Abstract
The paper focuses on recent achievements in the search for new chemical compounds able to inhibit multidrug resistance (MDR) mechanisms in Gram-positive pathogens. An analysis of the results of the search for new efflux pump inhibitors (EPIs) for Gram-positive bacteria, which have been performed over the last decade, indicates that almost all efforts are focused on the NorA (MFS) efflux pump in S. aureus. Considering the chemical structures of the NorA EPIs that have been identified, it can be observed that the most active agents belong to the families of compounds possessing conjugated double bonds, e.g., chalcones, piperine-like compounds, N-cinnamoylphenalkylamides or citral amide derivatives. Indole-, dihydronaphthyl-, 2-chloro-5-bromo-phenyl- or piperidine moieties seem to be profitable for the EPI properties, as well. These results, together with an increasing knowledge about a variety of efflux pumps that are involved in MDR of Gram-positive pathogens underline that further search for new EPIs should pay more attention to develop MDR efflux protein targets, including SMR, MATE, ABC or other members of the MFS family.
Keywords: bacterial multidrug resistance, MDR, efflux pump inhibitors, EPIs, NorA
1. Introduction
As a consequence of the intense fight against infections, bacteria have evolved through numerous defenses against antimicrobial agents [1]. The main mechanisms whereby the bacteria develop resistance to antimicrobial agents include enzymatic inactivation [2,3], modification of the drug target(s) [3,4], and reduction of intracellular drug concentration by changes in membrane permeability [3,5] or by the overexpression of efflux pumps [3,6]. With respect to efflux pumps, they provide a self-defense mechanism by which antibiotics are actively removed from the cell. For antibacterials, this results in sublethal drug concentrations at the active site that in turn may predispose the organism to the development of high-level target-based resistance [3,7]. Therefore, efflux pumps are viable antibacterial targets and identification and development of potent efflux pump inhibitors is a promising and valid strategy [3,8] which can restore the susceptibility of resistant strains to antibacterial agents that are substrates of efflux pumps [3,9]. The combination of a resistance inhibitor with an antibiotic has already proven its efficacy with the clavulanic acid (inhibitor of beta-lactamase)/amoxicillin association [10]. Predominantly, the world search for new tools to combat multidrug resistance (MDR) among bacterial pathogens is concentrated on Gram-negative bacteria aspects [11,12,13,14,15,16,17,18] because of their more complicated MDR mechanisms due to their double-membrane cells, which allow the expression of a tripartite efflux pump system such as AcrA/AcrB/TolC in Enterobacteriaceae [19,20,21] or MexA/ MexB/OprM in Pseudomonas aeruginosa [22,23,24]. Although the 3D-structures of protein components of the tripartite efflux pumps have been identified experimentally [25], which should simplify studies on inhibitor-binding pockets, the knowledge about the pump-inhibitor interactions is still not sufficient to involve the “protein-ligand drug design” approach in the search for new EPIs for Gram-negative pathogens. Results of recent microbiological- and medicinal chemistry studies allowed us to identify several chemical families of compounds inhibiting tripartite MFP/RND/OMF pump action [12,13,16,25] but it is hard to find a good pharmacophore model resulting from the studies that could be applicable in further design of new potent EPIs.
Indeed, various lines of evidence [1,3,10,26,27,28,29,30,31,32,33,34,35,36,37,38] have indicated a significant development of medicinal chemistry tools useful in the search for efflux pump inhibitors for Gram-positive pathogens. As multidrug resistant Gram-positive bacteria have been and still are a current therapeutic problem, it is of great importance to analyze the recent progress in the search for new tools to combat it. Thus, this paper focuses on recent achievements in the search for new chemical compounds able to inhibit MDR mechanisms in Gram-positive pathogens.
2. Efflux Pumps in Gram Positive Bacteria
Efflux pumps in Gram-positive bacteria belong to four unrelated families (Table 1): MFS (major facilitator superfamily), SMR (small multidrug resistance), ABC (ATP-binding cassette) and MATE (Multidrug And Toxic Compound Extrusion) [9,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54].
Table 1.
Efflux pumps in Gram positive bacteria and their role in antibiotics transport.
| Bacterial strain | Transport protein family | Efflux pump | Substrates |
|---|---|---|---|
| Staphylococcus aureus | MFS [9,41,42,43,44] | NorA | NOR, CPX |
| NorB | NOR, CPX, SPX | ||
| MdeA | Macrolides | ||
| MATE [45] | Tet38 | tetracyclines | |
| MepA | FQ, glycylcyclines | ||
| SMR [39,60,61] | Smr, QacG, QacH | - | |
| Staphylococcus spp | MFS [9,46,47] | Mef(A) | Macrolides |
| ABC [47,48,49] | MsrA | Macrolides, type B streptogramins | |
| Staphylococcus haemolyticus | MFS [42] | MdeA | Macrolides, lincosamides type A streptogramins |
| Staphylococcus lentus | [54] | FexA | - |
| Streptomyces coelicolor | MFS [38] | CmlR1, CmlR2 | Chloramphenicol |
| Streptomyces spp | MFS [50] | Cml, Cmlv, Cmr, Cmx, CmA | - |
| Streptococcus spp | MFS [9,45,47] | Mef(A) | Macrolides |
| Streptococcus pneumoniae | ABC [51] | Msr(D) | Macrolides, ketolides |
| MFS [52] | PmrA | NOR, CPX | |
| Clostridium difficile | MFS [53] | Cme | Erythromycin |
| MATE [45] | CdeA | FQ | |
| Bacillus subtilis | MFS [9,46] | LmrB | Lincosamides |
| Bmr, Bmr3, Blt | FQ | ||
| Bacillus glutamicum | MFS [9,46] | LmrB | Lincosamides |
NOR: norfloxacin; CPX: ciprofloxacin; SPX: sparfloxacin; FQ: flouroquinolones.
MFS transporters are typically composed of approx. 400 amino acids that are putatively arranged into 12 membrane-spanning helices, with a large cytoplasmic loop between helices six and seven [39,55,56]. The examples of MFS efflux pumps in Gram-positive bacteria are NorA, NorB, MdeA, Tet38 (Staphylococcus aureus), LmrB, Bmr, Bmr3, Blt (Bacillus subtilis), MefA (Streptococcus pyogenes), MefE (Streptococcus pneumoniae) or CmlR (Streptococcus coelicor) [39,55,57,58,59]. SMR transporters consist of approx. 110 amino acids and contain four transmembrane helices.
Owing to the small sizes of the proteins that belong to this family, they probably function as oligomeric complexes [39,59]. The examples of SMR efflux pumps in Gram-positive bacteria are EbrAB (Bacillus subtilis) or Smr, QacG, QacH (Staphylococcus aureus) [39,60,61]. MATE efflux proteins consist of 400–700 amino acids that form 12 transmembrane helices. All proteins of the MATE family exhibit almost 40% identity of their amino acid sequence. All genes that encode MATE proteins are derived from the same gene which was subsequently duplicated. An example of MATE efflux pump in Gram-positive bacteria is MepA protein found in Staphylococcus aureus [62,63]. MFS, SMR and MATE transporters use a transmembrane proton gradient as the driving force for transport [39,62,63,64,65]. The minimal structural organization of an ABC transporter includes the presence of four domains, i.e., two nucleotide binding domains (NBDs) and two transmembrane permease domains (TMDs). The TMDs usually consist of six transmembrane α-helices and form homo or heterodimers. Two NBDs bind ATP in the cytoplasmic side and cooperate with transmembrane domains [39,65,66].
