Abstract
The study of mutualistic plant and animal networks is an emerging field of ecological research. We reviewed progress in this field over the past 30 years. While earlier studies mostly focused on network structure, stability, and biodiversity maintenance, recent studies have investigated the conservation implications of mutualistic networks, specifically the influence of invasive species and how networks respond to habitat loss. Current research has also focused on evolutionary questions including phylogenetic signal in networks, impact of networks on the coevolution of interacting partners, and network influences on the evolution of interacting species. We outline some directions for future research, particularly the evolution of specialization in mutualistic networks, and provide concrete recommendations for environmental managers.
Keywords: Mutualistic networks, Coevolution, Speciation, Phylogenetic signal, Specialization
INTRODUCTION
Plant-animal mutualistic interactions, such as pollination and seed dispersal, have been regarded as one of the principle examples of coevolution and have long drawn the sustained attention of researchers (Fleming & Kress, 2013). The study of mutualistic interactions is part of the larger field of ecological networks, which also includes ‘antagonistic’ interactions between different species at varying trophic levels in the food chain (Pimm, 1982; Thompson, 2009). However, since the evolutionary forces that shape antagonistic networks may differ from those that shape mutualistic ones, we focused on mutualistic plant-animal interactions, a subject with now sufficient studies to stand on its own (Thompson, 2005; Thébault & Fontaine, 2010). Indeed, the published literature is so large and complex that is it useful to categorize the different kinds of questions that have been asked. Our review had the following objectives: (1) outline the major ecological and evolutionary questions about mutualistic networks that have become prominent in the last 30 years, and (2) point out lines of research where further development would be particularly fruitful.
BRIEF HISTORY OF RESEARCH ON PLANT-ANIMAL MUTUALISTIC NETWORKS
The idea of plant-animal interaction networks was first proposed 150 years ago with the publication of Darwin’s On the Origin of Species, who wrote “I am tempted to give one more instance showing how plants and animals, most remote in the scale of nature, are bound together by a web of complex relations” (Darwin, 1859, p74). A large number of studies have looked at specific mutualistic interactions between plants and animals, such as yucca moths and yuccas (Pellmyr, 2003), ants and Acacia (Janzen, 1966) and fig wasps and figs (Cook & Rasplus, 2003). However, the interactions among organisms are far more complex than pair-wise relationships, as many different organisms interact together in networks that include many species (Thompson, 2005). With more biological data available and the advance of algorithms for complex network analysis derived from physics and computer science, ecologists and evolutionary biologists have started to explore plant-animal mutualistic interactions from the network perspective (Guimarães et al, 2011; Olesen et al, 2007; Rezende et al, 2007a, b).
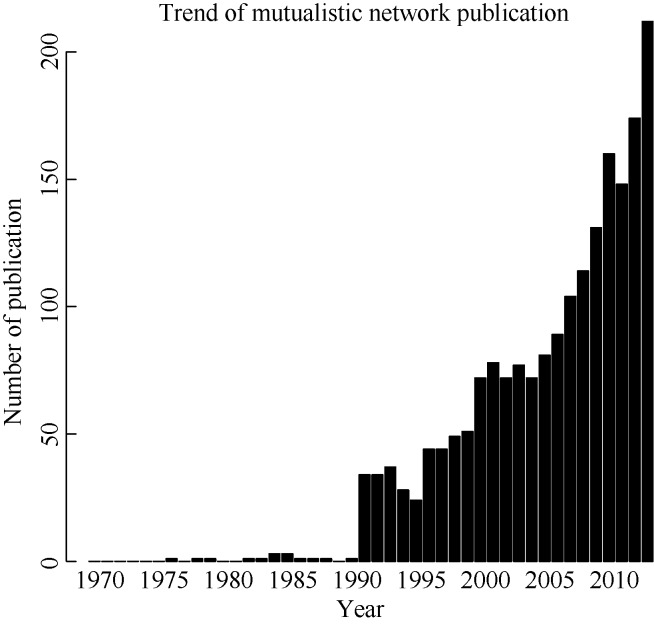
To study the growth in this literature, we searched the topics ‘mutualistic network’, ‘plant animal interaction’ or ‘mutualistic interaction’ with the timespan from 1970 to 2013 on Web of Science, and then further refined the results using the Web of Science ecology category. A total of 1 945 publications were closely related to the topics, with rapid growth year-to-year (Figure 1). In terms of the total number of publications in a year, most years had more publications than the year before. This trend is expected to keep on increasing annually in the future.
Figure 1.
Literature found on Web of Science by searching ‘mutualistic network’, ‘plant animal interaction’ or ‘mutualistic interaction’ with the time span 1970 to 2013, plotted against the date of publication Each bar represents the number of publications published in a single year. This search was conducted in June 2014.
