Abstract
Reproduction is the highest energy demand period for small mammals, during which both energy intake and expenditure are increased to cope with elevated energy requirements of offspring growth and somatic protection. Oxidative stress life history theory proposed that reactive oxygen species (ROS) were produced in direct proportion to metabolic rate, resulting in oxidative stress and damage to macromolecules. In the present study, several markers of oxidative stress and antioxidants activities were examined in brain, liver, kidneys, skeletal muscle and small intestine in non-lactating (Non-Lac) and lactating (Lac) KM mice. Uncoupling protein (ucps) gene expression was examined in brain, liver and muscle. During peak lactation, gross energy intake was 254% higher in Lac mice than in Non-Lac mice. Levels of H2O2 of Lac mice were 17.7% higher in brain (P<0.05), but 21.1% (P<0.01) and 14.5% (P<0.05) lower in liver and small intestine than that of Non-Lac mice. Malonadialdehyde (MDA) levels of Lac mice were significantly higher in brain, but lower in liver, kidneys, muscle and small intestine than that of Non-Lac mice. Activity of glutathione peroxidase (GSH-PX) was significantly decreased in brain and liver in the Lac group compared with that in the Non-Lac group. Total antioxidant capacity (T-AOC) activity of Lac mice was significantly higher in muscle, but lower in kidneys than Non-Lac mice. Ucp4 and ucp5 gene expression of brain was 394% and 577% higher in Lac mice than in Non-Lac mice. These findings suggest that KM mice show tissue-dependent changes in both oxidative stress and antioxidants. Activities of antioxidants may be regulated physiologically in response to the elevated ROS production in several tissues during peak lactation. Regulations of brain ucp4 and ucp5 gene expression may be involved in the prevention of oxidative damage to the tissue.
Keywords: Antioxidant, Energy intake, Lactation, Metabolic rate, Oxidative stress, Uncoupling protein
INTRODUCTION
Reproduction is the highest energy demand period for almost all mammals, during which sustained energy intake and expenditure are suggested to reach a ceiling, indicating that limited energy resources could be allocated between competing demands for reproduction and somatic protection (Speakman, 2008; Speakman & Garratt, 2014; Speakman & Król, 2005; 2011). Oxidative stress is suggested as a possible physiological cost of somatic protection that may limit investment on reproduction (Dowling & Simmons, 2009; Salmon et al, 2001; Selman et al, 2000; Xu et al, 2014). Oxidative stress life history theory proposed that free radicals, more specifically reactive oxygen species (ROS), were produced in direct proportion to metabolic rate as a consequence of the molecular functioning of mitochondria and the electron transport chain (Monaghan et al, 2009; Speakman & Garratt, 2014). An increase in metabolic rate in parallel with reproduction therefore leads to an elevation of ROS production (Dowling & Simmons, 2009; Selman et al, 2012; Speakman & Selman, 2011). If this is the case, there may be a trade-off in energy allocation between the investment in offspring growth and in physiological antioxidant against the elevated ROS levels.
However, data from several studies performed recently were less consistent and somewhat paradoxical, which might not be evident in the oxidative stress life history. The links between ROS production and rates of metabolism were not very strong in lactating females compared with that in their non-lactating counterparts (Fletcher et al, 2013; Garratt et al, 2012; Nussey et al, 2009; Speakman & Garratt, 2014; Xu et al, 2014). For example, Speakman & Garratt (2014) compared several markers indicative of oxidative stress between reproductive and non-reproductive animals in both laboratory and field animals, but observed that oxidative stress was unchanged, or was lower, in reproductive individuals in comparison with those that did not reproduce. These studies showed a weak positive correlation between oxidative damage and reproduction (Bergeron et al, 2011; Fletcher et al, 2013; Speakman & Garratt, 2014; Xu et al, 2014).
Mitochondria are the major producers of cellular ROS (Brand, 2000; Toime & Brand, 2010). There is a strong positive relationship between inner mitochondria membrane potential and ROS production, by which just a small increase in the membrane potential induces a large stimulation of ROS, and a small decrease is suggested to reduce ROS production (Boveris et al, 2006; Miwa & Brand, 2003). It has been suggested that UCPs (uncoupling proteins) partially uncouple mitochondria, and activate the proton conductance, leading to lowered proton motive force and decreased ROS production, consequently protecting against excessive superoxide production (Brand et al, 2004). Five mitochondrial UCPs exist: UCP2, expressed ubiquitously; UCP1, exclusively in brown adipose tissue; UCP3, predominantly in muscle; UCP4 and UCP5, in brain (Ježek, 2002). It is well established that lactating animals usually increase the rate of metabolism, but decrease ucps gene expression and/or protein content (Li & Wang, 2005; Martin et al, 1989; Pedraza et al, 2000; Xiao et al, 2004). However, it remains uncertain if changes of ucps expression are involved in ROS production and oxidative damage in lactating animals.
Kunming (KM) mice, a domestic laboratory rodent, are well known as a widely used mice model in the study of many field, including physiology and pharmacology. However, the oxidative stress and antioxidant, as well as UCPs expression, in several tissues related to digestion in KM mice during the peak lactation remain uncertain. The aim of this study was to examine the relationship between oxidative stress and reproduction in mice, using serial markers of both oxidative stress and antioxidants, including H2O2 and malonadialdehyde (MDA), as well as SOD, total antioxidant capacity (T-AOC), and glutathione peroxidase (GSH-PX). We manipulated female energy demand during lactation by altering female litter size at birth to 12 pups. This allowed us to test the relationship after correcting for the effect of litter size. In addition to oxidative stress and antioxidant, ucps gene expression in brain, liver and skeletal muscle was measured.
MATERIALS AND METHODS
Animals
Virgin female KM mice, aged 9-10 weeks, were obtained from the colony maintained in the animal house at Wenzhou University. Animals were housed individually in plastic cages (29 cm×18 cm×16 cm) with sawdust bedding, and maintained on a 12: 12 h light-dark cycle (lights on at 0800h) at a constant temperature of 21±1 °C. Animals were fed standard rodent chow (produced by Beijing KeAo FeedCo., Beijing, China) and water ad libitum. All experimental procedures were in compliance with the Animal Care and Use Committee of Wenzhou University.