The feature which distinguishes ABC transporters from the remaining families is the energy source for active extrusion of drugs, as it comes from the hydrolysis of ATP. Binding and hydrolysis of ATP triggers conformational changes in the transporter’s structure, which enable export of substrates [39,67]. The examples of ABC efflux pumps in Gram-positive bacteria are LmrA (Lactococcus lactis) or Rv1217c–Rv1218c (Mycobacterium tuberculosis) [39,55,68].
Multidrug resistance efflux pumps are recognized as an important component of resistance in both Gram-positive and Gram-negative bacteria. Some bacterial efflux pumps may be selective for one substrate or transport antibiotics of different classes, conferring a multiple drug resistance (MDR) phenotype. Efflux pumps inhibitors (EPIs) are promising therapeutic agents, as they should restore the activity of standard antibiotics. The efflux pump inhibitor-antibiotic combination is expected to increase the intracellular concentration of antibiotics that are expelled by efflux pumps, decrease the intrinsic bacterial resistance to antibiotics, reverse the acquired resistance associated with efflux pumps overexpression, and reduce the frequency of the emergence of resistant mutant strains [69].
Bypassing efflux pump activity may be achieved through a variety of different approaches: (1) by modifying the chemical design of previous antibiotics to reduce their respective affinity for binding sites and cavities located inside the pump transporter; (2) by increasing the influx of antibiotics, using membrane permeabilizers that subsequently increase the intracellular concentration of drugs; (3) by down-regulating the expression of efflux pump genes and/or decreasing the level of active efflux complexes in the bacterial envelope; (4) by collapsing the energy required to support the drug transport; (5) by inhibiting the functional assembly of efflux pump components; (6) by inserting a carefully-designed molecular plug inside the membrane channels responsible of antibiotic transport (inside the pump cavities or inside the exit channel component) or (7) by generating a dynamic competition, between a decoy- substrate and the antibiotic, during transport flux inside the pump [12].
3. Current Approaches in Search for EPIs in Gram Positive Bacteria
The methicillin-resistant Staphylococcus aureus (MRSA) is a major multidrug resistant Gram-positive bacteria that is a main cause of healthcare-associated infections (HAIs) resulting in a high death rate. MRSA is able to acquire resistance to various antibiotics, including tetracyclines, aminoglycosides and flouroquinolones. Studies on MDR efflux mechanisms in S. aureus indicated that NorA is predominant protein efflux pump [3]. For these two reasons, NorA in S. aureus is a frequently studied efflux pump as well as being the main protein target in the search for efflux pump inhibitors in the case of Gram-positive bacteria.
Recent decades have seen the production of a number of new chemical compounds belonging to various chemical families, which were investigated on their NorA EPI properties [3,10,26,27,28,29,30,31,32,33,34,35,36,37,38,70]. In the studies, an examination of the new compounds on their EPI properties have predominantly been based on: (1) a comparison of antibiotics efficacy in the presence- to that in the absence of the tested compound in the strain over-producing efflux pump and/or (2) the assays of inhibition of a substrate-efflux, mediated by the efflux pump, at various concentrations of the tested compound. In both types of assays, S. aureus SA 1199B was the most often used strain over-producing NorA efflux pump, and the wild strain S. aureus SA 1199 was involved as a reference one. Ciprofloxacin (CPX) is described as the most often used antibiotic, and ethidium bromide (EtBr) as the main reference substrate of NorA applied in the (real-time) efflux assays.
3.1. Plant-Derived NorA EPIs and Their Chemical Modifications
The role of phytochemistry in search for compounds inhibiting NorA of S.aureus is significant as it is reported to be an extremely varied series of plant-derived EPIs displaying different chemical properties, including flavones, isoflavones, acylated glycosides, porphyrin phaeophorbide A or kaempferol rhamnoside [34,70].
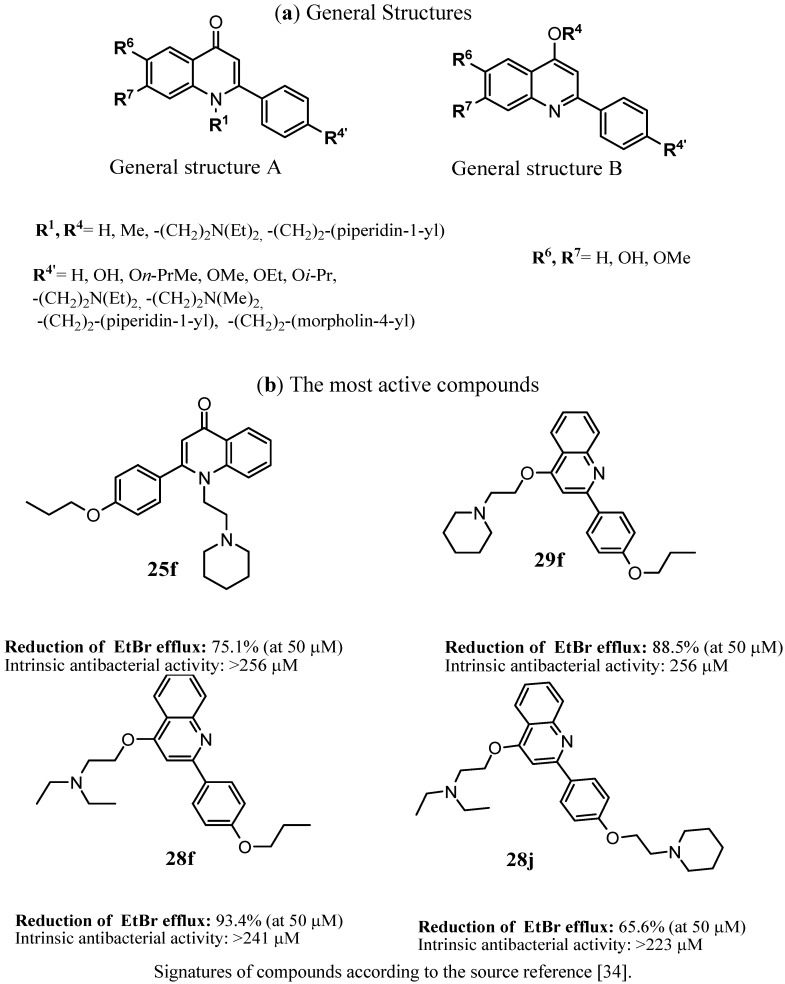
Chemical structures of the natural EPIs have been a good starting point for further modifications to search for new inhibitors with higher potency and better pharmacological profile. In this context, Sabatini et al. [34] described the synthesis of a series of 2-(4-propoxyphenyl)quinoline derivatives, that was designed on the basis of natural flavones nucleus (Figure 1). The compounds were investigated on their EPIs action in the ethidium bromide (EtBr) inhibition assays on strain S. aureus SA-1199B over-expressing norA. The most active compounds 25f, 28f, 28j and 29f displayed more than 65% inhibition of EtBr at their 50 µm concentration.
Figure 1.
The most active inhibitors of the S. aureus NorA efflux pump among 2-(4-propoxyphenyl)quinoline [34].