Tracing early literature, plant-animal mutualistic studies began to appear in the late 1970s, with an escalation in publications observed in the early 1990s. Most early studies were field experiments, which laid the foundation for later, more theoretical research. Jordano (1987) detailed the first well-recognized study on mutualistic networks from the theoretical perspective in American Naturalist. A rapid increase in publications on networks was observed from 2005 (Figure 1). Bascompte et al (2003) introduced an important technique for network analysis to measure the nestedness of the interaction network, opening new avenues in the field. Beyond studies on network architecture, increasing research has focused on the implications of network theory for ecology and evolution, and we address these two major kinds of studies separately below.
MUTUALISTIC NETWORKS IN ECOLOGICAL TIME
Mutualistic network properties
Plant-animal mutualistic networks can be described by interaction matrices, with plant species in the columns and animal species in the rows; we refer to an interaction between a plant and animal species as a “link”. According to the kind of values in the matrix cells, network matrices can be categorized into “weighted” networks (“quantitative” networks) and “unweighted” networks (“binary” networks or “qualitative” networks, as they only indicate whether a pair of species interact, not the intensity). Several network properties have been generated to describe the network structure, such as connectance and interaction strength (Table 1).
Table 1.
Glossary of main network terms and key references
| Metric | Definition |
|---|---|
| Asymmetry | Measures the imbalance in the interaction strength of two interacting species (Bascompte et al, 2006). It is defined as ASij=(bij-bji)/(bij+bji), where bji is the reciprocal dependence of species j on species i (bij see interaction strength, Bascompte et al, 2006; Blüthgen et al, 2007). |
| Binary network | In binary network matrix, the value is 0 or 1, if the interaction occurs, the value is 1, otherwise 0 (Jordano, 1987). |
| Connectance | Proportion of the realized interactions in all possible interactions (Yodzism 1980). In mutualistic networks, connectance (C) is: C=L/(IJ). L describes the number of realized links; I and J are the number of species of each bipartite network (Blüthgen et al, 2008). |
| Degree | Number of interactions a species has (Jordano et al, 2003). |
| Interaction strength | Interaction strength of species j on species i (bij) can be defined by the proportion of interactions between i and j (aij) of the total interactions recorded for i; thus |
| Modularity | Measures the degree to which the network is organized into clearly delimited modules (Olesen et al, 2007). Modularity (M): |
| Module | A set of weakly interlinked subsets of species that consist of strongly connected species (Olesen et al, 2007). |
| Nestedness | A nonrandom pattern of the network structure, which entails the tendency of specialized species to interact with a subset of the interaction partners of more generalized species. The nestedness temperature (T) measures the departure from a perfectly nested interaction matrix, ranging from 0 to 100, which indicates the degree of disorder. T=0 is defined for maximum nestedness: when rows and columns are ordered by decreasing number of links, links of each row and column exactly represent a subset of the previous ones. Nestedness can be defined as N=(100°-T)/100° (Bascompte et al, 2003). |
| Weighted network | Networks that include information on the intensity or weight of the interactions among nodes (Bascompte & Jordano, 2007). |
The topology of ecological interaction webs (such as mutualistic networks) holds important information for biodiversity, ecosystem stability and theories of coevolution (Bascompte et al, 2006; Jordano, 1987; Montoya et al, 2006). On the whole, mutualistic networks are neither a collection of pair-wise, highly specific interactions nor diffuse, random assemblages (Bascompte & Jordano, 2007). Rather, mutualistic networks have common, well-defined network architecture regardless of the type of mutualism, species composition, and geographic region (Bascompte et al, 2003). Several topological features are characteristic, including skewed distribution of links per species (i.e., a few species with many more interactions than expected by chance, and many species with a few interactions; Vázquez & Aizen, 2003; Jordano et al, 2003), the nested organization of the interaction matrix (Bascompte et al, 2003) and the frequent occurrence of asymmetric interactions (i.e., a plant species depending strongly on an animal species, the animal depending weakly on the plant; Bascompte et al, 2006). In addition to these properties, some networks are also modular (especially pollination networks; Olesen et al, 2007), whereby clusters of species interact more closely with each other than with species in other clusters or species outside the clusters. In comparison to antagonistic networks, mutualistic networks demonstrate high asymmetry and connectance, whereas antagonistic networks tend to be weakly connected, with many groups of species (“compartments”) interacting only within their group (Cagnolo et al, 2011; Krause et al, 2003; Prado & Lewinsohn, 2004; Thébault & Fontaine, 2010).
