | 41.14 (22.05)a,b | 54.58 (25.96)x | 51.03 (24.71)a | 66.00 (17.18) | 53.83 (25.28)b | 71.00 (17.09)x |
| n = 35 | n = 12 | n = 35 | n = 12 | n = 35 | n = 12 | n = 35 | n = 12 | |
| Trot | 40.63 (68.60) | 68.60 (18.19) | 38.43 (26.19)a | 67.10 (19.71) | 44.94 (27.69) | 71.00 (19.35) | 49.97 (27.63)a | 78.30 (15.83) |
| n = 35 | n = 10 | n = 35 | n = 10 | n = 35 | n = 10 | n = 35 | n = 10 | |
| Jump | 27.26 (2.24)a,b | 46.73 (27.24) | 31.49 (27.34)c | 49.18 (24.72) | 37.06 (29.13)a | 60.55 (28.65) | 40.43 (31.40)b,c | 60.55 (28.22) |
| n = 35 | n = 11 | n = 35 | n = 11 | n = 35 | n = 11 | n = 35 | n = 11 | |
| Upstairs | 38.93 (24.53)a | 64.27 (25.89) | 42.77 (22.16)b | 65.64 (20.20) | 45.30 (24.26) | 70.18 (23.56) | 49.83 (25.91)a,b | 67.64 (27.46) |
| n = 30 | n = 11 | n = 30 | n = 11 | n = 30 | n = 11 | n = 30 | n = 11 | |
| Downstairs | 37.87 (22.48)a | 57.27 (20.74) | 40.58 (22.35)b | 59.64 (17.51) | 43.77 (24.56)c | 63.45 (20.62) | 49.55 (25.28)a,b,c | 63.55 (23.52) |
| n = 31 | n = 11 | n = 31 | n = 11 | n = 31 | n = 11 | n = 31 | n = 11 | |
| Rising | 32.74 (23.16)a,b | 53.58 (23.80) | 36.66 (18.81)c | 56.25 (21.87)x | 42.31 (22.16)a | 65.08 (21.62) | 45.89 (23.31)b,c | 64.58 (25.15)x |
| n = 35 | n = 12 | n = 35 | n = 12 | n = 35 | n = 12 | n = 35 | n = 12 | |
| After rest | 26.88 (16.56)a,b | 50.67 (23.50) | 34.29 (21.09)c | 49.50 (23.44) | 41.26 (23.68)a | 62.42 (24.19) | 42.60 (24.38)b,c | 64.17 (26.17) |
| n = 35 | n = 12 | n = 35 | n = 12 | n = 35 | n = 12 | n = 35 | n = 12 | |
| After exercise | 28.54 (21.21)a,b | 36.42 (29.13)x,y | 31.57 (19.06)c,d | 43.75 (30.02)w,z | 40.77 (21.62)a,c | 60.25 (23.56)x,w | 42.91 (23.10)b,d | 61.92 (24.30)y,z |
| n = 35 | n = 12 | n = 35 | n = 12 | n = 35 | n = 12 | n = 35 | n = 12 | |
Subscripts separated by a comma denote separate comparisons.
Discussion
The purpose of this experiment was to determine if a combination of acupuncture and manual therapy could produce measurable improvement in the comfort and mobility of canine patients, as indicated by changes in blinded owner survey responses. From the initiation of the exercise modification program until immediately before the first treatment, none of the measured parameters showed statistically significant improvement. When the survey results of group A and group B during this period were compared, there was no statistical difference between them. This indicated that patients showed no statistically significant improvement in response to exercise restriction alone, regardless of whether they received 14 or 28 d of restricted exercise. However, 6 d after a single CAMT appointment (immediately before Treatment 2), significant improvement was seen in the dogs’ ability to walk, trot, jump, and rise from a lying position. Furthermore, the owners observed less stiffness after long rests or following exercise.
