Abstract
Pig-tailed macaques (Macaca nemistrina group) have been extensively used as non-human primate animal models for various human diseases in recent years, notably for AIDS research due to their sensitivity to HIV-1. Northern pig-tailed macaques (M. leonina) are distributed in China and other surrounding Southeast Asia countries. Although northern pig-tailed macaques have been bred on a large scale as experimental animals since 2012, the reference value of normal levels of leukocytes is not available. To obtain such information, 62 blood samples from male and female healthy northern pig-tailed macaques at different ages were collected. The normal range of major leukocyte subpopulations, such as T lymphocytes, B lymphocytes, natural killer (NK) cells, monocytes, and the expression levels of activation or differentiation related molecules (CD38, HLA-DR, CCR5, CD21, IgD, CD80 and CD86) on lymphocytes were analyzed by flow cytometry. The counts of B cells decreased with age, but those of CD8+ T cells and NK cells and the frequency of CD38+HLA-DR+CD4+ T cells were positively correlated with age. The counts of leukocyte subpopulations were higher in males than those in females except for CD4+ T cells. Males also showed higher expression levels of IgD and CD21 within B cells. This study provides basic data about the leukocyte subpopulations of northern pig-tailed macaques and compares this species with commonly used Chinese rhesus macaques (M. mulatta), which is meaningful for the biomedical application of northern pig-tailed macaques.
Keywords: Northern pig-tailed macaque, Flow cytometry, Leukocyte subpopulation, Age, Sex
Pig-tailed macaques (Macaca nemistrina group, PTM), rhesus macaques (M. mulatta, RM) and cynomolgus macaques (M. fascicularis, CM), all belonging to the Cercopithecidae family of Old World monkeys, have become more widely used models for AIDS pathogenesis in recent years (Lei et al, 2013; Zhu et al, 2010). As their name suggests, PTM are characterized by the short, semi-erect ‘pig-like’ tail and the flat vertex. They diverged from RM and CM approximately 5 million years ago, while the divergence between RM and CM was generated at one another 2.4 million years later (Baroncelli et al, 2008).
PTM have been widely employed as animal models of sexually transmitted diseases due to the similarities between the anatomic structure of the genital tract and the menstrual cycle with humans, the feature that they breed in allseasons and have a relatively large body size. By contrast, it is difficult to perform colposcopic examinations and multiple biopsies on CM as they have much smaller vaginal canals and cervix diameters than PTM. The reproductive physiology of RM is also quite different from humans as their constant breeding season (Patton et al, 2009). More importantly, PTM have the overriding advantages on their abilities in acute human immunodeficiency virus-1 (HIV-1) infection and exceptional susceptibility to simian immunodeficiency virus (SIV) and simian-human immunodeficiency virus (SHIV). In fact, PTM are the only reported species of Old World monkeys that could be infected by HIV-1 (Agy et al, 1992; Batten et al, 2006; Bosch et al, 2000).
Although they have been extensively applied in biomedical areas, such as cognitive neuroscience, pharmacology and infectious etiology, PTM did not obtain a clear taxonomic status until Gippoliti et al (2001) separated the initial taxonomic three subspecies (nemestrina, leonina, pagensis) of the M. nemistrina group at the species level. Sunda pig-tailed macaques (M. nemestrina) are found in the southern half of the Malay Peninsula (only just extending into southernmost Thailand), Borneo, Sumatra and Bangka Island. Mentawai macaques (M. pagensis) are located in the Mentawai islands. Northern pig-tailed macaques (M. leonina) range from about N8° in Peninsular Thailand through Burma and Indochina into Bangladesh, India extending north as far as to the Brahmaputra, and the southernmost of Yunnan, China (Malaivijitnond et al, 2012).
Due to the lack of primate models that can be productively infected by HIV-1, especially given that HIV-1 infection is more efficiently restricted by tripartite motif protein 5α (TRIM5α), apolipoprotein B mRNA-editing enzyme and catalytic polypeptide-like 3 (APOBEC3) proteins of macaques than those of humans, the pathogenic mechanisms of HIV-1 have not been fully elucidated. The early block to HIV-1 infection in monkey cells was relieved by interference with TRIM5α expression (Stremlau et al, 2004). In our previous study, we for the first time reported that northern PTM do not express TRIM5α but rather TRIM5-CypA fusion gene, in which the B30.2/ SPRY domain of TRIM5α is substituted by retrotransposed CypA in the 3'-UTR, contributing to the invalidation of restriction to HIV-1 replication in these animals (Kuang et al, 2009). Recent studies showed that PTM rather than RM or CM are susceptive to siman-tropic HIV-1 (stHIV-1), in which the HIV-1NL4-3 vif gene was replaced by the vif genes from SIVmac or HIV-2ROD to suppress the antiretroviral activity of several APOBEC3 proteins (Hatziioannou et al, 2009; Thippeshappa et al, 2011). Thus northern PTM may is a desirable animal model for AIDS studies.
Wild northern PTM mainly distribute in southwestern Yunnan, China and southeastern Tibet. Because of the scarcity of this primate species, they are under First Grade State Protection of China. In 2012, Kunming Primate Research Center, China, first introduced northern PTM from Vietnam for large-scaled breeding for laboratory use. However, the limited knowledge of their immune systems has restricted their applications. In this study, we determined the phenotypic characteristics of leukocytes in northern PTM ranging from 2 to 11 years of age to provide detailed immunological data.