Twenty-four female mice were assigned into two groups: 15 females were mated with males for 11 days, then males were moved out. 14 females became pregnant and gave birth (lactation, Lac, n=14) and the rest of females were not paired with males (non-lactation, Non-Lac, n=10). To correct for the effect of litter size, females were artificially regulated to raise 12 pups on parturition day. Body mass, food intake, energy intake, as well as litter size and litter mass were measured during the peak lactation (day 13-14 of lactation). Litters were weaned on day 17 of lactation.
Gross energy intake (GEI), digestive energy intake (DEI) and digestibility
GEI, DEI and digestibility were measured from day 13 to 14 of lactation, as described previously (Grodziński & Wunder, 1975; Liu et al, 2003). In detail, food was provided on the start of day 13, any uneaten food or food mixed within the bedding were collected along with any feces from each animal on the end of day 14 of lactation (over two days). They were separated manually after they were dried at 60 °C to constant mass. Gross energy contents of the food and feces were determined using a Parr 1281 oxygen bomb calorimeter (Parr Instrument, USA). GEI, DEI and digestibility were calculated using the following the equations (Grodziński & Wunder, 1975; Liu et al, 2003; Zhao et al, 2013, 2014a):
GEI (kJ/d)=[food provided (g/d)×dry matter content of food (%)-dry spillage of food and uneaten food]× gross energy content of food (kJ/g) (1)
DEI (kJ/d)=GEI-[dry feces mass (g/d)×gross energy content of feces (kJ/g)] (2)
Digestibility (%)=DEI/GEI×100% (3)
H2O2 and MDA
The female mice were euthanised by decapitation. Brain, leg skeletal muscle, liver and kidneys were separated and weighed (to 1 mg). The small intestine was also separated quickly and weighed (to 1 mg) after the contents were removed. All tissues were put into liquid nitrogen immediately after the weighing. Tissues were homogenized using ice-cold 0.9% NaCl solution. The homogenates were centrifuged at 3 000 g for 15 min and the supernatant was taken for later assay of the markers of oxidative stress and antioxidant enzymes. Levels of hydrogen peroxide (H2O2) were analyzed using commercial kits (Nanjing Jiancheng Bioengineering Institute) in accordance with the manufacturer’s instructions and guidelines. A pre-experiment performed on these tissues demonstrated that these assay kits were effective for KM mice. Level of H2O2 (405 nm) was expressed as mmol/g protein (Chen et al, 2014). The lipid peroxidation level was measured using the levels of malonadialdehyde (MDA), which is the end product of lipid peroxidation and reacts with thibabituric acid (TBA) as a thiobarbituric reactive species (TBARS) to produce a pink colored complex that has peak absorbance at 532 nm (Manivannan et al, 2013). Level of tissue MDA was expressed as nmol/mg protein (Chen et al, 2014).
Activity of SOD and T-AOC
Antioxidant enzymes activities, including SOD, and total antioxidant capacity (T-AOC) were determined using commercial kits (produced by Nanjing Jiancheng Bioengineering Institute), strictly according to the instructions. These kits were also sensitive for mouse tissues. 1 unit of SOD activity was defined as the amount of proteins causing 50% inhibition of the rate of nitroblue tetrazolium (NBT) reduction, and 1 unit of T-AOC activity was defined as the increment of 0.01 of absorbance OD value per min (Chen et al, 2014).
Activity of glutathione peroxidase (GSH-PX)
Activity of GSH-PX was also measured using a commercial kit (produced by Nanjing Jiancheng Bioengineering Institute) according to the instructions. The assay is based on the reaction of reduced glutathione (GSH) with H2O2, that produced oxidized glutathione (GSSG) and H2O. GSH reacted with disulfide generation sulfydryl reagent 5, 5'-dithio-bis (2 nitrobenzoic acid, DTNB), producing a 2-nitro-5-thiobenzoic acid a yellow colored compound, that was read at 412 nm. GSH-PX activity was calculated as the reduction rate of levels of GSH with the presence of GSH-PX relative to absence of GSH-PX. 1 unit of GSH-PX activity was defined as 1μmol of GSH consumption/min/mg protein at 37 °C. The protein concentration was determined by the method of Lowry (Lowry et al, 1951), using bovine serum albumin (BSA) as standard (Chen et al, 2014).
Real-time RT-qPCR analysis
Total RNA was prepared from brain, liver and skeletal muscle using TRIzol agent (TAKARA, Dalian, China). Real-time RT-qPCR analysis was carried out as described previously (Zhao et al, 2014b). Briefly, RNA concentration was determined spectrophotometrically, and 2 μg of total RNA was taken to synthesize cDNAs in a final reaction volume of 50 μLwith AMV Reverse Transcriptase (TAKARA) using random primer oligo (dT)18. The cDNA samples (2 μL) were used as a template for the subsequent PCR reaction using gene-specific primers, listed as follows: ucp2, forward, 5'-CCCAATGTTGCCCGTAAT-3' and reverse, 5'-CCCAAGCGGAGAAAGGAA-3'; ucp3, forward, 5'-GTTTACTGACAACTTCCCCT-3' and reverse, 5'-CTCCTGAGCCACCATCT-3'; ucp4, forward, 5'-GCCGAATAAT GAACCA AC-3' and reverse, 5'-ACCAAGGGGTCATTCTCA-3'; ucp5, forward, 5'-CGTTACTAAGACAGGCATCA-3' and reverse, 5'-ACACCCCTCCACAGACCC-3'. The final reaction volume of 20 μL contained 10 μL of 2× SYBR Premix EX Tag TM (TAKARA), 0.4 μL of forward prime and reverse primer (final concentration 0.2 μmol/L per primer) and 2 μL cDNA template. qPCR was performed using Roche LightCycler480 real-time qPCR system (Forrentrasse CH-6343 Rotkreuz, Switzerland). After an initial polymerase activation step at 95 °C for 60 s, amplification followed, by 40 cycles of (95 °C for 5 s, 55 °C for 30 s and 72 °C for 30 s) and the reaction finished by the built-in melt curve. All samples were quantified for relative quantity of gene expression by using actin expression as an internal standard, actin, forwards, 5'-CGTAAAGACCTCTATGCCAA-3' and reverse, 5'-GCGCAAGTTAGGTTTTGTC-3' (Zhao et al, 2014b).