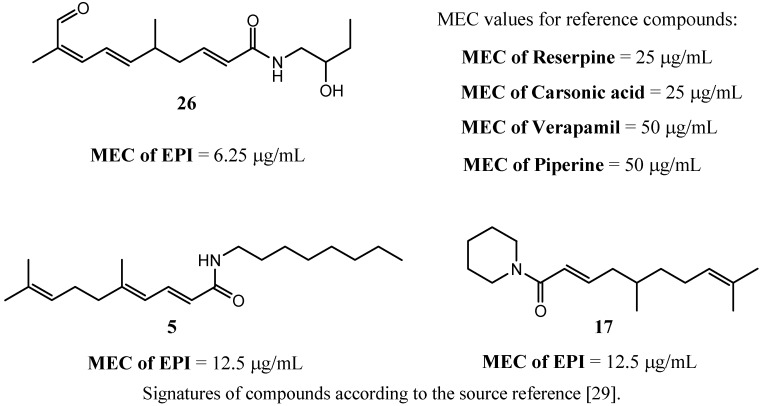
Thota et al. [29] described a series of citral derived amides that have been obtained from monoterpene citral or citronellal (Figure 2).
Figure 2.
Citral amide derivatives that potentiate MIC of ciprofloxacin on S. aureus 1199. The most active compounds found within the assays [29]. MEC value describes minimal effective concentration of the tested compounds.
The compounds were investigated on their abilities to decrease minimal inhibitory concentration of ciprofloxacin in the strains of S. aureus over-producing NorA pump. The EPI-action of the compounds was expressed as MEC-value (minimal dose of the tested compound that caused a decrease in the MIC of ciprofloxacin). As reference compounds, reserpine, carsonic acid, verapamil and piperine were used. The most active compound, 2,6,8-trienoic acid amide derivative 26 improved antimicrobial action of ciprofloxacin at the compound concentration 4–8 fold lower than that of reference inhibitors. Dienoic acid piperidide derivative (17) and 2,4,8-trienoic derivative of octylamide (5) were active at their doses 2–4 fold lower than those of reference EPIs (Figure 2) [29].
3.2. Chalcones and Alkenamide Inhibitors of the NorA in S. aureus
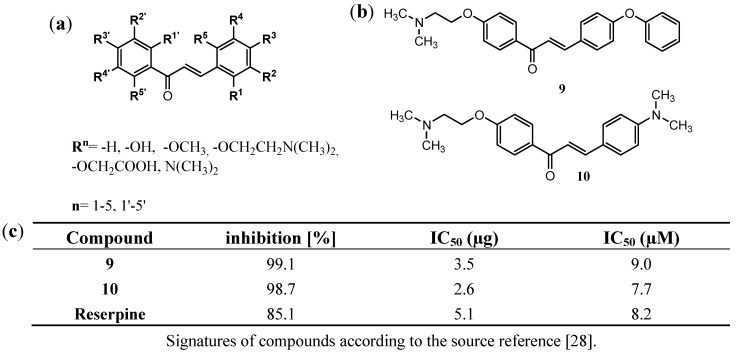
The newest work of Holler et al. [28] focuses on a series of 117 chalcone derivatives modified at each position of both phenyl rings (Figure 3a). The compounds were tested on their EPI activity against NorA mediated ethidium bromide efflux in real-time efflux assay (RTE) in the strain SA1199B. Five active compounds were found. Among them two N,N-dimethylaminoethoxyphenyl derivatives 9 and 10 (Figure 3b) were equipotent to reserpine, that was used as reference EPI in the studies. The compounds tested at their concentration of 20 µg/mL displayed almost total inhibition of EtBr efflux (Figure 3c).
Figure 3.
Chalcone inhibitors of the NorA efflux pump in S. aureus; (a) the general structure of the chalcone EPIs; (b) the most active inhibitors found in the real-time efflux (RTE) assay; (c) Inhibition of EtBr efflux in SA1199B at compounds concentration of 20 µg/mL for the most active chalcones 9 and 10 [28].
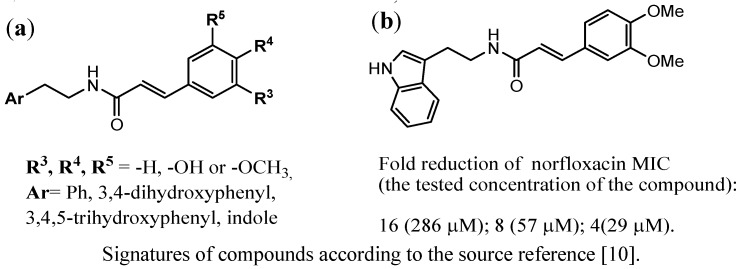
Michalet et al. [10] performed studies among N-cinnamoylphenalkylamide derivatives with (un)substituted phenyl or indole at one terminated fragment and (un)substituted phenyl at the opposite terminate fragment (Figure 4a).
Figure 4.
N-cinnamoylphenalkylamide derivatives investigated according to their efflux pump inhibitors (EPIs) action in the strain over-producing NorA pump; (a) general structures of the N-cinnamoylphenalkylamides; (b) the structure of the most active inhibitor [10].
In the case of both aromatic fragments electrodonated substituents were considered. The most active compound (Figure 4b), possessing indole moiety and 3,4-dimethoxycinnamoyl fragment, caused a 4-fold reduction of norfloxacin MIC at the lowest concentration of 29 µM.
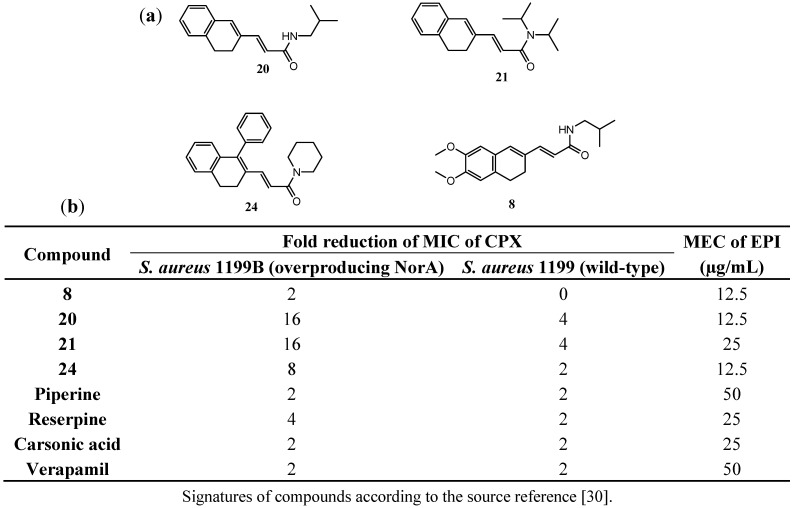
Compounds with similar topology of conjugated double bonds were obtained and investigated by Thota et al. [30], who found new EPIs among derivatives of 3,4-dihydronaphth-2-yl-propenoic acid (Figure 5). The compounds were examined according to their ability to improve the efficacy of ciprofloxacine in MDR strains of S. aureus (1199B and 1199). The most active compounds included chemical moieties of isobutylamide (8, 20), diisopropylamide (21) and piperidide (24). The compounds were able to reduce MIC of ciprofloxacine (CPX) in 2–16-fold at their concentrations 1–4 fold lower than those of reference EPI (Figure 5). The simple structure of 3-(3,4-dihydronaphth-2-yl)-propenoic acid isobutyl amide (20) was the most promising one, which caused a 16-fold reduction of MIC of CPX in strain 1199B and 4-fold in the case of 1199 strain. Its MEC value (12.5 µg/mL) was 4-fold lower than that of piperine and verapamil and 2-fold lower than that of reserpine and carsonic acid.
Figure 5.
Dihydronaphthalene inhibitors of the NorA efflux pump in S. aureus [30]. (a) Structures of the most active compounds (20, 21, 24 and 8); (b) Abilities of the most active compounds to potentiate antimicrobial action of ciprofloxacin (CPX) in comparison to reference compounds.