MATERIALS AND METHODS
Animals and sample collection
Cage-bred northern PTM were maintained according to the regulations approved by the American Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) at the Kunming Primate Research Center, Kunming Institute of Zoology, CAS. Ethylenediamine tetraacetic acid (EDTA) stabilized blood samples were collected from male (n=15) and female (n=14) juveniles (mean age=2.4 years (2-3 years)), as well as male (n=17) and female (n=16) adults (mean age=6.1 years (5-11 years)) without apparent external symptoms (acute infections, trauma and severe diarrhea) for flow cytometric analysis. For comparison purposes, data from age-appropriate male (n=9) and female (n=8) juvenile (mean age=2.4 years (2-3 years)), as well as male (n=14) and female (n=12) adult (mean age=7.5 years (5-10 years)) Chinese rhesus macaques (ChRM) were also collected. Animals’ age and sex information is shown in Table 1. All procedures were performed under the guidance of the Ethics Committee of Kunming Institute of Zoology.
Table 1.
Study group of northern pig-tailed macaques and Chinese rhesus macaques
| Age groups (years) | Northern pig-tailed macaques | Chinese rhesus macaques | ||||||
|---|---|---|---|---|---|---|---|---|
| Males | Females | Males | Females | |||||
| n | Age (years) | n | Age (years) | n | Age (years) | n | Age (years) | |
| Juvenile (2-3) | 15 | 2.3±0.5 | 14 | 2.5±0.5 | 9 | 2.3±0.5 | 8 | 2.5±0.5 |
| Adult (4-11) | 17 | 6.6±1.6 | 16 | 5.4±1.7 | 14 | 7.0±1.6 | 12 | 8.1±1.5 |
| Total | 32 | 4.6±2.5 | 30 | 4.0±2.0 | 23 | 5.2±2.6 | 20 | 5.9±3.0 |
Antibodies
Mouse anti-human CD molecule monoclonal and polyclonal antibodies that cross-reacted with PTM were used according to standard procedures and appropriate concentrations in this study. Anti-CD159a PE (clone Z199) mAb was obtained from Beckman Coulter. Anti-CD38 FITC (clone AT-1) mAb was obtained from STEMCELL. Streptomycin-PE-Cy7 was obtained from Biolegend. Anti-IgD biotin pAb used by conjunction with streptomycin-PE-Cy7 was obtained from Southern Biotech. Anti-CD14 APC (clone M5E2), anti-CD20 PerCP-Cy5. 5(clone 2H7), anti-CD21 APC (clone B-ly4), anti-CD3 APC-Cy7 (clone SP34-2), anti-CD4-PerCP-Cy5.5/ FITC (clone L200), anti-CD8α PE-Cy7 (clone RPA-T8), anti-CD80 PE (clone HB15e), anti-CD86 PE (clone FUN-1), anti-CCR5 PE (clone 3A9) and anti-HLA-DR APC (clone L243) mAbs were all obtained from BD Pharmigen.
Absolute quantification of major leukocyte subpopulations
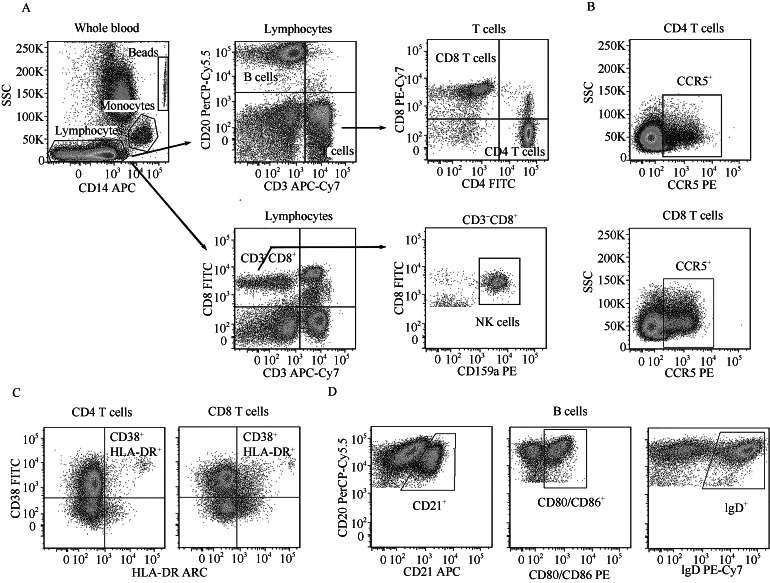
The methods of direct cell surface staining for whole blood and absolute number analysis were as described previously (Xia et al, 2009). Briefly, 50 μL of whole blood samples were added in TruCount tube (BD Biosciences) and incubated with fluorochrome-conjugated antibodies of CD3, CD4, CD8α, CD20, CD14 and CD159a mAbs for 30 min on ice. Erythrocytes were lysed with FACS lysing solution (BD Biosciences), and the samples were analyzed with BD FACSVerse cytometer directly. The absolute numbers of T cells (CD3+CD20-), CD4+ T cells (CD4+CD8a-CD3+), CD8+ T cells (CD4-CD8a+CD3+), B cells (CD3-CD20+), NK cells (CD3-CD8a+CD159a+) and monocytes (CD3-CD20-CD14+) were calculated using the following formula: Cell concentration (cell numbers/μL)=(events in gated region (n)×total number of TruCount beads (n))/(number of acquired beads (n)×sample volume (μL)) (Figure 1).
Figure 1.
Gating strategies for flow cytometric analysis
A: Gating strategies for T cells, B cells, NK cells and monocytes. CD14/side-scatter scattergram used for gating monocytes, TruCount beads and remaining lymphocytes. CD3/CD20 dot polt gated on lymphocytes used to count T and B cells. T cells were further divided into CD4+ and CD8+ T cells. NK cells were defined as CD3-CD8a+CD159a+ phenotype gating on lymphocytes. B: The expression levels of CCR5 were analyzed in each T cell subset. C: CD38+HLA-DR+ cells in each T cell subset were defined as activated cells. D: The expression levels of CD21, CD80/CD86 and IgD were analyzed in B cells.