Stability and diversity of mutualistic networks
The maintenance of stability in complex communities has been a long-standing debate since the classic work of May (1974). Most network studies have found that the properties of mutualistic networks contribute to their diversity and stability; for example, the asymmetric nature (i.e., asymmetric interactions and asymmetry in interaction strength) of mutualistic networks promotes community coexistence, which favors the persistence of biodiversity (Bascompte et al, 2006). Nestedness has been shown to reduce interspecific competition, and hence promote diversity and nested networks stability (Bastolla et al, 2009; Thébault & Fontaine, 2010). Other network properties, including community size, species degree, species strength, and symmetry of the interaction, may also positively contribute to stability (Okuyama & Holland, 2008), while modularity decreases stability (Thébault & Fontaine, 2010). Recent developments have questioned the idea that nestedness itself affects stability, suggesting that more simple features, such as species degree, are more important drivers (Feng & Takemoto, 2014; James et al, 2012; Jonhson et al, 2013; Staniczenko et al, 2013). A further complication is that other interaction types, such as antagonistic networks, may interact with mutualistic networks to affect overall community stability (Mougi & Kondoh, 2012).
Spatial-temporal variation of the mutualistic network
Studies have indicated that both spatial and temporal dimensions impose constraints on plant-animal mutualistic interactions and influence network patterns (Burkle & Alarcón, 2011). Nevertheless, some general network properties remain constant over time and space. For example, Plein et al (2013) found that none of the characteristics of the seed-dispersal networks (e.g., interaction diversity, interaction evenness, and network specialization) used in their study changed with landscape type (farmland, orchard, forest edge) or season. Other studies have shown that although seasonal species turnover exists in the network, general network patterns (such as nestedness, connectance and modularity) remain relatively constant (Dupont et al, 2009; Dupont & Olesen, 2012; Olesen et al, 2008, 2011; Petanidou et al, 2008; Plein et al, 2013). However, these studies were conducted over a relatively short time period (often two to four years), and further studies with longer time spans are needed to test these conclusions. Additional attention needs to be paid when comparing different networks or when pooling data from different networks for meta-analysis. Networks are only comparable in this manner if they are at the same time scales or over the same phenological periods; for example, mixing networks from different seasons may prove confusing (Burkle & Alarcón, 2011).
Conservation implications of mutualistic networks
One potential problem for networks in our changing world is that alien mutualists, including both plants and pollinators, can integrate into native pollination networks, and sometimes end up acting as super-generalist species (a few species that interact with an extremely large number of species) of the network (Aizen et al, 2008; Bartomeus et al, 2008; Olesen et al, 2002). Highly invaded networks exhibit weaker mutualism than less invaded networks, and the connectivity among native mutualists declines, although overall network connectivity may not change (Aizen et al, 2008) and other aspects of network structure such as nestedness are relatively robust to the introduction of invasive species (Vilà et al, 2009). However, the removal of invasive alien species from the network can change the network structure, imposing a pronounced effect on degree distribution and modularity of the network, leading to higher species loss, which could affect the evolution of the interaction network architecture (Valdovinos et al, 2009).
Habitat loss and fragmentation also have large, consistently negative effects on biodiversity (Fahrig, 2003). The extinction of species and loss of mutualistic partners may impose a cascading effect on mutualistic networks, which can consequently lead to greater biodiversity loss (Anderson et al, 2011). Several theoretical studies have looked at how mutualistic networks respond to such disturbances. For example, Fortuna & Bascompte (2006) used a modeling method to understand how mutualistic networks respond to habitat loss, and found that real communities started to decay sooner than random communities, although they persisted better at high levels of destruction. Pollination network field research also demonstrated that habitat loss not only leads to species loss, but also indirectly causes the reorganization of interspecific interactions in the local community (Spiesman & Inouye, 2013). The reduction in suitable habitats is associated with species loss, which is correlated with reduced nestedness and increased modularity (Tilman et al, 1994).
Given the work on how mutualistic networks respond to invasive species and anthropogenic disturbance, it is clear that network theory has important implications for conservation. For example, by looking at the network structure, we can identify the network’s susceptibility to alien species invasion (Olesen et al, 2002; Morales & Aizen, 2006). Network theory can also clarify the role of different species (i.e., species with different specialization degree) and their impacts on the whole network architecture and stability. For example, super-generalist species can greatly affect the overall topology of a network (Aizen et al, 2008; Hansen & Galetti, 2009), which has a direct consequence on the protection of the endangered system (Kiers et al, 2010) as these super-generalists can be targeted in conservation plans. Other issues that lead to species loss in the network, such as the effect of habitat loss on networks, are also important to conservation practice. Additionally, it has been suggested that the extinction of phylogenetically related species can lead to cascading coextinction events (Rezende et al, 2007a). Understanding this potential effect with the assistance of network theory could help conservationists make decisions on species priority.
Unfortunately, despite the theoretical advances in networks and their potential use in conservation, their actual implementation in conservation management is rare. There appears to be a communication failure among scientists, practitioners, and government officials that requires the assistance of all parties to resolve (Heleno et al, 2014).