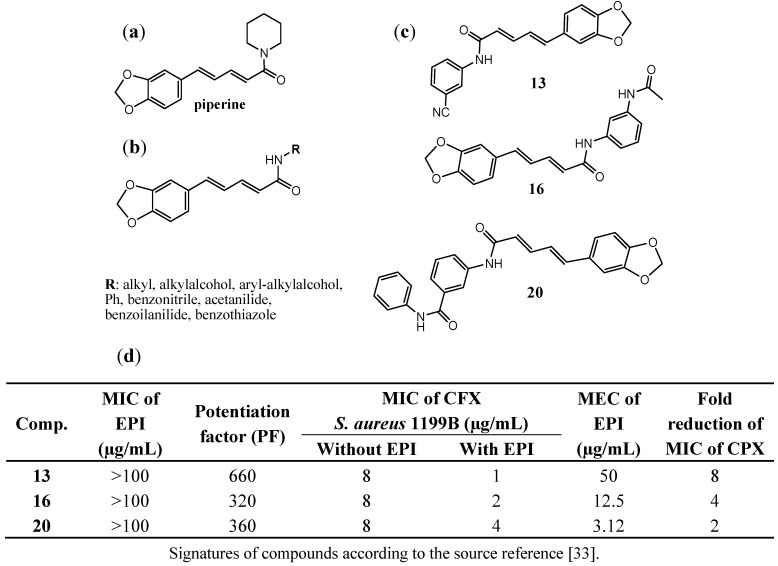
3.3. Piperine EPIs against NorA in S. aureus
Piperine (Figure 6a) is an alkaloid that was isolated from the fruits of Piper nigrum by H.C. Orsted in 1819. This natural compound displays properties of the inhibitors of some proteins important for metabolism and xenobiotics transport and it is used as a reference inhibitor in studies on new efflux pump inhibitors [29,30]. On the basis of the inhibitor properties of piperine, Nargotra et al. [33] designed and synthesized a series of piperine analogs, in which piperidine moiety was modified by replacement with other amines (Figure 6b). The new compounds were investigated according to their ability to reduce MIC of ciprofloxacin in S. aureus 1199B strain using eight different concentrations of the investigated compounds (0–50 µg/mL). Potentiation factor (PF) was calculated to express the potentiation of activity of ciprofloxacin in the presence of the tested new EPIs, reflected in the reduced MIC of combination compared to that of ciprofloxacin alone (Fig 6d). Three of the most promising EPIs were found (Figure 6c) in which the piperidine fragment of piperine was replaced with 3-aminobenzonitrile (13), N-(3-aminophenyl)acetamide (16) or 3-amino-N-phenylbenzamide (20). The highest potentiation factor was observed for the benzonitrile amide derivative (13), whereas the 3-amino-N-phenylbenzamide derivative (20) displayed EPIs activity at the lowest concentration with its MEC value of 3.12 µg/mL (Figure 6d).
Figure 6.
Piperine analogs that decrease MIC of ciprofloxacin in MDR S. aureus strain over-producing NorA (1199B); (a) structure of piperine; (b) the general structure of the tested compounds; (c) the most active compounds 13, 16 and 20; (d) Abilities of compounds 13, 16, 20 to increase efficacy of ciprofloxacin [33].
The series of compounds was used in quantitative structure-activity relationship studies (QSAR) to evaluate QSAR parameters that are responsible for NorA EPIs. In the QSAR studies, the authors considered seven categories of descriptors as follows: energy-state indices, electronic, information content, spatial, structural, thermodynamic and topological ones. On the basis of the obtained QSAR model, the descriptors of the partial negative surface area (Jurs-PNSA-1) and area of the molecular shadow in the XZ plane (Shadow_XZ) were identified as the most important parameters that contributed to the potentiation of EPI activity. An increase of Jurs-PNSA-1 is connected with the introduction of a polar group into the structure of a modified EPI, which is able to increase the partial negative surface area of the compound. This kind of modifications is postulated to be profitable for NorA efflux pump inhibitory properties. Similarly, changes of the placement of substitutuent(s) (ortho- meta- or para), which increase Shadow_XZ parameter, are profitable for the activity. Furthermore, the parameter of heat of formation (Hf) was demonstrated as a factor important for NorA inhibitory properties in S. aureus.
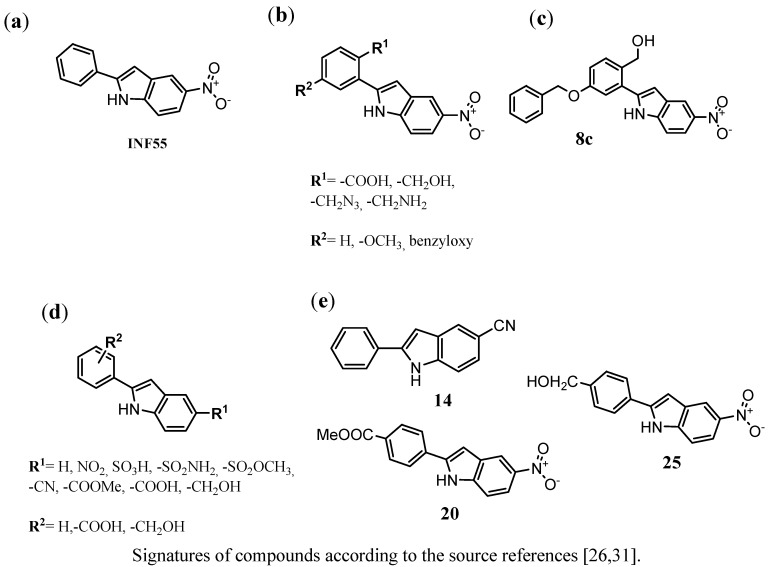
3.4. Indoles as Inhibitors of the NorA of S. aureus
Compound INF55, 5-nitro-2-phenyl-1H-indole (Figure 7a), was one of the first identified indole EPIs that was capable of producing 4-fold increase susceptibility of S. aureus to ciprofloxacin at its concentration of 1.5 g/mL.
Figure 7.
Derivatives or 2-aryl-1H-indoles that display NorA MDR inhibitory activity on S. aureus strains (K1758, 8325-4 and K2361); (a) lead structure INF55; (b) the general structure of the chemical group described by Samosorn et al. [31]; (c) the most active compound (8a) identified within the studies [31]; (d) the general structure of the chemical group described by Ambrus et al. [26]; (e) the most promising indoles-EPIs (14, 20 and 25) identified [26].