Phenotype analysis by flow cytometry
As shown in Figure 1, to determine CCR5+ T cells, activated (CD38+HLA-DR+) T cells, activated (CD80/ CD86+) B cells, resting (CD21+) B cells and IgD/IgM secreting (IgD+) B cells, 100 μL of fresh whole blood used for each analysis were lysed with FACS lysing solution for 10 min at room temperature, followed by washing and resuspension with Dulbecco’s phosphate-buffered saline (DPBS) with 2% of new-born calf serum and 0.09% sodium azide (staining buffer). The suspending leukocytes were then stained with the relevant directly conjugated mAbs for 30 min on ice and fixed using PBS containing 4% paraformaldehyde.
Flow cytometric acquisition was performed on the BD FACSVerse cytometer driven by the FACSuite software (version 1.0.3; BD). The acquired cell number was at least 100 000 CD3+ T cells or 50 000 CD20+ B cells. Analysis of the acquired data was performed using FlowJo software (version 7.6.1; TreeStar).
Statistical analysis
Statistical differences between two groups were determined by the nonparametric Mann-Whitney U rank sum test. The Spearman’s test was performed for correlation analysis. Two-tailed P<0.05 was considered statistically significant. Data were presented as mean±SD. All statistical analyses were performed via GraphPad Prism software (version 5.01; GraphPad Software).
RESULTS
The absolute number of leukocyte subpopulations in peripheral blood of northern PTM
The absolute number of T cells, CD4+ T cells, CD8+ T cells, B cells, monocytes and NK cells in different groups are listed in Table 2. Numbers of B cells were negatively correlated with age (r=-0.426, P<0.001). CD8+ T cell counts (r=0.336, P=0.008) and NK cell counts (r=0.329, P=0.01) were slightly increased with the increasing of age. Adult group (1 117±548/μL) had dramatically less B cells than juvenile group (1 782± 741/μL, P<0.001), however, no significant cell count differences of CD8+ T cells and NK cells were found between adult and juvenile groups.
Table 2.
Cell counts (cell numbers/µL) of leukocyte subpopulations in different age and sex groups
| Age (years) | Sex | T cells | CD4+ T cells | CD8+ T cells | B cells | NK cells | Monocytes |
|---|---|---|---|---|---|---|---|
| Juvenile | M | 3 433±1 469 | 1 886±874 | 1 209±533 | 1 751±698 | 548±411 | 442±319 |
| (2-3) | F | 3 524±1 322 | 1 933±701 | 1 295±633 | 1 815±809 | 421±368 | 577±330 |
| Adult | M | 3 642±1 254 | 1 941±662 | 1 399±597 | 1 455±526 | 836±741 | 636±412 |
| (4-11) | F | 2 306±701 | 1 303±491 | 828±304 | 757±281 | 340±240 | 414±235 |
| Total | M | 3 544±1 341 | 1 915±756 | 1 310±567 | 1 594±621 | 701±617 | 545±378 |
| F | 2 875±1 192 | 1 597±668 | 1 046±533 | 1 251±789 | 378±303 | 490±290 | |
| All | 3 220±1 305 | 1 761±727 | 1 182±562 | 1 428±722 | 563±510 | 518±337 | |
| Range | 1 141-6 635 | 564-3 794 | 321-3 001 | 378-3 282 | 66-3178 | 154-1 844 |
In adult groups, compared with females, males showed higher counts of T cells (male 3 544±1 341/μL, female 2 875±1 192/μL; P=0.032), CD8+ T cells (male 1310±56/μL, female 1 046±533/μL; P=0.026), B cells (male 1 594±621/μL, female 1 251±789/μL; P=0.016;) and NK cells (male 701±617/μL, female 378±303/μL; P=0.004). However, no significant differences of age or sex were found in their counts of CD4+ T cells and monocytes.
Expressions of activation or differentiation related molecules on lymphocytes of northern PTM
The expression values of activation or differentiation related markers on each lymphocyte subset in different groups were shown in Table 3. CD38+HLA-DR+ subset increased progressively with the increaseing of age in CD4+ T cells (r=0.256, P=0.045), and more CD38+HLA-DR+ subset within CD4+ T cells (juvenile 1.3±0.4%, adult 2.0±0.9%; P=0.006) as well as CD8+ T cells (juvenile 4.1±1.8%, adult 5.0±1.9%; P=0.02) were observed in adult groups.
Table 3.