Statistics
Data were expressed as mean±SE. and analyzed using SPSS 13.0 statistic software. Differences in body mass, DMI, GEI, DEI and digestibility, levels of H2O2 and MDA, activities of GSH-PX, SOD and T-AOC, as well as ucps gene expression between Non-Lac and Lac groups were examined using independent sample t tests. Correlations of H2O2, MDA, GSH-PX, SOD and T-AOC among different tissues were examined using Pearson’s correlation analysis. The level of significance was set at P<0.05.
RESULTS
Body mass, energy intake, litter size and litter mass
Body mass was significantly different between the two groups, and it was higher by 31.3% in Lac females than in their Non-Lac counterparts (Table 1). Lac females consumed significantly more food and produced more feces than Non-Lac females (Table 1). During peak lactation, GEI and DEI were 254% and 253% higher in the Lac group, respectively, than that in the Non-Lac group (Table 1). No difference was observed in digestibility between the two groups (Table 1). Litter size averaged 12 throughout lactation and mean litter mass was 97.5±2.6 g during peak lactation (Table 1).
Table 1.
Energy intake, digestibility and litter size and mass in non-lactating and lactating mice during peak lactation
| Non-Lac | Lac | t | P | |
|---|---|---|---|---|
| Body mass (g) | 41.8±1.0 | 54.9±0.9 | 9.48 | ** |
| DMI (kJ/d) | 7.0±0.3 | 25.0±0.8 | 16.75 | ** |
| GEI (kJ/d) | 124.0±5.4 | 438.9±14.5 | 16.75 | ** |
| GE of feces (kJ/d) | 28.5±1.1 | 102.2±3.5 | 16.44 | ** |
| DEI (kJ/d) | 95.5±4.5 | 336.7±11.8 | 15.78 | ** |
| Digestibility (%) | 76.9±0.5 | 76.7±0.5 | 0.30 | ns |
| Litter size | - | 12.0±0.7 | - | - |
| Litter mass (g) | - | 97.5±2.6 | - | - |
DMI: Dry matter intake; GEI: Gross energy intake; GE: Gross energy; DEI: Digestive energy intake; **: P<0.01; ns: Non-significance (P>0.05).
H2O2 and MDA
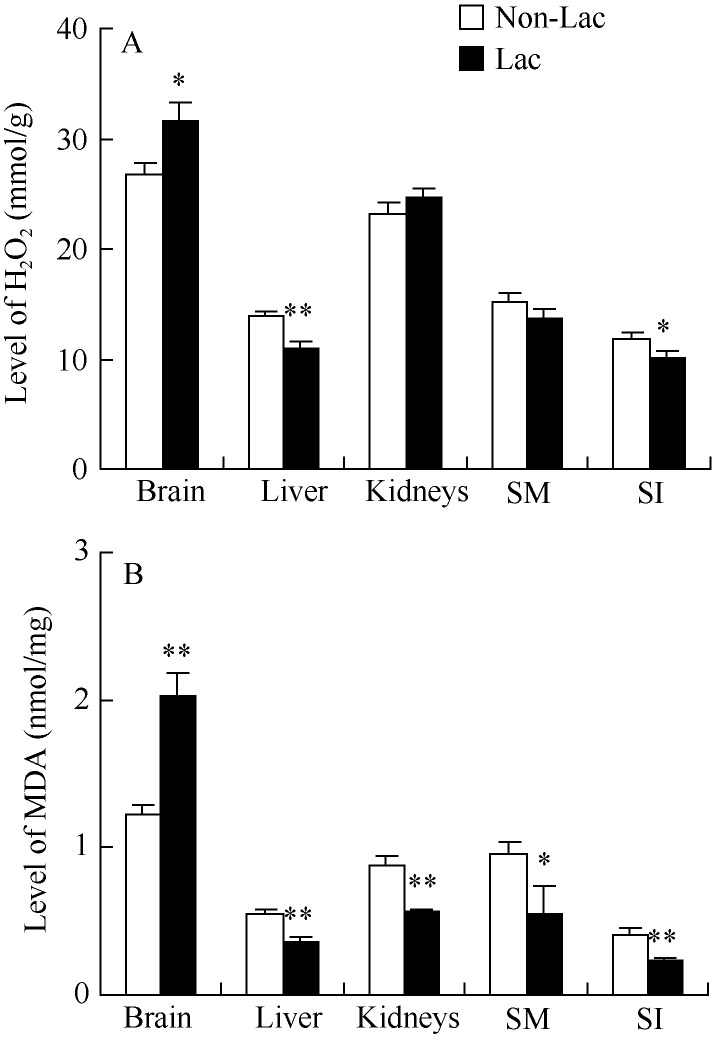
Brain showed 17.7% higher H2O2 levels in Lac than Non-Lac group (t22=2.34, P<0.05). Levels of H2O2 in liver and small intestine were significantly lower in Lac group than that in Non-Lac group (liver, t22=4.57, P<0.01; small intestine, t22=2.20, P<0.05). No statistical differences were observed in H2O2 levels between the two groups in kidneys (t22=1.14, P>0.05) and skeletal muscle (t22=2.20, P>0.05) (Figure 1A). There were significantly negative correlations of H2O2 between liver and brain and small intestine, and the relationships among other tissues were not significant (Table 2).
Figure 1.
Levels of H2O2 (A) and MDA (B) in different tissues in mice during peak lactation SM: Skeletal muscle; SI: Small intestine; *: P<0.05; **: P<0.01.
Table 2.