MUTUALISTIC NETWORK STUDIES FROM AN EVOLUTIONARY PERSPECTIVE
As discussed above, studies on network structural properties and their implication for ecology have been extensively investigated in recent years. More attention has been spent on investigating mutualistic networks from the ecological perspective than the evolutionary one. However, species are not independent entities but rather related to each other through common evolutionary histories. Phylogenetic constraints may influence mutualistic interactions, imprinting a phylogenetic signal (i.e., the tendency of phylogenetically similar species to have similar phenotypic attributes; Bascompte & Jordano, 2007) on network structure (Ives & Godfray, 2006; Rezende et al, 2007a, b). To understand plant-animal mutualistic networks, we must examine the phylogenetic signal of species’ positions (i.e., species centrality and their placement relative to modules; Olesen et al, 2007) in the network and observe which species form the network.
In addition, studies that have attempted to link network literature to explore the evolution of networks (e.g., Guimarães et al, 2011) remain scarce. The synthesis of network studies and phylogeny may change our understanding of the coevolutionary process. For example, recent work that combined phylogeny and pollination data found coevolution to be an important driver of species diversification (Van der Niet & Johnson, 2012). Despite some limitations of using phylogeny to explain the processes that influence speciation, work of this kind may nevertheless suggest novel directions towards understanding speciation, diversification, and biodiversity. Below, we discuss three topics in the evolution of mutualistic networks in which there has been recent research activity.
Phylogenetic signal in mutualistic networks
A revolutionary article on network evolution used phylogenetic methods for the first time to study how the interaction pattern was associated with phylogeny in mutualistic networks (Rezende et al, 2007a). By incorporating a large dataset of 36 plant-pollinator and 23 plant-frugivore mutualistic networks, they found that the phylogenetic signal in species degree (number of other species with which a species interacts) could be detected in more than one third of the networks. Meanwhile, the actual identity of interaction partners had a phylogenetic component in about half the interaction networks. Simulated extinction events triggered cascading coextinction, wherein phylogenetically related species went extinct together.
Having realized the importance of evolutionary history on network structure, more researchers now include phylogenetic studies in their work. For example, a recent study by Schleuning et al (2014) has further improved our understanding on network modularity. These researchers associated both weighted and binary network data with species traits and phylogenetic information to study how modularity is related with these ecological and evolutionary factors. The study followed the methodology of Olesen et al (2007), which identifies species connectedness within (z-score) and between (c-score) modules. The results showed that for both weighted and binary networks, no phylogenetic signal was detected in within-module degree (z), but significant phylogenetic signal was found in the c value: the tendency of a species to interact with species in other modules. Results also showed significant phylogenetic signal in species degree, concordant with the results of Rezende et al (2007a).
How do mutualistic networks affect coevolution of their species?
Guimarães et al (2011) combined a model for trait evolution with data from 20 plant-animal mutualistic networks to explore coevolution. They found that both evolution and coevolution contributed to the increase in convergence (e.g., trait similarities emerge in response to similar selective pressures) and complementarity (e.g., degree of trait matching between interactive partners) among traits within the network, and that coevolution significantly sped up the rate of trait evolution within networks (Guimarães et al, 2011). They also found that super-generalists facilitated trait evolution in mutualistic networks by greatly increasing complementarity (match of traits between partners) and convergence. Because super-generalists serve as connections between different modules, evolutionary and coevolutionary forces that affect them multiply throughout the whole network (Guimarães et al, 2011).
Thompson (2009) proposed a new hypothesis from an evolutionary perspective, in which plant-animal interaction networks act as vortexes, absorbing increasing numbers of species into the interaction network. As the web grows bigger, it has more capacity to hold diverse species. In this way, plant-animal interaction networks may promote the evolution of biodiversity. However, more evidence is needed to support or reject this hypothesis.
How could mutualistic networks affect the rate of evolution of participating species?
The above studies either combined data on mutualistic networks with the phylogeny of the interacting species to look at how evolutionary history influenced network properties or simulated how coevolution shaped the patterns of complementarity and convergence between species in networks. However, the question of the rate at which species with different specialization levels evolve and diverge (i.e., speciation) in mutualistic networks remains largely unexplored. As has been well studied, mutualistic networks have a wide range of species that differ in their connectedness to other species, with many species interacting with only a few species (“specialists”), and other species interacting with many other species (“generalists”). In the field of classical ecological evolution, many studies have compared the speciation rate between specialists and generalists (e.g., Colles et al, 2009; Fernández & Vrba, 2005); however, do different kinds of species in networks differ in their speed of evolutionary divergence?
We recently conducted a study along the lines of this question, asking if specialist and generalist frugivorous birds have different speeds of evolutionary divergence (Gu et al, 2015). A recent time-calibrated phylogeny of birds that included all extant species (Jetz et al, 2012) allowed such a study, as every species had an estimated time of divergence from its sister species. Using 16 seed dispersal networks in the published literature, we found that specialists (defined as per Olesen et al, 2007) had significantly shorter divergence times than did generalists. This result is somewhat surprising as most birds are thought to be rather unspecialized and not dependent on specific fruiting trees, in contrast to the very strong and sometimes obligate relationships found between pollinators and plant species (Blüthgen et al, 2007). We therefore treat the result quite tentatively, noting some confounding variables, such as specialists being generally rare, which could be driving the result. Nevertheless, we think the question is important, and believe future research should investigate this in other taxa such as insects and plants.