Expression levels (%) of activation or differentiation associated markers in different groups
| Age (years) | Sex | CD38+DR+ CD4+ T cells |
CD38+DR+ CD8+ T cells |
CCR5+ CD4+ T cells |
CCR5+ CD8+ T cells |
CD21+ B cells |
IgD+ B cells |
CD80/CD86+ B cells |
|---|---|---|---|---|---|---|---|---|
| Juvenile | M | 1.25±0.31 | 3.85±1.46 | 3.53±2.15 | 11.14±6.36 | 67.99±12.76 | 72.75±12.93 | 44.79±12.51 |
| (2-3) | F | 1.32±0.53 | 4.32±2.21 | 3.48±1.98 | 11.85±4.77 | 64.67±11.53 | 65.71±13.97 | 48.19±9.48 |
| Adult | M | 2.27±0.92 | 5.49±2.18 | 4.07±1.45 | 12.02±3.73 | 66.81±12.05 | 69.97±11.94 | 41.78±11.11 |
| (4-11) | F | 1.63±0.81 | 4.58±1.50 | 4.01±2.20 | 14.35±7.19 | 60.03±14.13 | 56.12±13.89 | 60.14±17.42 |
| Total | M | 1.79±0.86 | 4.72±2.03 | 3.82±1.81 | 11.61±5.07 | 67.37±12.20 | 71.27±12.29 | 43.19±11.69 |
| F | 1.49±0.70 | 4.46±1.84 | 3.76±2.08 | 13.19±6.21 | 62.19±12.98 | 60.60±14.53 | 54.57±15.30 | |
| All | 1.64±0.79 | 4.59±1.93 | 3.79±1.93 | 12.37±5.66 | 64.86±12.75 | 66.11±14.35 | 48.70±14.61 | |
| Range | 0.49-3.84 | 1.48-10.70 | 1.00-8.79 | 3.42-28.80 | 33.80-90.50 | 35.80-93.10 | 17.00-80.10 |
Males showed higher frequency of IgD+ B cells (male 71.3±12.3%, female 60.6±14.5%; P=0.006) but lower frequency of CD80/86+ B cells (male 43.2±11.7%, female 54.6±15.3%; P=0.004) than females. However, no statistically significant age and gender differences in the frequencies of CCR5+CD4+ T cells, CCR5+CD8+ T cells and CD21+B cells were found.
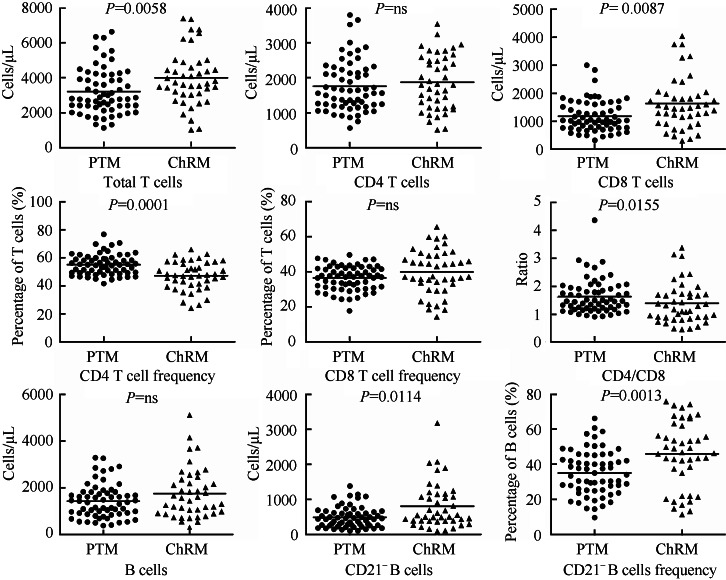
Comparisons of lymphocyte subpopulations between northern PTM and ChRM
As shown in Figure 2, a flow cytometric analysis was performed to study the differences in lymphocyte subpopulations between PTM and ChRM. Compared with ChRM, northern PTM exhibited less T cells (northern PTM 3 220±1 305/μL, ChRM 3 998±1 561/μL; P=0.006), less CD8+ T cells (northern PTM 1 182±562/μL, ChRM 1 634±921/μL; P=0.009), whereas higher frequencies of both CD4+ T cells (northern PTM 55.1±7.3%, ChRM 47.3±10.1%; P=0.0001) and CD21+ B cells (northern PTM 69.4±12.8% compared to 54.2±18.8%; P=0.001) as well as increased CD4/CD8 ratio (northern PTM 1.62±0.61, ChRM 1.38±0.72; P=0.016). Further analysis demonstrated that male northern PTM had less T cells (northern PTM 3 544±1 341/μL, ChRM 4498±1458/μL; P=0.03), less CD8+ T cells (northern PTM 1 310±567/μL, ChRM 1 985±991/μL; P=0.01; northern PTM 36.8±6.9%, ChRM 44.0±13.8%; P=0.013), but higher frequency of CD4+ T cells (northern PTM 54.3±7.2%, ChRM 45.9±11.0%; P=0.003) and CD4/CD8 ratio (northern PTM 1.58±0.65, ChRM 1.25±0.75; P=0.01) than male ChRM. Whereas, females showed lower frequency of CD21- B cells (northern PTM 37.8±13.0%, ChRM 54.6±15.4%; P<0.0001) compared to female ChRM.
Figure 2.
Comparisons between northern pig-tailed macaques and Chinese rhesus macaques
DISCUSSION
PTM group (M. nemestrina, M. leonine and M. pagensis) have served an important role in the field of biomedicine, notably in HIV/AIDS research, in recent years (Lei et al, 2013). However, no detailed taxonomic description of these animals was found in most studies that use the collectively called “pig-tailed macaques” (Agy et al, 1992; Batten et al, 2006; Bosch et al, 2000). We previously demonstrated that northern PTM express TRIM5-CypA fusion gene, resulting in its sensitivity to HIV-1 and improving the application value of these new laboratory animals in HIV/AIDS research (Kuang et al, 2009). We also provided the reference values of immunoglobulins, complements, C reactive protein (CRP), hematological and biochemical indexes of north PTM, and elucidated that gender, age and weight can influence these indexes (Pang et al, 2013; Zhang et al, 2014). However, the lack of the immune system characteristics of northern PTM may have restricted its application as AIDS animal models. Here we evaluated the absolute number and the expression of activation or differentiation related markers of major lymphocyte subpopulations in 62 male and female northern PTMs ranging from 2 to 11 years of age, and compared the lymphocyte subpopulations of PTM with those of the most widely used ChRM.