3.5. Other Chemical Groups of NorA EPIs
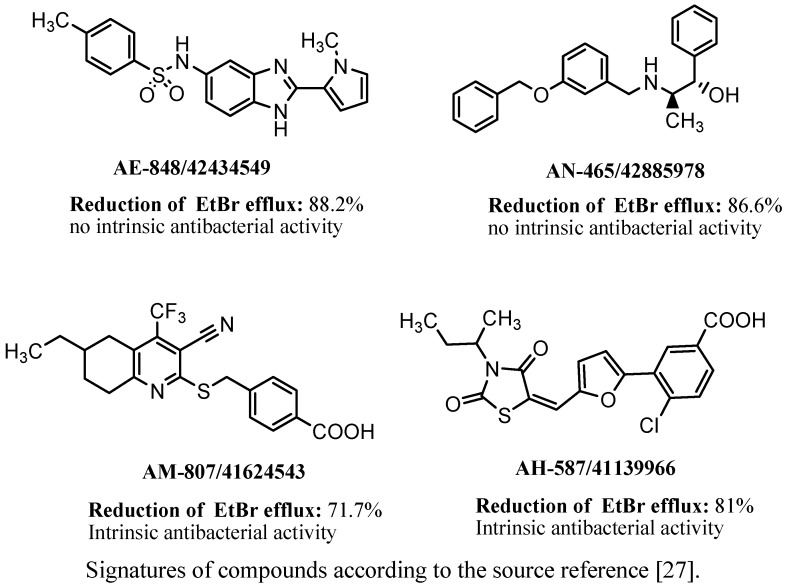
Brincat et al. [27] identified four novel inhibitors of the NorA efflux pump of S. aureus belonging to different chemical groups. Structures of the compounds were discovered on the basis of virtual screening that involved FLAP procedure and new methodology using GRID force field descriptors. The compounds created within the ligand-based design were evaluated on their NorA EPI properties in silico. The structures identified as active in silico were then synthesized and evaluated experimentally on their EtBr efflux inhibition in S. aureus strain SA-1199B as well as on their ability to potentiate the antibacterial effect of ciprofloxacin. In this group, the bezimidazole derivative AE-848/42434549 as well as the benzyloxybezylamine derivative AN-465/42885978 (Figure 8) demonstrated the highest EPIs properties as both of the compounds significantly inhibited EtBr efflux and did not display intrinsic antibacterial activities. Two other compounds, pyridine- and rhodanine derivatives (Figure 8), demonstrated significant inhibitory properties but their intrinsic antibacterial action was significant as well.
Figure 8.
Four NorA EPIs found by the use of virtual screening and their EPIs potency to inhibit EtBr efflux in SA1199B strain [27].
3.6. PSSRI-Based EPIs of NorA and MepA in S. aureus
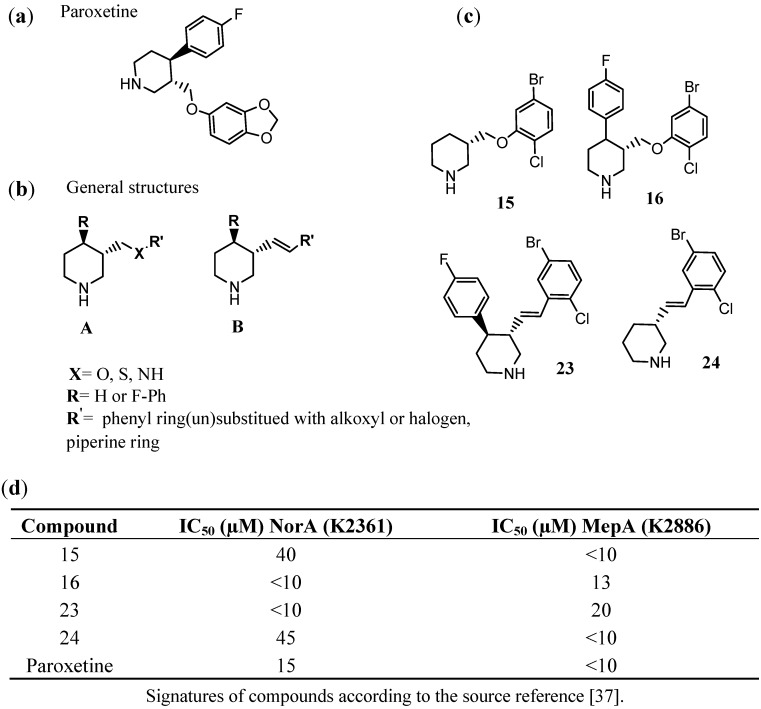
Phenylpiperidine selective serotonin reuptake inhibitors (PSSRIs) are able to block the function of some MDR efflux pumps. Paroxetine (Figure 9a) was one of the first identified PSSRI that inhibits both NorA- (MFS family) and MepA (MATE)-efflux pumps. On the basis of this structure, further chemical modifications were performed (Figure 9b) to search for new and more potent EPIs [35,37]. German et al. [37] obtained interesting results when replaced benzo[d] [1,3] dioxole moiety of paroxetine with 2-chloro-5-bromo-phenyl fragment (16, Figure 9c) and exchanged the phenoxyl fragment (ether linker) into arylidene one (23, Figure 9c). Both compounds (16, 23) displayed an increase of efflux inhibition of NorA comparing to that of paroxetine. A deletion of fluorophenyl substituent was also profitable to give selective EPIs properties for MepA (15 and 24, Figure 9c,d).
Figure 9.
Phenylpiperidine selective serotonin reuptake inhibitor (PSSRI)-based EPIs of S. aureus. (a) Paroxetine; (b) general structures (A and B) of the investigated piperidine derivatives; (c) the most promising EPIs found within the assays (15, 16, 23, 24) and their EPIs potency, expressed as IC50 values for inhibition of NorA (SA-K2361) or MepA (SA-K2886) in comparison to those of paroxetine [35,37].
3.7. Search for Inhibitors of Other Efflux Pumps of Gram-Positive Bacteria
Although most of the studies on efflux pump inhibitors for MDR Gram-positive bacteria are dedicated to NorA in S. aureus, a few lines of evidence are focused on other protein targets as well. Studies on MepA-inhibitors were mentioned in previous research [35,37]. Furthermore, Okandeji et al. [38] described C-capped dipeptides that inhibited chloramphenicol-specific efflux pumps, cmlR1 and cmlR2, in Streptomyces coelicolor, a strain of Gram-positive bacterium that is relative to the human pathogen M. tuberculosis.
4. Conclusions
An analysis of the results of the search for new efflux pump inhibitors for Gram-positive bacteria which have been performed for last decade indicates that almost all efforts are focused on the NorA efflux pump in S. aureus. Considering the chemical structures of the NorA EPIs that have been identified, it can be observed that the most active agents belong to the families of compounds possessing conjugated double bonds, e.g., chalcones, piperine-like compounds, N-cinnamoylphenalkylamides or citral amide derivatives. Indole-, dihydronaphthyl-, 2-chloro-5-bromo-phenyl- or piperidine moieties seem to be profitable for the EPI properties as well.
To date, no inhibitors of bacterial efflux pumps have been licensed for use in the treatment of bacterial infections in human or veterinary settings, although research continues. As far as Gram-positive bacteria are concerned, none of the efflux pump inhibitors have entered clinical trials yet [69,71]. Nevertheless, in the last decade, studies on MDR Gram-positive EPIs allowed us to identify new active agents of NorA in S. aureus with EPI-potency significantly higher than that of reference inhibitors. The active compounds give a new hope for their future therapeutic usage as antibiotics “adjuvants” and should be a subject of wider investigations, including their pharmacokinetic properties and toxic effects. In particular, further studies should be concentrated on the influence of the NorA agents on eucariotic efflux- and influx transport proteins with a special consideration of human transporters belonging to the ABC or SLC families. As human proteins expelling toxic substances out of tissues display significant similarities to those involved in bacterial MDR, bacterial EPIs probably inhibit human detoxification simultaneously with the inhibition of bacterial MDR. This aspect requires thorough research for each active bacterial EPI that is considered as a future “adjuvant” of antibiotics.
In contrast to NorA inhibitors, a population of EPIs active against other MDR efflux proteins of Gram-positive bacteria, which have been found during the last decade, is very small. These results, together with an increasing knowledge about a variety of efflux pumps that are involved in MDR of Gram-positive pathogens, seem to be an important challenge for current medicinal chemistry. They underline the opinion that, in the future, the search for new EPIs should pay more attention to developing MDR efflux protein targets, including SMR, MATE, ABC or other members of the MFS family.
Acknowledgments
Authors thank Leonard Amaral for invitation to this review. The work was inspired by COST action BM0701.