Correlations of H2O2, MDA, GSH-PX, SOD and T-AOC among different tissues in lactating mice
| Brain | Liver | Kidneys | SM | SI | ||
|---|---|---|---|---|---|---|
| H2O2 | Brain | 1 | -0.50* | -0.11 | -0.14 | 0.27 |
| Liver | 1 | -0.25 | 0.36 | -0.42* | ||
| Kidneys | 1 | -0.31 | -0.12 | |||
| SM | 1 | -0.03 | ||||
| SI | 1 | |||||
| MDA | Brain | 1 | -0.43* | 0.29 | -0.08 | -0.44* |
| Liver | 1 | 0.07 | 0.26 | 0.82** | ||
| Kidneys | 1 | 0.19 | 0.05 | |||
| SM | 1 | -0.02 | ||||
| SI | 1 | |||||
| GSH-PX | Brain | 1 | -0.19 | -0.09 | 0.08 | 0.15 |
| Liver | 1 | -0.17 | 0.22 | 0.32 | ||
| Kidneys | 1 | -0.01 | 0.47* | |||
| SM | 1 | -0.15 | ||||
| SI | 1 | |||||
| SOD | Brain | 1 | -0.16 | -0.46* | 0.04 | 0.01 |
| Liver | 1 | 0.08 | 0.09 | 0.09 | ||
| Kidneys | 1 | 0.06 | 0.04 | |||
| SM | 1 | -0.06 | ||||
| SI | 1 | |||||
| T-AOC | Brain | 1 | -0.13 | 0.11 | 0.29 | 0.30 |
| Liver | 1 | -0.13 | 0.21 | -0.16 | ||
| Kidneys | 1 | -0.04 | -0.12 | |||
| SM | 1 | 0.26 | ||||
| SI | 1 |
SM: Skeletal muscle; SI: Small intestine; *: P<0.05; **: P<0.01.
Levels of MDA in brain were significantly higher in Lac than in Non-Lac group (t22=4.69, P<0.01). However, MDA levels of Lac females were 32.7%, 36.7%, 42.3% and 39.4% lower in liver (t22=4.09, P<0.01), kidneys (t22=4.98, P<0.01), skeletal muscle (t22=2.14, P<0.05) and small intestine (t22=2.80, P<0.01) than that of Non-Lac females (Figure 1B). There were significant correlations of MDA levels between small intestine and brain and liver, as well as brain and liver (Table 2). No correlations were observed between H2O2 and MDA and litter mass in any tissues (Table 3).
Table 3.
Correlations of litter mass with H2O2, MDA, GSH-PX, SOD and T-AOC of different tissues in lactating mice
| Brain | Liver | Kidneys | SM | SI | |
|---|---|---|---|---|---|
| H2O2 | 0.13 | -0.29 | 0.03 | -0.30 | 0.36 |
| MDA | 0.23 | -0.34 | -0.33 | 0.07 | -0.37 |
| GSH-PX | -0.19 | -0.30 | 0.33 | 0.25 | -0.04 |
| SOD | -3.7 | -0.17 | 0.29 | -0.38 | 0.23 |
| T-AOC | 0.05 | -0.13 | 0.16 | 0.24 | -0.03 |
SM: Skeletal muscle; SI: Small intestine.
GSH-PX, SOD and T-AOC
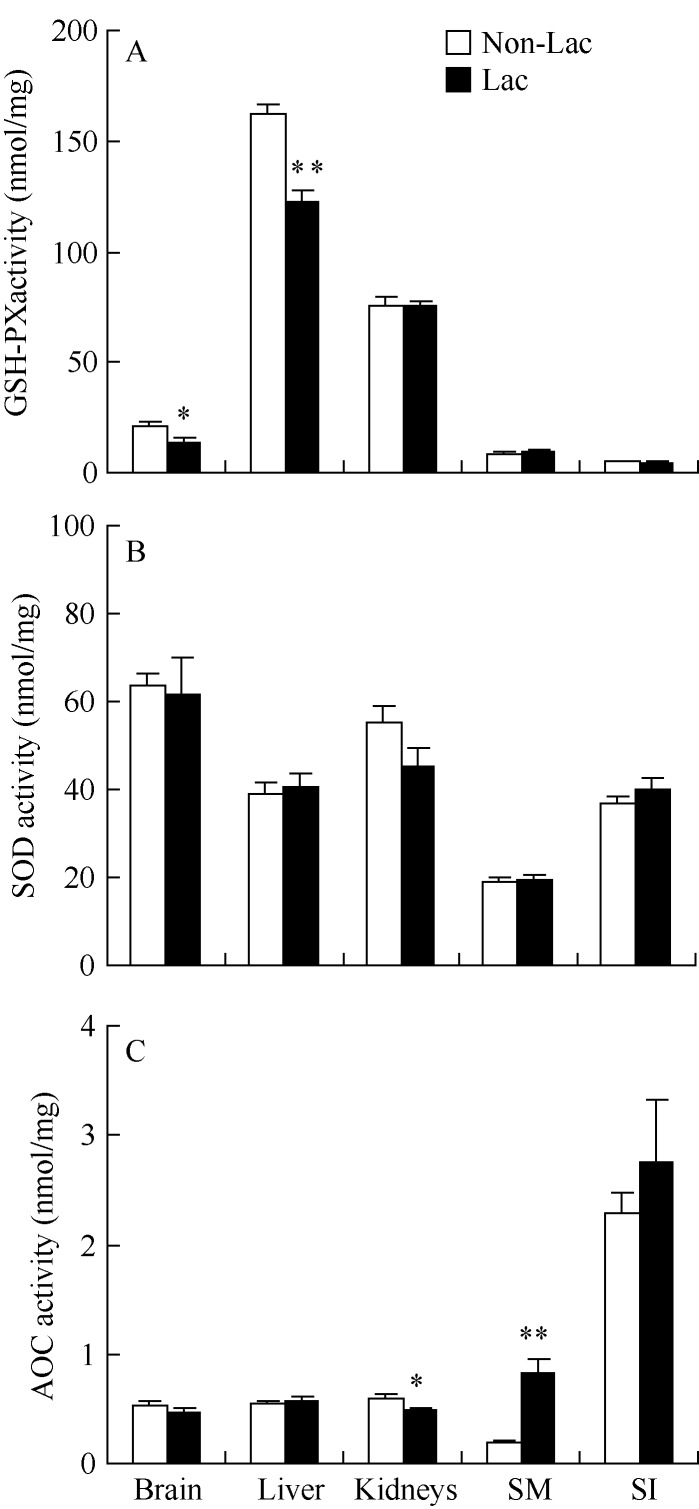
Activity of GSH-PX in the Lac females was significantly lower in the brain (t22=2.73, P<0.05) and liver (t22=5.65, P<0.01) than in Non-Lac females, while it did not differ between the two groups in kidneys (t22=0.08, P>0.05), skeletal muscle (t22=1.01, P>0.05) and small intestine (t22=0.49, P>0.05, Figure 2A). A significant correlation of GSH-PX was observed between kidneys and small intestine only (Table 2). There was no difference in levels of SOD between the two groups in any tissues (Figure 2B). No correlations were found in SOD among the several tissues except for significant relationship between brain and kidneys (Table 2). Activity of T-AOC did not differ between the two groups in brain (t22=1.52, P>0.05), liver (t22=0.43, P>0.05) and small intestine (t22=0.83, P>0.05), whereas it did in kidneys (t22=2.18, P<0.05) and skeletal muscle (t22=5.27, P<0.01, Figure 2C). No correlations were found in T-AOC activity among any tissues (Table 2). There were no correlations between litter mass and levels of GSH-PX, SOD and T-AOC in any tissues (Table 3).