Serving as a major risk factor of morbidity and mortality for many infections caused by various pathogens, age influences the immune system in both human and non-human primates (NHP) (High, 2004). Wiener et al (1990) found that the absolute number of both CD19+ B cells and CD3+ T cells decreased while the absolute number of CD4+ and CD8+ T cells and the CD4/CD8 ratio were well maintained with increasing age. The following Swedish OCTO immune longitudinal study showed decreased CD4 subset, increased CD8 subset, and a lower CD19+ B cell percentages in aged population (Ferguson et al, 1995). Fagnoni et al (1996) reported a decrease in the absolute number of both CD4+ and CD8+ T cells in people older than 60 years. However, no age correlations of the absolute or relative cell numbers of lymphocyte subpopulations were found in recent study (Klose et al, 2007). Although the results were contradictory in these studies, a widely accepted theory suggests that aging is characterized by the decline in B cell numbers and an accumulation of CD8+ T cells rather than a loss of CD4+ T cells in peripheral blood (Frasca et al, 2008; Koch et al, 2007). On the other hand, Studies have suggested that aging is associated with numerous alterations in innate immunity which is the first line of defense against pathogens and plays a key role in regulating the responses of adaptive immunity (Shaw et al, 2010). Positive correlations were observed between age and CD16+CD56+ NK cell numbers, and it is generally accepted that the CD56dim peripheral NK cell population expands with age (Jiao et al, 2009; Mahbub et al, 2011). Tollerud et al (1989) evaluated the influence of age, race, and gender on the immune system, and did not observe age related effects for CD14+ cells. However, other recent research reported that the number of monocytes was significantly higher in the elderly than in the young group (Della Bella et al, 2007).
Because they share greater genetic and physiological similarities with humans than rodent models, NHP has been used for biomedical research for several decades. Most of our understanding of the immune system in Old World monkeys comes from studies utilizing rhesus macaques (Messaoudi et al, 2011). However, only few reports focused on the age-related changes in the immune system of PTM, especially northern PTM. Asquith et al (2012) measured age-related changes in T cell homeostasis in Indian rhesus macaques (InRM) ranging from 1 to 30 years old, and indicated that the frequency of CD8+ T cells expands with age while the frequency of CD4+ T cells remains stable, whereas age-related changes of the absolute number of both CD4+ and CD8+ T cells were not found in InRM (Didier et al, 2012). The CD4/CD8 ratio in juvenile InRM is approximately 2, and starts to decline after the first 2 months of life, whereas no further decline is detected between 5 and 7 years of age (Dykhuizen et al, 2000). Similarly, B cells in RM are identified based on the expression of CD20 and HLA-DR, and show a sharp decrease during aging (Didier et al, 2012). Early studies indicated that the number of circulating CD56+CD16+ NK cells in RM reduces with age (Coe & Ershler, 2001). However, highly different from humans, NK cells in peripheral blood of macaques express high levels of CD8α and almost all of them express NKG2A, but do not express CD56, which make them a CD3-CD8α+ NKG2A+ phenotype (Hong et al, 2013). A recent study of rhesus macaques ranging from 2 to 24 years old did not find any correlation between the circulating NK cells and age (Didier et al, 2012). Though the frequency of circulating monocytes increases in InRM older than 5 years, the numbers of these cells reduces with aging (Asquith et al, 2012).
It has been reported that CD38+HLA-DR+ subset within CD8+ T cells has a strong positive correlation with HIV-1 disease progression and CD38+HLA-DR+ CD4+ T cells are highly susceptible to infection with R5-tropic virus because of elevated CCR5 expression (Kestens et al, 1992; Meditz et al, 2011). CCR5 plays an essential role in HIV pathogenesis as a main co-receptor responsible for viral transmission. CCR5+CD4+ T cells are the target of R5-tropic virus and peripheral blood CCR5+CD8+ T cells accumulate during HIV infection (Brenchley et al, 2004). In addition, a loss in the expression of CD21 and IgD, and increased levels of activation markers, including CD80 and CD86, on circulating B cells are suggested be linked to HIV viremia (Moir & Fauci, 2008). Klatt et al (2012) demonstrated that higher pre-infection levels of immune activation than RM may contribute to rapid disease progression in PTM. Thus it is also necessary to examine these in both healthy humans and laboratory animals. The frequency of CCR5+ cells within CD4+ T cells in northern PTM (3.79±1.93%) was the lowest compared to Sunda PTM (14.54±6.3%) and RM (7.76±4.8%) (Klatt et al, 2012). The frequency of CD38+HLA-DR+ cells was 4.4±2.5% for CD4+ T cells and 14.5±7.0% for CD8+ T cells in healthy human as compared with 1.64±0.79% and 4.59±1.93% for CD4+ and CD8+ T cells, respectively, from northern PTM and 3.03±2.84% for CD8+ T cells from rhesus macaques (Brignolo et al, 2004; Onlamoon et al, 2005). Elevated levels of CD21- B cells are believed to account for the poor proliferative responses of B cells. This population is significantly expanded in most patients with systemic lupus erythematosus (SLE) and HIV-1 and its normal level is approximately 15% in healthy people, 45% in CM, 46% in RM and 36% in northern PTM (Das et al, 2011; Kling et al, 2011; Zandieh et al, 2013). The B cells in the peripheral blood of healthy people have an average 75% IgD+ subset, while northern PTM have lower frequency (66%) (Klein et al, 1998).
ChRM infected with SIV commonly have lower plasma viral loads and develop AIDS more slowly than InRM, whereas PTM are susceptible to many lentiviral infections, including infection with SIV, HIV-1, HIV-2, SHIV and stHIV-1, and their progress to AIDS after SIV infection are also more rapid than RM (Ling et al, 2002). A comparison of lymphocytes between ChRM and northern PTM may provide clues to explaining this phenomenon. Previous studies demonstrated that ChRM experience significant age-related changes in leukocyte subpopulations, including decreased lymphocyte numbers, CD4+ T cell frequency and CD4/CD8 ratio, increased CD8+ T cell frequency, but stable monocytes (Xia et al, 2009; Zheng et al, 2014). Similarly, northern PTM showed increased numbers of NK cells and CD8+ T cells, reduced B cells, but stable CD4+ T cells and monocytes in our study. In addition, northern PTM also showed higher CD4+ T cell frequency but fewer CD8+ T cells and CD21- B cells than ChRM.