Conflict of Interest
The authors declare no conflict of interest.
References and Notes
- 1.Fournier Dit Chabert J., Marquez B., Neville L., Joucla L., Broussous S., Bouhours P., David E., Pellet-Rostaing S., Marquet B., Moreau N., et al. Synthesis and evaluation of new arylbenzo[b]thiophene and diarylthiophene derivatives as inhibitors of the NorA multidrug transporter of Staphylococcus aureus. Bioorg. Med. Chem. 2007;15:4482–4497. doi: 10.1016/j.bmc.2007.04.023. [DOI] [PubMed] [Google Scholar]
- 2.Bush K., Miller G.H. Bacterial enzymatic resistance: Beta-lactamases and aminoglycoside-modifying enzymes. Curr. Opin. Microbiol. 1998;1:509–515. doi: 10.1016/S1369-5274(98)80082-9. [DOI] [PubMed] [Google Scholar]
- 3.Sabatini S., Gosetto F., Serritella S., Manfroni G., Tabarrini O., Iraci N., Brincat J.P., Carosati E., Villarini M., Kaatz G.W., et al. Pyrazolo[4,3-c][1,2]benzothiazines 5,5-dioxide: A promising new class of Staphylococcus aureus NorA efflux pump inhibitors. J. Med. Chem. 2012;55:3568–3572. doi: 10.1021/jm201446h. [DOI] [PubMed] [Google Scholar]
- 4.Ruiz J. Mechanisms of resistance to quinolones: Target alterations, decreased accumulation and DNA gyrase protection. J. Antimicrob. Chemother. 2003;51:1109–1117. doi: 10.1093/jac/dkg222. [DOI] [PubMed] [Google Scholar]
- 5.Nikaido H. Molecular basis of bacterial outer membrane permeability revisited. Microbiol. Mol. Biol. Rev. 2003;67:593–656. doi: 10.1128/MMBR.67.4.593-656.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li X.Z., Nikaido H. Efflux-mediated drug resistance in bacteria: An update. Drugs. 2009;69:1555–1623. doi: 10.2165/11317030-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kohler T., Pechere J.C., Plesiat P. Bacterial antibiotic efflux systems of medical importance. Cell Mol. Life Sci. 1999;56:771–778. doi: 10.1007/s000180050024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mahamoud A., Chevalier J., Alibert-Franco S., Kern W.V., Pages J.M. Antibiotic efflux pumps in Gram-negative bacteria: The inhibitor response strategy. J. Antimicrob. Chemother. 2007;59:1223–1229. doi: 10.1093/jac/dkl493. [DOI] [PubMed] [Google Scholar]
- 9.Poole K., Lomovskaya O. Can efflux inhibitors really counter resistance? Drug Discov. Today. 2006;3:145–152. doi: 10.1016/j.ddtec.2006.06.011. [DOI] [Google Scholar]
- 10.Michalet S., Cartier G., David B., Mariotte A.M., Dijoux-franca M.G., Kaatz G.W., Stavri M., Gibbons S. N-Caffeoylphenalkylamide derivatives as bacterial efflux pump inhibitors. Bioorg. Med. Chem. Lett. 2007;17:1755–1758. doi: 10.1016/j.bmcl.2006.12.059. [DOI] [PubMed] [Google Scholar]
- 11.Mahamoud A., Chevalier J., Davin-Regli A., Barbe J., Pages J.M. Quinoline derivatives as promising inhibitors of antibiotic efflux pump in multidrug resistant Enterobacter aerogenes isolates. Curr. Drug Targets. 2006;7:843–847. doi: 10.2174/138945006777709557. [DOI] [PubMed] [Google Scholar]
- 12.Pages J.M., Amaral L., Fanning S. An original deal for new molecule: Reversal of efflux pump activity, a rational strategy to combat gram-negative resistant bacteria. Curr. Med. Chem. 2011;18:2969–2980. doi: 10.2174/092986711796150469. [DOI] [PubMed] [Google Scholar]
- 13.Pages J.M., Amaral L. Mechanisms of drug efflux and strategies to combat them: Challenging the efflux pump of Gram-negative bacteria. Biochim. Biophys. Acta. 2009;1794:826–833. doi: 10.1016/j.bbapap.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 14.Martins M., Dastidar S.G., Fanning S., Kristiansen J.E., Molnar J., Pages J.M., Schelz Z., Spengler G., Viveiros M., Amaral L. Potential role of non-antibiotics (helper compounds) in the treatment of multidrug-resistant Gram-negative infections: Mechanisms for their direct and indirect activities. Int. J. Antimicrob. Agents. 2008;31:198–208. doi: 10.1016/j.ijantimicag.2007.10.025. [DOI] [PubMed] [Google Scholar]
- 15.Nikaido H., Pages J.M. Broad-specificity efflux pumps and their role in multidrug resistance of Gram-negative bacteria. FEMS Microbiol. Rev. 2012;36:340–363. doi: 10.1111/j.1574-6976.2011.00290.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bolla J.M., Alibert-Franco S., Handzlik J., Chevalier J., Mahamoud A., Boyer G., Kiec-Kononowicz K., Pages J.M. Strategies for bypassing the membrane barrier in multidrug resistant Gram-negative bacteria. FEBS Lett. 2011;585:1682–1690. doi: 10.1016/j.febslet.2011.04.054. [DOI] [PubMed] [Google Scholar]
- 17.Handzlik J., Szymanska E., Chevalier J., Otrebska E., Kiec-Kononowicz K., Pages J.M., Alibert S. Amine-alkyl derivatives of hydantoin: New tool to combat resistant bacteria. Eur. J. Med. Chem. 2011;46:5807–5816. doi: 10.1016/j.ejmech.2011.09.032. [DOI] [PubMed] [Google Scholar]
- 18.Handzlik J., Szymanska E., Alibert S., Chevalier J., Otrebska E., Pekala E., Pages J.M., Kiec-Kononowicz K. Search for new tools to combat Gram-negative resistant bacteria among amine derivatives of 5-arylidenehydantoin. Bioorg. Med. Chem. 2013;21:135–145. doi: 10.1016/j.bmc.2012.10.053. [DOI] [PubMed] [Google Scholar]
- 19.Pradel E., Pages J.M. The AcrAB-TolC efflux pump contributes to multidrug resistance in the nosocomial pathogen Enterobacter aerogenes. Antimicrob. Agents Chemother. 2002;46:2640–2643. doi: 10.1128/AAC.46.8.2640-2643.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ghisalberti D., Masi M., Pages J.M., Chevalier J. Chloramphenicol and expression of multidrug efflux pump in Enterobacter aerogenes. Biochem. Biophys. Res. Commun. 2005;328:1113–1118. doi: 10.1016/j.bbrc.2005.01.069. [DOI] [PubMed] [Google Scholar]
- 21.Lavigne J.P., Sotto A., Nicolas-Chanoine M.H., Bouziges N., Bourg G., Davin-Regli A., Pages J.M. Membrane permeability, a pivotal function involved in antibiotic resistance and virulence in Enterobacter aerogenes clinical isolates. Clin. Microbiol. Infect. 2012;18:539–545. doi: 10.1111/j.1469-0691.2011.03607.x. [DOI] [PubMed] [Google Scholar]
- 22.Askoura M., Mottawea W., Abujamel T., Taher I. Efflux pump inhibitors (EPIs) as new antimicrobial agents against Pseudomonas aeruginosa. Libyan J. Med. 2011;6 doi: 10.3402/ljm.v6i0.5870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Drew D., Klepsch M.M., Newstead S., Flaig R., De Gier J.W., Iwata S., Beis K. The structure of the efflux pump AcrB in complex with bile acid. Mol. Membr. Biol. 2008;25:677–682. doi: 10.1080/09687680802552257. [DOI] [PubMed] [Google Scholar]