FUTURE DIRECTIONS AND CHALLENGES
A general challenge for studies of plant-animal mutualistic networks is the paucity of data sampling. Field observations are time-consuming, and certain habitats (e.g., canopies) are difficult to access. Fundamentally, there is a lack of properly trained personnel to make the observations. General problems for flora and insect taxonomy lie in the shortage of funding, the low Impact Factor index of taxonomic journals, low scientific regard of taxonomic knowledge, and a lack of investment and training programs for a new generation of taxonomists (Dangles et al, 2009; Ma, 2014; Rafael et al, 2009). These problems greatly hamper the development of plant-animal interaction network studies, leading to misidentification of species.
There are potential alternative methods of network construction, including DNA barcoding (Carcía-Robledo et al, 2013; Heber et al, 2004). For example, researchers recently found that DNA barcoding gave comparable results to direct observations in a plant-herbivore system (Carcía-Robledo et al, 2013). However, the authors only studied a small group of well-known taxa (with plants only from order Zingiberales and rolled-leaf beetles from only two genera, Cephaloleia and Chelobasis). They emphasized that DNA information is unavailable in public databases for many organisms that participate in plant-animal interaction networks. This is especially true for mutualistic networks, in which the organisms come from a wide range of taxa.
Additionally, the size of the network from different studies varies greatly, with total species ranging from a few dozen to several hundreds. This range in network size could reflect biological reality or could be due to differences in sampling effort. If the differences are due to sampling effort, these can be accounted for during analysis, as recently shown by Schleuning et al (2014). To answer large ecological or evolutionary questions, data from many different networks needs to be gathered together and a general protocol needs to be established (Heleno et al, 2014).
Another challenge of network studies is to find an effective way to quantify the network, which should reveal the real ecological processes behind the interactions. For example, for most existing pollination networks and seed dispersal networks, flower visitation and frugivory are recorded as proxies without evaluating the effectiveness of the ecological services, that is, how these services affect plant reproduction (Heleno et al, 2014). By reconsidering how networks are quantified, we can better understand the real ecological and evolutionary mechanisms. In addition, as weighted data are more informative than binary data (Barrat et al, 2004; Schleuning et al, 2014), we should encourage future studies to use weighted network data.
Further development of plant-animal mutualistic network theory requires more complete and informative datasets, which allow the evaluation of multiple mechanisms simultaneously (Vázquez et al, 2009). According to the geographic mosaic theory (Thompson, 2009), coevolution varies in time and space. Thus, network data with explicit spatio-temporal information are needed to provide more reliable and explanatory interpretations on both the ecological and evolutionary study of mutualistic networks. To answer questions of how networks affect the evolution of participating species, both comprehensive ecological information and phylogenetic information of participating species are required. We believe that field studies, particularly those that use the same systematic technique to look at networks in multiple study sites, and hence better understand consistency and variation in the qualities of the networks, are just as vital to the development of the field as theoretical studies.
New insights will undoubtedly arise as our knowledge about phylogeny increases. In pollination networks, for example, there are extremely large numbers of diverse pollinators from Diptera, Hymenoptera, Lepidoptera and Coleoptera. Yet only a small proportion of these species have been well studied taxonomically and phylogenetically. Time calibrated phylogenies for species in these groups are extremely limited (to some species from genus Bombus, family Apidae), and few of these species have DNA sequences accessible in GenBank. New developments in obtaining further phylogenetic information and better techniques will surely open new doors to analyses, just as Jetz et al (2012) did for our study (Gu et al, 2015).
Despite all the challenges in mutualistic network studies, there are many opportunities in this field. It is difficult to predict the directions of future research to come, after all who could have foreseen the network literature blossoming as it has over the last 30 years, but certainly phylogenetic and bar-coding techniques may ignite new possibilities. From our evolutionary perspective, it is clear that there are many questions yet unanswered about how the network phenomenon has influenced the evolution of participating species. We also believe that conservation is a priority. However, despite the rapidly accumulating research in this field, there is little consensus on how environmental managers should incorporate networks into their planning (Heleno et al, 2014). For example, should networks be used to evaluate habitat quality rather than individual species counts (Valiente-Banuet et al, 2014)? Are networks where invasive species are super-generalists irreparable or do remedial actions exist (Aizen et al, 2008; Valdovinos et al, 2009)? Further progress on these issues will demonstrate that our growing knowledge about ecological networks can be applied to solve environmental problems.