The sex effects on circulating lymphocytes have been reported in both humans and NHP. Uppal et al (2003) demonstrated that women have a higher number and frequency of CD4+ T cells, whereas men have a higher frequency of CD8+ T cells. However, no differences in circulating B cells, NK cells and CD14+ cells were found between men and women (Choong et al, 1995; Tollerud et al, 1989). However, the influence of sex on the leukocytes in macaques is less clear. Tryphonas et al (1996) revealed significant gender differences in the CD4+ T cell percentage (females> males) and CD4/CD8 ratio (females>males) of infant CM. Recent studies on circulating lymphocytes in InRM did not find differences between males and females (Asquith et al, 2012; Didier et al, 2012). However, our previous study revealed that female ChRM have higher counts of CD4+ T cells, CD8+ T cells, B cells and NK cells than males (Xia et al, 2009). In this study, northern PTM also showed numerous gender differences in leukocytes and activation markers, and males may have stronger immune response due to their higher levels of CD8+ T cells, B cells and NK cells than females. However, further work is required to determine the characteristics of immune function between males and females.
In summary, the present study not only provided data on the immunological characteristics of the leukocyte subpopulation, but also demonstrated age and sex effects on those in northern PTM. Moreover, the comparison of northern PTM with ChRM provided clues to understanding the big differences between these two species when infected with HIV-1 or SIV.
Acknowledgments
We thank Mr. Zhen-Fei Hu,Gui Li and Dong-Ti Huang of Kunming Primate Research Center for their assistance with the experiments.
Funding Statement
This work was supported by the National Special Science Research Program of China (2012CBA01305)the National Natural Science Foundation of China (81172876, U0832601, 81273251, U1202228)the Knowledge Innovation Program of CAS (KSCX2-EW-R-13) and the National Science and Technology Major Project (2013ZX10001-002, 2012ZX10001-007)
Footnotes
The authors have declared that no competing interests exist.
References
- [1].Agy MB, Frumkin LR, Corey L, Coombs RW, Wolinsky SM, Koehler J, Morton WR, Katze MG.1992. Infection of Macaca nemestrina by human immunodeficiency virus type-1.Science, 257(5066): 103-106. [DOI] [PubMed] [Google Scholar]
- [2].Asquith M, Haberthur K, Brown M, Engelmann F, Murphy A, Al-Mahdi Z, Messaoudi I.2012. Age-dependent changes in innate immune phenotype and function in rhesus macaques (Macaca mulatta).Pathobiology of Aging & Age Related Diseases, 2. [DOI] [PMC free article] [PubMed]
- [3].Baroncelli S, Negri D R, Michelini Z, Cara A.2008. Macaca mulatta, fascicularis and nemestrina in AIDS vaccine development. Expert Review of Vaccines, 7(9): 1419-1434. [DOI] [PubMed] [Google Scholar]
- [4].Batten CJ, De Rose R, Wilson KM, Agy MB, Chea S, Stratov I, Montefiori DC, Kent SJ.2006. Comparative evaluation of simian, simian-human, and human immunodeficiency virus infections in the pigtail macaque (Macaca nemestrina) model.AIDS Research and Human Retroviruses, 22(6): 580-588. [DOI] [PubMed] [Google Scholar]
- [5].Bosch ML, Schmidt A, Chen J, Florey MJ, Agy M, Morton WR.2000. Enhanced replication of HIV-1 in vivo in pigtailed macaques (Macaca nemestrina).Journal of Medical Primatology, 29(3-4): 107-113. [DOI] [PubMed] [Google Scholar]
- [6].Brenchley JM, Schacker TW, Ruff LE, Price DA, Taylor JH, Beilman GJ, Nguyen PL, Khoruts A, Larson M, Haase AT, Douek DC.2004. CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract.Journal of Experimental Medicine, 200(6): 749-759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Brignolo L, Spinner A, Yee JL, Lerche NW.2004. Subsets of T cells in healthy rhesus macaques (Macaca mulatta) infected with simian T-lymphotropic virus type 1.Comparative Medicine, 54(3): 271-274. [PubMed] [Google Scholar]
- [8].Choong ML, Ton SH, Cheong SK.1995. Influence of race, age and sex on the lymphocyte subsets in peripheral blood of healthy Malaysian adults.Annals of Clinical Biochemistry, 32(Pt 6): 532-539. [DOI] [PubMed] [Google Scholar]
- [9].Coe CL, Ershler WB.2001. Intrinsic and environmental influences on immune senescence in the aged monkey.Physiology & Behavior, 73(3): 379-384. [DOI] [PubMed] [Google Scholar]
- [10].Das A, Veazey RS, Wang XL, Lackner AA, Xu HB, Pahar B.2011. Simian immunodeficiency virus infection in rhesus macaques induces selective tissue specific B cell defects in double positive CD21+CD27+ memory B cells.Clinical Immunology, 140(3): 223-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Della Bella S, Bierti L, Presicce P, Arienti R, Valenti M, Saresella M, Vergani C, Villa ML.2007. Peripheral blood dendritic cells and monocytes are differently regulated in the elderly.Clinical Immunology, 122(2): 220-228. [DOI] [PubMed] [Google Scholar]