- 24.Sennhauser G., Bukowska M.A., Briand C., Grutter M.G. Crystal structure of the multidrug exporter MexB from Pseudomonas aeruginosa. J. Mol. Biol. 2009;389:134–145. doi: 10.1016/j.jmb.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 25.Pages J.M., Sandrine A.F., Mahamoud A., Bolla J.M., Davin-Regli A., Chevalier J., Garnotel E. Efflux pumps of gram-negative bacteria, a new target for new molecules. Curr. Top. Med. Chem. 2010;10:1848–1857. doi: 10.2174/156802610793176620. [DOI] [PubMed] [Google Scholar]
- 26.Ambrus J.I., Kelso M.J., Bremner J.B., Ball A.R., Casadei G., Lewis K. Structure-activity relationships of 2-aryl-1H-indole inhibitors of the NorA efflux pump in Staphylococcus aureus. Bioorg. Med. Chem. Lett. 2008;18:4294–4297. doi: 10.1016/j.bmcl.2008.06.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brincat J.P., Carosati E., Sabatini S., Manfroni G., Fravolini A., Raygada J.L., Patel D., Kaatz G.W., Cruciani G. Discovery of novel inhibitors of the NorA multidrug transporter of Staphylococcus aureus. J. Med. Chem. 2011;54:354–365. doi: 10.1021/jm1011963. [DOI] [PubMed] [Google Scholar]
- 28.Holler J.G., Slotved H.C., Molgaard P., Olsen C.E., Christensen S.B. Chalcone inhibitors of the NorA efflux pump in Staphylococcus aureus whole cells and enriched everted membrane vesicles. Bioorg. Med. Chem. 2012;20:4514–4521. doi: 10.1016/j.bmc.2012.05.025. [DOI] [PubMed] [Google Scholar]
- 29.Thota N., Koul S., Reddy M.V., Sangwan P.L., Khan I.A., Kumar A., Raja A.F., Andotra S.S., Qazi G.N. Citral derived amides as potent bacterial NorA efflux pump inhibitors. Bioorg. Med. Chem. 2008;16:6535–6543. doi: 10.1016/j.bmc.2008.05.030. [DOI] [PubMed] [Google Scholar]
- 30.Thota N., Reddy M.V., Kumar A., Khan I.A., Sangwan P.L., Kalia N.P., Koul J.L., Koul S. Substituted dihydronaphthalenes as efflux pump inhibitors of Staphylococcus aureus. Eur. J. Med. Chem. 2010;45:3607–3616. doi: 10.1016/j.ejmech.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 31.Samosorn S., Bremner J.B., Ball A., Lewis K. Synthesis of functionalized 2-aryl-5-nitro-1H-indoles and their activity as bacterial NorA efflux pump inhibitors. Bioorg. Med. Chem. 2006;14:857–865. doi: 10.1016/j.bmc.2005.09.019. [DOI] [PubMed] [Google Scholar]
- 32.Sangwan P.L., Koul J.L., Koul S., Reddy M.V., Thota N., Khan I.A., Kumar A., Kalia N.P., Qazi G.N. Piperine analogs as potent Staphylococcus aureus NorA efflux pump inhibitors. Bioorg. Med. Chem. 2008;16:9847–9857. doi: 10.1016/j.bmc.2008.09.042. [DOI] [PubMed] [Google Scholar]
- 33.Nargotra A., Sharma S., Koul J.L., Sangwan P.L., Khan I.A., Kumar A., Taneja S.C., Koul S. Quantitative structure activity relationship (QSAR) of piperine analogs for bacterial NorA efflux pump inhibitors. Eur. J. Med. Chem. 2009;44:4128–4135. doi: 10.1016/j.ejmech.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 34.Sabatini S., Gosetto F., Manfroni G., Tabarrini O., Kaatz G.W., Patel D., Cecchetti V. Evolution from a natural flavones nucleus to obtain 2-(4-Propoxyphenyl)quinoline derivatives as potent inhibitors of the S. aureus NorA efflux pump. J. Med. Chem. 2011;54:5722–5736. doi: 10.1021/jm200370y. [DOI] [PubMed] [Google Scholar]
- 35.Wei P., Kaatz G.W., Kerns R.J. Structural differences between paroxetine and femoxetine responsible for differential inhibition of Staphylococcus aureus efflux pumps. Bioorg. Med. Chem. Lett. 2004;14:3093–3097. doi: 10.1016/j.bmcl.2004.04.018. [DOI] [PubMed] [Google Scholar]
- 36.Abulrob A.N., Suller M.T., Gumbleton M., Simons C., Russell A.D. Identification and biological evaluation of grapefruit oil components as potential novel efflux pump modulators in methicillin-resistant Staphylococcus aureus bacterial strains. Phytochemistry. 2004;65:3021–3027. doi: 10.1016/j.phytochem.2004.08.044. [DOI] [PubMed] [Google Scholar]
- 37.German N., Kaatz G.W., Kerns R.J. Synthesis and evaluation of PSSRI-based inhibitors of Staphylococcus aureus multidrug efflux pumps. Bioorg. Med. Chem. Lett. 2008;18:1368–1373. doi: 10.1016/j.bmcl.2008.01.014. [DOI] [PubMed] [Google Scholar]
- 38.Okandeji B.O., Greenwald D.M., Wroten J., Sello J.K. Synthesis and evaluation of inhibitors of bacterial drug efflux pumps of the major facilitator superfamily. Bioorg. Med. Chem. 2011;19:7679–7689. doi: 10.1016/j.bmc.2011.10.011. [DOI] [PubMed] [Google Scholar]
- 39.Jarmula A., Oblak E., Wawrzycka D., Gutowicz J. Efflux-mediated antimicrobial multidrug resistance. Postepy Hig. Med. Dosw. (Online) 2011;65:216–227. doi: 10.5604/17322693.937011. [DOI] [PubMed] [Google Scholar]
- 40.Li X.Z., Nikaido H. Efflux-mediated drug resistance in bacteria. Drugs. 2004;64:159–204. doi: 10.2165/00003495-200464020-00004. [DOI] [PubMed] [Google Scholar]
- 41.Tseng T.T., Gratwick K.S., Kollman J., Park D., Nies D.H., Goffeau A., Saier M.H., Jr. The RND permease superfamily: An ancient, ubiquitous and diverse family that includes human disease and development proteins. J. Mol. Microbiol. Biotechnol. 1999;1:107–125. [PubMed] [Google Scholar]
- 42.Truong-Bolduc Q.C., Dunman P.M., Strahilevitz J., Projan S.J., Hooper D.C. MgrA is a multiple regulator of two new efflux pumps in Staphylococcus aureus. J. Bacteriol. 2005;187:2395–2405. doi: 10.1128/JB.187.7.2395-2405.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.DeMarco C.E., Cushing L.A., Frempong-Manso E., Seo S.M., Jaravaza T.A., Kaatz G.W. Efflux-related resistance to norfloxacin, dyes, and biocides in bloodstream isolates of Staphylococcus aureus. Antimicrob. Agents Chemother. 2007;51:3235–3239. doi: 10.1128/AAC.00430-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huang J., O'Toole P.W., Shen W., Amrine-Madsen H., Jiang X., Lobo N., Palmer L.M., Voelker L., Fan F., Gwynn M.N., et al. Novel chromosomally encoded multidrug efflux transporter MdeA in Staphylococcus aureus. Antimicrob. Agents Chemother. 2004;48:909–917. doi: 10.1128/AAC.48.3.909-917.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kaatz G.W., McAleese F., Seo S.M. Multidrug resistance in Staphylococcus aureus due to overexpression of a novel multidrug and toxin extrusion (MATE) transport protein. Antimicrob. Agents Chemother. 2005;49:1857–1864. doi: 10.1128/AAC.49.5.1857-1864.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Piddock L.J. Clinically relevant chromosomally encoded multidrug resistance efflux pumps in bacteria. Clin. Microbiol. Rev. 2006;19:382–402. doi: 10.1128/CMR.19.2.382-402.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pozzi G., Iannelli F., Oggioni M.R., Santagati M., Stefani S. Genetic elements carrying macrolide efflux genes in streptococci. Curr. Drug Targets Infect. Disord. 2004;4:203–206. doi: 10.2174/1568005043340641. [DOI] [PubMed] [Google Scholar]