Figure 2.
Activities of GSH-PX (A), SOD (B) and T-AOC (C) in different tissues in mice during peak lactation SM: Skeletal muscle; SI: Small intestine; *: P<0.05; *: P<0.01.
Ucps gene expression
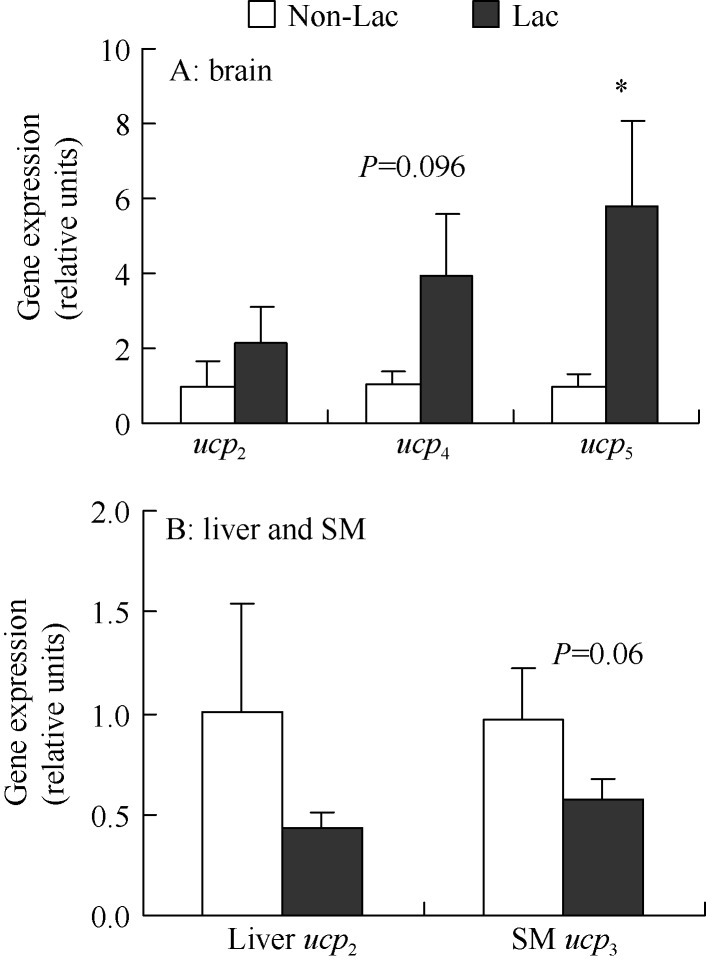
No significant difference was observed in brain ucp2 gene expression between Non-Lac and Lac groups (t26=0.97, P>0.05, Figure 3A). Ucp4 gene expression of Lac females was 394% higher than that in the Non-Lac control group (t26=1.73, P=0.096, Figure 3A). There was a significant difference in ucp5 gene expression between the two groups, and Lac females showed 577% higher ucp5 gene expression than the Non-Lac females (t26=2.06, P<0.05, Figure 3A). Ucp2 in liver did not differ between the two groups (t26=0.97, P>0.05, Figure 3B). Lac mice showed 41.4% lower ucp3 expression in skeletal muscle than the Non-Lac females (t26=1.94, P=0.06, Figure 3B).
Figure 3.
Gene expressions of ucp2, ucp4 and ucp5 in brain (A) and ucp2 in liver and ucp3 in skeletal muscle (B) in mice raising litters of 12 SM: Skeletal muscle; *: P<0.05.
DISCUSSION
Lactation is the most energetically demanding period encountered by small mammals, during which a female must allocate a large part of their energy to support their offspring, and also have to increase the energy utilised for maintenance (Speakman & Król, 2005). In the present study, female mice consumed 2.5-fold higher energy during peak lactation than non-reproductive females. This was consistent with results observed in the same strain of mouse (Zhao & Cao, 2009). Similarly, food intake during peak lactation was 3.4-fold higher in MF1 mice (Johnson et al, 2001), 1.2-fold in Brandt’s voles (Lasiopodomys brandtii) (Zhang and Wang, 2007), around 3-fold in Siberian hamsters (Phodopus sungorus) (Paul et al, 2010) and 2.1-fold in striped hamster (Cricetulus barabensis)(Zhao, 2011). Although, energy intake is notably increased in a majority of mammals during reproduction, it actually reaches a ceiling at peak lactation, called limitation on sustained energy intake (Hammond & Diamond, 1997; Speakman & Król, 2005, 2011). Life-history theory assumed that there was a trade-off between the allocation of limited resources to the competing demands of reproduction and somatic maintenance (Speakman & Garratt, 2014; Speakman & Król, 2005, 2011).
As mentioned above, the main energy expenditure during reproduction in lactating females is the energy utilised for milk production, the only energy resources that offspring could used for growth and activity (Speakman, 2008). In the current study, the weaned offspring were 97.5 g in KM mice. This was consistent with the average of litter mass in this strain of mouse (Zhao & Cao, 2009). That means lactating mice must export as much as 103kJ/d, on average, to support their offspring, accounting for 32.3% of daily gross energy intake (Zhao et al, 2010). Similarly, milk energy output accounts for 45.1% for MF1 mice (Johnson et al, 2001; Król & Speakman, 2003). It was assumed that the reproductive value would be higher if females invest more in their offspring (Speakman & Król, 2005, 2011). However, based on the life-history theory, animals do not reproduce maximally because this would reduce the probability of their own survival (Speakman & Król, 2005). One such possibility is oxidative stress, which has been suggested as a possible physiological cost of reproduction (Xu et al, 2014). The possible explanation is that the females usually increase their rate of metabolism during lactation, resulting in elevated generation of ROS that have potential to damage macromolecules unless quenched by antioxidants (Garratt et al, 2011).