- [12].Didier ES, Sugimoto C, Bowers LC, Khan IA, Kuroda MJ.2012. Immune correlates of aging in outdoor-housed captive rhesus macaques (Macaca mulatta).Immunity & Ageing, 9(1): 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Dykhuizen M, Ceman J, Mitchen J, Zayas M, MacDougall A, Helgeland J, Rakasz E, Pauza CD.2000. Importance of the CD3 marker for evaluating changes in rhesus macaque CD4/CD8 T-cell ratios.Cytometry, 40(1): 69-75. [DOI] [PubMed] [Google Scholar]
- [14].Fagnoni FF, Vescovini R, Mazzola M, Bologna G, Nigro E, Lavagetto G, Franceschi C, Passeri M, Sansoni P.1996. Expansion of cytotoxic CD8+ CD28- T cells in healthy ageing people, including centenarians.Immunology, 88(4): 501-507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Ferguson FG, Wikby A, Maxson P, Olsson J, Johansson B.1995. Immune parameters in a longitudinal study of a very old population of Swedish people: a comparison between survivors and nonsurvivors.The Journals of Gerontology. Series A: Biological Sciences and Medical Sciences, 50(6): B378-B382. [DOI] [PubMed] [Google Scholar]
- [16].Frasca D, Landin AM, Riley RL, Blomberg BB.2008. Mechanisms for decreased function of B cells in aged mice and humans.The Journal of Immunology, 180(5): 2741-2746. [DOI] [PubMed] [Google Scholar]
- [17].Gippoliti S.2001. Notes on the taxonomy of Macaca nemestrina leonina Blyth, 1863 (Primates: Cercopithecidae).Hystrix - Italian Journal of Mammalogy, 12(1): 51-54. [Google Scholar]
- [18].Hatziioannou T, Ambrose Z, Chung NPY, Piatak M Jr, Yuan F, Trubey CM, Coalter V, Kiser R, Schneider D, Smedley J, Pung R, Gathuka M, Estes JD, Veazey RS, KewalRamani VN, Lifson JD, Bieniasz PD.2009. A macaque model of HIV-1 infection.Proceedings of the National Academy of Sciences of the United States of America, 106(11): 4425-4429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].High KP.2004. Infection as a cause of age-related morbidity and mortality.Ageing Research Reviews, 3(1): 1-14. [DOI] [PubMed] [Google Scholar]
- [20].Hong HS, Rajakumar PA, Billingsley JM, Reeves RK, Johnson RP.2013. No monkey business: why studying NK cells in non-human primates pays off.Frontiers in Immunology, 4: 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Jiao Y, Qiu ZF, Xie J, Li DJ, Li TS.2009. Reference ranges and age-related changes of peripheral blood lymphocyte subsets in Chinese healthy adults.Science in China. Series C: Life Sciences, 52(7): 643-650. [DOI] [PubMed] [Google Scholar]
- [22].Kestens L, Vanham G, Gigase P, Young G, Hannet I, Vanlangendonck F, Hulstaert F, Bach BA.1992. Expression of activation antigens, HLA-DR and CD38, on CD8 lymphocytes during HIV-1 infection.AIDS, 6(8): 793-797. [DOI] [PubMed] [Google Scholar]
- [23].Klatt NR, Canary LA, Vanderford TH, Vinton CL, Engram JC, Dunham RM, Cronise HE, Swerczek JM, Lafont BAP, Picker LJ, Silvestri G, Brenchley JM.2012. Dynamics of simian immunodeficiency virus SIVmac239 infection in pigtail macaques.Journal of Virology, 86(2): 1203-1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Klein U, Rajewsky K, Kuppers R.1998. Human immunoglobulin (Ig) M+IgD+ peripheral blood B cells expressing the CD27 cell surface antigen carry somatically mutated variable region genes: CD27 as a general marker for somatically mutated (memory) B cells.Journal of Experimental Medicine, 188(9): 1679-1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Kling HM, Shipley TW, Norris KA.2011. Alterations in peripheral blood B-cell populations in SHIV89.6P-infected macaques (Macacca fascicularis).Comparative Medicine, 61(3): 269-277. [PMC free article] [PubMed] [Google Scholar]
- [26].Klose N, Coulibaly B, Tebit DM, Nauwelaers F, Spengler HP, Kynast-Wolf G, Kouyaté B, Kräusslich HG, Böhler T.2007. Immunohematological reference values for healthy adults in Burkina Faso.Clinical and Vaccine Immunology, 14(6): 782-784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Koch S, Larbi A, Ozcelik D, Solana R, Gouttefangeas C, Attig S, Wikby A, Strindhall J, Franceschi C, Pawelec G.2007. Cytomegalovirus infection: a driving force in human T cell immunosenescence.Annals of the New York Academy of Sciences, 1114: 23-35. [DOI] [PubMed] [Google Scholar]
- [28].Kuang YQ, Tang X, Liu FL, Jiang XL, Zhang YP, Gao GX, Zheng YT.2009. Genotyping of TRIM5 locus in northern pig-tailed macaques (Macaca leonina), a primate species susceptible to Human Immunodeficiency Virus type 1 infection.Retrovirology, 6: 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Lei AH, Pang W, Zhang GH, Zheng YT.2013. Use and research of pigtailed macaques in nonhuman primate HIV/AIDS models.Zoological Research, 34(2): 77-88. (in Chinese) [DOI] [PubMed] [Google Scholar]
- [30].Ling BH, Veazey RS, Luckay A, Penedo C, Xu KY, Lifson JD, Marx PA.2002. SIV(mac) pathogenesis in rhesus macaques of Chinese and Indian origin compared with primary HIV infections in humans.AIDS, 16(11): 1489-1496. [DOI] [PubMed] [Google Scholar]