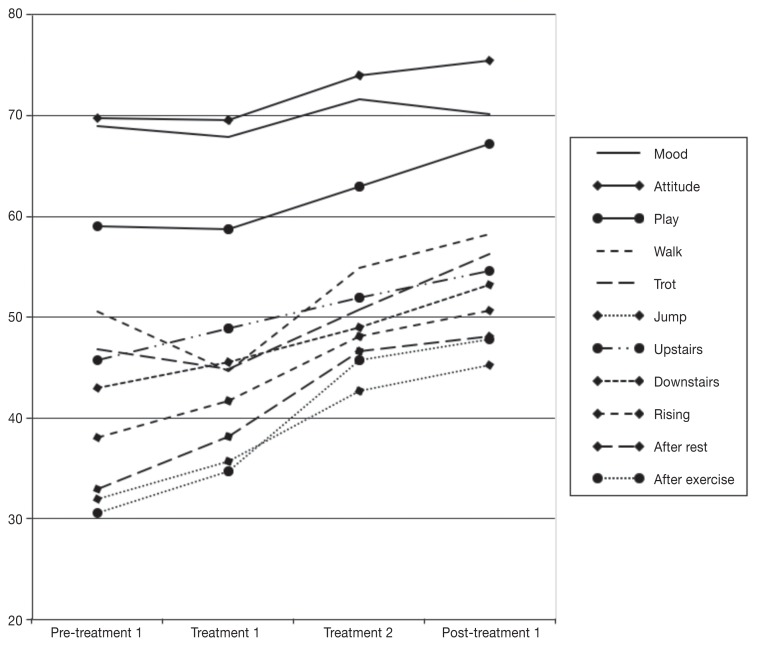
When the survey results from immediately before the first CAMT appointment are compared to those 8 d after the second CAMT appointment, patients showed significant increase in play behavior, and their ability to walk, trot, jump, descend stairs, and rise from a lying position. They also experienced less stiffness after rest or following exercise. Although improvements were also seen in mood, attitude, and ability to negotiate upstairs movement, these changes were not statistically significant. This pattern of findings suggests that any improvement due solely to exercise restriction was not sufficient to produce significantly better ratings of measured parameters by owners, as there were no significant differences between questionnaire responses collected at the initiation of the modified exercise program and those collected just before the first treatment. Furthermore, the differences between ratings collected immediately before the first treatment and 8 d following the second treatment suggest that the treatment was a causal factor in the improvements seen. The trend in the data is most clearly observed when the variables are compared visually (Figure 1).
Figure 1.
Mean owners’ ratings of behavioral variables across 4 assessment points.
Based on the parameters listed in Table 1, no contra-indications for CAMT referral were found, nor were any parameters deemed more likely to respond to treatment. It would appear that the patient’s individual response to treatment will ultimately determine the value of CAMT for each case. Based on these findings, if CAMT is going to help a given patient, it is reasonable to expect demonstrable improvement with just 2 treatments.
Dogs that presented with a history of a single leg lameness were given significantly higher survey item values by their owners at all 4 time points, relative to other dogs. This indicates that owners perceived that dogs with a single leg lameness were, on average, less debilitated than dogs without single leg lameness as a presenting complaint. It should be noted that dogs with a single leg lameness were no more or less likely to respond to CAMT than dogs that presented without this complaint.
Although the exercise restriction program was controlled for, was determined to not result in any significant improvement on its own, and was found not to be a cause for the clinical improvement following CAMT treatment, all patients were put on an exercise restriction program as part of the experimental design. Further research is required to determine the benefits of CAMT in the absence of an exercise restriction program.
Although this study demonstrated short-term benefit to CAMT lasting at least 8 d after the last treatment, further research is required to determine how long the benefits of CAMT are likely to last before additional treatments are required, if at all. A further limitation of this study is that it only sought to measure the effects of combined acupuncture and manual therapy, and made no attempt to isolate the benefits of either modality alone. As a result, no conclusions can be made about whether the observed benefits are solely due to 1 of the 2 modalities, or whether they reflect contributions from each, or perhaps even a synergistic response.
The purpose of this research was to determine whether CAMT has a therapeutic benefit relative to no treatment at all. Further research is required to determine if the benefits of CAMT are equal to those of other more established therapies such as pharmaceutical prescriptions. Further research would also be required to see if different practitioners produce the same clinical response. There is value in having future research projects include gait analysis equipment, such as a force plate or pressure mat in their methodology as this would allow for objective measures of gait quality following CAMT.
This experiment lacked an adequate sample size to perform a double crossover analysis but the design lends itself well to becoming a double crossover format for subsequent analyses. A larger sample size and a few additional variables would also allow for regression analysis to determine if dogs with certain presenting complaints, examination findings, or signalment variables, are more or less likely to respond to CAMT.
In summary, there appears to be good support for the hypothesis that 1 or 2 sessions of CAMT provides immediate short-term improvement in dogs’ comfort and mobility, as demonstrated by owner observed changes in play behavior, walking, trotting, jumping, descending stairs, rising from a lying position, and stiffness after rest or following exercise. This is the first paper to examine combined acupuncture and manual therapy in dogs. Veterinarians now have more information on which to form an evidence-based opinion on the value of CAMT. Because the owners were unaware of their dog’s treatment schedule and knew that not all appointments included treatment, this research was able to achieve a level of blinding that is difficult for human medical research to match. CVJ
Footnotes
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
This research was privately funded.