In the present study, we expected that females during peak lactation would elevate oxidative stress and antioxidant activity. In contrast to our expectation, several markers of oxidative stress did not increase in lactating mice compared with that in non-reproductive counterparts. MDA levels were significantly lower in liver, kidneys, skeletal muscle and small intestine in lactating females than non-reproductive controls, indicating that no evidence of increased oxidative stress was found in reproductive mice. Consistently, significant reduction of MDA and/or protein carbonyls in liver were observed in lactating Brandt’s voles (Xu et al, 2014) and Mongolian gerbils (Meriones unguiculatus) (Yang et al, 2013), and house mice (Mus musculus domesticus) (Garratt et al, 2011, 2013) compared with non-reproductive controls. In addition, Garratt and colleague found that reproductive animals did not have higher lipid peroxidation, which was even lower in inner tissues, like liver, indicating that no oxidative damage occurred to the females (Garratt et al, 2011). As a large proportion of energy was metabolized in lactating females directly to offspring in milk, showing significant increases in rate of energy metabolism, it was unlikely that the absence of oxidative damage was owing to an insufficient increase in metabolism (Garratt et al, 2011).
It has been suggested that oxidative stress occurs only when ROS production exceeds the capacities of protection, resulting in oxidative damage to macromolecules (Beckman & Ames, 1998; Selman et al, 2002). In the present study, activities of GSH-PX, SOD and T-AOC, the markers of antioxidants, in lactating mice were almost the same as that observed in non-reproductive mice. Organisms have a variety of defensive mechanisms that can protect against oxidative stress (Garratt et al, 2011). Physiological regulation of antioxidant systems are effective mechanisms to maintain the oxidant-antioxidant balance, and consequently may play important roles in preventing oxidative damage to macromolecules (Garratt et al, 2011). Actually, the potential associations between the both sides have been observed in the studies previously performed in house mice (Aloise et al, 2013; Garratt et al, 2011, 2013), Brandt’s voles (Xu et al, 2014), Mongolian gerbils (Yang et al, 2013), Wistar rats (Rattus norvegicus) (Davidović et al, 1999; Venditti et al, 2004) and short-tailed field voles (Microtus agrestis) (Selman et al, 2000). Among the different tissues in the current study, the brain was only one showing a significant increase in oxidative stress, indicated by levels of H2O2 and MDA, which might be partly caused by significant reduction of antioxidant of GSH-PX activity. The decreased GSH-PX activity might impair the oxidant-antioxidant balance. The data from the current study may also demonstrate in reproductive mice that antioxidants were regulated physiologically in response to ROS, by which oxidant-antioxidant balance occurs during peak lactation. In addition, in the present study tissue-dependent changes in both oxidative stress and antioxidants were observed in lactating KM mice. This might be due to the difference in the rate of metabolism among the different tissues. Unfortunately, the tissue-specific rate of metabolism was not measured in these tissues.
UCPs are suggested to uncouple mitochondria and lower proton motive force, and play roles in decreasing ROS production (Brand et al, 2004). It has been suggested that lactating animals usually decrease ucps gene expression in peripheral tissues, including brown adipose tissue, liver and skeletal muscle (Li & Wang, 2005; Martin et al, 1989; Pedraza et al, 2000; Xiao et al, 2004). In the present study, we observed that in lactating mice ucp5 gene expression of brain were 577% higher in lactating mice than non-reproductive mice (P<0.05), indicating potential roles of ucp5 in prohibition of ROS production. UCP5 passes protons through the inner mitochondrial membrane to the matrix, and thus performs the essential function of an uncoupler of oxidative phosphorylation (Ramsden et al, 2012). This process is accompanied by a reduction in oxidative stress, and consequentially exerts a protective influence on cells (Ramsden et al, 2012). In the present study, UCP5 expression was also up-regulated in the brain in lactating mice compared with that in non-lactating mice. This may reflect an adaptive response to the increased oxidative stress, indicated by the elevations of H2O2 and MDA, during peak lactation. However, the data from the present study were only associated with ucps gene expression in brain, liver and skeletal muscle, which might not draw a generally strong conclusion about the relationship between ROS production and UCPs expression in animals during peak lactation.
KM mice showed significantly higher energy intake and exported more energy in milk production during peak lactation than their non-reproductive counterparts. However, several markers of both oxidative stress and antioxidant activities were not significantly higher in liver, kidneys, skeletal muscle and small intestine in reproductive mice compared with that in controls. This was inconsistent with the prediction of the oxidative stress life history theory. Activities of antioxidants might be regulated physiologically in response to elevated ROS production in several tissues of lactating mice, by which oxidant-antioxidant balance might prevent oxidative damage to the tissues. Regulations of brain ucp4 and ucp5 gene expression might be involved in the prevention of oxidative damage to the tissue.
ACKNOWLEDGEMENTS
We thank Ally E Wilson, Institute of Biological and Environmental Sciences, University of Aberdeen, for her help with this study and the English correction of the manuscript.
Funding Statement
This work was supported by grants from Wenzhou University (14SK51A, 14SK52A), the National Natural Science Foundation of China (31270458) and grant from Zhejiang Province (pd2013374)
Contributor Information
Guo-Xiao ZHENG, College of Life and Environmental Science, Wenzhou University, Wenzhou 325035, China.
Jiang-Tao LIN, College of Life and Environmental Science, Wenzhou University, Wenzhou 325035, China.
Wei-Hong ZHENG, College of Life and Environmental Science, Wenzhou University, Wenzhou 325035, China.
Jing CAO, College of Life and Environmental Science, Wenzhou University, Wenzhou 325035, China.
Zhi-Jun ZHAO, College of Life and Environmental Science, Wenzhou University, Wenzhou 325035, China.