- [31].Mahbub S, Brubaker AL, Kovacs EJ.2011. Aging of the innate immune system: an update.Current Immunology Reviews, 7(1): 104-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Malaivijitnond S, Arsaithamkul V, Tanaka H, Pomchote P, Jaroenporn S, Suryobroto B, Hamada Y.2012. Boundary zone between northern and southern pig-tailed macaques and their morphological differences.Primates, 53(4): 377-389. [DOI] [PubMed] [Google Scholar]
- [33].Meditz AL, Haas MK, Folkvord JM, Melander K, Young R, McCarter M, MaWhinney S, Campbell TB, Lie Y, Coakley E, Levy DN, Connick E.2011. HLA-DR+ CD38+ CD4+ T lymphocytes have elevated CCR5 expression and produce the majority of R5-tropic HIV-1 RNA in vivo.Journal of Virology, 85(19): 10189-10200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Messaoudi I, Estep R, Robinson B, Wong SW.2011. Nonhuman primate models of human immunology.Antioxidants and Redox Signaling, 14(2): 261-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Moir S, Fauci AS.2008. Pathogenic mechanisms of B-lymphocyte dysfunction in HIV disease.Journal of Allergy and Clinical Immunology, 122(1): 12-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Onlamoon N, Tabprasit S, Suwanagool S, Louisirirotchanakul S, Ansari AA, Pattanapanyasat K.2005. Studies on the potential use of CD38 expression as a marker for the efficacy of anti-retroviral therapy in HIV-1-infected patients in Thailand.Virology, 341(2): 238-247. [DOI] [PubMed] [Google Scholar]
- [37].Pang W, Lü LB, Wang Y, Li G, Huang DT, Lei AH, Zhang GH, Zheng YT.2013. Measurement and analysis of hematology and blood chemistry parameters in northern pig-tailed macaques (Macaca leonina).Zoological Research, 34(2): 89-96. (in Chinese) [DOI] [PubMed] [Google Scholar]
- [38].Patton DL, Sweeney YT, Paul KJ.2009. A summary of preclinical topical microbicide rectal safety and efficacy evaluations in a pigtailed macaque model.Sexually Transmitted Diseases, 36(6): 350-356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Shaw AC, Joshi S, Greenwood H, Panda A, Lord JM.2010. Aging of the innate immune system.Current Opinion in Immunology, 22(4): 507-513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Stremlau M, Owens CM, Perron MJ, Kiessling M, Autissier P, Sodroski J.2004. The cytoplasmic body component TRIM5α restricts HIV-1 infection in Old World monkeys.Nature, 427(6977): 848-853. [DOI] [PubMed] [Google Scholar]
- [41].Thippeshappa R, Polacino P, Yu Kimata MT, Siwak EB, Anderson D, Wang WM, Sherwood L, Arora R, Wen M, Zhou P, Hu SL, Kimata JT.2011. Vif substitution enables persistent infection of pig-tailed macaques by human immunodeficiency virus type 1.Journal of Virology, 85(8): 3767-3779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Tollerud DJ, Clark JW, Brown LM, Neuland CY, Pankiw-Trost LK, Blattner WA, Hoover RN.1989. The influence of age, race, and gender on peripheral blood mononuclear-cell subsets in healthy nonsmokers.Journal of Clinical Immunology, 9(3): 214-222. [DOI] [PubMed] [Google Scholar]
- [43].Tryphonas H, Lacroix F, Hayward S, Izaguirre C, Parenteau M, Fournier J.1996. Cell surface marker evaluation of infant Macaca monkey leukocytes in peripheral whole blood using simultaneous dual-color immunophenotypic analysis.Journal of Medical Primatology, 25(2): 89-105. [DOI] [PubMed] [Google Scholar]
- [44].Uppal SS, Verma S, Dhot PS.2003. Normal values of CD4 and CD8 lymphocyte subsets in healthy indian adults and the effects of sex, age, ethnicity, and smoking.Cytometry. Part B: Clinical Cytometry, 52(1): 32-36. [DOI] [PubMed] [Google Scholar]
- [45].Wiener D, Shah S, Malone J, Lowell N, Lowitt S, Rowlands DT Jr.1990. Multiparametric analysis of peripheral blood in the normal pediatric population by flow cytometry.Journal of Clinical Laboratory Analysis, 4(3): 175-179. [DOI] [PubMed] [Google Scholar]
- [46].Xia HJ, Zhang GH, Wang RR, Zheng YT.2009. The influence of age and sex on the cell counts of peripheral blood leukocyte subpopulations in Chinese rhesus macaques.Cellular & Molecular Immunology, 6(6): 433-440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Zandieh A, Izad M, Fakhri M, Amirifard H, Khazaeipour Z, Harirchian MH.2013. Cytometric profiling in various clinical forms of multiple sclerosis with respect to CD21+, CD32+, and CD35+ B and T cells.Translational Neurodegeneration, 2(1): 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Zhang XL, Pang W, Deng DY, Lü LB, Feng Y, Wang Y, Zheng YT.2014. Analysis of immunoglobulin, complements and CRP levels in serum of captive northern pig-tailed macaques (Macaca leonina).Zoological Research, 35(3): 196-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Zheng HY, Zhang MX, Pang W, Zheng YT.2014. Aged Chinese rhesus macaques suffer severe phenotypic T- and B-cell aging accompanied with sex differences.Experimental Gerontology, 55: 113-119. [DOI] [PubMed] [Google Scholar]
- [50].Zhu L, Zhang GH, Zheng YT.2010. Application studies of animal models in evaluating safety and efficacy of HIV-1 microbicides.Zoological Research, 31(1): 66-76. (in Chinese) [DOI] [PubMed] [Google Scholar]