References
- 1.Still J. Analgesic effects of acupuncture in thoracolumbar disc disease in dogs. J Small Anim Pract. 1989;30:298–301. [Google Scholar]
- 2.Bolliger C, DeCamp CE, Stajich M, et al. Gait analysis of dogs with hip dysplasia treated with gold bead implantation acupuncture. Vet Comp Orthop Traumatol. 2002;2:116–122. [Google Scholar]
- 3.Hielm-Bjorkman A, Raekallio M, Kuusela E, Saarto E, Markkola A, Tulamo RM. Double blind evaluation of implants of gold wire at acupuncture points in the dog as a treatment for osteoarthritis induced by hip dysplasia. Vet Rec. 2001;149:452–456. doi: 10.1136/vr.149.15.452. [DOI] [PubMed] [Google Scholar]
- 4.Han HJ, Yoon HY, Kim JY, et al. Clinical effect of additional electroacupuncture on thoracolumbar intervertebral disc herniation in 80 paraplegic dogs. Am J Chin Med. 2010;38:1015–25. doi: 10.1142/S0192415X10008433. [DOI] [PubMed] [Google Scholar]
- 5.Kapatkin AS, Tomasic M, Beech J, et al. Effects of electrostimulated acupuncture on ground reaction forces and pain scores in dogs with chronic elbow joint arthritis. J Am Vet Med Assoc. 2006;228:1350–1354. doi: 10.2460/javma.228.9.1350. [DOI] [PubMed] [Google Scholar]
- 6.Janssens LA. Trigger point therapy. Probl Vet Med. 1992;4:117–124. [PubMed] [Google Scholar]
- 7.Li N, Tian F, Wang C, et al. Therapeutic effect of acupuncture and massage for shoulder-hand syndrome in hemiplegia patients: A clinical two-center randomized controlled trial. J Tradit Chin Med. 2012;32:343–349. doi: 10.1016/s0254-6272(13)60035-7. [DOI] [PubMed] [Google Scholar]
- 8.Norrbrink C, Lundeberg T. Acupuncture and massage therapy for neuropathic pain following spinal cord injury: An exploratory study. Acupunct Med. 2011;29:108–115. doi: 10.1136/aim.2010.003269. [DOI] [PubMed] [Google Scholar]
- 9.Shin BC, Ha CH, Song YS, Lee MS. Effectiveness of combining manual therapy and acupuncture on temporomandibular joint dysfunction: A retrospective study. Am J Chin Med. 2007;35:203–208. doi: 10.1142/S0192415X07004746. [DOI] [PubMed] [Google Scholar]
- 10.Li X, Zhou K, Zhang E, et al. Therapeutic effect of electroacupuncture, massage and blocking therapy on external humeral epicondylitis. J Tradit Chin Med. 2014;34:261–266. doi: 10.1016/s0254-6272(14)60088-1. [DOI] [PubMed] [Google Scholar]
- 11.Shekelle PG, Adams AH, Chassin MR, Hurwitz EL, Brook RH. Spinal manipulation for low-back pain. Ann Intern Med. 1992;117:590–598. doi: 10.7326/0003-4819-117-7-590. [DOI] [PubMed] [Google Scholar]
- 12.Wall R. Myofascial pain syndrome in dogs. In: Egger CM, Love L, Doherty T, editors. Pain Management in Veterinary Practise. 1st ed. Ames, Iowa: Blackwell Publishing; 2014. pp. 161–170. [Google Scholar]
- 13.Angeli AL, Luna SPL. Aquapuncture improves metabolic capacity in thoroughbred horses. J Equine Vet Sci. 2008;28:525–531. [Google Scholar]
- 14.Ulett GA, Han S, Han JS. Electroacupuncture: Mechanisms and clinical application. Biol Psychiatry. 1998;44:129–138. doi: 10.1016/s0006-3223(97)00394-6. [DOI] [PubMed] [Google Scholar]
- 15.Bronfort G, Haas M, Evans RL, Bouter LM. Efficacy of spinal manipulation and mobilization for low back pain and neck pain: A systematic review and best evidence synthesis. Spine J. 2004;4:335–356. doi: 10.1016/j.spinee.2003.06.002. [DOI] [PubMed] [Google Scholar]
- 16.Gross AR, Hoving JL, Haines TA, et al. A Cochrane review of manipulation and mobilization for mechanical neck disorders. Spine. 2004;29:1541–1548. doi: 10.1097/01.brs.0000131218.35875.ed. [DOI] [PubMed] [Google Scholar]
- 17.Hurwitz EL, Aker PD, Adams AH, Meeker WC, Shekelle PG. Manipulation and mobilization of the cervical spine: A systematic review of the literature. J Spinal Disord Tech. 1996;21:1746–1759. doi: 10.1097/00007632-199608010-00007. [DOI] [PubMed] [Google Scholar]