REFERENCES
- [1].Aloise King ED, Garratt M, Brooks R.2013. Manipulating reproductive effort leads to changes in female reproductive scheduling but not oxidative stress.Ecology and Evolution, 3(12): 4161-4171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Beckman KB, Ames BN.1998. The free radical theory of aging matures.Physiological Reviews, 78(2): 547-581. [DOI] [PubMed] [Google Scholar]
- [3].Bergeron P, Careau V, Humphries MM, Réale D, Speakman JR, Garant D.2011. The energetic and oxidative costs of reproduction in a free-ranging rodent.Functional Ecology, 25(5): 1063-1071. [Google Scholar]
- [4].Boveris A, Valdez LB, Zaobornyj T, Bustamante J.2006. Mitochondrial metabolic states regulate nitric oxide and hydrogen peroxide diffusion to the cytosol.Biochimica et Biophysica Acta (BBA) - Bioenergetics, 1757(5-6): 535-542. [DOI] [PubMed] [Google Scholar]
- [5].Brand MD.2000. Uncoupling to survive? The role of mitochondrial inefficiency in ageing.Experimental Gerontology, 35(6-7): 811-820. [DOI] [PubMed] [Google Scholar]
- [6].Brand MD, Buckingham JA, Esteves TC, Green K, Lambert AJ, Miwa S, Murphy MP, Pakay JL, Talbot DA, Echtay KS.2004. Mitochondrial superoxide and aging: uncoupling-protein activity and superoxide production.Biochemical Society Symposium, 71: 203-213. [DOI] [PubMed] [Google Scholar]
- [7].Chen KX, Wang CM, Wang GY, Zhao ZJ.2014. Energy budget, oxidative stress and antioxidant in striped hamster acclimated to moderate cold and warm temperatures.Journal of Thermal Biology, 44: 35-40. [DOI] [PubMed] [Google Scholar]
- [8].Davidović V, Đjokić I, Petrović N, Đurašević S, Cvijić G.1999. Activity of antioxidant enzymes in rat skeletal muscle and brown fat: effect of cold and propranolol.Journal of Thermal Biology, 24(5-6): 385-389. [Google Scholar]
- [9].Dowling DK, Simmons LW.2009. Reactive oxygen species as universal constraints in life-history evolution.Proceedings of the Royal Society B: Biological Sciences, 276(1663): 1737-1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Fletcher QE, Selman C, Boutin S, McAdam AG, Woods SB, Seo AY, Leeuwenburgh C, Speakman JR, Humphries MM.2013. Oxidative damage increases with reproductive energy expenditure and is reduced by food-supplementation.Evolution, 67(5): 1527-1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Garratt M, Pichaud N, Aloise King ED, Brooks RC.2013. Physiological adaptations to reproduction. I. Experimentally increasing litter size enhances aspects of antioxidant defence but does not cause oxidative damage in mice.The Journal of Experimental Biology, 216(Pt 15): 2879-2888. [DOI] [PubMed] [Google Scholar]
- [12].Garratt M, Vasilaki A, Stockley P, McArdle F, Jackson M, Hurst JL.2011. Is oxidative stress a physiological cost of reproduction? An experimental test in house mice.Proceedings of the Royal Society B: Biological Sciences, 278(1708): 1098-1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Garratt M, McArdle F, Stockley P, Vasilaki A, Beynon RJ, Jackson MJ, Hurst JL.2012. Tissue-dependent changes in oxidative damage with male reproductive effort in house mice.Functional Ecology, 26(2): 423-433. [Google Scholar]
- [14].Grodziński W, Wunder BA.1975. Ecological energetics of small mammals. In: Golley EB, Petrusewicz K, Ryszkowski L. Small Mammals: Their Productivity and Population Dynamics. Cambridge: Cambridge University Press, 173-204. [Google Scholar]
- [15].Hammond KA, Diamond J.1997. Maximal sustained energy budgets in humans and animals.Nature, 386(6624): 457-462. [DOI] [PubMed] [Google Scholar]
- [16].Ježek P.2002. Possible physiological roles of mitochondrial uncoupling proteins-UCPn.The International Journal of Biochemistry & Cell Biology, 34(10): 1190-1206. [DOI] [PubMed] [Google Scholar]
- [17].Johnson MS, Thomson SC, Speakman JR.2001. Limits to sustained energy intake I. Lactation in the laboratory mouse Mus musculus.The Journal of Experimental Biology, 204(11): 1925-1935. [DOI] [PubMed] [Google Scholar]
- [18].Król E, Speakman JR.2003. Limits to sustained energy intake VII. Milk energy output in laboratory mice at thermoneutrality.The Journal of Experimental Biology, 206(23): 4267-4281. [DOI] [PubMed] [Google Scholar]
- [19].Li XS, Wang DH.2005. Suppression of thermogenic capacity during reproduction in primiparous brandt’s voles (Microtus brandtii).Journal of Thermal Biology, 30(6): 431-436. [Google Scholar]
- [20].Liu H, Wang DH, Wang ZW.2003. Energy requirements during reproduction in female Brandt’s voles (Microtus brandtii).Journal of Mammalogy, 84(4): 1410-1416. [Google Scholar]
- [21].Lowry OH, Rosebrough NJ, Farr AL, Randall RJ.1951. Protein measurement with the Folin phenol reagent.Journal of Biological Chemistry, 193: 265-275. [PubMed] [Google Scholar]
- [22].Manivannan J, Sinha S, Ghosh M, Mukherjee A.2013. Evaluation of multi-endpoint assay to detect genotoxicity and oxidative stress in mice exposed to sodium fluoride.Mutation Research/Genetic Toxicology and Environmental Mutagenesis, 751(1): 59-65. [DOI] [PubMed] [Google Scholar]
- [23].Martin I, Giralt M, Viñas O, Iglesias R, Mampel T, Villarroya F.1989. Adaptative decrease in expression of the mRNA for uncoupling protein and subunit II of cytochrome c oxidase in rat brown adipose tissue during pregnancy and lactation.Biochemical Journal, 263(3): 965-968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Miwa S, Brand MD.2003. Mitochondrial matrix reactive oxygen species production is very sensitive to mild uncoupling.Biochemical Society Transactions, 31(6): 1300-1301. [DOI] [PubMed] [Google Scholar]
- [25].Monaghan P, Metcalfe NB, Torres R.2009. Oxidative stress as a mediator of life history trade-offs: mechanisms, measurements and interpretation.Ecology Letters, 12(1): 75-92. [DOI] [PubMed] [Google Scholar]