REFERENCES
- [1].Aizen MA, Morales CL, Morales JM.2008. Invasive mutualists erode native pollination webs.PLoS Biology, 6(2): e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Anderson SH, Kelly D, Ladley JJ, Molloy S, Terry J.2011. Cascading effects of bird functional extinction reduce pollination and plant density.Science, 331(6020): 1068-1071. [DOI] [PubMed] [Google Scholar]
- [3].Barrat A, Barthélemy M, Pastor-Satorras R, Vespignani A.2004. The architecture of complex weighted networks.Proceedings of the National Academy of Sciences of the United States of America, 101(11): 3747-3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Bartomeus I, Vilà M, Santamaría L.2008. Contrasting effects of invasive plants in plant-pollinator networks.Oecologia, 155(4): 761-770. [DOI] [PubMed] [Google Scholar]
- [5].Bascompte J, Jordano P.2007. Plant-animal mutualistic networks: the architecture of biodiversity.Annual Review of Ecology, Evolution, and Systematics, 38: 567-593. [Google Scholar]
- [6].Bascompte J, Jordano P, Melian CJ, Olesen JM.2003. The nested assembly of plant-animal mutualistic networks.Proceedings of the National Academy of Sciences of the United States of America, 100(16): 9383-9387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Bascompte J, Jordano P, Olesen JM.2006. Asymmetric coevolutionary networks facilitate biodiversity maintenance.Science, 312(5772): 431-433. [DOI] [PubMed] [Google Scholar]
- [8].Bastolla U, Fortuna MA, Pascual-García A, Ferrera A, Luque B, Bascompte J.2009. The architecture of mutualistic networks minimizes competition and increases biodiversity.Nature, 458(7241): 1018-1020. [DOI] [PubMed] [Google Scholar]
- [9].Blüthgen N, Menzel F, Hovestadt T, Fiala B, Blüthgen N.2007. Specialization, constraints, and conflicting interests in mutualistic networks.Current Biology, 17(4): 341-346. [DOI] [PubMed] [Google Scholar]
- [10].Burkle LA, Alarcón R.2011. The future of plant-pollinator diversity: understanding interaction networks across time, space, and global change.American Journals of Botany, 98(3): 528-538. [DOI] [PubMed] [Google Scholar]
- [11].Cagnolo L, Salvo A, Valladares G.2011. Network topology: patterns and mechanisms in plant-herbivore and host-parasitoid food webs.Journal of Animal Ecology, 80(2): 342-351. [DOI] [PubMed] [Google Scholar]
- [12].Carcía-Robledo C, Erickson DL, Staines CL, Erwin TL, Kress WJ.2013. Tropical plant-herbivore networks: reconstructing species interactions using DNA barcodes.PLoS ONE, 8(1): e52967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Colles A, Liow LH, Prinzing A.2009. Are specialists at risk under environmental change? Neoecological, paleoecological and phylogenetic approaches.Ecology Letters, 12(8): 849-863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Cook JM, Rasplus JY.2003. Mutualists with attitude: Coevolving fig wasps and figs.Trends in Ecology & Evolution, 18(5): 241-248. [Google Scholar]
- [15].Dangles O, Barragán A, Cárdenas RE, Onore G, Keil C.2009. Entomology in Ecuador: recent developments and future challenges.Annales de la Société entomologique de France (N.S.), 45(4): 424-436. [Google Scholar]
- [16].Darwin C.1859. On the Origin of Species by Means of Natural Selection, or the Preservation of Favoured Races in the Struggle for Life. London, UK:John Murrey. [PMC free article] [PubMed] [Google Scholar]
- [17].Dupont YL, Olesen JM.2012. Stability of modular structure in temporal cumulative plant-flower-visitor networks.Ecological Complexity, 11: 84-90. [Google Scholar]
- [18].Dupont YL, Padrón B, Olesen JM, Petanidou T.2009. Spatio-temporal variation in the structure of pollination networks.Oikos, 118(8): 1261-1269. [Google Scholar]
- [19].Fahrig L.2003. Effects of habitat fragmentation on biodiversity.Annual Review of Ecology, Evolution, and Systematics, 34: 487-515. [Google Scholar]
- [20].Feng WF, Takemoto K.2014. Heterogeneity in ecological mutualistic networks dominantly determines community stability.Scientific Reports, 4: 5912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Fernández MH, Vrba ES.2005. Macroevolutionary processes and biomic specialization: testing the resource-use hypothesis.Evolutionary Ecology, 19(3): 199-219. [Google Scholar]
- [22].Fleming TH, Kress WJ.2013. The Ornaments of Life: Coevolution and Conservation in the Tropics. Chicago: University of Chicago Press. [Google Scholar]