- 48.Reynolds E., Ross J.I., Cove J.H. Msr(A) and related macrolide/streptogramin resistance determinants: incomplete transporters? Int. J. Antimicrob. Agents. 2003;22:228–236. doi: 10.1016/S0924-8579(03)00218-8. [DOI] [PubMed] [Google Scholar]
- 49.Portillo A., Ruiz-Larrea F., Zarazaga M., Alonso A., Martinez J.L., Torres C. Macrolide resistance genes in Enterococcus spp. Antimicrob. Agents Chemother. 2000;44:967–971. doi: 10.1128/AAC.44.4.967-971.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schwarz S., Kehrenberg C., Doublet B., Cloeckaert A. Molecular basis of bacterial resistance to chloramphenicol and florfenicol. FEMS Microbiol. Rev. 2004;28:519–542. doi: 10.1016/j.femsre.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 51.Robertson G.T., Doyle T.B., Lynch A.S. Use of an efflux-deficient streptococcus pneumoniae strain panel to identify ABC-class multidrug transporters involved in intrinsic resistance to antimicrobial agents. Antimicrob. Agents Chemother. 2005;49:4781–4783. doi: 10.1128/AAC.49.11.4781-4783.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yoshida H., Bogaki M., Nakamura S., Ubukata K., Konno M. Nucleotide sequence and characterization of the Staphylococcus aureus norA gene, which confers resistance to quinolones. J. Bacteriol. 1990;172:6942–6949. doi: 10.1128/jb.172.12.6942-6949.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pumbwe L., Piddock L.J. Identification and molecular characterisation of CmeB, a Campylobacter jejuni multidrug efflux pump. FEMS Microbiol. Lett. 2002;206:185–189. doi: 10.1111/j.1574-6968.2002.tb11007.x. [DOI] [PubMed] [Google Scholar]
- 54.Kehrenberg C., Schwarz S. fexA, a novel Staphylococcus lentus gene encoding resistance to florfenicol and chloramphenicol. Antimicrob. Agents Chemother. 2004;48:615–618. doi: 10.1128/AAC.48.2.615-618.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Borges-Walmsley M.I., McKeegan K.S., Walmsley A.R. Structure and function of efflux pumps that confer resistance to drugs. Biochem. J. 2003;376:313–338. doi: 10.1042/BJ20020957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Borges-Walmsley M.I., Walmsley A.R. The structure and function of drug pumps. Trends Microbiol. 2001;9:71–79. doi: 10.1016/S0966-842X(00)01920-X. [DOI] [PubMed] [Google Scholar]
- 57.Paulsen I.T., Brown M.H., Skurray R.A. Proton-dependent multidrug efflux systems. Microbiol. Rev. 1996;60:575–608. doi: 10.1128/mr.60.4.575-608.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Poole K. Efflux-mediated antimicrobial resistance. J. Antimicrob. Chemother. 2005;56:20–51. doi: 10.1093/jac/dki171. [DOI] [PubMed] [Google Scholar]
- 59.Markham P.N., Neyfakh A.A. Efflux-mediated drug resistance in Gram-positive bacteria. Curr. Opin. Microbiol. 2001;4:509–514. doi: 10.1016/S1369-5274(00)00243-5. [DOI] [PubMed] [Google Scholar]
- 60.Kikukawa T., Nara T., Araiso T., Miyauchi S., Kamo N. Two-component bacterial multidrug transporter, EbrAB: Mutations making each component solely functional. Biochim. Biophys. Acta. 2006;1758:673–679. doi: 10.1016/j.bbamem.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 61.Putman M., van Veen H.W., Konings W.N. Molecular properties of bacterial multidrug transporters. Microbiol. Mol. Biol. Rev. 2000;64:672–693. doi: 10.1128/MMBR.64.4.672-693.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wasaznik A., Grinholc M., Bielawski K.P. Active efflux as the multidrug resistance mechanism. Postepy Hig. Med. Dosw. (Online) 2009;63:123–133. [PubMed] [Google Scholar]
- 63.Omote H., Hiasa M., Matsumoto T., Otsuka M., Moriyama Y. The MATE proteins as fundamental transporters of metabolic and xenobiotic organic cations. Trends Pharmacol. Sci. 2006;27:587–593. doi: 10.1016/j.tips.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 64.Van Bambeke F., Balzi E., Tulkens P.M. Antibiotic efflux pumps. Biochem. Pharmacol. 2000;60:457–470. doi: 10.1016/S0006-2952(00)00291-4. [DOI] [PubMed] [Google Scholar]
- 65.Kumar A., Schweizer H.P. Bacterial resistance to antibiotics: Active efflux and reduced uptake. Adv. Drug Deliv. Rev. 2005;57:1486–1513. doi: 10.1016/j.addr.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 66.Lubelski J., Konings W.N., Driessen A.J. Distribution and physiology of ABC-type transporters contributing to multidrug resistance in bacteria. Microbiol. Mol. Biol. Rev. 2007;71:463–476. doi: 10.1128/MMBR.00001-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zgurskaya H.I., Nikaido H. Multidrug resistance mechanisms: Drug efflux across two membranes. Mol. Microbiol. 2000;37:219–225. doi: 10.1046/j.1365-2958.2000.01926.x. [DOI] [PubMed] [Google Scholar]
- 68.Wang K., Pei H., Huang B., Zhu X., Zhang J., Zhou B., Zhu L., Zhang Y., Zhou F.F. The Expression of ABC Efflux Pump, Rv1217c-Rv1218c, and Its Association with Multidrug Resistance of Mycobacterium tuberculosis in China. Curr. Microbiol. 2013;66:222–226. doi: 10.1007/s00284-012-0215-3. [DOI] [PubMed] [Google Scholar]
- 69.Zechini B., Versace I. Inhibitors of multidrug resistant efflux systems in bacteria. Recent. Pat. Antiinfect. Drug Discov. 2009;4:37–50. doi: 10.2174/157489109787236256. [DOI] [PubMed] [Google Scholar]
- 70.Holler J.G., Christensen S.B., Slotved H.C., Rasmussen H.B., Guzman A., Olsen C.E., Petersen B., Molgaard P. Novel inhibitory activity of the Staphylococcus aureus NorA efflux pump by a kaempferol rhamnoside isolated from Persea lingue Nees. J. Antimicrob. Chemother. 2012;67:1138–1144. doi: 10.1093/jac/dks005. [DOI] [PubMed] [Google Scholar]
- 71.ClinicalTrials.gov. A service of the US National Institutes of Health. [(accessed on 31 January 2013)]. Available online: www.clinicaltrials.gov.