- [26].Nussey DH, Pemberton JM, Pilkington JG, Blount JD.2009. Life history correlates of oxidative damage in a free-living mammal population.Functional Ecology, 23(4): 809-817. [Google Scholar]
- [27].Paul MJ, Tuthill C, Kauffman AS, Zucker I.2010. Pelage insulation, litter size, and ambient temperature impact maternal energy intake and offspring development during lactation.Physiology &Behavior, 100(2): 128-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Pedraza N, Solanes G, Carmona MC, Iglesias R, Viñas O, Mampel T, Vazquez M, Giralt M, Villarroya F.2000. Impaired expression of the uncoupling protein-3 gene in skeletal muscle during lactation: fibrates and troglitazone reverse lactation-induced downregulation of the uncoupling protein-3 gene.Diabetes, 49(7): 1224-1230. [DOI] [PubMed] [Google Scholar]
- [29].Ramsden DB, Ho PWL, Ho JWM, Liu HF, So DHF, Tse HM, Chan KH, Ho SL.2012. Human neuronal uncoupling proteins 4 and 5 (UCP4 and UCP5): structural properties, regulation, and physiological role in protection against oxidative stress and mitochondrial dysfunction.Brain and Behavior, 2(4): 468-478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Salmon AB, Marx DB, Harshman LG.2001. A cost of reproduction in Drosophila melanogaster: stress susceptibility.Evolution, 55(8): 1600-1608. [DOI] [PubMed] [Google Scholar]
- [31].Selman C, McLaren JS, Himanka MJ, Speakman JR.2000. Effect of long-term cold exposure on antioxidant enzyme activities in a small mammal.Free Radical Biology and Medicine, 28(8): 1279-1285. [DOI] [PubMed] [Google Scholar]
- [32].Selman C, Blount JD, Nussey DH, Speakman JR.2012. Oxidative damage, ageing, and life-history evolution: where now?. Trends in Ecology & Evolution, 27(10): 570-577. [DOI] [PubMed] [Google Scholar]
- [33].Selman C, Grune T, Stolzing A, Jakstadt M, McLaren JS, Speakman JR.2002. The consequences of acute cold exposure on protein oxidation and proteasome activity in short-tailed field voles, microtus agrestis.Free Radical Biology and Medicine, 33(2): 259-265. [DOI] [PubMed] [Google Scholar]
- [34].Speakman JR.2008. The physiological costs of reproduction in small mammals.Philosophical Transactions of the Royal Society B: Biological Sciences, 363(1490): 375-398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Speakman JR, Król E.2005. Limits to sustained energy intake IX: a review of hypotheses.Journal of Comparative Physiology B, 175(6): 375-394. [DOI] [PubMed] [Google Scholar]
- [36].Speakman JR, Król E.2011. Limits to sustained energy intake. XIII. Recent progress and future perspectives.The Journal of Experimental Biology, 214(2): 230-241. [DOI] [PubMed] [Google Scholar]
- [37].Speakman JR, Selman C.2011. The free-radical damage theory: accumulating evidence against a simple link of oxidative stress to ageing and lifespan.BioEssays, 33(4): 255-259. [DOI] [PubMed] [Google Scholar]
- [38].Speakman JR, Garratt M.2014. Oxidative stress as a cost of reproduction: Beyond the simplistic trade-off model.BioEssays, 36(11): 93-106. [DOI] [PubMed] [Google Scholar]
- [39].Toime LJ, Brand MD.2010. Uncoupling protein-3 lowers reactive oxygen species production in isolated mitochondria.Free Radical Biology and Medicine, 49(4): 606-611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Venditti P, De Rosa R, Portero-Otin M, Pamplona R, Di Meo S.2004. Cold-induced hyperthyroidism produces oxidative damage in rat tissues and increases susceptibility to oxidants.The International Journal of Biochemistry & Cell Biology, 36(7): 1319-1331. [DOI] [PubMed] [Google Scholar]
- [41].Xiao XQ, Grove KL, Grayson BE, Smith MS.2004. Inhibition of uncoupling protein expression during lactation: role of leptin.Endocrinology, 145(2): 830-838. [DOI] [PubMed] [Google Scholar]
- [42].Xu YC, Yang DB, Speakman JR, Wang DH.2014. Oxidative stress in response to natural and experimentally elevated reproductive effort is tissue dependent.Functional Ecology, 28(2): 402-410. [Google Scholar]
- [43].Yang DB, Xu YC, Wang DH, Speakman JR.2013. Effects of reproduction on immuno-suppression and oxidative damage, and hence support or otherwise for their roles as mechanisms underpinning life history trade-offs, are tissue and assay dependent.The Journal of Experimental Biology, 216(22): 4242-4250. [DOI] [PubMed] [Google Scholar]
- [44].Zhang XY, Wang DH.2007. Thermogenesis, food intake and serum leptin in cold-exposed lactating Brandt’s voles Lasiopodomys brandtii.The Journal of Experimental Biology, 210(3): 512-521. [DOI] [PubMed] [Google Scholar]
- [45].Zhao ZJ, Cao J.2009. Effect of fur removal on the thermal conductance and energy budget in lactating Swiss mice.The Journal of Experimental Biology, 212(16): 2541-2549. [DOI] [PubMed] [Google Scholar]
- [46].Zhao ZJ, Chi QS, Cao J, Han YD.2010. The energy budget, thermogenic capacity and behavior in Swiss mice exposed to a consecutive decrease in temperatures.The Journal of Experimental Biology, 213(23): 3988-3997. [DOI] [PubMed] [Google Scholar]
- [47].Zhao ZJ.2011. Relationship between reproductive output and basal metabolic rate in striped hamster (Cricetulus barabensis).Acta Theriologica Sinica, 31(1): 69-78. (in Chinese) [Google Scholar]
- [48].Zhao ZJ, Wei WT, Li MZ, Cao J.2013. Body mass, energy budget and leptin of mice under stochastic food restriction and refeeding.Zoological Research, 34(6): 574-581. [PubMed] [Google Scholar]
- [49].Zhao ZJ, Liu YA, Xing YJ, Zhang ML, Ni XY, Cao J.2014a. The role of leptin in striped hamsters subjected to food restriction and refeeding.Zoological Research, 35(4): 262-271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Zhao ZJ, Chen KX, Liu YA, Wang CM, Cao J.2014b. Decreased circulating leptin and increased neuropeptide Y gene expression are implicated in food deprivation-induced hyperactivity in striped hamsters, Cricetulus barabensis.Hormones and Behavior, 65(4): 355-362. [DOI] [PubMed] [Google Scholar]