- [23].Fortuna MA, Bascompte J.2006. Habitat loss and the structure of plant-animal mutualistic networks.Ecology Letter, 9(3): 281-286. [DOI] [PubMed] [Google Scholar]
- [24].Gu H, Goodale E, Chen J.2015. Does the role that frugivorous bird species play in seed dispersal networks influence the speed of evolutionary divergence? Global Ecology and Conservation, 3: 121-128. [Google Scholar]
- [25].Guimarães PR Jr, Jordano P, Thompson JN.2011. Evolution and coevolution in mutualistic networks.Ecology Letters, 14(9): 877-885. [DOI] [PubMed] [Google Scholar]
- [26].Hansen DM, Galetti M.2009. The forgotten megafauna.Science, 324(5923): 42-43. [DOI] [PubMed] [Google Scholar]
- [27].Hebert PDN, Penton EH, Burns JM, Janzen DH, Hallwachs W.2004. Ten species in one: DNA barcoding reveals cryptic species in the neotropical skipper butterfly Astraptes fulgerator.Proceedings of the National Academy of Sciences of the United States of America, 101(41): 14812-14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Heleno R, Garcia C, Jordano P, Traveset A, Gómes JM, Blüthgen N, Memmott J, Moora M, Cerdeira J, Rodrígurz-Echeverría S, Freitas H, Olesen JM.2014. Ecological networks: delving into the architecture of biodiversity. Biology Letters,10(1), doi: 10.1098/rsbl.2013.1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Ives AR, Godfray HCJ.2006. Phylogenetic analysis of trophic associations.The American Naturalist, 168(1): E1-E14. [DOI] [PubMed] [Google Scholar]
- [30].James A, Pitchford JW, Plank MJ.2012. Disentangling nestedness from models of ecological complexity.Nature, 487(7406): 227-230. [DOI] [PubMed] [Google Scholar]
- [31].Janzen DH.1966. Coevolution of mutualism between ants and acacias in central America.Evolution, 20(3): 249-275. [DOI] [PubMed] [Google Scholar]
- [32].Jetz W, Thomas GH, Joy JB, Hartmann K, Mooers AO.2012. The global diversity of birds in space and time.Nature, 491(7424): 444-448. [DOI] [PubMed] [Google Scholar]
- [33].Jonhson S, Domínguez-García V, Muñoz MA.2013. Factors determining nestedness in complex networks.PLoS ONE, 8(9): e74025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Jordano P.1987. Patterns of mutualistic interactions in pollination and seed dispersal: connectance, dependence asymmetries, and coevolution.The American Naturalist, 129(5): 657-677. [Google Scholar]
- [35].Jordano P, Bacompte J, Olesen JM.2003. Invariant properties in coevolutionary networks of plant-animal interactions.Ecology Letters, 6(1): 69-81. [Google Scholar]
- [36].Kiers ET, Palmer TM, Ives AR, Bruno JF, Bronstein JL.2010. Mutualisms in a changing world: an evolutionary perspective.Ecology Letters, 13(12): 1459-1474. [DOI] [PubMed] [Google Scholar]
- [37].Krause AE, Frank KA, Mason DM, Ulanowicz RE, Taylor WW.2003. Compartments revealed in food-web structure.Nature, 426(6964): 282-285. [DOI] [PubMed] [Google Scholar]
- [38].Ma JS.2014. Current status and challenges of Chinese plant taxonomy.Chinese Science Bulletin, 59(6): 510-521. (in Chinese) [Google Scholar]
- [39].May RM.1974. Stability and Complexity in Model Ecosystems. Princeton: Princeton University Press. [Google Scholar]
- [40].Montoya JM, Pimm SL, Solé RV.2006. Ecological networks and their fragility.Nature, 442(7100): 259-264. [DOI] [PubMed] [Google Scholar]
- [41].Morales CL, Aizen MA.2006. Invasive mutualisms and the structure of plant-pollinator interactions in the temperate forests of north-west Patagonia, Argentina.Journal of Ecology, 94(1): 171-180. [Google Scholar]
- [42].Mougi A, Kondoh M.2012. Diversity of interaction types and ecological community stability.Science, 337(6092): 349-351. [DOI] [PubMed] [Google Scholar]
- [43].Okuyama T, Holland JN.2008. Network structural properties mediate the stability of mutualistic communities.Ecology Letters, 11(3): 208-216. [DOI] [PubMed] [Google Scholar]
- [44].Olesen JM, Eskildsen LI, Venkatasamy S.2002. Invasion of pollination networks on oceanic islands: importance of invader complexes and endemic super generalists.Diversity and Distributions, 8(3): 181-192. [Google Scholar]
- [45].Olesen JM, Stefanescu C, Traveset A.2011. Strong, longy-term temporal dynamics of an ecological network.PLoS ONE, 6(11): e26455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Olesen JM, Bascompte J, Dupont YL, Jordano P.2007. The modularity of pollination networks.Proceedings of the National Academy of Sciences of the United States of America, 104(50): 19891-19896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Olesen JM, Bascompte J, Elberling H, Jordano P.2008. Temporal dynamics in a pollination network.Ecology, 89(6): 1573-1582. [DOI] [PubMed] [Google Scholar]