- 18.Bronfort G, Haas M, Evans R, Leninger B, Triano J. Effectiveness of manual therapies: The UK evidence report. Chiropr Osteopat. [Last accessed February 10, 2016]. p. 18. Available from: http://www.chiromt.com/content/articles/10.1186/1746-1340-18-3. [DOI] [PMC free article] [PubMed]
- 19.Rubinstein SM, Terwee CB, Assendelft WJ, de Boer MR, van Tulder MW. Spinal manipulative therapy for acute low back pain. Spine. 2012;38:158–177. doi: 10.1097/BRS.0b013e31827dd89d. [DOI] [PubMed] [Google Scholar]
- 20.Cherkin DC, Sherman KJ, Richard AD, Shekelle PG. A review of the evidence for the effectiveness, safety and cost of acupuncture, massage therapy and spinal manipulation for back pain. Ann Intern Med. 2003;138:898–907. doi: 10.7326/0003-4819-138-11-200306030-00011. [DOI] [PubMed] [Google Scholar]
- 21.Bialosky JE, Bishop MD, Price DD, Robinson ME, George SZ. The mechanisms of manual therapy in the treatment of musculoskeletal pain: A comprehensive model. Man Ther. 2009;14:531–538. doi: 10.1016/j.math.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schmid A, Brunner F, Wright A, Bachmann LM. Paradigm shift in manual therapy? Evidence for a central nervous system component in the response to passive cervical joint mobilization. Man Ther. 2008;13:387–396. doi: 10.1016/j.math.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 23.Hurwitz EL, Morgenstern H, Harber P, Kominski GF, Yu F, Adams AH. A randomized trial of chiropractic manipulation and mobilization for patients with neck pain: Clinical outcomes from the UCLA neck-pain study. Am J Public Health. 2002;92:1634–1641. doi: 10.2105/ajph.92.10.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gross A, Miller J, D’Sylvia J, et al. Manipulation or mobilisation for neck pain. Cochrane Database Syst Rev. 2010;1:1–108. doi: 10.1002/14651858.CD004249.pub3. [DOI] [PubMed] [Google Scholar]
- 25.Manheimer E, White A, Berman B, Forys K, Ernst E. Meta-analysis: Acupuncture for low back pain. Ann Intern Med. 2005;142:651–663. doi: 10.7326/0003-4819-142-8-200504190-00014. [DOI] [PubMed] [Google Scholar]
- 26.Van Tulder MW, Cherkin DC, Berman B, Lao L, Koes BW. The effectiveness of acupuncture in the management of acute and chronic low back pain. Spine. 1999;24:1113–1123. doi: 10.1097/00007632-199906010-00011. [DOI] [PubMed] [Google Scholar]
- 27.Habacher G, Pittler MH, Ernst E. Effectiveness of acupuncture in veterinary medicine: Systematic review. J Vet Intern Med. 2006;20:480–488. doi: 10.1892/0891-6640(2006)20[480:eoaivm]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 28.Hielm-Bjorkman AK, Rita H, Tulamo RM. Psychometric testing of the Helsinki chronic pain index by completion of a questionnaire in Finnish by owners of dogs with chronic sins of pain caused by osteoarthritis. Am J Vet Res. 2009;70:727–734. doi: 10.2460/ajvr.70.6.727. [DOI] [PubMed] [Google Scholar]
- 29.Hielm-Bjorkman AK, Kuusela E, Liman A, et al. Evaluation of methods for assessment of pain associated with chronic osteoarthritis in dogs. J Am Vet Med Assoc. 2003;222:1552–1558. doi: 10.2460/javma.2003.222.1552. [DOI] [PubMed] [Google Scholar]
- 30.Wiseman-Orr ML, Nolan AM, Reid J, Scott EM. Development of a questionnaire to measure the effect of chronic pain on health related quality of life in dogs. Am J Vet Res. 2004;65:1077–1084. doi: 10.2460/ajvr.2004.65.1077. [DOI] [PubMed] [Google Scholar]
- 31.Hudson JT, Slater MR, Taylor L, Scott HM, Kerwin SC. Assessing repeatability and validity of a visual analogue scale questionnaire for use in assessing pain and lameness in dogs. Am J Vet Res. 2004;65:1634–1643. doi: 10.2460/ajvr.2004.65.1634. [DOI] [PubMed] [Google Scholar]
- 32.Jamieson S. Likert scales: How to (ab)use them. Med Educ. 2004;38:1212–1218. doi: 10.1111/j.1365-2929.2004.02012.x. [DOI] [PubMed] [Google Scholar]