- [48].Pellmyr O.2003. Yuccas, yucca moths, and coevolution: a review.Annals of the Missouri Botanical Garden, 90(1): 35-55. [Google Scholar]
- [49].Petanidou T, Kallimanis AS, Tzanopoulos J, Sgardelis SP, Pantis JD.2008. Long-term observation of a pollination network: fluctuation in species and interactions, relative invariance of network structure and implications for estimates of specialization.Ecology Letters, 11(6): 564-575. [DOI] [PubMed] [Google Scholar]
- [50].Pimm SL.1982. Food Webs. London: Chapman &Hall. [Google Scholar]
- [51].Plein M, Längsfeld L, Neuschulz EL, Schultheiß C, lngmann L, Töpfer T, Böhning-Gaese K, Schleuning M.2013. Constant properties of plant-frugivore networks despite fluctuations in fruit and bird communities in space and time.Ecology, 94(6): 1296-1306. [DOI] [PubMed] [Google Scholar]
- [52].Prado PI, Lewinsohn TM.2004. Compartments in insect-plant associations and their consequences for community structure.Journal of Animal Ecology, 73(6): 1168-1178. [Google Scholar]
- [53].Rafael JA, Aguiar AP, Amorim DS.2009. Knowledge of insect diversity in Brazil: challenges and advances.Neotropical Entomology, 38(5): 565-570. [DOI] [PubMed] [Google Scholar]
- [54].Rezende EL, Lavabre JE, Guimarães PR, Jordano J, Bascompte J.2007a. Non-random coextinctions in phylogenetically structured mutualistic networks.Nature, 448(7156): 925-928. [DOI] [PubMed] [Google Scholar]
- [55].Rezende EL, Jordano P, Bascompt J.2007b. Effects of phenotypic complementarity and phylogeny on the nested structure of mutualistic networks.Oikos, 116(11): 1919-1929. [Google Scholar]
- [56].Schleuning M, Ingmann L, Strauß R, Fritz SA, Dalsgaard B, Dehling DM, Plein M, Saavedra F, Sandel B, Svenning JC, Böhning-Gaese K, Dormann CF.2014. Ecological, historical and evolutionary determinants of modularity in weighted seed-dispersal networks.Ecology Letters, 17(4): 454-463. [DOI] [PubMed] [Google Scholar]
- [57].Spiesman BJ, Inouye BD.2013. Habitat loss alters the architecture of plant-pollinator interaction networks.Ecology, 94(12): 2688-2696. [DOI] [PubMed] [Google Scholar]
- [58].Staniczenko PPA, Kopp JC, Allesina S.2013. The ghost of nestedness in ecological networks.Nature Communications, 4: 1391. [DOI] [PubMed] [Google Scholar]
- [59].Thébault E, Fontaine C, 2010. Stability of ecological communities and the architecture of mutualistic and trophic networks.Science, 329(5993): 853-856. [DOI] [PubMed] [Google Scholar]
- [60].Thompson JN.2005. The Geographic Mosaic of Coevolution. Chicago: University of Chicago Press, 442 pp. [Google Scholar]
- [61].Thompson JN.2009. The coevolving web of life.The American Naturalist, 173(2): 125-140. [DOI] [PubMed] [Google Scholar]
- [62].Tilman D, May RM, Lehman CL, Nowak MA.1994. Habitat destruction and the extinction debt.Nature, 371(6492): 65-66. [Google Scholar]
- [63].Valdovinos FS, Ramos-Jiliberto R, Flores JD, Espinoza C, López G.2009. Structure and dynamics of pollination networks: the role of alien plants.Oikos, 118(8): 1190-1200. [Google Scholar]
- [64].Valiente-Banuet A, Aizen MA, Alcántara JM, Arroyo J, Cocucci A, Galetti M, García MB, García D, Gómez JM, Jordano P, Medel R, Navarro L, Obeso JR, Oviedo R, Ramírez N, Rey PJ, Traveset A, Verdú M, Zamora R.2014. Beyond species loss: the extinction of ecological interactions in a changing world.Functional Ecology, doi: 10.1111/1365-2435.12356. [Google Scholar]
- [65].Van der Niet T, Johnson SD.2012. Phylogenetic evidence for pollinator-driven diversification of angiosperms.Trends in Ecology and Evolution, 27(6): 353-361. [DOI] [PubMed] [Google Scholar]
- [66].Vázquez DP, Aizen MA.2003. Null model analyses of specialization in plant-pollinator interactions.Ecology, 84(9): 2493-2501. [Google Scholar]
- [67].Vázquez DP, Blüthgen N, Cagnolo L, Chacoff NP.2009. Uniting pattern and process in plant-animal mutualistic networks: a review.Annals of Botany, 103(9): 1445-1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Vilà M, Bartomeus I, Dietzsch AC, Petanidou T, Steffan-Dewenter I, Stout JC, Tscheulin T.2009. Invasive plant integration into native plant-pollinator networks across Europe.Proceedings of the Royal Society B: Biological Sciences, 276(1674): 3887-3893. [DOI] [PMC free article] [PubMed] [Google Scholar]